Abstract
Guangdong, Guangxi and Yunnan are the three provinces in China that yield the most brown sugar, a brown-red colored solid or powdered sugar product made from sugar cane. In the present study, the differences between odor compounds of brown sugar from Guangdong, Guangxi, and Yunnan provinces in China were compared and analyzed by gas chromatography-olfactometry-mass spectrometry (GC-O-MS). A total of 80 odor compounds, including 5 alcohols, 9 aldehydes, 8 phenols, 21 acids, 14 ketones, 5 esters, 12 pyrazines, and 6 other compounds, were detected. The fingerprint analysis of the brown sugar odor compounds showed 90% similarity, indicating a close relationship among the odor properties of brown sugar in each province. Moreover, the orthogonal partial least squares discriminant analysis (OPLS-DA) was performed to identify the compounds contributing to the volatile classification of the brown sugar from three provinces, which confirmed that OPLS-DA could be a potential tool to distinguish the brown sugar of three origins.
Keywords: non-centrifugal cane sugar (NCS), GC-O-MS, fingerprint, orthogonal partial least squares discriminant analysis (OPLS-DA)
1. Introduction
Brown sugar, a traditional sweetener with a distinctive flavor, is mainly made from sugarcane through extraction, clarification, and boiling [1]. It is also called non-centrifugal cane sugar (NCS), which does not separate molasses, so it retains the original flavor and nutrients of sugarcane. Brown sugar is rich in flavonoids and phenols that may act as antioxidants and, therefore, exert benefits on organisms [2,3,4]. Furthermore, it exerts immunomodulatory, cytoprotective, anti-carcinogenic, and anti-cancer properties [5].
A study on the physicochemical properties and storage stability of brown sugar revealed darker color, increased water content and water activity, but decreased glucose and fructose contents due to the Maillard reaction [6]. Similarly, a study on the odor components of brown sugar revealed that acetaldehyde, 2-methylbutyraldehyde, 3-methylbutyraldehyde, 2,6-dimethylpyrazine, nonanal, 2,6-diethylpyrazine, 2,3,5-trimethylpyrazine, furfural, 2,3-dimethylpyrazine, decanal, and 2-acetylpyrrole were the primary components based on their relative concentration [7]. Juliana et al. [8] extracted a total of six odor compounds from brown sugar beverages through simultaneous steam distillation-solvent extraction using a mixture of diethyl ether-pentane (1:1, w/w) as the solvent. Of the six components, 2-methylpyrazine was the key aroma compound in this beverage. Our previous research has proved that heating of syrup was the primary production step affecting the brown sugar flavor because of the production of a large number of pyrazine compounds [9].
Brown sugar has a green and a strong caramel aroma. Some aroma compounds are inherent in sugarcane, while others are produced by microbial metabolism and Maillard reaction. Sugarcane varieties, growing regions, processing methods, storage conditions and other factors will affect the flavor of brown sugar [10]. The composition and concentration of odor compounds and nutrients in sugarcane from different producing areas are different, which leads to great differences in the flavor composition of brown sugar. However, it is difficult to distinguish the origin of brown sugars only by sensory evaluation. As an intuitive and reproducible method, GC-MS analysis has been effectively applied in origin differentiation studies [11]. Li et al. [12] and Zhao et al. [13] used GC-MS to analyze the volatile odor compounds of ham and rice, respectively, and the results proved that GC-MS played an important role in food odor analysis and origin identification.
Previous studies on brown sugar mostly focused on the identification of key aroma, and there is no study on the flavor differences of brown sugar in different regions. Guangdong, Guangxi and Yunnan are the three major producing areas of brown sugar in China. To the best of our knowledge, the discrimination of brown sugar according to origin has not been reported previously. Therefore, the purpose of this study is to (1) identify the odor compounds of the 18 brown sugar samples from Guangdong, Guangxi, and Yunnan using LLE/GC-O-MS; (2) determine the key odor compounds in brown sugar by calculating OAV; (3) establish the fingerprints of brown sugar from three different origins and (4) find out the compounds that cause the difference using OPLS, so as to provide the basis for selecting brown sugar from different regions when producing foods with different flavor characteristics.
2. Results and Discussion
2.1. Volatile Aroma Components Analysis
A total of 80 odor compounds, including 5 alcohols, 9 aldehydes, 8 phenols, 21 acids, 14 ketones, 5 esters, 12 pyrazines, and 6 other compounds, were detected in 18 samples from three different regions (Table 1). The brown sugar samples from Guangdong, Guangxi and Yunnan contained 72, 60 and 75 odor compounds, respectively. There are four kinds of alcohols in all three regions, but the types of acid compounds are quite different, with Guangdong and Yunnan containing 20 and 19 acid compounds, respectively, while Guangxi contained only 12 acid compounds. The types of pyrazines, aldehydes, ketones and phenols in the three regions are very close. By comparing the odor compounds in the three regions, it was found that the unique odor compounds of the brown sugar samples in Guangdong were 2-acetyl-5-methylpyrazine, 2-methylbutanoic acid and 3-phenylpropionic acid; the unique odor compound in Guangxi was propylene glycol; and the unique odor compounds in Yunnan were 1,3-dimethoxy-2-hydroxybenzene, 3-hydroxyl-2-methyl-4H-pyran-4-one, 3-methyl-1,2-cyclopentanedione, 4-methylpentanoic acid and γ-butyrolactone. These unique odor compounds are expected to be important indicators to distinguish the origin of brown sugar samples.
Table 1.
Volatile aroma components of brown sugars from different producing areas.
| No. | Compounds | Odor description | RI | Identification | Guangdong (ng/g) | Guangxi (ng/g) | Yunnan (ng/g) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guangdong1 | Guangdong2 | Guangdong3 | Guangdong4 | Guangdong5 | Guangdong6 | Guangxi1 | Guangxi2 | Guangxi3 | Guangxi4 | Guangxi5 | Guangxi6 | Yunnan1 | Yunnan2 | Yunnan3 | Yunnan4 | Yunnan5 | Yunnan6 | |||||
| 1 | 2,3-butanediol | fruit, onion | 1568 | MS/RI/O | 265.09 ± 24.94 | 184.68 ± 20.31 | 76.15 ± 9.36 | 209.60 ± 19.39 | 83.16 ± 6.00 | 179.33 ± 13.56 | 296.97 ± 15.27 | - | 114.44 ± 10.07 | 256.59 ± 21.35 | 81.45 ± 5.45 | 1019.88 ± 47.28 | 173.87 ± 12.84 | 256.31 ± 27.60 | 466.38 ± 31.83 | 410.50 ± 35.41 | 165.52 ± 4.61 | 50.48 ± 2.32 |
| 2 | propylene glycol | sweet | 1603 | MS/RI/O | - | - | - | - | - | - | - | - | 75.58 ± 12.71 | - | - | - | - | - | - | - | - | - |
| 3 | furfuryl alcohol | burnt | 1644 | MS/RI | 474.08 ± 15.80 | 480.63 ± 13.93 | 604.00 ± 40.01 | 130.47 ± 16.31 | 131.31 ± 6.74 | 535.66 ± 48.23 | 2006.99 ± 23.40 | 1502.75 ± 148.15 | 675.84 ± 18.94 | 1027.58 ± 93.40 | 205.87 ± 11.41 | 409.98 ± 34.42 | 881.75 ± 15.61 | 786.48 ± 36.72 | 681.50 ± 47.48 | 589.18 ± 31.74 | 84.99 ± 5.94 | 581.65 ± 39.11 |
| 4 | 5-methyl furfuryl alcohol | sweet, caramellic | 1705 | MS/RI | - | - | - | - | - | 295.98 ± 33.01 | 779.98 ± 180.99 | 147.60 ± 7.97 | - | 303.36 ± 121.91 | 87.16 ± 26.45 | 325.62 ± 12.36 | - | - | - | - | - | 189.50 ± 59.19 |
| 5 | benzyl alcohol | sweet, flower | 1865 | MS/RI | 118.05 ± 16.92 | 153.98 ± 11.55 | 299.03 ± 22.63 | - | - | 17.22 ± 1.07 | - | - | - | - | - | - | - | 169.70 ± 13.38 | - | 380.32 ± 22.02 | 100.30 ± 7.22 | - |
| Content of total alcohols | 857.22 | 819.29 | 979.18 | 340.07 | 214.47 | 1028.19 | 3083.94 | 1650.35 | 865.86 | 1587.53 | 374.48 | 1755.48 | 1055.62 | 1212.49 | 1147.88 | 1380 | 350.81 | 821.63 | ||||
| 6 | hexanal | grass, tallow, fat | 1075 | MS/RI/O | 399.76 ± 21.53 | 279.98 ± 19.85 | 313.08 ± 16.32 | 308.33 ± 6.52 | 150.05 ± 4.55 | 260.23 ± 19.05 | 230.54 ± 19.85 | 57.51 ± 4.66 | 130.21 ± 11.55 | 294.62 ± 15.77 | 209.07 ± 19.25 | 182.99 ± 15.58 | 207.86 ± 7.53 | 36.23 ± 2.97 | 231.15 ± 20.79 | 241.62 ± 17.27 | 155.67 ± 9.51 | 182.12 ± 16.88 |
| 7 | furfural | bread, almond, sweet | 1455 | MS/RI | - | - | - | 50.05 ± 3.69 | - | - | 97.89 ± 3.70 | 111.40 ± 5.84 | 95.82 ± 6.77 | - | 108.26 ± 5.01 | 161.29 ± 9.46 | - | - | - | - | - | 80.62 ± 4.36 |
| 8 | (E)-2-nonenal | cucumber, fat, green | 1507 | MS/RI/O | 56.20 ± 2.12 | 95.35 ± 2.05 | 68.21 ± 4.20 | 71.00 ± 3.80 | 36.96 ± 3.69 | 85.51 ± 2.13 | - | 59.88 ± 0.21 | 34.18 ± 3.20 | 60.66 ± 2.67 | 73.78 ± 5.50 | 79.64 ± 8.90 | 44.27 ± 2.08 | 70.81 ± 6.62 | 109.32 ± 12.26 | 80.28 ± 8.59 | - | - |
| 9 | benzaldehyde | almond, caramel | 1514 | MS/RI/O | 57.98 ± 6.17 | 109.82 ± 6.24 | 50.92 ± 3.04 | - | - | 168.28 ± 3.28 | - | - | 46.19 ± 5.23 | 102.28 ± 7.28 | 52.62 ± 7.13 | 94.52 ± 2.68 | - | 49.57 ± 4.48 | 69.14 ± 6.60 | 58.67 ± 4.52 | 54.31 ± 1.43 | - |
| 10 | 5-methylfurfural | almond, caramel | 1560 | MS/RI | 65.10 ± 5.32 | 60.46 ± 3.14 | 76.48 ± 2.04 | - | - | - | 121.80 ± 7.66 | 58.28 ± 2.64 | 71.86 ± 6.37 | 48.57 ± 3.84 | 27.43 ± 1.07 | 84.62 ± 4.31 | - | 74.75 ± 5.59 | 89.67 ± 8.14 | 65.65 ± 4.47 | - | 32.39 ± 2.98 |
| 11 | 2-hydroxymethyl-5-furfural | cardboard | 2512 | MS/RI | 527.94 ± 1.84 | 543.97 ± 14.99 | 840.13 ± 26.60 | 204.68 ± 16.87 | - | - | - | 835.97 ± 5.84 | 965.27 ± 12.27 | - | 387.49 ± 6.35 | 559.31 ± 6.19 | - | 182.63 ± 3.23 | 826.44 ± 9.41 | 600.61 ± 18.03 | - | 806.60 ± 45.30 |
| 12 | 4-hydroxy-3-methoxybenzaldehyde | vanilla | 2520 | MS/RI | 1163.20 ± 28.06 | 938.21 ± 12.55 | 659.00 ± 12.82 | - | 930.42 ± 38.08 | 1330.74 ± 17.04 | 1123.39 ± 38.79 | 1146.19 ± 38.02 | 1256.95 ± 36.57 | 796.62 ± 26.25 | 707.06 ± 24.88 | 813.25 ± 12.95 | 282.85 ± 23.12 | 337.09 ± 7.33 | 1009.19 ± 14.03 | 694.32 ± 19.89 | 562.95 ± 26.69 | 851.26 ± 25.56 |
| 13 | 3,5-dimethoxy-4-hydroxybenzaldehyde | sweet, cocoa, nutty | 2905 | MS/RI/O | 4115.06 ± 183.57 | 3970.11 ± 230.98 | 3258.76 ± 103.02 | 4530.25 ± 115.07 | 1861.44 ± 137.60 | 1393.28 ± 152.55 | 5558.03 ± 199.50 | - | - | - | - | - | 4106.21 ± 220.69 | 2356.17 ± 61.68 | 4986.34 ± 98.27 | 1882.87 ± 82.12 | 1255.94 ± 14.65 | 3220.19 ± 164.10 |
| 14 | 4-hydroxybenzaldehyde | creamy, musty | 2908 | MS/RI | - | - | - | - | - | 1990.86 ± 53.37 | - | 6374.49 ± 82.32 | 6982.29 ± 125.16 | - | 3014.51 ± 138.30 | - | - | - | - | - | - | - |
| Content of total aldehydes | 6385.24 | 5997.9 | 5266.58 | 5164.31 | 2978.87 | 5228.9 | 7131.65 | 8643.72 | 9582.77 | 1302.75 | 4580.22 | 1975.62 | 4641.19 | 3107.25 | 7321.25 | 3624.02 | 2028.87 | 5173.18 | ||||
| 15 | 2,6-di-tert-butyl-4-methylphenol | camphor | 1904 | MS/RI/O | 6272.71 ± 42.00 | 6702.74 ± 109.26 | 6540.27 ± 49.48 | 5238.74 ± 73.22 | 4705.80 ± 38.67 | 5559.88 ± 55.06 | 6468.03 ± 79.39 | 7106.41 ± 74.89 | 6733.20 ± 53.34 | 5149.51 ± 72.96 | 5177.12 ± 46.93 | 7033.33 ± 25.56 | 6293.40 ± 47.17 | 5659.68 ± 67.47 | 7638.60 ± 69.28 | 7938.76 ± 88.45 | 5115.72 ± 52.86 | 3851.64 ± 64.59 |
| 16 | 4-ethenyl-2-methoxyphenol | clove, curry | 2168 | MS/RI | 3571.11 ± 211.98 | 3749.85 ± 126.32 | 3296.40 ± 110.64 | 3640.61 ± 173.25 | 2405.55 ± 54.02 | 3822.00 ± 212.87 | 3438.47 ± 193.67 | 1672.14 ± 135.59 | 4249.96 ± 176.21 | 4588.34 ± 344.50 | 1419.61 ± 117.81 | 2766.58 ± 282.45 | 3400.23 ± 161.64 | 3112.76 ± 217.96 | 4595.74 ± 345.42 | 3222.71 ± 211.17 | 2317.34 ± 178.28 | 1808.79 ± 133.86 |
| 17 | 2,4-di-tert-butylphenol | phenolic | 2292 | MS/RI | 644.13 ± 80.76 | 456.81 ± 31.92 | 1052.57 ± 17.01 | 765.57 ± 39.53 | 332.60 ± 19.04 | 947.69 ± 13.41 | 1292.16 ± 26.55 | - | 1359.89 ± 78.31 | 467.47 ± 30.26 | 323.04 ± 21.63 | 1207.83 ± 109.14 | 446.63 ± 20.55 | 684.35 ± 35.98 | 675.77 ± 60.09 | 705.89 ± 49.64 | 498.28 ± 14.81 | 558.50 ± 25.05 |
| 18 | 2-methoxy-4-propenylphenol | flower | 2250 | MS/RI | - | - | - | - | 149.00 ± 15.25 | - | - | - | - | - | - | - | - | - | - | - | - | 391.82 ± 19.80 |
| 19 | (E)-4-propenyl-2-methoxyphenol | flower | 2315 | MS/RI | 630.45 ± 15.19 | 622.41 ± 11.80 | 689.57 ± 19.91 | - | - | 372.06 ± 21.68 | 605.14 ± 18.49 | - | - | - | - | - | 619.56 ± 48.92 | 563.39 ± 38.89 | 907.11 ± 87.87 | 662.54 ± 23.60 | 434.79 ± 35.66 | - |
| 20 | 4-allyl-2,6-dimethoxyphenol | sweet, flower | 2510 | MS/RI/O | - | - | - | - | - | 72.86 ± 9.71 | 440.73 ± 32.29 | - | 243.43 ± 15.50 | - | - | - | 129.33 ± 13.43 | - | - | 269.60 ± 18.11 | 132.49 ± 14.66 | - |
| 21 | 4-ethenyl-2,6-dimethoxy-phenol | animal leather | 2541 | MS/RI | 893.16 ± 23.65 | 799.51 ± 17.03 | 916.48 ± 36.36 | 655.80 ± 23.22 | 321.18 ± 28.46 | 835.89 ± 38.06 | 1183.04 ± 31.14 | 803.61 ± 72.23 | 1461.19 ± 29.31 | 1365.67 ± 76.92 | 746.52 ± 42.58 | 634.98 ± 13.65 | 572.43 ± 51.73 | 807.85 ± 70.45 | 1249.40 ± 36.45 | 1069.77 ± 73.55 | 718.19 ± 34.31 | 1237.55 ± 79.52 |
| 22 | 2-methoxy-4-acetylphenol | vanilla | 2640 | MS/RI/O | - | - | - | - | 352.34 ± 24.92 | - | 693.73 ± 55.31 | 808.51 ± 34.53 | 638.98 ± 11.43 | 1094.03 ± 62.69 | 935.46 ± 64.81 | 584.08 ± 42.12 | 855.15 ± 69.12 | - | - | - | 669.33 ± 59.70 | 649.86 ± 47.29 |
| Content of total phenols | 12,011.56 | 12,331.32 | 12,495.29 | 10,300.72 | 8266.47 | 11,610.38 | 14,121.3 | 10,390.67 | 14,686.65 | 12,665.02 | 8601.75 | 12,226.8 | 12,316.73 | 10,828.03 | 15,066.62 | 13,869.27 | 9886.14 | 8498.16 | ||||
| 23 | acetic acid | sour | 1415 | MS/RI/O | 10,184.79 ± 464.53 | 10,757.27 ± 305.67 | 2778.13 ± 46.39 | 6350.81 ± 222.21 | 6432.09 ± 175.47 | 5900.64 ± 582.42 | 6650.69 ± 245.63 | 4210.80 ± 330.79 | 2977.92 ± 249.84 | 10,999.92 ± 407.67 | 5047.46 ± 287.21 | 11,141.62 ± 393.70 | 4237.56 ± 172.97 | 1068.38 ± 53.52 | 12,748.83 ± 163.89 | 9209.62 ± 249.19 | 5570.25 ± 363.18 | 10,334.03 ± 261.79 |
| 24 | formic acid | acetic, astringent, fruity | 1489 | MS/RI/O | 249.89 ± 11.00 | 123.64 ± 7.91 | 219.04 ± 9.56 | 195.29 ± 11.26 | 84.47 ± 6.03 | 96.96 ± 8.18 | - | - | - | - | - | - | 91.75 ± 13.52 | - | 389.25 ± 20.61 | 426.15 ± 37.81 | - | 286.39 ± 23.78 |
| 25 | propanoic acid | pungent, rancid, soy | 1526 | MS/RI/O | 821.24 ± 27.91 | 619.01 ± 37.09 | 644.68 ± 16.61 | 396.79 ± 26.64 | 303.37 ± 5.15 | 260.37 ± 16.25 | 332.73 ± 19.43 | 147.19 ± 14.68 | 329.19 ± 2.45 | 457.22 ± 9.98 | 280.06 ± 14.46 | 653.58 ± 28.53 | 425.26 ± 19.38 | 299.24 ± 25.79 | 1143.61 ± 18.50 | 994.93 ± 49.00 | 515.40 ± 13.38 | 344.61 ± 26.73 |
| 26 | 2-methylpropionic acid | rancid, butter, cheese | 1563 | MS/RI | 519.47 ± 21.24 | 325.82 ± 19.73 | 264.85 ± 9.33 | 120.82 ± 9.90 | 166.03 ± 2.88 | 4.00 ± 0.24 | - | - | 88.45 ± 8.92 | 278.92 ± 25.60 | 272.22 ± 18.48 | 322.66 ± 11.67 | - | 153.86 ± 29.30 | 621.60 ± 24.32 | 768.20 ± 37.42 | 342.05 ± 10.18 | - |
| 27 | butanoic acid | rancid, cheese, sweat | 1607 | MS/RI/O | 2660.81 ± 89.68 | 2644.03 ± 91.30 | 614.21 ± 9.49 | 2513.84 ± 26.28 | 1227.91 ± 39.85 | 1421.39 ± 43.73 | 810.96 ± 56.86 | 192.43 ± 16.79 | 1269.77 ± 80.47 | 1178.36 ± 29.86 | 168.86 ± 8.63 | 440.72 ± 17.86 | 1266.55 ± 13.63 | 837.56 ± 53.38 | 4204.64 ± 71.97 | 2118.54 ± 37.34 | 1154.60 ± 33.74 | - |
| 28 | 3-methylbutanoic acid | sweat, acid, rancid | 1665 | MS/RI/O | 1633.63 ± 125.32 | 1076.75 ± 113.20 | 645.70 ± 71.56 | 306.69 ± 15.93 | - | 12.90 ± 0.34 | 271.65 ± 16.23 | 116.04 ± 14.73 | 218.33 ± 20.09 | 884.46 ± 15.52 | 810.07 ± 53.66 | 710.73 ± 29.15 | 1123.49 ± 87.06 | 566.92 ± 16.79 | 1390.95 ± 38.95 | 1609.20 ± 36.46 | - | - |
| 29 | 2-methylbutanoic acid | cheese, sweat | 1651 | MS/RI/O | - | - | - | - | - | 373.25 ± 28.50 | - | - | - | - | - | - | - | - | - | - | - | - |
| 30 | 2-methylpentanoic acid | buttery, creamy | 1728 | MS/RI/O | - | - | - | 183.26 ± 5.52 | - | 37.89 ± 3.86 | - | - | - | - | - | - | - | - | - | - | 302.40 ± 19.20 | - |
| 31 | 4-methylpentanoic acid | pungent cheese | 1820 | MS/RI/O | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 119.80 ± 6.83 | 56.28 ± 4.95 |
| 32 | hexanoic acid | sweat | 1826 | MS/RI/O | 300.51 ± 7.89 | 597.73 ± 14.47 | 487.13 ± 17.43 | 280.13 ± 27.04 | 283.56 ± 12.23 | 435.09 ± 35.20 | 280.31 ± 18.91 | - | 155.31 ± 12.61 | 453.98 ± 23.33 | 119.62 ± 11.23 | 112.67 ± 9.39 | 580.84 ± 13.70 | 441.59 ± 28.07 | 727.74 ± 38.34 | 728.69 ± 15.97 | 383.25 ± 13.93 | 78.31 ± 3.80 |
| 33 | octanoic acid | sweat, cheese | 2083 | MS/RI/O | 228.77 ± 6.47 | 267.60 ± 21.47 | - | - | - | - | - | - | - | - | - | - | - | 262.06 ± 13.93 | 252.12 ± 24.62 | 487.06 ± 38.82 | - | - |
| 34 | nonanoic acid | green, fat | 2147 | MS/RI/O | - | - | - | - | - | 413.10 ± 28.63 | - | - | - | - | - | - | - | - | - | - | - | 227.94 ± 12.15 |
| 35 | levulinic acid | acidic, sweet, creamy | 2312 | MS/RI | 587.58 ± 22.24 | 584.31 ± 18.74 | 589.85 ± 16.89 | - | - | - | - | - | 306.63 ± 20.02 | - | 338.16 ± 17.34 | 530.63 ± 35.66 | - | - | 653.12 ± 14.73 | 424.09 ± 35.11 | - | - |
| 36 | benzoic acid | urine | 2392 | MS/RI/O | 3080.65 ± 130.04 | 3564.46 ± 241.54 | 4062.78 ± 303.13 | 2709.59 ± 234.74 | 1609.97 ± 79.53 | 1795.59 ± 129.38 | 3194.93 ± 194.09 | 1982.81 ± 136.80 | 2736.59 ± 235.52 | 3573.53 ± 145.40 | 1480.40 ± 134.07 | 2837.50 ± 175.53 | 2965.81 ± 197.60 | 1857.28 ± 192.93 | 4446.03 ± 192.35 | 4751.21 ± 272.41 | 1872.63 ± 65.71 | 2262.00 ± 138.28 |
| 37 | dodecanoic acid | metal | 2517 | MS/RI | 555.78 ± 15.49 | 708.53 ± 18.03 | 575.78 ± 16.57 | 215.12 ± 19.98 | - | - | - | - | - | - | - | - | - | 438.26 ± 33.22 | 750.95 ± 39.27 | 504.14 ± 42.56 | 222.18 ± 21.86 | - |
| 38 | phenylacetic acid | honey, flower | 2551 | MS/RI/O | 1468.96 ± 56.27 | 1173.77 ± 75.04 | 1187.97 ± 92.84 | 880.89 ± 63.45 | 730.30 ± 47.81 | 47.37 ± 3.63 | 1267.41 ± 64.32 | - | 945.30 ± 61.58 | 1180.95 ± 82.76 | 741.94 ± 40.05 | 1353.01 ± 89.13 | 526.12 ± 26.32 | 903.11 ± 25.21 | 1487.08 ± 19.13 | 1976.49 ± 49.24 | 747.98 ± 54.78 | 606.57 ± 35.96 |
| 39 | 3-phenylpropionic acid | balsamic | 2650 | MS/RI/O | - | - | - | 156.03 ± 11.33 | - | 10.79 ± 0.95 | - | - | - | - | - | - | - | - | - | - | - | - |
| 40 | tetradecanoic acid | sweet, spicy, carnation | 2674 | MS/RI | 821.53 ± 67.39 | 1273.66 ± 98.64 | 457.82 ± 19.58 | - | - | 173.22 ± 16.83 | - | - | - | - | - | - | - | 498.30 ± 17.36 | 975.39 ± 21.75 | 631.33 ± 10.41 | 252.25 ± 17.50 | - |
| 41 | pentadecanoic acid | waxy | 2784 | MS/RI/O | 904.37 ± 37.76 | 1205.19 ± 44.08 | 867.02 ± 48.07 | 423.32 ± 20.01 | - | 806.02 ± 71.41 | 586.50 ± 17.13 | - | - | - | - | - | - | 733.11 ± 17.63 | 1093.45 ± 76.47 | 886.36 ± 59.46 | 381.02 ± 26.80 | - |
| 42 | 3-phenyl-2-propenoic acid | balsam, sweet, storax | 2815 | MS/RI | - | 502.70 ± 18.02 | - | - | 329.63 ± 27.74 | 555.52 ± 31.40 | 693.22 ± 19.57 | - | - | 1366.71 ± 46.48 | 904.32 ± 31.86 | 502.43 ± 39.39 | - | - | 871.20 ± 27.75 | 491.13 ± 25.65 | 416.66 ± 18.74 | - |
| 43 | n-hexadecanoic acid | fatty | 2903 | MS/RI | 11,549.66 ± 283.92 | 12,914.61 ± 160.72 | 8039.99 ± 159.00 | 6586.75 ± 270.87 | 4432.49 ± 192.77 | 8965.43 ± 247.84 | 6422.49 ± 176.26 | 13,536.74 ± 152.68 | 3748.63 ± 233.13 | 17,908.48 ± 240.92 | 5811.71 ± 96.26 | 3458.70 ± 197.36 | 9174.60 ± 92.17 | 6027.10 ± 231.08 | 15,086.68 ± 176.07 | 9036.63 ± 202.89 | 3926.92 ± 280.51 | 4354.01 ± 214.68 |
| Content of total carboxylic acids | 35,567.64 | 38,339.08 | 21,434.95 | 21,319.33 | 15,599.82 | 21,309.53 | 20,510.89 | 20,186.01 | 12,776.12 | 38,282.53 | 15,974.82 | 22,064.25 | 20,391.98 | 14,086.77 | 46,842.64 | 35,043.77 | 16,207.39 | 18,550.14 | ||||
| 44 | 2-methyl-4,5-dihydro-3(2H)-furanone | nutty, creamy | 1253 | MS/RI/O | 341.22 ± 28.42 | 1177.87 ± 50.20 | 513.32 ± 23.30 | 276.67 ± 4.52 | 70.39 ± 4.46 | 327.17 ± 28.81 | 202.04 ± 17.85 | 291.29 ± 28.07 | 93.57 ± 6.69 | 712.73 ± 89.42 | 141.35 ± 12.32 | 173.07 ± 3.56 | 701.59 ± 29.98 | 1141.42 ± 76.00 | 391.82 ± 12.71 | 578.35 ± 18.67 | 726.04 ± 22.75 | 1389.22 ± 47.00 |
| 45 | 3-hydroxy-2-butanone | butter, cream | 1272 | MS/RI | 91.84 ± 7.28 | 126.67 ± 7.48 | 124.74 ± 9.98 | 103.62 ± 3.31 | - | 163.28 ± 2.03 | 253.98 ± 9.70 | 145.05 ± 8.21 | 47.80 ± 1.02 | 123.45 ± 4.66 | 32.46 ± 4.69 | 68.52 ± 1.15 | 97.36 ± 7.88 | 74.92 ± 5.14 | 79.07 ± 3.53 | 80.41 ± 3.66 | 56.30 ± 2.47 | 105.69 ± 8.71 |
| 46 | 1-hydroxy-2-propanone | sweet | 1287 | MS/RI/O | 457.94 ± 39.12 | 582.30 ± 18.77 | 605.26 ± 9.93 | 438.74 ± 12.52 | 206.31 ± 12.44 | 652.59 ± 47.59 | 841.13 ± 59.74 | 715.56 ± 23.75 | 503.38 ± 2.41 | 505.10 ± 33.50 | 220.33 ± 5.58 | 211.55 ± 20.44 | 458.82 ± 7.71 | 360.04 ± 29.17 | 382.45 ± 17.01 | 461.70 ± 11.83 | 355.89 ± 14.98 | 438.74 ± 30.24 |
| 47 | 1-hydroxy-2-butanone | oily, alcoholic | 1375 | MS/RI/O | 39.22 ± 2.39 | 111.06 ± 5.97 | 41.37 ± 3.74 | 43.91 ± 3.57 | - | 16.70 ± 0.68 | - | 76.40 ± 7.75 | 82.88 ± 4.40 | 62.58 ± 4.71 | - | 24.26 ± 1.31 | 56.34 ± 6.77 | 46.29 ± 6.89 | 63.27 ± 6.83 | 56.43 ± 3.28 | - | - |
| 48 | 1-acetoxy-2-propanone | fruity, nutty | 1451 | MS/RI/O | 118.22 ± 4.77 | 230.25 ± 15.43 | 187.71 ± 12.77 | 168.77 ± 15.67 | - | 66.52 ± 6.06 | 218.30 ± 12.12 | 159.99 ± 17.64 | 115.45 ± 11.12 | 245.41 ± 19.90 | 139.08 ± 6.91 | 235.11 ± 24.82 | 176.14 ± 11.14 | 191.49 ± 11.15 | 132.61 ± 7.14 | 177.62 ± 9.60 | 125.30 ± 4.14 | 131.45 ± 4.25 |
| 49 | 4,5-dihydro-5-methyl-2(3H)-furanone | sweet, cocoa, woody | 1590 | MS/RI | 65.34 ± 7.38 | 113.07 ± 5.93 | 89.27 ± 2.10 | - | - | - | - | - | - | - | - | - | - | - | - | 90.38 ± 5.27 | - | - |
| 50 | 2(5H)-furanone | buttery | 1727 | MS/RI/O | 475.08 ± 10.84 | 581.86 ± 9.05 | 479.55 ± 13.75 | - | - | 145.49 ± 10.40 | 394.39 ± 11.56 | 265.72 ± 18.69 | 286.88 ± 27.60 | 221.84 ± 19.51 | 203.23 ± 15.77 | 363.54 ± 28.70 | - | 232.91 ± 14.77 | 712.75 ± 37.46 | 266.08 ± 24.57 | - | 154.22 ± 8.91 |
| 51 | 3-methyl-1,2-cyclopentanedione | sweet, maple, bready | 1781 | MS/RI/O | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 361.67 ± 24.53 |
| 52 | 2-hydroxy-3-methyl-2-cyclopenten-1-one | caramellic | 1807 | MS/RI/O | 468.60 ± 24.21 | 482.48 ± 34.60 | 707.42 ± 68.00 | 283.22 ± 26.02 | 182.61 ± 11.36 | 588.56 ± 57.85 | 879.99 ± 59.16 | 726.67 ± 69.62 | 745.18 ± 66.72 | 519.02 ± 24.12 | 186.46 ± 22.90 | 476.39 ± 46.95 | 681.71 ± 49.56 | 637.34 ± 38.58 | 716.33 ± 14.99 | 684.63 ± 28.94 | 248.07 ± 2.51 | - |
| 53 | 3-hydroxyl-2-methyl-4H-pyran-4-one | caramel | 1931 | MS/RI | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 201.59 ± 23.74 | 94.06 ± 7.11 |
| 54 | 2(3H)-furanone | cotton candy | 2002 | MS/RI/O | 974.60 ± 62.46 | 721.47 ± 64.38 | 1069.93 ± 92.52 | 351.84 ± 39.07 | 292.08 ± 13.56 | 470.52 ± 30.39 | 631.95 ± 22.11 | 615.99 ± 36.85 | 655.84 ± 55.61 | 705.84 ± 16.73 | 496.09 ± 31.24 | 977.50 ± 56.20 | 560.98 ± 8.63 | 596.89 ± 59.04 | 1123.58 ± 46.28 | 1324.65 ± 17.01 | 397.11 ± 16.87 | 323.38 ± 29.23 |
| 55 | 2,5-dimethyl-4-hydroxy-3(2H)-furanone | caramel | 2012 | MS/RI/O | 271.76 ± 26.35 | 326.77 ± 19.05 | 454.38 ± 18.37 | 230.03 ± 13.34 | 123.31 ± 4.96 | 731.60 ± 15.54 | 2168.59 ± 55.50 | 1158.64 ± 13.07 | 298.58 ± 23.33 | 471.80 ± 39.52 | 69.64 ± 3.26 | 144.05 ± 13.78 | 415.29 ± 14.57 | 861.94 ± 90.58 | 625.51 ± 26.54 | 529.53 ± 18.15 | - | 141.69 ± 16.00 |
| 56 | 4-hydroxy-5-methyl-3-(2H)-furanone | caramel | 2113 | MS/RI/O | - | - | - | 181.54 ± 21.75 | - | 19.69 ± 1.35 | 612.25 ± 48.80 | - | - | - | - | - | - | - | - | - | - | 99.22 ± 7.93 |
| 57 | 4-hydroxyacetophenone | sweet | 2958 | MS/RI/O | 1461.87 ± 17.59 | 1892.62 ± 25.11 | 1469.22 ± 34.48 | - | - | - | 1546.24 ± 57.40 | - | - | - | - | - | - | 1656.02 ± 57.98 | 2234.53 ± 34.42 | 1370.88 ± 77.57 | 1132.23 ± 64.94 | - |
| Content of total ketones | 4765.69 | 6346.42 | 5742.17 | 2078.34 | 874.7 | 3182.12 | 7748.86 | 4155.31 | 2829.56 | 3567.77 | 1488.64 | 2673.99 | 3148.23 | 5799.26 | 6461.92 | 5620.66 | 3242.53 | 3239.34 | ||||
| 58 | dimethyl butanedioate | sweet, fruity, green | 1558 | MS/RI | - | - | - | - | - | - | - | - | - | 590.16 ± 16.86 | - | - | 120.03 ± 12.32 | - | - | - | - | - |
| 59 | γ-butyrolactone | caramel, sweet | 1647 | MS/RI | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 193.04 ± 34.38 |
| 60 | dimethyl glutarate | floral | 1687 | MS/RI | - | - | - | - | - | - | - | - | - | 2030.19 ± 99.86 | - | - | 1039.76 ± 40.69 | - | - | - | - | - |
| 61 | benzyl benzoate | balsamic, oil, herb | 2592 | MS/RI | - | - | - | 205.81 ± 17.70 | - | 503.82 ± 28.75 | 621.78 ± 52.33 | - | 503.64 ± 41.22 | - | 431.15 ± 37.87 | 482.89 ± 31.13 | 510.18 ± 36.70 | 381.49 ± 27.23 | 682.59 ± 34.84 | 514.18 ± 27.27 | - | - |
| 62 | dibutyl phthalate | faint odor | 2705 | MS/RI | 1264.25 ± 33.84 | 1113.96 ± 65.74 | 810.31 ± 68.93 | 1061.36 ± 22.53 | 979.13 ± 65.98 | 373.31 ± 25.22 | 1089.36 ± 16.24 | - | 1202.60 ± 47.72 | 977.92 ± 29.04 | 735.97 ± 19.95 | - | 2530.75 ± 52.67 | - | 1333.62 ± 38.03 | 2536.41 ± 162.39 | 955.08 ± 69.59 | 1694.09 ± 93.37 |
| Content of total esters | 1264.25 | 1113.96 | 810.31 | 1267.17 | 979.13 | 877.13 | 1711.14 | 0 | 1706.24 | 3598.27 | 1167.12 | 482.89 | 4200.72 | 381.49 | 2016.21 | 3050.59 | 955.08 | 1887.13 | ||||
| 63 | 2-methylpyrazine | popcorn | 1259 | MS/RI | 366.92 ± 30.48 | 662.33 ± 40.09 | 159.11 ± 11.85 | 402.41 ± 7.65 | 112.41 ± 5.11 | 854.10 ± 77.77 | 651.18 ± 27.24 | 500.27 ± 17.30 | 235.82 ± 8.38 | 561.03 ± 42.71 | 76.46 ± 1.56 | 46.44 ± 5.46 | 289.74 ± 9.95 | 196.65 ± 14.01 | 172.90 ± 15.26 | 203.79 ± 21.19 | 88.40 ± 1.38 | - |
| 64 | 2,5-dimethylpyrazine | cocoa, nutty, roast beef | 1321 | MS/RI/O | 924.49 ± 34.20 | 1938.05 ± 101.57 | 111.73 ± 10.50 | 963.30 ± 10.96 | 201.81 ± 14.82 | 1827.76 ± 35.72 | 1343.97 ± 86.27 | 989.09 ± 53.50 | 1309.65 ± 15.59 | 1748.65 ± 121.67 | 88.92 ± 6.07 | 70.87 ± 2.23 | 1114.25 ± 44.64 | 703.42 ± 34.33 | 419.79 ± 28.03 | 430.64 ± 21.63 | 375.15 ± 3.73 | 93.56 ± 8.17 |
| 65 | 2,6-dimethylpyrazine | nutty, cocoa, roast beef | 1326 | MS/RI/O | 682.76 ± 22.04 | 1035.34 ± 80.61 | 144.76 ± 15.74 | 983.96 ± 18.81 | 72.49 ± 6.85 | 1094.56 ± 26.51 | 1444.15 ± 48.60 | 1045.87 ± 46.22 | 800.00 ± 42.14 | 946.87 ± 86.05 | 68.92 ± 14.85 | 85.72 ± 11.44 | 693.95 ± 41.35 | 273.74 ± 19.98 | 230.77 ± 22.95 | 289.35 ± 16.10 | 139.77 ± 2.60 | 208.04 ± 17.25 |
| 66 | 2,3-dimethylpyrazine | nutty, cocoa, meat | 1343 | MS/RI | 125.46 ± 9.27 | 231.83 ± 13.14 | 56.13 ± 2.20 | - | 38.84 ± 3.35 | 325.66 ± 36.06 | 253.09 ± 14.24 | 142.65 ± 9.66 | 72.47 ± 5.15 | 216.09 ± 18.81 | 10.89 ± 1.38 | - | 152.68 ± 11.65 | 73.56 ± 4.48 | 75.96 ± 6.30 | 70.09 ± 5.01 | 42.31 ± 1.02 | - |
| 67 | 2-ethyl-6-methylpyrazine | roasted hazelnut | 1382 | MS/RI | 69.27 ± 2.41 | 157.44 ± 5.95 | - | 92.61 ± 1.87 | 13.81 ± 2.78 | 88.18 ± 9.04 | 165.64 ± 5.86 | 114.68 ± 7.43 | 58.06 ± 6.05 | 167.14 ± 12.11 | 14.91 ± 1.33 | 19.91 ± 1.53 | 153.89 ± 9.44 | 66.03 ± 4.67 | 52.17 ± 4.62 | 46.08 ± 2.79 | 21.31 ± 0.76 | 5.15 ± 0.38 |
| 68 | 2-ethyl-5-methylpyrazine | fruit, sweet | 1376 | MS/RI | 55.63 ± 6.94 | 227.21 ± 18.30 | - | - | - | - | 151.24 ± 10.05 | 139.24 ± 7.77 | 123.72 ± 3.04 | 178.42 ± 6.03 | - | 21.87 ± 0.65 | 88.57 ± 8.00 | 105.86 ± 13.64 | 129.60 ± 16.42 | 77.52 ± 8.13 | - | - |
| 69 | 2,3,5-trimethylpyrazine | roast, potato, must | 1405 | MS/RI/O | - | - | - | 180.76 ± 15.90 | 35.78 ± 3.42 | 306.68 ± 21.34 | 249.04 ± 14.64 | 250.84 ± 14.85 | 40.86 ± 3.21 | - | 46.57 ± 3.30 | - | - | 125.18 ± 7.11 | - | - | - | 33.86 ± 3.99 |
| 70 | 2,5-dimethyl-3-ethylpyrazine | potato, roast | 1445 | MS/RI/O | 151.21 ± 12.91 | 315.82 ± 12.23 | - | 157.83 ± 2.51 | - | 338.49 ± 32.43 | - | - | - | - | - | - | 109.14 ± 7.37 | 201.75 ± 16.35 | 138.27 ± 14.70 | 119.00 ± 1.38 | - | - |
| 71 | 2,6-dimethyl-3-ethylpyrazine | potato | 1455 | MS/RI/O | - | - | - | - | 79.78 ± 4.61 | 186.16 ± 12.73 | 412.18 ± 36.51 | 272.34 ± 16.48 | 203.53 ± 6.05 | 286.15 ± 18.85 | - | - | - | - | - | - | 82.88 ± 8.05 | - |
| 72 | 2-methyl-6-vinylpyrazine | hazelnut | 1487 | MS/RI | - | - | - | - | - | 177.67 ± 24.80 | 688.17 ± 39.07 | 665.08 ± 41.61 | 311.55 ± 14.08 | - | 181.63 ± 8.49 | 550.08 ± 34.15 | - | 133.21 ± 8.77 | - | - | - | - |
| 73 | 2-acetyl-5-methylpyrazine | popcorn | 1664 | MS/RI/O | - | - | - | 156.34 ± 13.27 | - | 218.85 ± 16.63 | - | - | - | - | - | - | - | - | - | - | - | - |
| 74 | 2-acetyl-6-methylpyrazine | coffee, cocoa, popcorn | 1673 | MS/RI/O | 251.42 ± 21.79 | 419.45 ± 28.68 | - | 141.33 ± 9.04 | - | 245.73 ± 14.16 | 183.55 ± 23.20 | 144.23 ± 7.83 | 121.93 ± 11.22 | 218.58 ± 16.46 | - | - | - | 408.61 ± 9.22 | - | 113.67 ± 8.19 | 96.94 ± 7.01 | - |
| Content of total pyrazines | 2627.16 | 4987.47 | 471.73 | 3078.54 | 554.92 | 5663.84 | 5542.21 | 4264.29 | 3277.59 | 4322.93 | 488.3 | 794.89 | 2602.22 | 2288.01 | 1219.46 | 1350.14 | 846.76 | 340.61 | ||||
| 75 | 2-acetylpyrrole | nutty, walnut, bread | 1947 | MS/RI | 679.69 ± 54.14 | 856.53 ± 39.09 | 1676.37 ± 66.60 | 531.22 ± 38.42 | 111.84 ± 5.12 | 791.31 ± 37.88 | 2227.41 ± 88.13 | 2964.65 ± 58.72 | 2703.33 ± 101.11 | 747.17 ± 33.20 | 461.32 ± 47.83 | 1263.31 ± 41.84 | 855.32 ± 80.10 | 1610.00 ± 150.67 | 942.57 ± 99.06 | 1761.17 ± 84.12 | 1298.42 ± 59.86 | 971.33 ± 70.56 |
| 76 | 2-acetylfuran | balsamic | 1490 | MS/RI/O | - | - | - | - | - | 5.27 ± 0.85 | - | 231.24 ± 17.91 | 103.74 ± 7.62 | - | 37.99 ± 2.05 | 72.73 ± 1.11 | - | - | - | - | - | 54.65 ± 3.48 |
| 77 | (+)-limonene | citrus, mint | 1201 | MS/RI | - | - | - | - | 48.67 ± 3.12 | - | 210.31 ± 21.01 | - | - | - | 71.37 ± 2.85 | 99.25 ± 2.63 | 69.37 ± 7.74 | 69.29 ± 5.81 | 89.42 ± 6.55 | 51.53 ± 3.80 | - | - |
| 78 | phenylethylene | balsamic, gasoline | 1247 | MS/RI/O | 651.29 ± 54.83 | 613.81 ± 14.74 | 573.68 ± 15.42 | 451.99 ± 11.84 | 160.07 ± 12.09 | 457.61 ± 37.97 | 546.32 ± 24.83 | 469.44 ± 28.01 | 511.72 ± 22.34 | 984.77 ± 64.37 | 984.42 ± 73.21 | 1025.24 ± 14.21 | 500.79 ± 9.91 | 459.13 ± 34.72 | 564.51 ± 15.57 | 601.32 ± 18.81 | 471.01 ± 19.63 | 162.20 ± 23.00 |
| 79 | methyl sulfoxide | garlic | 1576 | MS/RI | 151.90 ± 19.28 | 176.80 ± 8.92 | 63.28 ± 7.19 | 89.59 ± 3.82 | 49.16 ± 4.14 | 2.89 ± 1.67 | - | - | - | - | - | - | - | 178.52 ± 18.72 | 237.91 ± 15.70 | 207.83 ± 13.45 | 151.60 ± 18.19 | 42.30 ± 4.61 |
| 80 | 1,3-dimethoxy-2-hydroxybenzene | medicine, phenol, smoke | 2296 | MS/RI | - | - | - | - | - | - | - | - | - | - | - | - | 1138.32 ± 54.20 | - | - | - | - | - |
| Content of other compounds | 1482.88 | 1647.14 | 2313.33 | 1072.8 | 369.74 | 1257.08 | 2984.04 | 3665.33 | 3318.79 | 1731.94 | 1555.1 | 2460.53 | 2563.8 | 2316.94 | 1834.41 | 2621.85 | 1921.03 | 1230.48 | ||||
| Total identified/detected | 64,961.64 | 71,582.58 | 49,513.54 | 44,621.28 | 29,838.12 | 50,157.17 | 62,834.03 | 52,955.68 | 49,043.58 | 67,058.74 | 34,230.43 | 44,434.45 | 50,920.49 | 40,020.24 | 81,910.39 | 66,560.3 | 35,438.61 | 39,740.67 | ||||
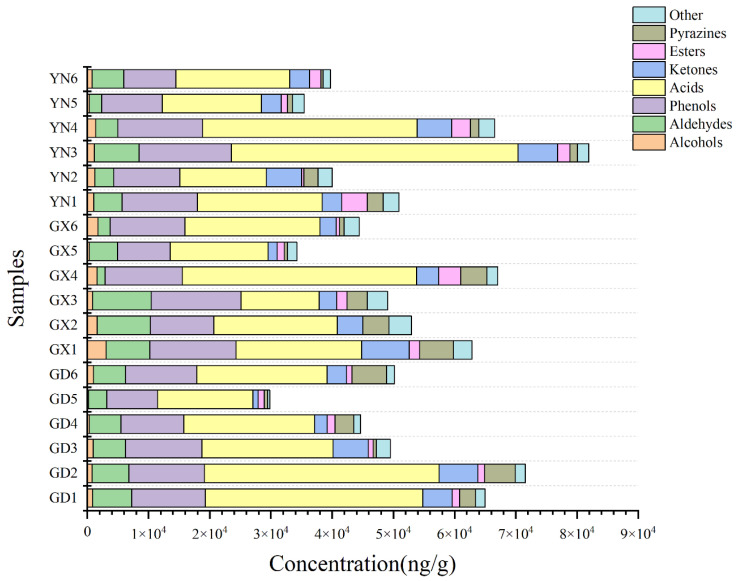
The average contents of odor compounds in brown sugar samples from the three regions are shown in Figure 1. It can be seen that the highest contents of acid compounds were found in all three regions with 25,595.06, 21,632.44 and 25,187.12 ng/g, followed by phenolic compounds with average contents of 111,69.29, 12,115.37 and 11,744.16 ng/g. In contrast, alcohols, esters, pyrazines and ketones had lower contents.
Figure 1.
The average content of different kinds of compounds in three regions.
2.2. Analysis of Key Aroma Compounds in Brown Sugar Samples
A total of 46 aroma-active compounds were identified in 18 brown sugar samples by olfactometry, including 4 alcohols, 4 aldehydes, 3 phenols, 15 acids, 11 ketones, 7 pyrazines, and 2 other compounds. According to the odor properties of the aroma active compounds, these compounds can be classified into nine types: sweet/caramel, fruity, green/grassy, sour, sweaty/cheese, nutty, roasted, fatty and potato, which indicated that the aroma profile of brown sugar was the result of the synergistic effect of various odors.
In fact, it is the OAV of the aroma compound, and not its amount, that determines the contribution of the aroma compound. Aroma activity is generally defined as compounds with OAVs greater than 1 [14]. Therefore, the calculation of OAV was carried out for aroma compounds that can be sniffed (Table 2). Among the 18 brown sugar samples, 26 compounds with OAV >1 were considered as the key aroma active compounds of the brown sugar samples in this study and contributed to the overall flavor.
Table 2.
OAV of key odor compounds in brown sugar.
| No. | Compounds a | OT (ng/g) b | GD1 | GD2 | GD3 | GD4 | GD5 | GD6 | GX1 | GX2 | GX3 | GX4 | GX5 | GX6 | YN1 | YN2 | YN3 | YN4 | YN5 | YN6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | pentadecanoic acid | 500 | 2 | 2 | 2 | 1 | - | 2 | 1 | - | - | - | - | - | - | 1 | 2 | 2 | 1 | - |
| 2 | 2-methylbutanoic acid | 20 | - | - | - | - | - | 19 | - | - | - | - | - | - | - | - | - | - | - | - |
| 3 | 3-methylbutanoic acid | 1.8 | 908 | 598 | 359 | 170 | - | 7 | 151 | 64 | 121 | 491 | 450 | 395 | 624 | 315 | 773 | 894 | - | - |
| 4 | 4-methylpentanoic acid | 1.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 63 | 30 |
| 5 | acetic acid | 13 | 783 | 827 | 214 | 489 | 495 | 454 | 512 | 324 | 229 | 846 | 388 | 857 | 326 | 82 | 981 | 708 | 428 | 795 |
| 6 | benzoic acid | 1000 | 3 | 4 | 4 | 3 | 2 | 2 | 3 | 2 | 3 | 4 | 1 | 3 | 3 | 2 | 4 | 5 | 2 | 2 |
| 7 | butanoic acid | 20 | 133 | 132 | 31 | 126 | 61 | 71 | 41 | 10 | 63 | 59 | 8 | 22 | 63 | 42 | 210 | 106 | 58 | - |
| 8 | hexanoic acid | 4.8 | 63 | 125 | 101 | 58 | 59 | 91 | 58 | - | 32 | 95 | 25 | 23 | 121 | 92 | 152 | 152 | 80 | 16 |
| 9 | nonanoic acid | 1.6 | - | - | - | - | - | 258 | - | - | - | - | - | - | - | - | - | - | - | 142 |
| 10 | octanoic acid | 22 | 10 | 12 | - | - | - | - | - | - | - | - | - | - | - | 12 | 11 | 22 | - | - |
| 11 | phenylacetic acid | 17 | 86 | 69 | 70 | 52 | 43 | 3 | 75 | - | 56 | 69 | 44 | 80 | 31 | 53 | 87 | 116 | 44 | 36 |
| 12 | hexanal | 1.4 | 286 | 200 | 224 | 220 | 107 | 186 | 165 | 41 | 93 | 210 | 149 | 131 | 148 | 26 | 165 | 173 | 111 | 130 |
| 13 | (E)-2-nonenal | 0.19 | 296 | 502 | 359 | 374 | 195 | 450 | - | 315 | 180 | 319 | 388 | 419 | 233 | 373 | 575 | 423 | - | - |
| 14 | 3,5-dimethoxy-4-hydroxybenzaldehyde | 1900 | 2 | 2 | 2 | 2 | 1 | 1 | 3 | - | - | - | - | - | 2 | 1 | 3 | 1 | 1 | 2 |
| 15 | benzaldehyde | 60 | 1 | 2 | 1 | - | - | 3 | - | - | 1 | 2 | 1 | 2 | - | 1 | 1 | 1 | 1 | - |
| 16 | 3-methyl-1,2-cyclopentanedione | 26 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 14 |
| 17 | 2-hydroxy-3-methyl-2-cyclopenten-1-one | 10 | 47 | 48 | 71 | 28 | 18 | 59 | 88 | 73 | 75 | 52 | 19 | 48 | 68 | 64 | 72 | 68 | 25 | - |
| 18 | 2,5-dimethyl-4-hydroxy-3(2H)-furanone | 1.6 | 170 | 204 | 284 | 144 | 77 | 457 | 1355 | 724 | 187 | 295 | 44 | 90 | 260 | 539 | 391 | 331 | - | 89 |
| 19 | 4-hydroxy-5-methyl-3-(2H)-furanone | 500 | - | - | - | <1 | - | <1 | 1 | - | - | - | - | - | - | - | - | - | - | <1 |
| 20 | phenylethylene | 37 | 18 | 17 | 16 | 12 | 4 | 12 | 15 | 13 | 14 | 27 | 27 | 28 | 14 | 12 | 15 | 16 | 13 | 4 |
| 21 | furfuryl alcohol | 1415 | <1 | <1 | <1 | <1 | <1 | <1 | 1 | 1 | <1 | 1 | <1 | <1 | 1 | 1 | <1 | <1 | <1 | <1 |
| 22 | 2,3,5-trimethylpyrazine | 23 | - | - | - | 8 | 2 | 13 | 11 | 11 | 2 | - | 2 | - | - | 5 | - | - | - | 1 |
| 23 | 2,5-dimethylpyrazine | 80 | 12 | 24 | 1 | 12 | 3 | 23 | 17 | 12 | 16 | 22 | 1 | 1 | 14 | 9 | 5 | 5 | 5 | 1 |
| 24 | 2,6-dimethyl-3-ethylpyrazine | 0.04 | - | - | - | - | 1995 | 4654 | 10,305 | 6809 | 5088 | 7154 | - | - | - | - | - | - | 2072 | - |
| 25 | 2,6-dimethylpyrazine | 250 | 3 | 4 | 1 | 4 | <1 | 4 | 6 | 4 | 3 | 4 | <1 | <1 | 3 | 1 | 1 | 1 | 1 | 1 |
| 26 | 2-acetyl-6-methylpyrazine | 300 | 1 | 1 | - | <1 | - | 1 | 1 | <1 | <1 | 1 | - | - | - | 1 | - | <1 | <1 | - |
a Volatile compounds that can be smelled at sniffer port. b Odor thresholds were referenced in a book, named: odor thresholds compilations of odor threshold values in air, water and other media.
Alcohols: Among the four alcohols that can be sniffed, only furfuryl alcohol had OAV >1 and was only found in Guangxi and Yunnan. The content of furfuryl alcohol in Guangxi and Yunnan was 971.50 and 392.70 ng/g, respectively, and it contributed sweet, toast and caramel aroma to brown sugar. Sugar and amino acids react readily at elevated temperatures to form this compound [15]. The furfuryl alcohol contained in soy sauce has been considered to be one of the main components responsible for its odor, exhibiting a caramel scent, which contributes to the overall flavor of the sample [16].
Aldehydes: Among the aldehydes, there are four aldehydes with OAV >1, namely hexanal, (E)-2-nonenal, 3,5-dimethoxy-4-hydroxybenzaldehyde and benzaldehyde. (E)-2-nonenal and hexanal are probably oxidation products of polyunsaturated fatty acids [17], with high OAV due to their higher concentration and lower odor threshold, and are key aroma compounds among aldehydes, contributing to the green odor of brown sugar. The average content of benzaldehyde in Guangdong was higher than that in Guangxi and Yunnan, and it may be the degradation product of phenylalanine [14], contributing nutty and caramel aromas to the brown sugar. 3,5-dimethoxy-4-hydroxybenzaldehyde showed close OAV in Guangdong and Yunnan, and was higher than that in Guangxi, contributing sweet and nutty aroma to brown sugars. According to Chen, Song, Li, Chen, Wang, Che, Zhang and Zhao [9], 3,5-dimethoxy-4-hydroxybenzaldehyde is formed during brown sugar production, and the difference in content might be related to the raw materials and processing technology.
Ketones: Four ketones with OAV >1 were found in brown sugar samples, including 3-methyl-1,2-cyclopentanedione, 2-hydroxy-3-methyl-2-cyclopenten-1-one, 2,5-dimethyl-4-hydroxy-3(2H)-furanone, and 4-hydroxy-5-methyl-3(2H)-furanone. 2,5-Dimethyl-4-hydroxy-3(2H)-furanone has the highest OAV and contributes a strong caramel flavor to brown sugar, which is most likely formed by the Maillard reaction through deoxy sugars and is most abundant in strawberries [18,19]. 2-Hydroxy-3-methyl-2-cyclopenten-1-one has a strong caramel aroma and is one of the key odor compounds that contribute to the caramel odor in black tea, soy sauce and molasses [20,21,22]. 3-Methyl-1,2-cyclopentanedione was detected only in Yunnan brown sugar with OAV=14, which contributed sweet and bready aroma to Yunnan brown sugar. 2,5-Dimethyl-4-hydroxy-3(2H)-furanone was detected in all the three regions’ samples, but the OAV was greater than 1 only in Guangxi brown sugar, which was caused by its high concentration in Guangxi brown sugar.
Pyrazines: Many products possess a distinctive aroma resulting from pyrazines, which are special Maillard reaction compounds [23,24]. Pyrazine is formed by condensing two α-aminocarbonyl compounds and forming a dihydropyrazine, which oxidizes spontaneously to form the pyrazine [23,25,26]. Among the twelve pyrazines detected in the eighteen samples, there are five kinds of pyrazines with OAV greater than 1, namely 2,3,5-trimethylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethyyl-3-ethylpyrazine, 2,6-dimethyl-3-ethylpyrazine and 2-acetyl-6-methylpyrazine. 2,6-Dimethyl-3-ethylpyrazine exhibited the highest OVA due to its low threshold (OT=0.04 ng/g), contributing a strong roasted potato flavor to brown sugar. 2,5-Dimethylpyrazine and 2,6-dimethylpyrazine were previously reported to be key odor compounds in coffee, exhibiting strong roasted and nutty aroma [27].
Acids: A total of 21 kinds of acid compounds were detected in 18 brown sugars, among which the OAV of 11 kinds of acid compounds was greater than 1. Acetic acid, one of the most abundant compounds in brown sugar, had the highest OAV and contributed sour aroma to the samples. 2-Methylbutanoic acid and 3-methylbutanoic acid exhibited a sour aroma and had been reported to be the key aroma components in Japanese sweet rice wine, which played an important role in the overall flavor of sweet rice wine [28]. Benzoic acid, however, has an unpleasant urine-like odor, which may be caused by phenylalanine under the action of phenylalanine ammonia-lyase in plants [29].
2.3. Fingerprint Analysis of Sugar Products from Three Different Regions
A food fingerprint can be defined as molecular markers that indicate a characteristic state or condition of food, thus enabling more accurate product identification [30]. Each sample is regarded as a multidimensional space vector. If two samples are more similar, their space will be closer, and the angle between the two samples’ space vectors will be smaller, which leads the cosine of the angle between the two vectors to move closer to 1. Therefore, the similarity of samples can be expressed by the cosine of the included angle. On the contrary, if the difference between the two samples is greater, the cosine of the included angle becomes smaller. In this study, the samples were determined by GC-O-MS, and the odor-active compounds were selected for fingerprint and similarity evaluation.
It is worth mentioning that the similarity of samples becomes higher when the similarity or the cosine of the angle is above 90%. As depicted in Table 3 and Figure 2, of the six samples in Guangdong, except for Guangdong3, the similarity and cosine of the included angle of the other five samples were above 90%. This indicated that the odor properties of Guangdong3 were quite different than the other five samples, which might have happened due to different processing technology.
Table 3.
Fingerprint results of brown sugar from each producing area.
| No. | Compounds | Guangdong | Guangxi | Yunnan | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guangdong1 | Guangdong2 | Guangdong3 | Guangdong4 | Guangdong5 | Guangdong6 | Guangxi1 | Guangxi2 | Guangxi3 | Guangxi4 | Guangxi5 | Guangxi6 | Yunnan1 | Yunnan2 | Yunnan3 | Yunnan4 | Yunnan5 | Yunnan6 | ||
| 1 | 2,3-butanediol | 265.09 | 184.68 | 76.15 | 209.6 | 83.16 | 179.33 | 296.97 | 0 | 114.44 | 256.59 | 81.45 | 1019.88 | 173.87 | 256.31 | 466.38 | 410.5 | 165.52 | 50.48 |
| 2 | propylene glycol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 75.58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | hexanal | 399.76 | 279.98 | 313.08 | 308.33 | 150.05 | 260.23 | 230.54 | 57.51 | 130.21 | 294.62 | 209.07 | 182.99 | 207.86 | 36.23 | 231.15 | 241.62 | 155.67 | 182.12 |
| 4 | (E)-2-nonenal | 56.2 | 95.35 | 68.21 | 71 | 36.96 | 85.51 | 0 | 59.88 | 34.18 | 60.66 | 73.78 | 79.64 | 44.27 | 70.81 | 109.32 | 80.28 | 0 | 0 |
| 5 | benzaldehyde | 57.98 | 109.82 | 50.92 | 0 | 0 | 168.28 | 0 | 0 | 46.19 | 102.28 | 52.62 | 94.52 | 0 | 49.57 | 69.14 | 58.67 | 54.31 | 0 |
| 6 | 3,5-dimethoxy-4-hydroxybenzaldehyde | 4115.06 | 3970.11 | 3258.76 | 4530.25 | 1861.44 | 1393.28 | 5558.03 | 0 | 0 | 0 | 0 | 0 | 4106.21 | 2356.17 | 4986.34 | 1882.87 | 1255.94 | 3220.19 |
| 7 | 2,6-di-tert-butyl-4-methylphenol | 6272.71 | 6702.74 | 6540.27 | 5238.74 | 4705.8 | 5559.88 | 6468.03 | 7106.41 | 6733.2 | 5149.51 | 5177.12 | 7033.33 | 6293.4 | 5659.68 | 7638.6 | 7938.76 | 5115.72 | 3851.64 |
| 8 | 4-allyl-2,6-dimethoxyphenol | 0 | 0 | 0 | 0 | 0 | 72.86 | 440.73 | 0 | 243.43 | 0 | 0 | 0 | 129.33 | 0 | 0 | 269.6 | 132.49 | 0 |
| 9 | 2-methoxy-4-acetylphenol | 0 | 0 | 0 | 0 | 352.34 | 0 | 693.73 | 808.51 | 638.98 | 1094.03 | 935.46 | 584.08 | 855.15 | 0 | 0 | 0 | 669.33 | 649.86 |
| 10 | acetic acid | 10,184.79 | 10,757.27 | 2778.13 | 6350.81 | 6432.09 | 5900.64 | 6650.69 | 4210.8 | 2977.92 | 10,999.92 | 5047.46 | 11,141.62 | 4237.56 | 1068.38 | 12,748.83 | 9209.62 | 5570.25 | 10,334.03 |
| 11 | methanoic acid | 249.89 | 123.64 | 219.04 | 195.29 | 84.47 | 96.96 | 0 | 0 | 0 | 0 | 0 | 0 | 91.75 | 0 | 389.25 | 426.15 | 0 | 286.39 |
| 12 | propanoic acid | 821.24 | 619.01 | 644.68 | 396.79 | 303.37 | 260.37 | 332.73 | 147.19 | 329.19 | 457.22 | 280.06 | 653.58 | 425.26 | 299.24 | 1143.61 | 994.93 | 515.4 | 344.61 |
| 13 | butanoic acid | 2660.81 | 2644.03 | 614.21 | 2513.84 | 1227.91 | 1421.39 | 810.96 | 192.43 | 1269.77 | 1178.36 | 168.86 | 440.72 | 1266.55 | 837.56 | 4204.64 | 2118.54 | 1154.6 | 0 |
| 14 | 3-methylbutanoic acid | 1633.63 | 1076.75 | 645.7 | 306.69 | 0 | 12.9 | 271.65 | 116.04 | 218.33 | 884.46 | 810.07 | 710.73 | 1123.49 | 566.92 | 1390.95 | 1609.2 | 0 | 0 |
| 15 | 2-methylbutanoic acid | 0 | 0 | 0 | 0 | 0 | 373.25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 2-methylpentanoic acid | 0 | 0 | 0 | 183.26 | 0 | 37.89 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 302.4 | 0 |
| 17 | 4-methylpentanoic acid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 119.8 | 56.28 |
| 18 | hexanoic acid | 300.51 | 597.73 | 487.13 | 280.13 | 283.56 | 435.09 | 280.31 | 0 | 155.31 | 453.98 | 119.62 | 112.67 | 580.84 | 441.59 | 727.74 | 728.69 | 383.25 | 78.31 |
| 19 | octanoic acid | 228.77 | 267.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 262.06 | 252.12 | 487.06 | 0 | 0 |
| 20 | nonanoic acid | 0 | 0 | 0 | 0 | 0 | 413.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 227.94 |
| 21 | benzoic acid | 3080.65 | 3564.46 | 4062.78 | 2709.59 | 1609.97 | 1795.59 | 3194.93 | 1982.81 | 2736.59 | 3573.53 | 1480.4 | 2837.5 | 2965.81 | 1857.28 | 4446.03 | 4751.21 | 1872.63 | 2262 |
| 22 | phenylacetic acid | 1468.96 | 1173.77 | 1187.97 | 880.89 | 730.3 | 47.37 | 1267.41 | 0 | 945.3 | 1180.95 | 741.94 | 1353.01 | 526.12 | 903.11 | 1487.08 | 1976.49 | 747.98 | 606.57 |
| 23 | 3-phenylpropionic acid | 0 | 0 | 0 | 156.03 | 0 | 10.79 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | pentadecanoic acid | 904.37 | 1205.19 | 867.02 | 423.32 | 0 | 806.02 | 586.5 | 0 | 0 | 0 | 0 | 0 | 0 | 733.11 | 1093.45 | 886.36 | 381.02 | 0 |
| 25 | 2-methyl-4,5-dihydro-3(2H)-furanone | 341.22 | 1177.87 | 513.32 | 276.67 | 70.39 | 327.17 | 202.04 | 291.29 | 93.57 | 712.73 | 141.35 | 173.07 | 701.59 | 1141.42 | 391.82 | 578.35 | 726.04 | 1389.22 |
| 26 | 1-hydroxy-2-propanone | 457.94 | 582.3 | 605.26 | 438.74 | 206.31 | 652.59 | 841.13 | 715.56 | 503.38 | 505.1 | 220.33 | 211.55 | 458.82 | 360.04 | 382.45 | 461.7 | 355.89 | 438.74 |
| 27 | 1-hydroxy-2-butanone | 39.22 | 111.06 | 41.37 | 43.91 | 0 | 16.7 | 0 | 76.4 | 82.88 | 62.58 | 0 | 24.26 | 56.34 | 46.29 | 63.27 | 56.43 | 0 | 0 |
| 28 | 1-acetoxy-2-propanone | 118.22 | 230.25 | 187.71 | 168.77 | 0 | 66.52 | 218.3 | 159.99 | 115.45 | 245.41 | 139.08 | 235.11 | 176.14 | 191.49 | 132.61 | 177.62 | 125.3 | 131.45 |
| 29 | 2(5H)-furanone | 475.08 | 581.86 | 479.55 | 0 | 0 | 145.49 | 394.39 | 265.72 | 286.88 | 221.84 | 203.23 | 363.54 | 0 | 232.91 | 712.75 | 266.08 | 0 | 154.22 |
| 30 | 3-methyl-1,2-cyclopentanedione | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 361.67 |
| 31 | 2-hydroxy-3-methyl-2-cyclopenten-1-one | 468.6 | 482.48 | 707.42 | 283.22 | 182.61 | 588.56 | 879.99 | 726.67 | 745.18 | 519.02 | 186.46 | 476.39 | 681.71 | 637.34 | 716.33 | 684.63 | 248.07 | 0 |
| 32 | 2(3H)-furanone | 974.6 | 721.47 | 1069.93 | 351.84 | 292.08 | 470.52 | 631.95 | 615.99 | 655.84 | 705.84 | 496.09 | 977.5 | 560.98 | 596.89 | 1123.58 | 1324.65 | 397.11 | 323.38 |
| 33 | 2,5-dimethyl-4-hydroxy-3(2H)-furanone | 271.76 | 326.77 | 454.38 | 230.03 | 123.31 | 731.6 | 2168.59 | 1158.64 | 298.58 | 471.8 | 69.64 | 144.05 | 415.29 | 861.94 | 625.51 | 529.53 | 0 | 141.69 |
| 34 | 4-hydroxy-5-methyl-3-(2H)-furanone | 0 | 0 | 0 | 181.54 | 0 | 19.69 | 612.25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 99.22 |
| 35 | 4-hydroxyacetophenone | 1461.87 | 1892.62 | 1469.22 | 0 | 0 | 0 | 1546.24 | 0 | 0 | 0 | 0 | 0 | 0 | 1656.02 | 2234.53 | 1370.88 | 1132.23 | 0 |
| 36 | 2,5-dimethylpyrazine | 924.49 | 1938.05 | 111.73 | 963.3 | 201.81 | 1827.76 | 1343.97 | 989.09 | 1309.65 | 1748.65 | 88.92 | 70.87 | 1114.25 | 703.42 | 419.79 | 430.64 | 375.15 | 93.56 |
| 37 | 2,6-dimethylpyrazine | 682.76 | 1035.34 | 144.76 | 983.96 | 72.49 | 1094.56 | 1444.15 | 1045.87 | 800 | 946.87 | 68.92 | 85.72 | 693.95 | 273.74 | 230.77 | 289.35 | 139.77 | 208.04 |
| 38 | 2,3,5-trimethylpyrazine | 0 | 0 | 0 | 180.76 | 35.78 | 306.68 | 249.04 | 250.84 | 40.86 | 0 | 46.57 | 0 | 0 | 125.18 | 0 | 0 | 0 | 33.86 |
| 39 | 2,5-dimethyl-3-ethylpyrazine | 151.21 | 315.82 | 0 | 157.83 | 0 | 338.49 | 0 | 0 | 0 | 0 | 0 | 0 | 109.14 | 201.75 | 138.27 | 119 | 0 | 0 |
| 40 | 2,6-dimethyl-3-ethylpyrazine | 0 | 0 | 0 | 0 | 79.78 | 186.16 | 412.18 | 272.34 | 203.53 | 286.15 | 0 | 0 | 0 | 0 | 0 | 0 | 82.88 | 0 |
| 41 | 2-acetyl-5-methylpyrazine | 0 | 0 | 0 | 156.34 | 0 | 218.85 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 42 | 2-acetyl-6-methylpyrazine | 251.42 | 419.45 | 0 | 141.33 | 0 | 245.73 | 183.55 | 144.23 | 121.93 | 218.58 | 0 | 0 | 0 | 408.61 | 0 | 113.67 | 96.94 | 0 |
| 43 | 2-acetylfuran | 0 | 0 | 0 | 0 | 0 | 5.27 | 0 | 231.24 | 103.74 | 0 | 37.99 | 72.73 | 0 | 0 | 0 | 0 | 0 | 54.65 |
| 44 | phenylethylene | 651.29 | 613.81 | 573.68 | 451.99 | 160.07 | 457.61 | 546.32 | 469.44 | 511.72 | 984.77 | 984.42 | 1025.24 | 500.79 | 459.13 | 564.51 | 601.32 | 471.01 | 162.2 |
| Cosine of included angle | 0.9879 | 0.9888 | 0.8855 | 0.9800 | 0.9750 | 0.9643 | 0.9031 | 0.9463 | 0.9152 | 0.9527 | 0.9776 | 0.9671 | 0.9439 | 0.8155 | 0.9815 | 0.9839 | 0.9822 | 0.9189 | |
| Similarity | 0.9850 | 0.9859 | 0.8562 | 0.9752 | 0.9781 | 0.9553 | 0.8824 | 0.9373 | 0.8980 | 0.9445 | 0.9762 | 0.9664 | 0.9300 | 0.7655 | 0.9773 | 0.9799 | 0.9787 | 0.9138 | |
Figure 2.
Fingerprint of brown sugar from Guangdong, Guangxi and Yunnan.
The cosine of the included angle of six samples in Guangxi was above 90%, and the similarity of Guangxi3 was just less than 90% (89.80%). This result indicated that the odor properties of these six samples in Guangxi were similar, without much difference
Of the six samples in Yunnan, only Yunnan2 had similarity and cosine of included angle lower than 90%, while the other five samples had similarity and cosine of included angle higher than 90%. This result indicated that the odor attributes of the other five samples were similar, but Yunnan2 had significant differences with them.
2.4. Verification of Fingerprint
In order to verify whether the fingerprint method is suitable for the analysis of brown sugar, the verification was carried out. Fingerprint verification includes three parts: stability experiment, precision experiment, and repeatability experiment. Following the sample preparation described in Section 2.4, a brown sugar sample was selected and analyzed by GC-MS after 0, 2, 4, 8, 16, and 24 h. Furthermore, the relative standard deviations (RSD) of the relative retention times (RT) and relative peak areas of the odor-active compounds were calculated. The results showed that the RSD of the relative RT of the odor-active compounds was less than 0.3%, and the RSD of the relative peak areas was less than 5%, indicating that the samples were stable within 24 h and met the requirements of the fingerprint method.
A brown sugar sample was extracted and concentrated with the organic solvent, and then the concentration was injected six times consecutively to calculate the RSD of relative RT and relative peak area of the odor-active compounds. These results showed that the RSD of the relative RT of the odor active compounds was less than 0.5%, and the RSD of the relative peak area was less than 6%, indicating that the precision of the instrument was good and met the requirements of the fingerprint method.
Five brown sugar samples were extracted and analyzed for their odor compounds, followed by the RSD of relative RT and relative peak area of the odor active compounds analysis. The results showed that the RSD of relative RT was less than 0.3%, and the RSD of the relative peak area was less than 7%, indicating that they had good repeatability and met the requirements of the fingerprint method.
2.5. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
The fingerprinting analysis of samples from the three origins of Guangdong, Guangxi, and Yunnan revealed that the majority of samples within each province had similar odor types. In addition, a supervised OPLS-DA multivariate statistical analysis method was used to establish a statistical model in order to distinguish odor compounds between Guangdong and Guangxi, Guangdong and Yunnan, and Guangxi and Yunnan.
By conducting OPLS-DA analysis on the brown sugar, a variable importance of projection diagram (VIP) of the model was obtained. A VIP is a vector that summarizes the contribution of a variable to the explanation of the model. Variables with a VIP >1 are generally considered to contribute to the explanation of the model [31,32]. The samples were assessed as independent variables, and the OPLS-DA model was fitted automatically.
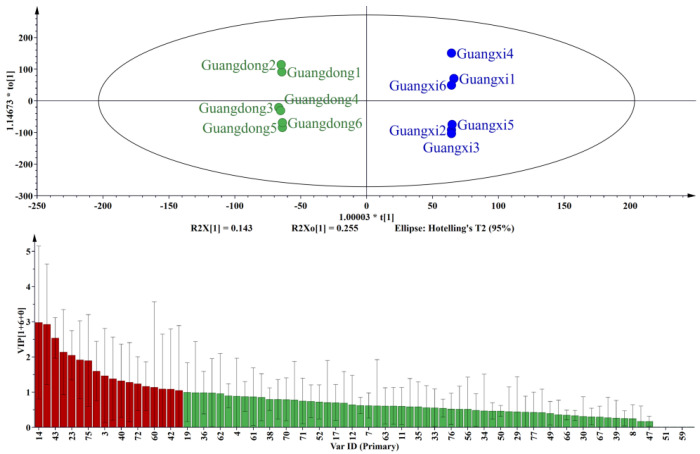
The OPLS-DA and VIP results (Figure 3) indicate that the brown sugars from Guangdong and Guangxi were well separated. The brown sugar from Guangdong and Guangxi showed the greatest degree of separation and low intra-group differences, facilitating an accurate exploration of the differences in composition. VIP diagram elucidated that 4-hydroxybenzaldehyde, 3,5-dimethoxy-4-hydroxybenzaldehyde, n-hexadecanoic acid, butanoic acid, acetic acid, 2-methoxy-4-acetylphenol, 2-acetylpyrrole, pentadecanoic acid, furfuryl alcohol, 4-hydroxyacetophenone, etc., were the main contributors to the distinction between Guangdong and Guangxi samples. These compounds were basically aldehydes, acids, ketones, and phenols. Among these, 3,5-dimethoxy-4-hydroxybenzaldehyde and 4-hydroxybenzaldehyde played an important role in classifying Guangdong and Guangxi. 4-Hydroxybenzaldehyde and 3,5-dimethoxy-4-hydroxybenzaldehyde presented a pleasant nutty and creamy odor. Previously, 4-hydroxybenzaldehyde and 3,5-dimethoxy-4-hydroxybenzaldehyde were identified as the major volatile constituents in brown sugars [33]. Acetic acid is also one of the key compounds that can distinguish brown sugar from two provinces. Acetate is a well-known product of the thermal degradation of saccharides, and it is primarily formed during the early stage of the Maillard reaction, under neutral and alkaline conditions. Acetic acid is formed exclusively by hydrolytic cleavage of β-dicarbonyl in hexose-based systems [34].
Figure 3.
OPLS-DA analysis and VIP diagram of brown sugar in Guangdong and Guangxi.
As shown in Figure 4, OPLS-DA analysis and VIP results indicate that the brown sugars from Guangdong and Yunnan are distinguishable. The principal compounds contributing to this distinction include n-hexadecanoic acid, acetic acid, dibutylphthalate, 2-acetylpyrrole, 2,5-dimethylpyrazine, and 2-methylpyrazine. Of the compounds with VIP greater than 1, pyrazine compounds appeared, which indicated that pyrazine compounds played a significant role in distinguishing brown sugar between Guangdong and Yunnan. The average content of pyrazines in Guangdong and Yunnan was 2897.28 ng/g and 1441.20 ng/g, respectively, and the pyrazine contents in Guangdong samples were higher than in Yunnan. These compounds could impart a popcorn, nutty, and roasted aroma to brown sugar.
Figure 4.
OPLS-DA analysis and VIP diagram of brown sugar in Guangdong and Yunnan.
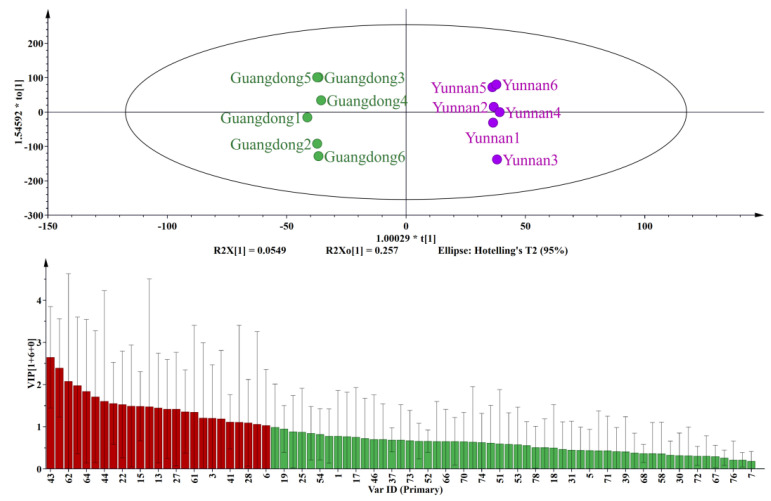
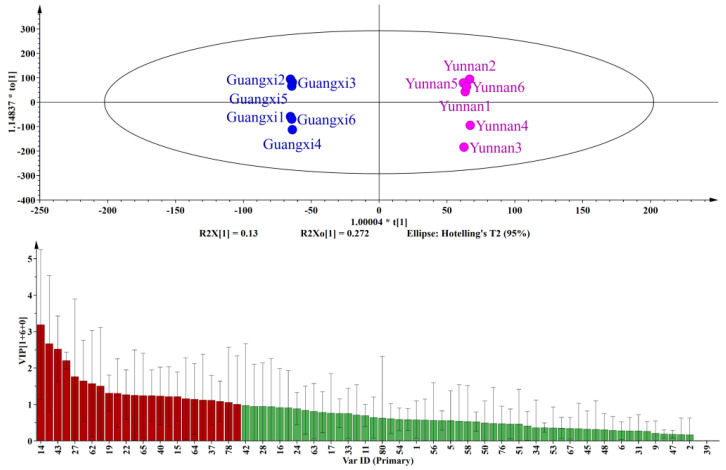
Based on the VIP diagram and OPLS-DA analysis of brown sugar between Guangxi and Yunnan (Figure 5), they were well separated. A number of compounds contributed to the differentiation between the two provinces, including 4-hydroxybenzaldehyde, 3,5-dimethoxy-4-hydroxybenzaldehyde, n-hexadecanoic acid, acetic acid, butanoic acid, and 4-hydroxyacetophenone. Of these volatile compounds, the contribution of 4-hydroxybenzaldehyde was the greatest. The average content of 4-hydroxybenzaldehyde in Guangxi was 2728.55 ng/g, while the samples from Guangxi had no odor compounds. The average contents of 3,5-dimethoxy-4-hydroxybenzaldehyde in Guangxi and Yunnan were 926.34 ng/g and 2967.95 ng/g and the contents in Yunnan were significantly higher than in Guangxi. Perhaps these compounds play an important role in distinguishing the sugars from Guangxi and Yunnan.
Figure 5.
OPLS-DA analysis and VIP diagram of brown sugar in Guangxi and Yunnan.
3. Materials and Methods
3.1. Materials
Eighteen brown sugar samples from Guangdong, Guangxi and Yunnan were provided by COFCO. These samples were stored in a refrigerator at −80 °C before analysis.
3.2. Standards and Reagents
Ether (purity > 99%), dichloromethane (purity > 99%), anhydrous sodium sulfate, 2-methyl-3-heptanone (purity > 99%) and n-alkane (C7-C30) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and carrier gas (helium) was purchased from Beijing AP Baif Gases Industry Co., Ltd. (Beijing, China).
3.3. Extraction of Odor Compounds from Sugars
The odor compounds in brown sugar were extracted by a liquid–liquid extraction (LLE) method according to Chen et al. [33]. In brief, 50.00 g of brown sugar was placed in a triangular flask, 50 mL of distilled water was added to dissolve the brown sugar, then, 50 mL of ether, 50 mL of dichloromethane and 5 μL of internal standard 2-methyl-3-heptanone (81.6 mg/mL) were added, and the mixture was magnetically stirred at 1000 rpm for 10 min. After centrifugation (Hitachi, Japan) for 30 min at 10,000 rpm, the extract containing the volatile aroma compounds was separated by a funnel. Subsequently, 150.0 g anhydrous sodium sulfate was added to the extract and put into a refrigerator at 4 °C to remove water for 12 h, and filtered with a filter paper. A gentle nitrogen stream was used to concentrate the volume into 100 μL, and the odor compounds were extracted and stored at −80 °C for further analysis.
3.4. GC-O-MS
Three well-trained panelists conducted a GC-O analysis of the concentrated distillate. The panelists were recruited from Beijing Technology and Business University’s Molecular Sensory Laboratory. To identify and describe the aroma characteristics of the reference compounds, they smelled several concentrations of reference compounds in model solutions 2 h per day before analysis. The training lasted for one month. For the GC-O analysis, wet gas was delivered to the nose using a blank capillary column to improve the sensitivity of the panelists. The aroma perceptions, intensity, and RT were recorded by the panelists. If two or more panelists detected the aroma, an aroma-active compound was identified [35].
To determine the volatile aroma profile of sugars, an Agilent 7890A gas chromatograph (GC) coupled with an Agilent 5977B mass spectrometer (MS) and a sniffing port (Gerstel, Germany) was used. The aroma extract (1 μL) was injected into a DB-Wax column (60 m × 0.25 mm i.d., film thickness 0.25 μm, Agilent J&W) through splitless mode, and the flow rate of the helium carrier gas was maintained at 1.7 mL/min. The oven temperature was initially programmed at 40 °C, further raised to 100 °C at a rate of 4 °C/min, following a gradual increase up to 200 °C at a rate of 3 °C/min for 5 min, and after achieving an ultimate temperature of 230 °C at a rate of 3 °C/min, it was maintained for 10 min. The interface and ion source were set at 250 °C and 230 °C, respectively, while the electron-impact ionization was set at 70 eV, the acquisition range (m/z) at 35–350 amu, and the scan rate at 1.77 scans/s. The transmission line temperature of the olfactory detection port (ODP) was maintained at 235 °C.
3.5. Qualitative Analysis
The ionization of a molecule in a vacuum produces a characteristic group of ions of different masses. The plot of relative abundance versus mass of these ions constitutes a mass spectrum. The spectrum can be used to identify the molecule. The unknowns were identified by comparing the fragments with the National Institute of Standards and Technology (NIST) MS Spectral Library (Version 2020), by comparing the odor percepts with the database (http://www.thegoodscentscompany.com) and by calculating the linear retention indices (LRIs) using a homologous series of n-alkanes (C7-C30). The use of multiple methods can increase the accuracy of qualitative results. Using the internal standard area, the resulting peaks were calibrated, and the aroma compound contents were expressed as nanograms per gram of sample [10].
3.6. Odor Activity Value (OAV)
In order to evaluate the contribution of each odorant to the overall aroma of brown sugar, the OAV (ratio of concentration to its odor threshold) was calculated [36]. These threshold values were derived from the literature in water [37].
3.7. Statistical Analysis
All experiments in this study were conducted in triplicates, and the data were expressed as mean ± standard deviation. The bar graph was drawn by OriginPro 2022 (OriginLab Corp., Northampton, MA, USA), the OPLS-DA analysis was conducted by SIMCA 14.1 (MKS Instruments, Andover, MA, USA), and the tables were organized by Microsoft Excel 2021 (Microsoft Corp., Redmond, WA, USA).
4. Conclusions
In summary, a total of 80 odor compounds, including 5 alcohols, 9 aldehydes, 8 phenols, 21 acids, 14 ketones, 5 esters, 12 pyrazines, and 6 other compounds, were detected in 18 brown sugar samples from three different provinces. The fingerprint analysis showed 90% similarity, indicating a close relationship among the odor components of brown sugars from each province without much difference. Further, the stability, accuracy, and repeatability of the fingerprint method were verified, and speculated that the method could meet the requirements of the fingerprint. In the future, fingerprint might have wider applications due to its characteristic of distinguishing geographical origin and food adulteration. Additionally, the OPLS-DA was employed to identify the tracing of brown sugar and to identify the compounds contributing to brown sugars’ volatile classification. The results demonstrated that 4-hydroxybenzaldehyde, 3,5-dimethoxy-4-hydroxybenzaldehyde, n-hexadecanoic acid, and acetic acid were the essential components in distinguishing the sugars from Guangdong, Guangxi, and Yunnan, validating the efficiency of OPLS-DA.
Acknowledgments
The present work was supported by China Oil and Food Import and Export Corporation (COFCO). No project number.
Abbreviations
| GC-O-MS | gas chromatography-olfactometry-mass spectrometry |
| LLE | liquid–liquid extraction |
| NCS | non-centrifugal cane sugar |
| COFCO | China Oil and Food Import and Export Corporation |
| GC | gas chromatography |
| MS | mass spectrometer |
| ODP | olfactory detection port |
| RSD | relative standard deviation |
| RT | retention time |
| RI | retention index |
| OPLS-DA | orthogonal partial least squares discriminant analysis |
| VIP | variable importance of projection |
| OT | odor threshold |
Author Contributions
Methodology, H.S.; Software, Y.Z. and W.L.; Writing—original draft preparation, E.C.; Writing—review and editing, H.S. and S.Z.; Supervision, H.S. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
Funding Statement
COFCO Nutrition and Health Research Institute Co., Ltd.: No number.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jader R., Fabian V., John E., Sebastian E., Oscar M. Thermal performance evaluation of production technologies for non-centrifuged sugar for improvement in energy utilization. Energy. 2018;152:858–865. doi: 10.1016/j.energy.2018.03.127. [DOI] [Google Scholar]
- 2.Feng S., Luo Z., Zhang Y., Zhong Z., Lu B. Phytochemical contents and antioxidant capacities of different parts of two sugarcane (Saccharum officinarum L.) cultivars. Food Chem. 2014;151:452–458. doi: 10.1016/j.foodchem.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.S., Ramalingam S., Jo I.G., Kwon Y.S., Bahuguna A., Oh Y.S., Kwon O.J., Kim M. Comparative study of the physicochemical, nutritional, and antioxidant properties of some commercial refined and non-centrifugal sugars. Food Res. Int. 2018;109:614–625. doi: 10.1016/j.foodres.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 4.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005;81:215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 5.EFSA Panel on Dietetic Products N. Allergies, Scientific Opinion on the substantiation of health claims related to various food (s)/food constituent (s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1489. [Google Scholar]
- 6.Asikin Y., Kamiya A., Mizu M., Takara K., Tamaki H., Wada K. Changes in the physicochemical characteristics, including flavour components and Maillard reaction products, of non-centrifugal cane brown sugar during storage. Food Chem. 2014;149:170–177. doi: 10.1016/j.foodchem.2013.10.089. [DOI] [PubMed] [Google Scholar]
- 7.Huang S.R., Hang F.X., Wei C.B., Xie C.F., Li K. Analysis of volatile aroma components in brown sugar. China Condiment. 2019;44:146–151. [Google Scholar]
- 8.Juliana M.G., Paulo C.N., Francisco J.H., Alvaro O., Coralia O. Physicochemical and sensory (aroma and colour) characterisation of a non-centrifugal cane sugar (“panela”) beverage. Food Chem. 2017;228:7–13. doi: 10.1016/j.foodchem.2017.01.134. [DOI] [PubMed] [Google Scholar]
- 9.Chen E., Song H., Li Y., Chen H., Wang B., Che X., Zhang Y., Zhao S. Analysis of aroma components from sugarcane to non-centrifugal cane sugar using GC-O-MS. RSC Adv. 2020;10:32276–32289. doi: 10.1039/D0RA05963C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asikin Y., Takahara W., Takahashi M., Hirose N., Ito S., Wada K. Compositional and Electronic Discrimination Analyses of Taste and Aroma Profiles of Non-Centrifugal Cane Brown Sugars. Food Anal. Methods. 2017;10:1844–1856. doi: 10.1007/s12161-016-0746-5. [DOI] [Google Scholar]
- 11.Cheng H., Wu W., Chen J., Haibo P., Xu E., Chen S., Ye X., Chen J. Establishment of anthocyanin fingerprint in black wolfberry fruit for quality and geographical origin identification. LWT. 2022;157:113080. doi: 10.1016/j.lwt.2022.113080. [DOI] [Google Scholar]
- 12.Li W., Chen Y.P., Blank I., Li F., Li C., Liu Y. GC × GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021;142:110222. doi: 10.1016/j.foodres.2021.110222. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q., Xi J., Xu D., Jin Y., Wu F., Tong Q., Yin Y., Xu X. A comparative HS-SPME/GC-MS-based metabolomics approach for discriminating selected japonica rice varieties from different regions of China in raw and cooked form. Food Chem. 2022;385:132701. doi: 10.1016/j.foodchem.2022.132701. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann T., Schieberle P. Formation of aroma-active Strecker-aldehydes by a direct oxidative degradation of Amadori compounds. J. Agric. Food Chem. 2000;48:4301–4305. doi: 10.1021/jf000076e. [DOI] [PubMed] [Google Scholar]
- 15.Moon J.K., Shibamoto T. Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. J. Agric. Food Chem. 2009;57:5823–5831. doi: 10.1021/jf901136e. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Guo M., Song H., Meng Q. Characterization of key aroma compounds in traditional Chinese soy sauce through the molecular sensory science technique. Lwt. 2020;128:109413. doi: 10.1016/j.lwt.2020.109413. [DOI] [Google Scholar]
- 17.Liu Y., He C., Song H. Comparison of SPME versus SAFE processes for the analysis of flavor compounds in watermelon juice. Food Anal. Methods. 2018;11:1677–1689. doi: 10.1007/s12161-018-1153-x. [DOI] [Google Scholar]
- 18.Raab T., López-Ráez J.A., Klein D., Caballero J.L., Moyano E., Schwab W., Muñoz-Blanco J. FaQR, Required for the Biosynthesis of the Strawberry Flavor Compound 4-Hydroxy-2,5-Dimethyl-3-(2H)-Furanone, Encodes an Enone Oxidoreductase. Plant. Cell. 2006;18:1023–1037. doi: 10.1105/tpc.105.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roscher R., Herderich M., Steffen J.-P., Schreier P., Schwab W. 2,5-Dimethyl-4-hydroxy-3-[2H]-furanone 6′O-malonyl-β-d-glucopyranoside in strawberry fruits. Phytochemistry. 1996;43:155–159. doi: 10.1016/0031-9422(96)00216-6. [DOI] [PubMed] [Google Scholar]
- 20.Kumazawa K., Masuda H., Nishimura O., Kato T. Identification of potent aroma components in brewed black tea. J. Jpn. Soc. Food Sci. Technol. Jpn. 1998;45:728–734. doi: 10.3136/nskkk.45.728. [DOI] [Google Scholar]
- 21.Lee K.E., Lee S.M., Choi Y.H., Hurh B.S., Kim Y.S. Comparative volatile profiles in soy sauce according to inoculated microorganisms. Biosci. Biotechnol. Biochem. 2013;77:2192–2200. doi: 10.1271/bbb.130362. [DOI] [PubMed] [Google Scholar]
- 22.Kallio H. Proceedings of the 5th International Flavor Conference, Porto Carras, Greece, 1987. J-GLOBAL; Tokyo, Japan: 1988. [(accessed on 2 August 2022)]. In Comparison and characteristics of aroma compounds from maple and birch syrups; pp. 241–248. Available online: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=200902006786988250. [Google Scholar]
- 23.Adams A., Polizzi V., Van Boekel M., De Kimpe N. Formation of Pyrazines and a Novel Pyrrole in Maillard Model Systems of 1,3-Dihydroxyacetone and 2-Oxopropanal. J. Agric. Food Chem. 2008;56:2147–2153. doi: 10.1021/jf0726785. [DOI] [PubMed] [Google Scholar]
- 24.Scalone G.L.L., Cucu T., De Kimpe N., De Meulenaer B. Influence of Free Amino Acids, Oligopeptides, and Polypeptides on the Formation of Pyrazines in Maillard Model Systems. J. Agric. Food Chem. 2015;63:5364–5372. doi: 10.1021/acs.jafc.5b01129. [DOI] [PubMed] [Google Scholar]
- 25.Cha J., Debnath T., Lee K.G. Analysis of α-dicarbonyl compounds and volatiles formed in Maillard reaction model systems. Sci. Rep. 2019;9:5325. doi: 10.1038/s41598-019-41824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vernin G., Parkanyi C. Occurrence and Formation of Heterocyclic Compounds. Ellis Horwood; New York, NY, USA: 1982. pp. 192–198. [Google Scholar]
- 27.Pickard S., Becker I., Merz K.H., Richling E. Determination of the alkylpyrazine composition of coffee using stable isotope dilution–gas chromatography–mass spectrometry (SIDA-GC-MS) J. Agric. Food Chem. 2013;61:6274–6281. doi: 10.1021/jf401223w. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko S., Kumazawa K. Aroma compounds in Japanese sweet rice wine (Mirin) screened by aroma extract dilution analysis (AEDA) Biosci. Biotechnol. Biochem. 2015;79:484–487. doi: 10.1080/09168451.2014.980218. [DOI] [PubMed] [Google Scholar]
- 29.Gonda I., Davidovich-Rikanati R., Bar E., Lev S., Jhirad P., Meshulam Y., Wissotsky G., Portnoy V., Burger J., Schaffer A.A., et al. Differential metabolism of L–phenylalanine in the formation of aromatic volatiles in melon (Cucumis melo L.) fruit. Phytochemistry. 2018;148:122–131. doi: 10.1016/j.phytochem.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Medina S., Pereira J.A., Silva P., Perestrelo R., Câmara J.S. Food fingerprints-A valuable tool to monitor food authenticity and safety. Food Chem. 2019;278:144–162. doi: 10.1016/j.foodchem.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 31.Beatriz G.P., Eriksson L., Trygg J. Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS) J. Chemom. 2014;28:623–632. [Google Scholar]
- 32.Huang B., Chen T., Xiao S., Zha Q., Luo P., Wang Y., Cui X., Liu L., Zhou H. A new approach for authentication of four ginseng herbs and their related products based on the simultaneous quantification of 19 ginseng saponins by UHPLC-TOF/MS coupled with OPLS-DA. RSC Adv. 2017;7:46839–46851. doi: 10.1039/C7RA06812C. [DOI] [Google Scholar]
- 33.Chen E., Song H., Zhao S., Liu C., Tang L., Zhang Y. Comparison of odor compounds of brown sugar, muscovado sugar, and brown granulated sugar using GC-O-MS. LWT. 2021;142:111002. doi: 10.1016/j.lwt.2021.111002. [DOI] [Google Scholar]
- 34.Davidek T., Gouézec E., Devaud S., Blank I. Origin and yields of acetic acid in pentose-based Maillard reaction systems. Ann. N. Y. Acad Sci. 2008;1126:241–243. doi: 10.1196/annals.1433.053. [DOI] [PubMed] [Google Scholar]
- 35.Yang F., Liu Y., Wang B., Song H., Zou T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. Lwt. 2021;137:110478. doi: 10.1016/j.lwt.2020.110478. [DOI] [Google Scholar]
- 36.Zhai X., Granvogl M. Characterization of the key aroma compounds in two differently dried Toona sinensis (A. Juss.) Roem. by means of the molecular sensory science concept. J. Agric. Food Chem. 2019;67:9885–9894. doi: 10.1021/acs.jafc.8b06656. [DOI] [PubMed] [Google Scholar]
- 37.Van Gemert L.J. Compilations of Odour Threshold Values in Air, Water and Other Media. 2nd ed. Oliemans, Punter & Partners BV; Utrecht, The Netherlands: 2011. [(accessed on 2 August 2022)]. p. 485. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201300091148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.