Abstract
The mechanistic understanding of the physiology and interactions of microorganisms in starter cultures is critical for the targeted improvement of fermented milk products, such as yogurt, which is produced by Streptococcus thermophilus in co-culture with Lactobacillus delbrueckii subsp. bulgaricus. However, the use of complex growth media or milk is a major challenge for quantifying metabolite production, consumption, and exchange in co-cultures. This study developed a synthetic medium that enables the establishment of defined culturing conditions and the application of flow cytometry for measuring species-specific biomass values. Time courses of amino acid concentrations in mono-cultures and co-cultures of L. bulgaricus ATCC BAA-365 with the proteinase-deficient S. thermophilus LMG 18311 and with a proteinase-positive S. thermophilus strain were determined. The analysis revealed that amino acid release rates in co-culture were not equivalent to the sum of amino acid release rates in mono-cultures. Data-driven and pH-dependent amino acid release models were developed and applied for comparison. Histidine displayed higher concentrations in co-cultures, whereas isoleucine and arginine were depleted. Amino acid measurements in co-cultures also confirmed that some amino acids, such as lysine, are produced and then consumed, thus being suitable candidates to investigate the inter-species interactions in the co-culture and contribute to the required knowledge for targeted shaping of yogurt qualities.
Keywords: microbial interactions, co-culture, Lactobacillus bulgaricus, Streptococcus thermophilus, milk, amino acid metabolism, metabolite exchange, flow cytometry, pH-dependent modeling, proteolytic activity
1. Introduction
Dairy products have been a part of the human diet since ancient times [1]. Detailed identification and analysis of fermented milk products began in the twentieth century [2]. Efforts are ongoing to develop tools to examine lactic acid bacteria [3,4,5,6]. Yogurt, which is currently an important part of the cuisine of many cultures, will be a critical dietary component in the future. Therefore, the identification and determination of novel co-culture compositions that impart improved technological and organoleptic properties are active areas of research in the food industry [7]. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus are the key species that drive yogurt production [2].
To meet the changing market demands, there is a need to understand the interaction between S. thermophilus and L. bulgaricus during milk fermentation and to make use of this knowledge to design improved food products [8]. Despite significant progress in the past, the current state of understanding still shows white spots [2].
In the last 15 years, metabolomics [9,10] and transcriptomics [11,12,13] have been widely applied to understand the physiology of S. thermophilus and L. bulgaricus in mono-culture and co-culture. Previous studies provide insights into the metabolites exchanged between the strains and elucidated the characteristic gene expression patterns. However, these datasets have provided a limited scope to assign contextual functionalities to metabolites [12,13,14].
Screening various combinations of S. thermophilus and L. bulgaricus strains in co-cultures is a time-consuming and costly process. Thus, only a small subset of all possible combinations and conditions has been investigated. To overcome this limitation, mathematical modeling approaches, such as community flux balance analysis, have been used to predict the performance of co-cultures [15]. Although the mathematical modeling approach enables the estimation of flux distributions in underdetermined systems, a minimum number of experimental measurements is required to limit the solution space. Additionally, the stoichiometry of interactions must be understood for the application of mathematical approaches. Both constraints require reliable and representative experimental datasets as a prerequisite for flux balance modeling [16].
Understanding of the complex metabolic interactions between S. thermophilus and L. bulgaricus, including the exchange of peptides and amino acids, is currently limited [2]. One key feature is the strong proteolytic activity of L. bulgaricus, which enhances the production of peptides and amino acids that become available for S. thermophilus, enabling growth [13]. However, some S. thermophilus strains exhibit proteolytic activity. Consequently, the question that arises is whether and what differences in this inter-species interaction exist when proteolytic and non-proteolytic S. thermophilus are combined with L. bulgaricus in co-cultures.
Acidification, a marker for lactic acid formation, may serve as an easy-to-follow readout once mono-cultures and co-cultures can be cultured under comparable conditions. Limited information is available on amino acid production and consumption [9] and potential amino acid depletion, which may trigger amino acid biosynthesis [12,13].
Milk is traditionally used as a growth medium for S. thermophilus and L. bulgaricus cultivations in the production of yogurt. S. thermophilus and L. bulgaricus produce lactic acid from lactose, which imparts an acidic taste and inhibits the growth of microbes, including S. thermophilus and L. bulgaricus [17,18]. However, milk composition is highly variable. Furthermore, milk comprises several complex ingredients that interfere with the sensitivity of analytical methods, such as high-performance liquid chromatography (HPLC) and mass spectrometry. Additionally, the acidification of milk leads to an increase in viscosity, which impairs the sensitivity of the analytical methods [19].
To overcome these intrinsic analytical barriers, this study developed a synthetic medium supplemented with amino acids (SMaa) to allow the growth of S. thermophilus and L. bulgaricus in mono-cultures, which enabled the analysis of individual growth characteristics. The synthetic medium may be supplemented with casein (SMcas) instead of amino acids to investigate the proteolytic abilities of S. thermophilus and L. bulgaricus in mono-cultures. The medium allows for investigation of the interactions between S. thermophilus and L. bulgaricus by excluding individual components that are likely to be exchanged. An important effect of the symbiotic relationship between S. thermophilus and L. bulgaricus is the faster acidification during milk fermentation [13]. Therefore, this study investigated this feature by co-cultivating the strains in SMcas.
This study presents a new medium and comparable datasets of S. thermophilus and L. bulgaricus in mono-culture and co-culture conditions, providing useful insights into essential amino acid production and consumption. Our results demonstrate that the patterns and levels of amino acid release and consumption in co-cultures are different from those of mono-cultures. These findings are essential for data-driven modeling and testing hypotheses on the induction of basic regulatory mechanisms in cells.
2. Materials and Methods
2.1. Strains and Cultivation Conditions
Lactobacillus delbrueckii subsp. bulgaricus strains (LB.1 = ATCC BAA-365, LB.2, LB.3, and LB.4) were provided by Chr. Hansen A/S and stored at −70 °C in Man–Rogosa–Sharpe (MRS) (69966 MRS Broth, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) containing 20% (v/v) glycerol. For cultivation, the total cell suspension in the cryotube (1 mL) was transferred into 15 mL of MRS supplemented with 14.3 g L–1 lactose and incubated for 6–8 h at 40 °C [20,21,22,23]. After washing twice with 0.9% NaCl solution, the cell pellet was resuspended in 200 µL of 0.9% NaCl to inoculate the preculture containing SMaa. The preculture was cultured at 40 °C and gently stirred with a 10 mm magnetic bar at 400 rpm for 14–18 h until the pH was between 5 and 6.
Streptococcus thermophilus strains (ST.1, ST.2, ST.3, and ST.4 = LMG 18311) were provided by the industrial partner (Chr. Hansen) and stored at −70 °C in M17 (56156 M-17 Broth, Sigma-Aldrich Chemie GmbH, Steinheim, DE, USA) containing 20% (v/v) glycerol. The cells in the cryotube were washed twice with 0.9% NaCl solution. Then, the cell pellet was resuspended in 200 µL of 0.9% NaCl to inoculate the preculture containing SMaa. The preculture was cultured at 40 °C and gently stirred with a 10 mm magnetic bar at 400 rpm for 2–6 h until the pH was between 5 and 6.
Calculated amounts of biomass from L. bulgaricus and S. thermophilus precultures were washed twice with 0.9% NaCl solution and the cell pellets were resuspended in 200 µL 0.9% NaCl to inoculate the main culture. The main culture was carried out in SMaa or SMcas as indicated in Table 1.
Table 1.
Composition of synthetic medium (SM).
| Category | Compound | Concentration [g L−1] | CAS Number |
|---|---|---|---|
| - | Di-potassium hydrogen phosphate | 2.5 | 7758-11-4 |
| Potassium dihydrogen phosphate | 3 | 7778-77-0 | |
| Sodium acetate | 1 | 127-09-3 | |
| Ammonium citrate tribasic | 0.6 | 3458-72-8 | |
| Manganese sulfate monohydrate | 0.02 | 10034-96-5 | |
| Iron(II) sulfate heptahydrate | 0.00132 | 7782-63-0 | |
| Calcium chloride dihydrate | 0.08745 | 10035-04-8 | |
| Tween 80 | 1 mL L−1 | 9005-65-6 | |
| D-Lactose monohydrate | 15.75 | 10039-26-6 | |
| Magnesium sulfate heptahydrate | 0.2 | 10034-99-8 | |
| Urea | 0.12 | 57-13-6 | |
| nucleobases | Adenine | 0.01 | 73-24-5 |
| Guanine | 0.01 | 73-40-5 | |
| Uracil | 0.01 | 66-22-8 | |
| Xanthine | 0.01 | 69-89-6 | |
| vitamins | Biotin | 0.0002 | 58-85-5 |
| Folic acid | 0.0002 | 59-30-3 | |
| Pyridoxal hydrochloride | 0.001 | 65-22-5 | |
| Riboflavin | 0.0005 | 83-88-5 | |
| Thiamine chloride hydrochloride | 0.0005 | 67-03-8 | |
| Nicotinamide | 0.0005 | 98-92-0 | |
| Cyanocobalamin | 0.0005 | 68-19-9 | |
| 4-Aminobenzoic acid | 0.0005 | 150-13-0 | |
| D-Pantothenic acid hemicalcium salt | 0.004 | 137-08-6 | |
| DL-6,8-thioctic acid | 0.0005 | 1077-28-7 | |
| trace elements | Ammonium molybdate tetrahydrate | 0.0000037 | 12054-85-2 |
| Cobalt(II) chloride hexahydrate | 0.000007 | 7791-13-1 | |
| Boric acid | 0.000025 | 10043-35-3 | |
| Copper(II) sulfate pentahydrate | 0.0000025 | 7758-99-8 | |
| Zinc sulfate heptahydrate | 0.0000029 | 7446-20-0 | |
| amino acids | L-Alanine | 0.1 | 56-41-7 |
| L-Arginine | 0.317 | 74-79-3 | |
| L-Asparagine monohydrate | 0.343 | 5794-13-8 | |
| L-Aspartic acid | 0.499 | 56-84-8 | |
| L-Cysteine hydrochloride monohydrate | 0.3 | 7048-04-6 | |
| L-Glutamic acid | 0.331 | 56-86-0 | |
| L-Glutamine | 0.29 | 56-85-9 | |
| Glycine | 0.16 | 56-40-6 | |
| L-Histidine monohydrochloride monohydrate | 0.273 | 5934-29-2 | |
| L-Isoleucine | 0.361 | 73-32-5 | |
| L-Leucine | 0.6 | 61-90-5 | |
| L-Lysine | 0.351 | 56-87-1 | |
| L-Methionine | 0.119 | 63-68-3 | |
| L-Phenylalanine | 0.34 | 63-91-2 | |
| L-Proline | 0.921 | 147-85-3 | |
| L-Serine | 0.359 | 56-45-1 | |
| L-Threonine | 0.3 | 72-19-5 | |
| L-Tryptophan | 0.102 | 73-22-3 | |
| L-Tyrosine | 0.12 | 60-18-4 | |
| L-Valine | 0.468 | 72-18-4 | |
| casein | Casein | 2 | 9005-46-3 |
The SM contains all listed compounds, except amino acids and casein. SM supplemented with amino acids (SMaa) contains all listed compounds, except casein. SM supplemented with casein (SMcas) contains all listed compounds, except amino acids.
The preculture (SMaa) and main culture (SMaa or SMcas) were cultured in crimp-top serum bottles, which were pretreated by flushing with 80% N2 and 20% CO2 for 10 min at 400 rpm. Growth was monitored by measuring the optical density (OD) (λ = 600 nm) using a photometer (Amersham Bioscience, Ultrospec 10 cell density meter) or flow cytometry.
2.2. Acidification Measurements
The pH was measured offline using a pH meter (SevenEasyTM, Mettler Toledo, Columbus, OH, USA) connected to a pH electrode (InLab Semi-Micro, Mettler Toledo, Columbus, OH, USA).
2.3. Medium Preparation
2.3.1. Complex Media
MRS (69966 MRS Broth, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was dissolved in Milli-Q water and the pH of the medium was adjusted to 6.5 using 2 M NaOH. Then, the medium was filtered using a 0.22-μm filter (ROTILABO®, PVD, Carl Roth GmbH & Co. KG, Karlsruhe, Germany) and sterile polysorbate 80 (CAS-Nr.: 9005-65-6, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was added according to the manufacturer’s instructions.
M17 (56156 M17 Broth, Sigma-Aldrich Chemie GmbH, Steinheim, DE, USA) was prepared following the manufacturer’s instructions and autoclaved.
2.3.2. Semi-Synthetic Medium
A sterile 5× basal solution containing di-potassium hydrogen phosphate, potassium dihydrogen phosphate, sodium acetate, ammonium citrate, manganese sulfate, iron(II) sulfate, and Tween 80 was prepared as indicated in Table 1. Sterile lactose, magnesium sulfate, urea, nucleobases, and amino acids were added to the solution. After the pH was set to 6.5 with 1 M HCl, trace elements, vitamins, calcium chloride, and casein were added. The serum bottle was sealed, crimped, and flushed with sterile 80% N2 and 20% CO2 for 10 min at 400 rpm.
The casein stock solution was prepared in a beaker containing glass beads (3 mm in diameter), which were covered with a thin layer of 200 µL of Tween 80. Next, 100 mL of water containing 0.26 g L−1 CaCl2 was added, and the solution was stirred slowly overnight, followed by autoclaving for 5 min at 121 °C.
2.4. Cell Dry Weight (DW)
A glass vial (1 mL, VWR) was dried at 105 °C for at least 36 h, cooled at 20 °C for at least 1 h, and weighed. Aliquots of 1 mL of culture samples in SMaa were washed thrice with Milli-Q water (40 °C) in a 1.5-mL reaction tube (Eppendorf), resuspended in 300 µL of Milli-Q water, and transferred into a dried glass vial. The reaction tube was rinsed with 200 µL of Milli-Q water, and the water was transferred to the glass vial. The glass vial was dried at 105 °C for at least 36 h, cooled at 20 °C overnight in a desiccator, and weighed to calculate the cell dry weight. The correlation between optical density, flow cytometry data (events mL−1), and cell dry weight (gDW L−1) was as follows: for LB.1, 1 gDW L−1 = 0.17101671 × 10−7 * events mL−1 = 0.2527 × OD600 nm; for ST.1, 1 gDW L−1 = 0.01970622 × 10−7 * events mL−1 = 0.2075 × OD600 nm; for ST.4. 1 gDW L−1 = 0.043115 × 10−7 * events mL−1 = 0.243 × OD600 nm.
2.5. Biomass Measurements Using Flow Cytometry
Samples for flow cytometry analysis were prepared as described previously [3]. The cell suspension (100 µL) was diluted 10-fold with Tris-HCl (1.3 M) EDTA (0.13 M) buffer (pH 8) and incubated for 10 min on a shaker (Eppendorf Thermomixer 5436, Hamburg, Germany) at 1200 rpm and 50 °C. Next, the cell suspension was incubated with 1× SYBR™Green I nucleic acid gel stain concentrate (Thermo Fisher Scientific, Waltham, MA, USA) for at least 10 min at 20 °C in the dark. The sample was filtered through a filter (Partec CellTrics® 30 µM mesh filter size, Sysmex, Germany) into a polystyrene tube immediately before measurements and analyzed using a flow cytometer (BD Accuri™ C6; BD Bioscience, Franklin Lakes, NJ, USA) equipped with four fluorescence detectors (FL1 533/30 nm, FL2 585/40 nm, FL3 > 670 nm, and FL4 675/25 nm), two scatter detectors, a blue laser (488 nm), and a red laser (640 nm). Sterile Milli-Q water was used as the sheath fluid. The instrument performance was monitored weekly with BDTM CS&T RUO Beads. The threshold settings, FSC-H 500 and FL1-H 500, a limit of 25 μL, and the slow flow rate of 14 μL/min were used for the analysis of the samples.
The log-transformed FL1-A and FSC-H signals were used to enumerate the total number of events in a sample. The flow cytometry data of the first 10,000 events of the pure medium sample were used for a one-class support vector machine (SVM) classifier implemented in MATLAB® using the command ‘fitcsvm’ to identify and remove signal from medium in samples. Additionally, the lower background data were removed using a linear line as the gate, resulting in a cleaned dataset. Linear correlations between cleaned flow cytometric data and the dry weight of cells cultured in SMaa were fitted to the measured data from LB.1, ST1, and ST.4 cultures (Figure S8). To determine the transferability of the linear correlation between flow cytometric data and cell dry weight from cells cultured in SMaa to cells cultured in SMcas, a 1:1 mixture (v/v) of both samples was prepared and measured using flow cytometry. Additionally, each sample was individually analyzed using flow cytometry. The calculated sum of the number of cell events cultured in SMaa and the number of cell events cultured in SMcas resulted in the same number of cell events in the measured mixture, indicating transferability (Figure S8).
Cell dry weight in co-cultures was calculated using the same method with determined transferability (Figure S9). The strain-specific cell events of S. thermophilus and L. bulgaricus in co-culture were estimated using manual classification or SVM classification depending on the pH of the sample (Figure S10). Manual classification was achieved by separating the flow cytometry data using a line (the log-transformed FSC-H signal was plotted against the log-transformed FL1-A signal and separated by a linear line). The data points above and below the line represent L. bulgaricus and S. thermophilus, respectively. Classification of strains in co-culture using SVM was achieved using the log-transformed FSC-H and FL1-A signals of mono-culture datasets. Background data were removed to optimize SVM parameters in MATLAB® using the command ‘fitcsm’ (Figure S11).
2.6. Quantification of Fermentation Products
The culture sample (0.5 mL) was centrifuged for 3 min at 20,000× g and 4 °C. The supernatant was stored at −70 °C.
Sugars (lactose, glucose, galactose) and organic acids (lactate, succinate, formate) were quantified using the Agilent 1200 series HPLC system equipped with an RI detector [24]. Before analysis, the supernatant was incubated with 4 M NH3 and 1.2 M MgSO4 solutions, followed by an incubation for 15 min with 0.1 M H2SO4 to precipitate phosphate. Isocratic separation was achieved using a Rezex ROA organic acid H (8%) column (300 × 7.8 mm, 8 μm; Phenomenex) protected by a Phenomenex guard carbo-H column (4 × 3.0 mm) at 50 °C. The HPLC conditions were as follows: mobile phase, 5 mM H2SO4 solution; constant flow rate, 0.4 mL min−1. Absolute concentrations were obtained by standard-based external calibration, and rhamnose was used as an internal standard (1 g L–1) to correct measurement variability.
Amino acid concentrations were determined by an Agilent 1200 series instrument (Agilent Technologies) [24]. Bicratic separation was achieved by an Agilent Zorbax Eclipse Plus C18 column (250 by 4.6 mm, 5 µm), which was protected by an Agilent Zorbax Eclipse Plus C18 guard column (12.5 by 4.6 mm, 5 µm). After automatic precolumn derivatization with ortho-phthaldialdehyde, fluorometric detection (excitation at 230 nm and emission at 450 nm) was carried out. The elution buffer consisted of a polar phase (10 mM Na2HPO4, 10 mM Na2B4O7, 0.5 mM NaN3, pH 8.2) and a nonpolar phase (45% [v/v] acetonitrile, 45% [v/v] methanol). The quantification of amino acids was achieved by standard-based external calibration, and 4-aminobutanoic acid was used as an internal standard at 100 µM to correct for analyte variability.
2.7. Total Amino Acid Composition in the Supernatant
The culture sample (0.3 mL) was centrifuged for 3 min at 20,000× g and 4 °C. The supernatant was stored at −70 °C. The supernatant (200 µL) was incubated with 300 µL of 32% HCl at 100 °C for 24 h, cooled at 20 °C for at least 1 h, slowly mixed with 490 µL of 6.23 M NaOH, and stored at −20 °C until quantification of amino acid concentrations by HPLC analysis.
2.8. Calculation of Amino Acid Production Rates
Individual biomass-specific amino acid production rates qaa [mol gDW−1 h−1] were calculated for each amino acid in a differential manner at 1 h intervals. The average biomass cx [gDW L−1] in the period Δt [h], and the net amount of produced amino acids Δcaa [mol L−1] (Equation (1)) were considered.
| (1) |
2.9. Fitting of Gaussian Models to pH-Dependent Amino Acid Production Rate
The release of amino acids strongly relies on enzymatic proteolysis. As the proteolytic activity depends on various enzymes with each contributing to an individual optimum pH [25,26], integral activities may be described by the superposition of Gaussian activity distributions. However, exact values for pH optima were not available. Additionally, de novo biosynthesis may occur, albeit to a minor extent. Consequently, the Gaussian model was considered a suitable proxy for the observed amino acid ‘production’ profiles. Parameter regression was achieved by fitting the pH-dependent qaa of the L. bulgaricus LB.1 mono-culture (Figure S13) using Equation (2) [27].
| (2) |
where qaa is the amino acid production rate [mol gDW−1 h−1]; n is the number of pH optima to fit; and a, b, and c are regression parameters coding for the shape of the curve. MATLAB ® was used for fitting. The consideration of a single pH dependency is not always sufficient. Then, overlaying Gaussian models considering two pH optima were used to improve the model prediction quality (Figure S13).
2.10. Simulation of Amino Acid Concentrations
Changes of biomass, substrate, and product concentrations were described in a process model assuming batch operation modes by balancing biomass (Equation (3)), substrate (Equation (4)), and product (Equation (5)) within the system boundary.
| (3) |
| (4) |
| (5) |
The amino acid production kinetics were integrated into the process model to predict caa(t). The simulation time steps Δt considered the mean pH and biomass values as indicated in Equation (6).
| (6) |
The feasibility of this approach was demonstrated for the mono-culture of L. bulgaricus LB.1 (Figure S12).
2.11. Uncertainty Analysis
Metabolite concentrations, pH, OD, flow cytometric data, and dry weight values were analyzed using Microsoft® Excel. Mean and standard deviation were calculated using duplicates and triplicates (STABW.S) in Microsoft® Excel. All experimental results are expressed as the mean of three biological replicates with experimental errors unless otherwise stated.
3. Results
3.1. Medium Development
The main objectives for preparing the SMcas were as follows: (a) enabling the growth of both species in mono-culture, (b) enabling the growth of both species in co-culture, and (c) potential metabolites that may be exchanged [2,3,6,10,12,13,14,28,29] were excluded if growth was not affected. To obtain this medium, previously reported defined growth medium compositions of S. thermophilus [30,31] and L. bulgaricus [21,32] were compiled, resulting in a long list of constituents. This list was further reduced to achieve a lean growth medium to fulfil the demands (a–c). Medium acidification, which mirrors growth-coupled lactate formation, was used as a readout to verify the ability of the strains to grow with different modifications in the medium. Oleic acid, pyruvic acid, formic acid, orotic acid, niacin, spermine, ascorbic acid, thioglycolate, and 2′-deoxyguanosine, which were used in the growth medium by Chervaux et al. [32] but not by Grobben et al. [21], were excluded from the medium because they are not essential for the growth of L. bulgaricus. Additionally, we evaluated whether the addition of orotic acid is essential since it was considered to be an important component of the growth medium by Otto et al. [30] and Letort et al. [31]. Growth analysis of L. bulgaricus and S. thermophilus in the medium lacking orotic acid revealed culture acidification. The omission of biotin, thiamine, aminobenzoic acid, and thioctic acid did not result in the acidification in S. thermophilus culture but promoted the acidification in L. bulgaricus culture. Furthermore, urea was not excluded from the medium because it has previously been established that it increases the buffer capacity of the medium [31] and provides carbon dioxide and ammonia [3].
Studies using SMcas revealed the ability of three proteinase-positive S. thermophilus (ST.1, ST.2, and ST.3) strains and the four L. bulgaricus strains to acidify the medium. The proteinase-negative S. thermophilus ST.4 was not able to acidify SMcas and required access to free amino acids provided in SMaa (Figure S1).
Protocooperation between L. bulgaricus and S. thermophilus in co-culture has industrial relevance [2]. Co-culture benefits from the rapid exchange of metabolites, leading to accelerated acidification [13]. The effect of this protocooperation in the co-culture was observed in SMcas in the form of a faster acidification rate and a lower final pH (Figure S2).
3.2. Growth and Amino Acid Release in L. bulgaricus Mono-Culture
L. bulgaricus hydrolyzes amino acids from casein through its cell wall proteinase PrtB, which is complemented by other intracellular and extracellular peptidase activities [12,13,33,34]. Therefore, peptides and free amino acids can be utilized by S. thermophilus. Furthermore, amino acid depletion may upregulate amino acid biosynthesis in co-cultures [12,13]. Hence, a key step in understanding cellular responses to extracellular amino acid depletion is to monitor amino acid release and uptake.
L. bulgaricus LB.1 was cultured in SMcas as a mono-culture. The biomass of the culture increased from 0.05 to 0.6 gDW L–1, whereas the pH decreased from 6.4 to 4.3 (Figure 1). Lactose was consumed, glucose was initially secreted (up to 1.4 mM) and then consumed, and galactose, lactate, formate, and succinate were produced (Figure S7) in the culture, indicating metabolic activity.
Figure 1.
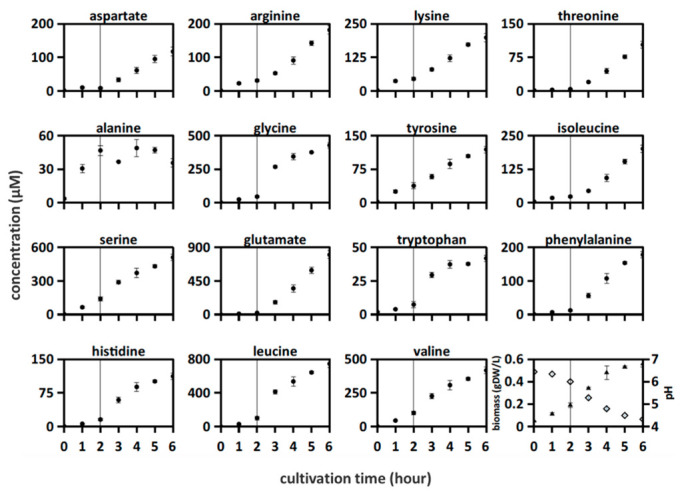
Amino acid concentrations were measured in Lactobacillus bulgaricus LB.1 culture in synthetic medium supplemented with casein (SMcas). The line indicates a change in increasing amino acid concentration profiles after 2 h. Downright: biomass (triangle) and pH (rhomb) measurements.
The following two patterns of amino acid release were observed (Figure 1): accumulation of alanine, serine, lysine, tyrosine, and valine from the beginning of culturing; other amino acids began to increase after 2 h. A previous study suggested that this lag time indicates cellular adaptation to casein through upregulation of proteolytic activity [9]. The initial release of tyrosine, arginine, serine, leucine, and valine indicates active proteolytic activity from the beginning of culturing as they might not be produced de novo from L. bulgaricus [13,35].
3.3. Growth and Amino Acid Release in Proteinase-Positive S. thermophilus Mono-Culture
The dynamics of amino acid release and uptake in the proteinase-positive S. thermophilus ST.1, amino acid concentrations were measured over a culturing period of 14 h (Figure 2). The following three distinct phases were identified: 0–5 h, increase of some amino acid concentrations but no change in biomass and pH; 5–10 h, acidification, biomass increase, and decrease of some amino acid concentrations while others kept increasing; 10–15 h, acidification, biomass decrease, and uptake and release of amino acids. The concentration of all analyzed amino acids increased at some time point. Additionally, the pH decreased from 6.6 to 4.7, whereas the biomass increased from 0.03 gDW L−1 to 0.1 gDW L−1 (Figure 2). Furthermore, 12 out of the 15 amino acids were consumed at some points in time. Moreover, the concentrations of some amino acids exhibited an oscillating release-consumption-release profile (e.g., serine and leucine). After 12 h, almost all lactose was consumed (30 mM), which was accompanied by the production of large amounts of glucose (22 mM) and lactate (30 mM) (Figure S3).
Figure 2.
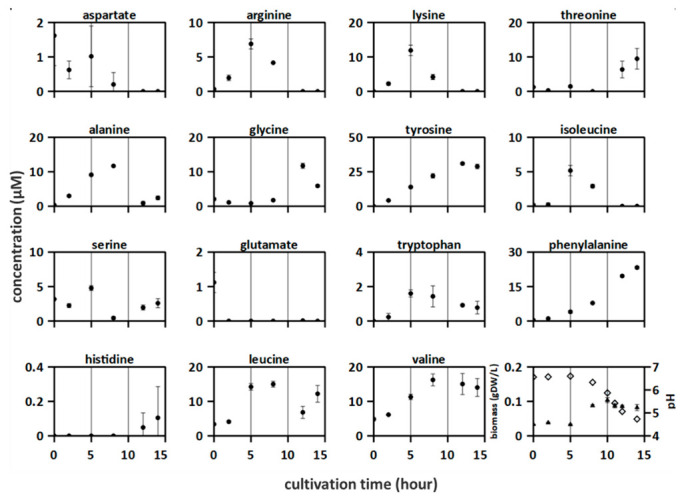
Amino acid concentrations were measured in proteinase-positive S. thermophilus ST.1 culture in synthetic medium supplemented with casein (SMcas). The lines indicate three phases according to the growth. Downright: biomass (triangle) and pH (rhomb) measurements.
3.4. Growth and Amino Acid Release in the Co-Culture of Proteinase-Positive S. thermophilus and L. bulgaricus
Next, the amino acid concentrations in an L. bulgaricus LB.1—proteinase-positive S. thermophilus ST.1 co-culture were examined. The strains could grow in both SMcas (Figure 1 and Figure 2) and SMaa (Figures S4 and S6), indicating their ability to utilize casein and free amino acids. As shown in Figure 3, the concentration of all amino acids increased during cultivation at some point. The concentrations of aspartate, arginine, lysine, alanine, and isoleucine began to decrease after approximately 2 h. Meanwhile, the decrease in glycine concentration was delayed until 4 h. The following two phases were observed in amino acid release (Figure 3), growth, and acidification (Figure 4): 0–4 h, pH decreased from 6.4 to 4.7 while the growth of both strains was weak (Figure 4); 4–7 h, the biomass of L. bulgaricus increased from 0.05 gDW L−1 to 0.22 gDW L−1. Additionally, the consumption of 30 mM lactose, the production of 57 mM lactate, and the secretion (up to 10 mM) and uptake of glucose were observed (Figure S5).
Figure 3.
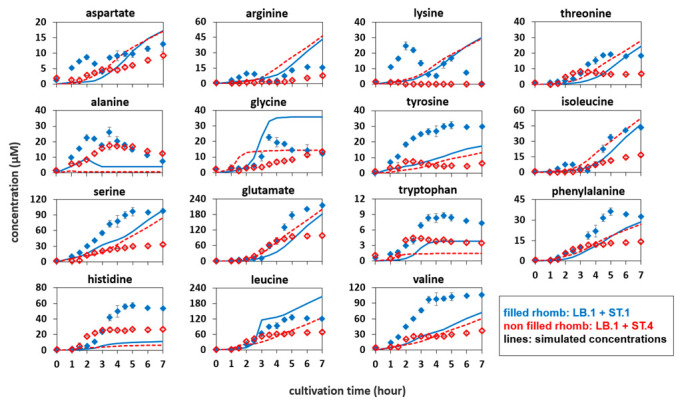
Amino acid concentrations in different co-cultures. (filled) Lactobacillus bulgaricus LB.1 co-cultured with proteinase-positive Streptococcus thermophilus ST.1 in synthetic medium supplemented with casein (SMcas). (non-filled) L. bulgaricus LB.1 co-cultured with proteinase-negative S. thermophilus ST.4 in SMcas. (line) Simulated amino acid concentration released from L. bulgaricus LB.1 in LB.1–ST.1 co-culture. (dashed line) Simulated amino acid concentration released from L. bulgaricus LB.1 in LB.1–ST.4 co-culture.
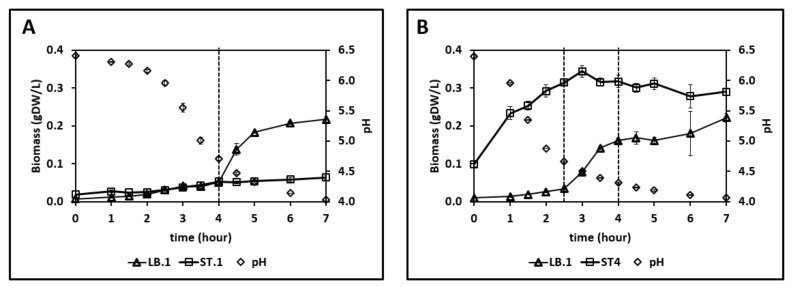
Figure 4.
Strain-specific biomass profiles measured by flow cytometry and pH measurements in (A) LB.1–ST.1 (initial biomass fraction of 1:2 (LB:ST)) and (B) LB.1–ST.4 (initial biomass fraction 1:10 (LB:ST)) co-cultures in synthetic medium supplemented with casein (SMcas).
3.5. Growth and Amino Acid Release in the Co-Culture of Proteinase-Negative S. thermophilus and L. bulgaricus
Next, the effects of replacement of proteinase-positive S. thermophiles ST.1 with proteinase-negative S. thermophilus ST.4 on the amino acid availability and the nutrient needs in the co-culture with L. bulgaricus LB.1 were examined. ST.4 could not grow in SMcas but could grow in SMaa (Figures S4 and S6). Therefore, a higher biomass fraction of S. thermophilus ST.4 was inoculated to avoid the anticipated overgrow of L. bulgaricus.
Figure 4B shows the following three phases: 0–2.5 h, increased biomass of S. thermophilus ST.4; 2.5–4 h, dominant growth of L. bulgaricus LB.1; 4–7 h, decreased biomass of S. thermophilus ST.4 even as L. bulgaricus LB.1 continued to grow. Hence, the presence of L. bulgaricus LB.1 enables the growth of S. thermophilus ST.4 in SMcas, which is consistent with previous findings [12]. Additionally, 25 mM of lactose was consumed and 58 mM of lactate was produced (Figure S5). Interestingly, lactose consumption severely slowed down after the growth stop of ST.4, while lactate formation continued. Furthermore, the concentrations of arginine (0–5 h), isoleucine (0–3 h), and lysine (0–7 h) decreased. Overall, the amino acid concentration in the proteinase-negative S. thermophilus ST.4—L. bulgaricus co-culture was lower than that in the proteinase-positive S. thermophilus ST.1—L. bulgaricus LB.1 co-culture.
3.6. Simulation of Amino Acid Concentrations to Compare Mono- and Co-Culture Cultivations
To indicate the changes in the amino acid profile when S. thermophilus was added to the L. bulgaricus culture, a Gaussian model of amino acid release dependent on pH and biomass was generated (see Methods). This model enables the simulation of the amount of amino acids released solely from L. bulgaricus in co-culture, which could not be identified in the mixed culture. Hence, the comparison between the simulation and measured data will indicate if the amino acid release activity differs between mono-culture and co-culture.
Amino acid profiles of L. bulgaricus mono-culture (Figure 1) were used to fit the Gaussian qaa models. Figure 3 compares the simulated amino acid profiles of L. bulgaricus with the measured amino acid profiles of the co-cultures, reflecting the results of the mixed culture interaction.
Generally, the amino acid concentrations in the proteinase-positive S. thermophilus ST.1—L. bulgaricus co-culture were higher than those in the simulated amino acid time courses of L. bulgaricus in mono-culture, with the exception of glycine and leucine.
By way of analogy, Figure 3 shows the difference between the measured amino acid concentrations in the S. thermophilus ST.4—L. bulgaricus co-culture and the simulated amino acid concentrations released from L. bulgaricus. Here, most of the measured amino acid profiles, except for alanine, tryptophan, and histidine, were lower than those of the simulated courses. This indicates increased uptake of amino acids, likely via the proteinase-negative S. thermophilus ST.4, which can only feed on amino acids and peptides released from L. bulgaricus but not from casein.
4. Discussion
4.1. Amino Acids Are Consumed by L. bulgaricus and S. thermophilus
In this study, amino acids were consumed by L. bulgaricus and S. thermophilus cultured in SMcas in both mono-culture (Figure 1 and Figure 2) and co-culture (Figure 3). This is in accordance with [22]. Amino acids were consumed even in the presence of peptide-bound amino acids (Table S1). For example, lysine was consumed in the S. thermophilus ST.1—L. bulgaricus LB.1 co-culture after 4 h (Figure 3), although at least 230 µM of lysine bound to proteins and peptides was available (Table S1).
This indicates that amino acid transporters are active and enable the strains to exchange amino acids that are produced through casein hydrolysis or biosynthesis [36,37]. Hence, it allows interaction [29,38,39]. Additionally, this enables the manipulation of S. thermophilus and L. bulgaricus cultivations in biotechnological processes by adding amino acids, such as lysine [40].
4.2. Amino Acids Can Accumulate in Cultivations with L. bulgaricus and S. thermophilus
L. bulgaricus LB.1 could accumulate all analyzed amino acids (Figure 1). Some of these amino acids accumulated from the beginning of culturing, indicating basal proteolytic activity although the strain was precultured under SMaa conditions. This suggests that L. bulgaricus LB.1 releases more amino acids from casein or/and produces amino acids than it is needed for growth and that amino acids become available for other strains [41]. The accumulation of amino acids indicates that extracellular peptidases are highly active [42], unusable amino acids are separated from peptides to gain posteriorly required amino acids, or proton-coupled amino acid secretion supports the maintenance of intracellular pH during acidification [43]. The poor release of amino acids in a S. thermophilus ST.1 cultivation reflects its low activity of peptidases [26,44].
4.3. Differences between Co-Cultures with Different S. thermophilus Strains
The proteinase-negative S. thermophilus ST.4—L. bulgaricus LB.1 co-culture yielded lower amino acid concentrations than the proteinase-positive S. thermophilus ST.1—L. bulgaricus LB.1 co-culture. This phenotype can be attributed to the increased growth of S. thermophilus ST.4 (Figure 4), which results in an enhanced demand for amino acids [45]. In addition, this observation is consistent with the lack of protease activity of S. thermophilus ST.4 (Figure 3). The depletion of arginine, lysine, and isoleucine observed in this study can upregulate peptidases or amino acid biosynthesis, which is consistent with the hypothesis of previous studies [9,12,13].
4.4. Co-Culture Is Not the Sum of Mono-Cultures
The proteinase-positive S. thermophilus ST.1—L. bulgaricus LB.1 co-culture yielded higher amino acid concentrations than the simulated concentration of amino acids released from only L. bulgaricus LB.1 (Figure 3). In particular, histidine was rarely released in the presumably histidine auxotroph S. thermophilus ST.1 mono-culture (Figure 2) [46] but was detected in high amounts in the S. thermophilus ST.1—L. bulgaricus LB.1 co-culture. The interaction between the two species may trigger metabolic changes in the strains, resulting in the rearrangement of metabolic fluxes [6,35,47]. Future studies must identify these co-culture triggers that serve as stimuli for basic metabolic adjustments.
The amount of amino acid released from the co-culture was higher than the individual sums of the amounts of amino acid released from the mono-cultures. This might be a consequence of an upregulated proteolytic system in L. bulgaricus LB.1 and S. thermophilus ST.1. Alternatively, individual biosynthetic pathways might be stimulated in co-culture but not in mono-culture [46,48]. Previous studies have alluded to the upregulation of histidine biosynthesis [12,13].
4.5. Stimulatory Effects of Branched-Chain Amino Acid (BCAA) Depletion
Previous studies have hypothesized that BCAA availability is limited in the S. thermophilus—L. bulgaricus co-cultures due to the upregulation of BCAA permease in L. bulgaricus [13] and BCAA biosynthesis in S. thermophilus [12,13,49]. In this study, the levels of isoleucine, but not those of valine or leucine, were temporarily depleted in the co-cultures (Figure 3). Furthermore, the release of BCAA in the L. bulgaricus LB.1 mono-culture was similar to that reported in a previous study [9], which revealed that the proteolytic activity of L. bulgaricus promotes the excess release of BCAA from casein. In the LB.1 mono-culture, the final concentration of isoleucine (200 µM) was lower than that of valine (417 µM) and leucine (746 µM). This indicated isoleucine as a potential candidate for depletion. Additionally, low concentrations of isoleucine (up to 5 µM), leucine (up to 15 µM), and valine (up to 16 µM) were observed in the protease-positive S. thermophilus ST.1 mono-culture, indicating its ability to release BCAA from casein or biosynthesize BCAA [36,46]. However, the levels of isoleucine, leucine, and valine were lower than those in L. bulgaricus. Hence, isoleucine depletion is plausible and may result in the upregulation of BCAA permease in L. bulgaricus and BCAA biosynthesis in S. thermophilus, respectively.
4.6. Arginine and Lysine Depletion in Co-Cultures
Arginine and lysine concentrations were limited in the proteinase-negative S. thermophilus ST.4—L. bulgaricus LB.1 co-culture and oscillated in the proteinase-positive S. thermophilus ST.1—L. bulgaricus LB.1 co-culture (Figure 3). Previous studies [12,13] have reported the upregulation of arginine biosynthesis in S. thermophilus co-cultured with L. bulgaricus. Hence, our results support the hypothesis that low arginine concentrations might influence physiological responses [50], such as the upregulation of arginine biosynthesis in S. thermophilus.
5. Conclusions
In this work, we developed a synthetic medium that supports the growth of the dairy organisms S. thermophilus and L. bulgaricus in mono- and co-culture, which enables the quantitative monitoring of growth as well as substrate consumption and metabolite production dynamics. Amino acid release profiles in co-culture were not the sum of amino acid release profiles in mono-cultures. Additionally, the amino acid release profiles were not similar in co-cultures with different strain combinations. Amino acid depletion was observed in S. thermophilus—L. bulgaricus co-cultures, which may provide an explanation for the induced expression of proteolytic enzymes.
The uptake of several amino acids was observed during growth. Knowledge of co-culture-specific consumption rates for peptide and amino acid uptake along with release rates of amino acids provides a tool for determining yogurt quality and useful insights into cellular fitness for further strain and process optimization. Understanding cellular amino acid needs may enable a quantitative and detailed understanding of interactions in yogurt cultures.
Acknowledgments
The authors thank the ERA CoBioTech for funding of the “YogurtDesign” project, and the members of the “YogurtDesign” consortium for productive collaboration and fruitful discussions (Bas Teusink, Julia Lischke, Sebastian Mendoza Farias, Ursula Kummer, Ana Sofia Figueiredo, Tamara Bendig, Petri-Jaan Lahtvee, Regina Maruste, Gintare Liudziute, Ana Rute Neves), as well as Attila Teleki, Martina Schweikert, Alexander Dietrich, and Marion Fleischer for technical support.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms10091771/s1: Table S1: Concentrations of amino acids bound to extracellular peptides; Figure S1: Acidification of medium; Figure S2: Acidification of synthetic medium supplemented with casein (SMcas); Figure S3: Extracellular metabolite concentrations in ST.1 culture; Figure S4: Biomass and pH; Figure S5: Extracellular metabolite concentrations in co-cultures; Figure S6: Amino acid concentration in co-cultures; Figure S7: Extracellular metabolite concentrations in LB.1 culture; Figure S8: Correlations between flow cytometric data and cell dry weight; Figure S9: Enumeration of total cell events; Figure S10: Classification of flow cytometric data; Figure S11: Code for support vector machine (SVM) training; Figure S12: Amino acid concentrations in LB.1 culture; Figure S13: Fitted Gaussian model for aspartate; Figure S14: Fitted Gaussian model for glutamate; Figure S15: Fitted Gaussian model for serine; Figure S16: Fitted Gaussian model for histidine; Figure S17: Fitted Gaussian model for glycine; Figure S18: Fitted Gaussian model for threonine; Figure S19: Fitted Gaussian model for arginine; Figure S20: Fitted Gaussian model for alanine; Figure S21: Fitted Gaussian model for tyrosine; Figure S22: Fitted Gaussian model for valine; Figure S23: Fitted Gaussian model for tryptophan; Figure S24: Fitted Gaussian model for phenylalanine; Figure S25: Fitted Gaussian model for isoleucine; Figure S26: Fitted Gaussian model for leucine; Figure S27: Fitted Gaussian model for lysine.
Author Contributions
Funding acquisition, R.T.; investigation, A.U. and F.E.; flow cytometry methodology, A.U., S.M. and S.W.; HPLC analysis, M.L.; medium development, A.U., M.L., M.L.J., P.G. and A.A.Z.; simulation, A.U.; visualization, A.U.; supervision, A.A.Z. and R.T.; writing—original draft, A.U.; writing—review and editing, A.U., P.G., A.A.Z. and R.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Bundesministerium für Bildung und Forschung BMBF (Funding Number: 031B0596B).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marco M.L., Heeney D., Binda S., Cifelli C.J., Cotter P.D., Foligné B., Gänzle M., Kort R., Pasin G., Pihlanto A., et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Markakiou S., Gaspar P., Johansen E., Zeidan A.A., Neves A.R. Harnessing the Metabolic Potential of Streptococcus thermophilus for New Biotechnological Applications. Curr. Opin. Biotechnol. 2020;61:142–152. doi: 10.1016/j.copbio.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Arioli S., della Scala G., Remagni M.C., Stuknyte M., Colombo S., Guglielmetti S., de Noni I., Ragg E., Mora D. Streptococcus thermophilus Urease Activity Boosts Lactobacillus delbrueckii subsp. Bulgaricus Homolactic Fermentation. Int. J. Food Microbiol. 2017;247:55–64. doi: 10.1016/j.ijfoodmicro.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Blasche S., Kim Y., Mars R.A.T., Machado D., Maansson M., Kafkia E., Milanese A., Zeller G., Teusink B., Nielsen J., et al. Metabolic Cooperation and Spatiotemporal Niche Partitioning in a Kefir Microbial Community. Nat. Microbiol. 2021;6:196–208. doi: 10.1038/s41564-020-00816-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schöpping M., Gaspar P., Neves A.R., Fanzén C.J., Zeidan A.A. Identifying the Essential Nutritional Requirements of the the Probiotic Bacteria Bifidobacterium animalis and Bifidobacterium longum Using Genome-Scale Modeling. NPJ Syst. Biol. Appl. 2021;7:1–15. doi: 10.1038/s41540-021-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Settachaimongkon S., Nout M.J.R., Antunes Fernandes E.C., Hettinga K.A., Vervoort J.M., van Hooijdonk T.C.M., Zwietering M.H., Smid E.J., van Valenberg H.J.F. Influence of Different Proteolytic Strains of Streptococcus thermophilus in Co-Culture with Lactobacillus delbrueckii subsp. Bulgaricus on the Metabolite Profile of Set-Yoghurt. Int. J. Food Microbiol. 2014;177:29–36. doi: 10.1016/j.ijfoodmicro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Johansen E. Use of Natural Selection and Evolution to Develop New Starter Cultures for Fermented Foods. Annu. Rev. Food Sci. Technol. 2018;9:411–428. doi: 10.1146/annurev-food-030117-012450. [DOI] [PubMed] [Google Scholar]
- 8.Chandan R.C., Gandhi A., Shah N.P. Yogurt: Historical Background, Health Benefits, and Global Trade. Elsevier Inc.; Amsterdam, The Netherlands: 2017. [DOI] [Google Scholar]
- 9.Liu E., Zheng H., Hao P., Konno T., Kume H., Oda M., Suzuki K., Ji Z. Acquisition of Amino Acids by Lactobacillus delbrueckii subsp. Bulgaricus 2038 When Grown in the Presence of Casein. Int. Dairy J. 2014;35:145–152. doi: 10.1016/j.idairyj.2013.11.006. [DOI] [Google Scholar]
- 10.de Souza Oliveira R.P., Torres B.R., Perego P., de Oliveira M.N., Converti A. Co-Metabolic Models of Streptococcus thermophilus in Co-Culture with Lactobacillus bulgaricus or Lactobacillus acidophilus. Biochem. Eng. J. 2012;62:62–69. doi: 10.1016/j.bej.2012.01.004. [DOI] [Google Scholar]
- 11.Herve-Jimenez L., Guillouard I., Guedon E., Gautier C., Boudebbouze S., Hols P., Monnet V., Rul F., Maguin E. Physiology of Streptococcus thermophilus during the Late Stage of Milk Fermentation with Special Regard to Sulfur Amino-Acid Metabolism. Proteomics. 2008;8:4273–4286. doi: 10.1002/pmic.200700489. [DOI] [PubMed] [Google Scholar]
- 12.Herve-Jimenez L., Guillouard I., Guedon E., Boudebbouze S., Hols P., Monnet V., Maguin E., Rul F. Postgenomic Analysis of Streptococcus thermophilus Cocultivated in Milk with Lactobacillus delbrueckii subsp. Bulgaricus: Involvement of Nitrogen, Purine, and Iron Metabolism. Appl. Environ. Microbiol. 2009;75:2062–2073. doi: 10.1128/AEM.01984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sieuwerts S., Molenaar D., van Hijum S.A.F.T., Beerthuyzen M., Stevens M.J.A., Janssen P.W.M., Ingham C.J., de Bok F.A.M., de Vos W.M., van Hylckama Vlieg J.E.T. Mixed-Culture Transcriptome Analysis Reveals the Molecular Basis of Mixed-Culture Growth in Streptococcus thermophilus and Lactobacillus bulgaricus. Appl. Environ. Microbiol. 2010;76:7775–7784. doi: 10.1128/AEM.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendes F., Sieuwerts S., de Hulster E., Almering M.J.H., Luttik M.A.H., Pronk J.T., Smid E.J., Bron P.A., Daran-Lapujadea P. Transcriptome-Based Characterization of Interactions between Saccharomyces cerevisiae and Lactobacillus delbrueckii subsp. Bulgaricus in Lactose-Grown Chemostat Cocultures. Appl. Environ. Microbiol. 2013;79:5949–5961. doi: 10.1128/AEM.01115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branco dos Santos F., de Vos W.M., Teusink B. Towards Metagenome-Scale Models for Industrial Applications-the Case of Lactic acid bacteria. Curr. Opin. Biotechnol. 2013;24:200–206. doi: 10.1016/j.copbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Somerville V., Grigaitis P., Battjes J., Moro F., Teusink B. Use and Limitations of Genome-Scale Metabolic Models in Food Microbiology. Curr. Opin. Food Sci. 2022;43:225–231. doi: 10.1016/j.cofs.2021.12.010. [DOI] [Google Scholar]
- 17.Chen S., Niu H., Wu Y., Sun J., Han X., Zhang L. Influence of Lactic Acid on Cell Cycle Progressions in Lactobacillus bulgaricus During Batch Culture. Appl. Biochem. Biotechnol. 2021;193:912–924. doi: 10.1007/s12010-020-03459-8. [DOI] [PubMed] [Google Scholar]
- 18.Russell J.B., Diez-Gonzalez F. The Effects of Fermentation Acids on Bacterial Growth. Adv. Microb. Physiol. 1998;39:228–234. doi: 10.1016/s0065-2911(08)60017-x. [DOI] [PubMed] [Google Scholar]
- 19.Douwenga S., Janssen P., Teusink B. A Centrifugation-Based Clearing Method Allows High-Throughput Acidification and Growth-Rate Measurements in Milk. J. Dairy Sci. 2021;8:8530–8540. doi: 10.3168/jds.2020-20108. [DOI] [PubMed] [Google Scholar]
- 20.Radke-Mitchell L., Sandine W.E. Associative Growth and Differential Enumeration of Streptococcus thermophilus and Lactobacillus bulgaricus: A Review. J. Food Prot. 1984;47:245–248. doi: 10.4315/0362-028X-47.3.245. [DOI] [PubMed] [Google Scholar]
- 21.Grobben G.J., Sikkema J., Smith M.R., de Bont J.A.M. Production of Extracellular Polysaccharides by Lactobacillus delbrueckii ssp. Bulgaricus NCFB 2772 Grown in a Chemically Defined Medium. J. Appl. Bacteriol. 1995;79:103–107. doi: 10.1111/j.1365-2672.1995.tb03130.x. [DOI] [Google Scholar]
- 22.Liu E., Zheng H., Shi T., Ye L., Konno T., Oda M., Shen H., Ji Z.S. Relationship between Lactobacillus bulgaricus and Streptococcus thermophilus under Whey Conditions: Focus on Amino Acid Formation. Int. Dairy J. 2016;56:141–150. doi: 10.1016/j.idairyj.2016.01.019. [DOI] [Google Scholar]
- 23.Radke-Mitchell L.C., Sandine W.E. Influence of Temperature on Associative Growth of Streptococcus thermophilus and Lactobacillus bulgaricus. J. Dairy Sci. 1986;69:2558–2568. doi: 10.3168/jds.S0022-0302(86)80701-9. [DOI] [PubMed] [Google Scholar]
- 24.Buchholz J., Schwentner A., Brunnenkan B., Gabris C., Grimm S., Gerstmeir R., Takors R., Eikmanns B.J., Blombacha B. Platform Engineering of Corynebacterium glutamicum with Reduced Pyruvate Dehydrogenase Complex Activity for Improved Production of L-Lysine, l-Valine, and 2-Ketoisovalerate. Appl. Environ. Microbiol. 2013;79:5566–5575. doi: 10.1128/AEM.01741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunji E.R.S. The Proteolytic Systems of Lactic acid bacteria. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Serrano G.M., García-Garibay J.M., Cruz-Guerrero A.E., Gómez-Ruiz L.D.C., Ayala-Niño A., Castañeda-Ovando A., González-Olivares L.G. Proteolytic System of Streptococcus thermophilus. J. Microbiol Biotechnol. 2018;28:1581–1588. doi: 10.4014/jmb.1807.07017. [DOI] [PubMed] [Google Scholar]
- 27.Hetényi K., Németh Á., Sevella B. Role of PH-Regulation in Lactic Acid Fermentation: Second Steps in a Process Improvement. Chem. Eng. Processing: Process Intensif. 2011;50:293–299. doi: 10.1016/j.cep.2011.01.008. [DOI] [Google Scholar]
- 28.Crittenden R.G., Martinez N.R., Playne M.J. Synthesis and Utilisation of Folate by Yoghurt Starter Cultures and Probiotic Bacteria. Int. J. Food Microbiol. 2003;80:217–222. doi: 10.1016/S0168-1605(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 29.Sieuwerts S., de Bok F.A.M., Hugenholtz J., van Hylckama Vlieg J.E.T. Unraveling Microbial Interactions in Food Fermentations: From Classical to Genomics Approaches. Appl. Environ. Microbiol. 2008;74:4997–5007. doi: 10.1128/AEM.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto R., Tenbrink B., Veldkamp H., Konings W.N. The Relation between Growth-Rate and Electrochemical Proton Gradient of Streptococcus-Cremoris. FEMS Microbiol. Lett. 1983;16:69–74. doi: 10.1111/j.1574-6968.1983.tb00261.x. [DOI] [Google Scholar]
- 31.Letort C., Juillard V. Development of a Minimal Chemically-Defined Medium for the Exponential Growth of Streptococcus thermophilus. J. Appl. Microbiol. 2001;91:1023–1029. doi: 10.1046/j.1365-2672.2001.01469.x. [DOI] [PubMed] [Google Scholar]
- 32.Chervaux C., Ehrlich S.D., Maguin E. Physiological Study of Lactobacillus delbrueckii subsp. Bulgaricus Strains in a Novel Chemically Defined Medium. Society. 2000;66:5306–5311. doi: 10.1128/AEM.66.12.5306-5311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Courtin P., Monnet V., Rul F. Cell-Wall Proteinases PrtS and PrtB Have a Different Role in Streptococcus thermophilus/Lactobacillus bulgaricus Mixed Cultures in Milk. Microbiology (N Y) 2002;148:3413–3421. doi: 10.1099/00221287-148-11-3413. [DOI] [PubMed] [Google Scholar]
- 34.Liu E., Hao P., Konno T., Yu Y., Oda M., Zheng H., Ji Z. Amino Acid Biosynthesis and Proteolysis in Lactobacillus bulgaricus Revisited: A Genomic Comparison. Computat. Mol. Biosci. 2012;2012:61–77. doi: 10.4236/cmb.2012.23006. [DOI] [Google Scholar]
- 35.van de Guchte M., Penaud S., Grimaldi C., Barbe V., Bryson K., Nicolas P., Robert C., Oztas S., Mangenot S., Couloux A., et al. The Complete Genome Sequence of Lactobacillus bulgaricus Reveals Extensive and Ongoing Reductive Evolution. Proc. Natl. Acad. Sci. 2006;103:9274–9279. doi: 10.1073/pnas.0603024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hols P., Hancy F., Fontaine L., Grossiord B., Prozzi D., Leblond-Bourget N., Decaris B., Bolotin A., Delorme C., Ehrlich S.D., et al. New Insights in the Molecular Biology and Physiology of Streptococcus thermophilus Revealed by Comparative Genomics. FEMS Microbiol. Rev. 2005;29:435–463. doi: 10.1016/j.femsre.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Zheng H., Liu E., Hao P. In Silico Analysis of Amino Acid Biosynthesis and Proteolysis in Lactobacillus delbrueckii subsp. Bulgaricus 2038 and the Implications for Bovine Milk Fermentation. Biotechnol. Lett. 2012;34:1545–1551. doi: 10.1007/s10529-012-1006-4. [DOI] [PubMed] [Google Scholar]
- 38.Smid E.J., Lacroix C. Microbe-Microbe Interactions in Mixed Culture Food Fermentations. Curr. Opin. Biotechnol. 2013;24:148–154. doi: 10.1016/j.copbio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Rau M.H., Gaspar P., Jensen M.L., Geppel A., Neves A.R., Zeidan A.A. Genome-Scale Metabolic Modeling Combined with Transcriptome Profiling Provides Mechanistic Understanding of Streptococcus thermophilus CH8 Metabolism. Appl. Environ. Microbiol. 2022;88:1–18. doi: 10.1128/aem.00780-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y., Li Y. Soybean Peptides Promote Yoghurt Fermentation and Quality. Biotechnol. Lett. 2020;42:1927–1937. doi: 10.1007/s10529-020-02912-2. [DOI] [PubMed] [Google Scholar]
- 41.Kliche T., Li B., Bockelmann W., Habermann D., Klempt M., de Vrese M. Screening for Proteolytically Active Lactic acid bacteria and Bioactivity of Peptide Hydrolysates Obtained with Selected Strains. Appl. Microbiol. Biotechnol. 2017;101:7621–7633. doi: 10.1007/s00253-017-8369-3. [DOI] [PubMed] [Google Scholar]
- 42.Liu E., Zheng H., Hao P., Konno T., Yu Y., Kume H., Oda M., Ji Z.S. A Model of Proteolysis and Amino Acid Biosynthesis for Lactobacillus delbrueckii subsp. Bulgaricus in Whey. Curr. Microbiol. 2012;65:742–751. doi: 10.1007/s00284-012-0214-4. [DOI] [PubMed] [Google Scholar]
- 43.Hutkins R.W., Nannen N.L. pH Homeostasis in Lactic acid bacteria. J. Dairy Sci. 1993;76:2354–2365. doi: 10.3168/jds.S0022-0302(93)77573-6. [DOI] [Google Scholar]
- 44.Rajagopal S.N., Sandine W.E. Associative Growth and Proteolysis of Streptococcus thermophilus and Lactobacillus bulgaricus in Skim Milk. J. Dairy Sci. 1990;73:894–899. doi: 10.3168/jds.S0022-0302(90)78745-0. [DOI] [Google Scholar]
- 45.Beshkova D.M., Simova E.D., Frengova G.I., Simov Z.I., Adilov E.F. Production of Amino Acids by Yogurt Bacteria. Biotechnol. Prog. 1998;14:963–965. doi: 10.1021/bp980082j. [DOI] [PubMed] [Google Scholar]
- 46.Pastink M.I., Teusink B., Hols P., Visser S., de Vos W.M., Hugenholtz J. Genome-Scale Model of Streptococcus thermophilus LMG18311 for Metabolic Comparison of Lactic acid bacteria. Appl. Environ. Microbiol. 2009;75:3627–3633. doi: 10.1128/AEM.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wintermute E.H., Silver P.A. Dynamics in the Mixed Microbial Concourse. Genes Dev. 2010;24:2603–2614. doi: 10.1101/gad.1985210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao P., Zheng H., Yu Y., Ding G., Gu W., Chen S., Yu Z., Ren S., Oda M., Konno T., et al. Complete Sequencing and Pan-Genomic Analysis of Lactobacillus delbrueckii subsp. Bulgaricus Reveal Its Genetic Basis for Industrial Yogurt Production. PLoS ONE. 2011;6:e15964. doi: 10.1371/journal.pone.0015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garault P., Letort C., Juillard V., Monnet V. Branched-Chain Amino Acids and Purine Biosynthesis: Two Pathways Essential for Optimal Growth of Streptococcus thermophilus in Milk. Lait. 2001;81:83–90. doi: 10.1051/lait:2001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arioli S., Roncada P., Salzano A.M., Deriu F., Corona S., Guglielmetti S., Bonizzi L., Scaloni A., Mora D. The Relevance of Carbon Dioxide Metabolism in Streptococcus thermophilus. Microbiology. 2009;155:1953–1965. doi: 10.1099/mic.0.024737-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.