Abstract
Human trypanosomiasis affects nearly eight million people worldwide, causing great economic and social impact, mainly in endemic areas. T. cruzi and T. brucei are protozoan parasites that present efficient mechanisms of immune system evasion, leading to disease chronification. Currently, there is no vaccine, and chemotherapy is effective only in the absence of severe clinical manifestations. Nevertheless, resistant phenotypes to chemotherapy have been described in protozoan parasites, associated with cross-resistance to other chemically unrelated drugs. Multidrug resistance is multifactorial, involving: (i) drug entry, (ii) activation, (iii) metabolism and (iv) efflux pathways. In this context, ABC transporters, initially discovered in resistant tumor cells, have drawn attention in protozoan parasites, owing to their ability to decrease drug accumulation, thus mitigating their toxic effects. The discovery of these transporters in the Trypanosomatidae family started in the 1990s; however, few members were described and functionally characterized. This review contains a brief history of the main ABC transporters involved in resistance that propelled their investigation in Trypanosoma species, the main efflux modulators, as well as ABC genes described in T. cruzi and T. brucei according to the nomenclature HUGO. We hope to convey the importance that ABC transporters play in parasite physiology and chemotherapy resistance.
Keywords: ABC transporters, sleeping sickness, chagas disease, T. brucei, T. cruzi
1. Introduction
The Trypanosoma genus comprises several species of protozoan parasites of vertebrates that have the following characteristics: a region containing mitochondrial DNA, called the kinetoplast (Kinetoplastida class); a single flagellum (Trypanosomatidae family); and a heteroxenic evolutionary cycle in which it presents itself in one of the evolutionary forms as a trypomastigote in the vertebrate host [1]. Some peculiar characteristics of its cellular biology include: the presence of a flagellar pocket, located close to its single and large mitochondria, where its flagellum comes from, and which is also known for intense endo/exocytosis activity; the presence of glycosomes, which are peroxisome-like organelles that perform glycolysis; a cell membrane supported by a layer of microtubules, highly decorated by specific antigens that are critical for its survival; and proliferation by binary fission, though gene recombination is demonstrated and explains their great genomic complexity [2]. Most species have a heteroxenic life cycle, in which terrestrial vertebrate parasites have part of their cycle in the digestive tract of hematophagous insects and aquatic vertebrate parasites in leeches [3]. Two species of Trypanosoma are etiological agents of human disease, causing a great social and economic impact in endemic areas. T. cruzi is the etiological agent of American trypanosomiasis or Chagas’ disease and T. brucei, the etiological agent of African trypanosomiasis or Sleeping sickness. These two tropical diseases affect roughly eight million people worldwide and are responsible for over 12,000 deaths annually [4]. According to the World Health Organization (WHO), the estimated cost of medical treatment of these diseases exceed 500 million dollars a year (from 2015 to 2030). In addition, infected patients can present morbidities, resulting in damage to the local economy due to absence from work, decreased productivity and medical and chemotherapy expenses [5]. Vector control is the main way to reduce transmission, since there is no vaccine for preventive use in humans. Chemotherapy often has a high cure rate, especially in the early stages of diseases. With the disease progression and, consequently, parasite migration from the blood to the organs of tropism, the limited local bioavailability of the drug leads to a reduction in treatment efficiency. In addition, antitrypanocidal treatment is not able to reverse severe tissue damage caused by the presence of the parasites. Thus, depending on the stage of the disease, chemotherapy can be only reductive. Furthermore, it presents high toxicity leading to adverse effects that can make adherence difficult or prevent patients from continuing treatment [6]. Additionally, resistance to treatment has been reported in these species and results from multifactorial mechanisms, among which we can highlight the presence of ABC transporters [7]. ABC transporters work together to maintain stress response pathways and cellular detoxification and are involved in the multidrug resistance (MDR) phenotype. Although ABC transporters were described in these flagellated protozoa in the early 1990s, little is known about their physiological role and participation in treatment resistance. This review aims to summarize the findings on ABC proteins in these two diseases of epidemiological relevance, emphasizing their participation in chemotherapy resistance.
2. ABC Transporters
The ABC (ATP-binding cassette) comprises one of the largest families of proteins in living beings, presenting conserved ABC domains that bind and hydrolyze ATP. This energy is coupled to different cellular processes, including not only the transport of various molecules, but also housekeeping functions [8]. A typical ABC protein presents a nucleotide-binding domain (NBD), also known as an ABC module or ATPase. NBDs are present in a subgroup of the superfamily of P-loop NTPases, which depend on magnesium ions for catalysis. Each NBD has a core of 200 amino acids with two subdomains: the larger RecA-like subdomain, also found in other P-loop ATPases, and α-helical subdomain, unique to ABC proteins. NBDs are mainly identified by three highly conserved motifs: loop A or Walker A, loop P or Walker B and ABC signature or C motif, whose sequences and functions was summarized by Beek et al., 2014 [9]. A typical functional ABC transporter consists of two transmembrane domains (TMD) and two NBDs, and often additional domains connected in different ways [10]. These domains are encoded either as individual proteins or, in varying degrees of fusion, they are expressed as so-called half-size (TMD–NBD or NBD–TMD) or full-size (TMD–NBD–TMD–NBD or NBD–TMD–NBD–TMD) transporters [11]. In the case of half-size transporters, it is assumed that they form dimers or oligomers to carry out the transport of molecules.
All ABC proteins can be divided into three classes by function: classes 1 and 3 are involved with the transport of molecules across biological membranes and class 2 is involved with housekeeping functions [12]. Class 3 contains three types of transporters that are mostly associated with the exclusive function of importing molecules. The three types of ABC importers are found only in prokaryotes. Class 1 is involved with the function of importing and exporting molecules and is present in prokaryotes and eukaryotes [9,13]. To avoid confusion in the nomenclature of ABC members, the scientific community adopted the designation of subfamilies approved by the Human Genome Organization (HUGO). First, seven subfamilies from A to G were created based on sequence homology, phylogenetic relationship and domain organization of eukaryotic ABC proteins (mainly human, mouse and zebrafish genomes). It should be noted that ABCE and ABCF are not transporters; they have two NBDs and are involved in the control of mRNA translation. The enigmatic H subfamily was originally discovered by three genes from Drosophila, and then other genes were discovered in other organisms such as zebrafish and other insects, though they are absent in mammals and plants [14]. Later, subfamily I was created to harbor prokaryotic-like ABCs found in plants that are not present in most animal genomes [15]. In 2020, Thomas and colleagues proposed a new nomenclature for ABC proteins, supported by quantitative analyses using transmembrane helix scores based on the paired structural alignment of TMDs, now organizing its members into seven types of structures. Most eukaryote ABC transporters (subfamily A–G), presenting 6+6 transmembrane helix organization in TMDs, would form types IV and V [16].
One of the most studied ABC proteins is ABCB1, previously known as P-glycoprotein (P-gp or PGP, with P being for permeability) or multidrug resistance protein 1 (MDR1), the first ABC transporter cloned and characterized by its ability to confer resistance to colchicine, the main alkaloid of the poisonous plant meadow saffron, in Chinese hamster ovarian cells [17]. Although ABC was first described in tumor cells, ABCB1 is also expressed in normal tissues, mainly the blood–brain barrier, liver, kidney, intestine, placenta and adrenal, where it participates in the bioavailability of molecules and cellular detoxification [18,19]. ABCB1 can transport a wide variety of substrates, which vary in size, structure and function. Most are weakly amphipathic and relatively hydrophobic, generally containing aromatic rings and a positively charged nitrogen atom [20]. ABCB1 interacts with its substrates through Van der Waals and hydrophobic bonds. Each substrate appears to define a unique niche in the binding cavity by an induced adjustment mechanism [21]. The question remains as to whether the substrate removal would occur in the external aqueous phase (vacuum cleaner model) or whether it would just be translocated from the inner to the outer lipid layer (flippase model) [22]. It is likely that the two models could coexist, according to the size and hydrophobicity of the substrates.
Sixteen years after the discovery of ABCB1, the protein ABCC1 was identified in lung tumor cell lines resistant to anthracyclines, a class of key molecules in neoplastic chemotherapy [23]. The ABCC1 protein was involved in drug transport, being named the Multidrug Resistance Associated Protein 1 (MRP1) [24]. The ABCC1 transporter has a larger structure than the others, thanks to the presence of an extra module with five transmembrane helices, located in the N-terminal portion [25]. The functioning of the transporter is similar to that described for ABCB1 and the extra module does not have a known function; however, it has been proposed that it participates in three-dimensional organization [26]. The ABCC1 transporter shows ubiquitous expression in human tissues [27] and can mediate the cellular efflux of a variety of physiological organic anions, xenobiotics and their metabolites, with many of them co-transported or conjugated to glutathione (GSH), glucuronide or sulfate [28]. GSH is a tripeptide antioxidant and the main non-protein thiol of cells, critical for maintaining the redox state and several cellular processes including drug and free radical detoxification and apoptosis. GSSG is the oxidized dimeric form of GSH, which tends to accumulate in cells under conditions of oxidative stress. Due to their pro-oxidant activities, the maintenance of low GSSG levels and of an appropriate GSH:GSSG ratio are critical for normal cellular functions [29]. As the transport of GSH and GSSG can be carried out by ABCC1, this transporter has an important contribution to redox homeostasis and oxidative stress [27,30].
In 1998, Doyle and colleagues discovered the so-called breast cancer resistance protein (BCRP) and cloned it from a breast cancer cell resistant to doxorubicin, an anthracycline with antitumor and antibiotic properties [31]. In the same year, Allikmets and colleagues characterized a new ABC transporter gene highly expressed in the human placenta and named it ABC placental protein (ABCP) [32]. Additionally, Miyake and co-workers (1998) discovered and cloned two genes designated MXR1-2, which codifies mitoxantrone resistance protein 1 and 2, from several human strains resistant to mitoxantrone, another known anticancer drug [33]. The gene sequences and structure of BCRP, ABCP and MXR were almost identical and represented the same transporter, from now on renamed ABCG2, according to the HUGO nomenclature [34]. ABCG2 is a reverse half-size transporter with an NBD–TMD arrangement, which must form homodimers [35] or homotetramers [36] in order to be functional. ABCG2 recognizes a wide variety of molecules, both positively and negatively charged, organic anions and sulfated conjugates [37]. Similar to the other transporters mentioned, it plays an important role in cell detoxification [38]. Interestingly, ABCG2 is highly expressed in human stem cells, this expression being reduced with differentiation. Thus, ABCG2 is a marker for the “side population” phenotype, since the expression of other transporters was not observed in stem cells [39]. The physiological role of ABCG2 in these cells has not yet been fully determined, although it appears to be involved in the transport of perforins [40]. Similar to ABCB1, ABCG2 is also expressed by cells from the placenta, liver, kidney, intestine and blood–brain barrier [41].
ABC Modulators
Drugs can interact with ABC transporters in several ways. Briefly, an ABC agonist would be a substrate, that is, a drug that is transported by the protein, or an activator of ATPase activity. On the other hand, an ABC antagonist would be a drug capable of inhibiting or reducing drug transport or ATPase activity directly. As the transport function is dependent on ATP hydrolysis, generally the inhibition of the first has a direct impact on the second and vice-versa. An ABC substrate may also be an inhibitor, which prevents the binding of other drugs (competitive inhibition). This type of inhibition will depend on (i) the binding site(s) of the molecule, since ABC transporters have several binding sites for their substrates that may (or may not) overlap, and (ii) the affinity for the transporter [42]. Additionally, drugs can produce a bimodal effect on ATPase activity, where low concentrations stimulate ATPase activity and high concentrations inhibit it, as seen for verapamil (VP) in ABCB1 (see later). Nonetheless, an inhibitor of ATPase activity binds at the catalytic site, acting as a non-competitive inhibitor of transport, a well-known example is vanadate [43]. The association of vanadate with ATP in one of the NBDs results in trapping of the complex and complete abolition of ATPase activity. It is noteworthy that agents such as sodium azide, an inhibitor of the enzyme cytochrome C oxidase, or dinitrophenol and carbonyl cyanide m-chlorophenyl hydrazine (known as CCCP), which inhibit oxidative phosphorylation via decoupling, do not directly interact with ABC transporters but are also able to decrease drug efflux by ATP depletion [44].
Among the mechanisms of modulation of the MDR phenotype, the inhibition of ABCB1 is one of the most observed and was first demonstrated with VP, a calcium channel blocker, in 1981 [45]. In that study, VP increased the in vivo and in vitro cytotoxicity of leukemia cells resistant to vinca alkaloids. However, low concentrations of VP greatly stimulated the ATPase activity of ABCB1 purified and reconstituted in a lipid bilayer [46]. Ledwitch et al. (2016) demonstrated that the bimodal effect is attributable to the VP binding at two distinct sites in TMD [47]. At low concentrations, VP binds only at the external site, promoting a conformational change (from the open state to the intermediate state), which promotes a partial approximation of NBDs, reducing the energy barrier for ATP hydrolysis and substrate transport (stimulation). At high concentrations, VP occupies both sites and stabilizes the intermediate state, preventing the total approach of NBDs (closed state), reducing VP-induced ATP hydrolysis and substrate transport (inhibition). As VP is a substrate, its inhibition is also competitive, due to occlusion of substrate binding sites [48]. VP is also a known stimulator of ABCC1-mediated GSH transport [49]. Perrotton et al. (2007) demonstrated that VP isomers bind directly to ABCC1 [48]. Only the S isomer interferes with the GSH binding site, increasing the affinity of the transporter for tripeptide and being responsible for its rapid depletion. This effect is known as collateral sensitivity, as cells with an MDR phenotype are more sensitive to the drug, in this case VP, than parental cells. The R isomer competitively inhibits the binding of other drugs, promoting the accumulation of other drugs and reversing the MDR phenotype.
Currently, zosuquidar (known before as LY335979) is a difluoro-cyclopropyl dibenzosuberane derivative and one of most potent and selective third-generation inhibitors of ABCB1, capable of inhibiting in vitro and in vivo transport at nanomolar concentrations and reversing the MDR phenotype [50]. To date, there is no evidence that zosuquidar is able to modulate the efflux of ABCC1 and ABCG2 [51]. There is a great overlap of ABCB1 and cytochrome P450 isoforms substrates. Zosuquidar is able to inhibit some of these isoforms such as CYP3A; however, it has an affinity that is 60 times lower than ABCB1 [52].
Neyfakh (1988) showed that MDR cells had a reduced accumulation of fluorescent dyes compared to sensitive cells and that the use of inhibitors of the MDR phenotype restored normal accumulation [53]. The results introduced rhodamine 123 (rho 123), a cationic lipophilic dye, as a good fluorescent substrate for ABCB1 for efflux analysis by flow cytometry and fluorescence microscopy, since the use of anti-ABC protein antibodies does not always represent functionality of the transporter. Thus, a simple cell labeling procedure could be used to detect resistant cells and study the MDR phenotype. In the following years, other molecules were described and many ABCB1 modulators were found, as can be seen in the paper from Silva et al. (2015) [20].
The efflux activity from ABCC1 transporters can be measured using 5(6)-carboxyfluorescein diacetate (CFDA) dye. It is an acetoxymethyl ester derivative able to cross the plasma membrane efficiently. In the intracellular medium, CFDA is deacetylated by nonspecific esterases. After hydrolysis, the dye gives rise to the fluorescent substrate carboxyfluorescein, which is charged and retained in intact cells and quickly transported by ABCC1, but not by ABCB1 [54]. MK-571 is a potent and selective antagonist of cysteinyl leukotriene receptor 1, synthesized from the receptor backbone and proposed to attenuate the effects of bronchoconstriction induced by leukotriene signaling that occurs in diseases such as asthma [55]. Gekele et al. (1995) showed that MK-571 competitively inhibits the transport of leukotriene LTC4 and other organic anions transported by ABCC1, sensitizing MDR cells overexpressing the transporter [56]. It is noteworthy that the ABCC subfamily has 13 members, nine of which are called MRPs because they mediate the MDR phenotype by drug extrusion [57]. Unfortunately, MK-571 has the same effect on C subfamily members that transport LTC4, being able to inhibit almost, if not all, MRP members. Nevertheless, it does not affect ABCB1 transport [20,27]. As ABCC1 transport is dependent on GSH, its depletion is able to inhibit/reduce efflux. Depletion of GSH levels can be achieved by treatment with buthionine sulfoximine (BSO), an inhibitor of the GSH biosynthesis pathway, or by thiol alkylating agents such as N-ethylmaleimide (NEM) or iodoacetic acid [58,59]. A list of the modulators of ABCC member transport can be found in the paper by Zhou et al. (2008) [60].
For ABCG2, rho 123 and mitoxantrone are the most commonly used fluorescent substrates [61]. Both are also transported by ABCB1, whose MDR phenotype overlaps considerably with ABCG2 [20,37]. However, two known mutations that occur in the ABCG2 gene during the course of drug selection can alter substrate specificity. For instance, the wild-type ABCG2 gene has an arginine residue at position 482 and cells overexpressing wild-type ABCG2 are capable of extruding mitoxantrone. Nonetheless, cells containing a threonine or glycine residue at the same position are able to transport not only mitoxantrone but also rho 123 [62]. For that reason, mitoxantrone appears to be a better substrate. Fumitremorgin C (FTC) is an organic heteropentacyclic compound that is a prenylated indole alkaloid derived from some fungi such as Aspergillus fumigatus. FTC is able to selectively reverse ABCG2-transporter-induced resistance [63,64]. At subtoxic concentrations it has limited, if any, effects on ABCB1 and ABCC transporters. Fumitremorgins have known neurotoxic effects such as tremors and convulsions in animals [65,66], and perhaps for this reason it has not been tested in patients. A tetracyclic analogue of FTC, known as Ko143, proved to be one of the most specific inhibitors among those known for ABCG2 without showing toxicity in mice [67]. However, at higher concentrations it can interact with both ABCB1 and ABCC1 in vitro [68]. Szafraniec et al. (2014) and Peña-Solárzono et al. (2017) summarized the other ABCG2 modulators tested [37,69]. The fluorescent substrates and ABC inhibitors are listed in Table 1.
Table 1.
Fluorescent substrates and ABC inhibitors for efflux analysis.
| ABCB1 | ABCC1 | ABCG2 | |
|---|---|---|---|
| FLUORESCENT SUBSTRATE | Rhodamine 123 | CFDA | Mitoxantrone |
| SELECTIVE INHIBITOR | Zosuquidar | MK-571 | Ko-143 |
| ATPASE ACTIVITY INHIBITOR | Vanadate | ||
| ATP DEPLETION | Sodium azide, dinitrophenol, CCCP | ||
| THIOL DEPLETION | Iodoacetic acid, N-ethylmaleimide, Buthionine sulfoximine |
||
CCCP: Carbonyl cyanide m-chlorophenyl hydrazine; MK-571: L-660711, 5-(3-(2-(7-Chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid; Ko143: (3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino [1′,2′:1,6]pyrido [3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester.
3. Human Pathogens of the Trypanosoma Genus
3.1. T. cruzi
Chagas disease or American trypanosomiasis is a zoonosis caused by the protozoan T. cruzi, named in honor of Dr. Oswaldo Cruz. In 1909, the disease was discovered by Dr. Carlos Chagas who published a study with a detailed description of the life cycle of the parasite, vectors and animals that act as hosts, and the clinical characteristics of the disease [70,71]. The WHO estimates that six to eight million individuals are chronically infected with T. cruzi in the world, most of them in Latin America, where the disease is endemic [5]. Nevertheless, there is an increasing number of cases in non-endemic regions, such as in European countries, Australia and North America, as a result of migratory processes and alternative routes of disease transmission. The United States has the largest number of infected people outside of the endemic area, with an estimated 300,000 people with Chagas disease living in the country [72].
After an incubation period of approximately two weeks, Chagas disease has an acute phase lasting about two months, characterized by high parasitemia. The acute phase is recognized in few infected individuals, as most cases are asymptomatic or have non-specific symptoms. In the absence of treatment, the infection progresses to the chronic phase [73]. Most individuals remain asymptomatic over the years, without any detectable anatomical and/or physiological alterations, although they present positive serology for T. cruzi, characterizing the indeterminate form of Chagas disease. Nonetheless, some infected people may have cardiac, digestive or mixed symptoms, hallmarking the determinate form. These clinical manifestations usually appear between 10 and 30 years after the initial acute infection and affect about 30% of patients. The cardiac form is the most expressive, due to its frequency and severity, causing sudden death in two-thirds of deaths [74,75]. In 1967, nifurtimox was the first oral drug used in the treatment of the acute phase of Chagas disease. Benznidazole is a nitroimidazole derivative synthesized by Wineholt and Liebman in 1972 and the first-choice treatment for Chagas disease thanks to its better efficacy and safety profile [76]. Both drugs are effective in the acute phase and present reduced efficacy with disease progression or presence of severe clinical manifestations [75]. Benznidazole is an oral drug that requires prolonged treatment and presents side effects that are generally dermatological such as itching and rashes, frequent in 30% to 50% of cases, responding well to antihistamines. However, peripheral neuropathy, common in up to 30% of cases, and rare bone marrow depression can develop in patients and lead to immediate discontinuation of treatment [77].
The life cycle of T. cruzi is complex and involves morphologically distinct developmental stages, alternating between a triatomine invertebrate vector, also known as a kissing bug, and a mammalian vertebrate host. Epimastigotes are the extracellular replicative forms found in the triatomine’s gut, where they differentiate into infective metacyclic trypomastigotes. Amastigotes are replicative forms found in mammalian nucleated cells that differentiate into bloodstream trypomastigotes [78,79]. T. cruzi is a diploid organism and its genome is distributed in pairs of homologous chromosomes that vary in size and number between lineages, indicating high genomic plasticity [80,81]. In 2009, an intraspecific nomenclature for the parasite was proposed by a committee that recognized six genetic lineages or discrete typing units (DTUs), named TcI to TcVI. The strains belonging to the TcI and TcII clade are recognized as pure, while TcV and TcVI are heterozygous hybrids, possibly of the TcII and TcIII parents. TcIII and TcIV are homozygous hybrids and their evolutionary line is still a matter of debate. A seventh DTU was identified as a monophyletic lineage in bats, and is called Tcbat [82]. In 2016, Breniere et al. investigated 137 papers and found a total of 6343 strains of T. cruzi distributed in the seven DTUs from 158 host species [83].
In 1987, Filardi and Brener demonstrated the existence of naturally susceptible and resistant strains to the drugs benznidazole and nifurtimox, which could explain the epidemiologic variations in the effectiveness of chemotherapy in infected vertebrate hosts [84]. The first evidence of the involvement of ABC transporters in drug resistance came from the study by Neal et al. (1989) who developed parasites of strain X10, clone 1, resistant to nifurtimox in vitro [85]. The nifurtimox-resistant parasites had a half maximal inhibitory concentration (IC50) value of 19.6 µM, while the parental parasites had 7 µM. Treatment with VP alone had no effect on the IC50 value, but the combination of nifurtimox with 1 µM of VP reduced the IC50 of resistant parasites to 2.4 µM.
In 1994, Dallagiovanna et al. were the first to identify sequences homologous to the NBDs from PGPA genes of Leishmania tarentolae resistant to methotrexate, used to treat certain types of cancer [86], in the Y strain of T. cruzi [87]. Using the same probe that identified LtPGPA, the authors isolated gene fragments that were sequenced and designated as TcPgp1 and TcPgp2, though the predicted amino acid sequence showed more identity with ABCC1 than ABCB1 [87,88]. Nucleotide sequence analysis of the TcPgp2 gene revealed an open reading frame (ORF) of 4,602 bp that encoded a 1,534 amino acid polypeptide with an estimated molecular mass of approximately 170 kDa [88]. The structure is similar to that of other ABC proteins, divided into two halves containing six transmembrane helices that make up the TMD and an NBD (Figure 1). The gene is equally transcribed between evolutionary forms, but mRNAs were identified only in the stages of epimastigotes and trypomastigotes, suggesting a post-transcriptional regulation. Due to the difficulty of transfecting TcPgp2 in epimastigotes of the T. cruzi Y strain, Dallagiovanna et al. (1996) performed the transfection in L. tropica to evaluate the role of this gene in drug resistance [88]. The transfection resulted in the overexpression of TcPgp2 transcripts; however, there was no difference in the IC50 value in relation to parental cells for different drugs such as benznidazole, nifurtimox, puromycin and daunorubicin. At that time, human ABCC1 showed itself as a transporter of compounds conjugated to GSH, depending on the molecule for efflux of metabolites and drugs. Because of the gene sequence identity of Leishmania PGPA, and hence TcPgp2, with human ABCC1 [87], it was suspected that these transporters in protozoa could be transporters of trypanothione [T(SH)2], or GSH-conjugated compounds. In trypanosomatids, the main non-protein thiol is T(SH)2 that has two GSH molecules linked to a polyamine chain, usually spermidine [89].
Figure 1.
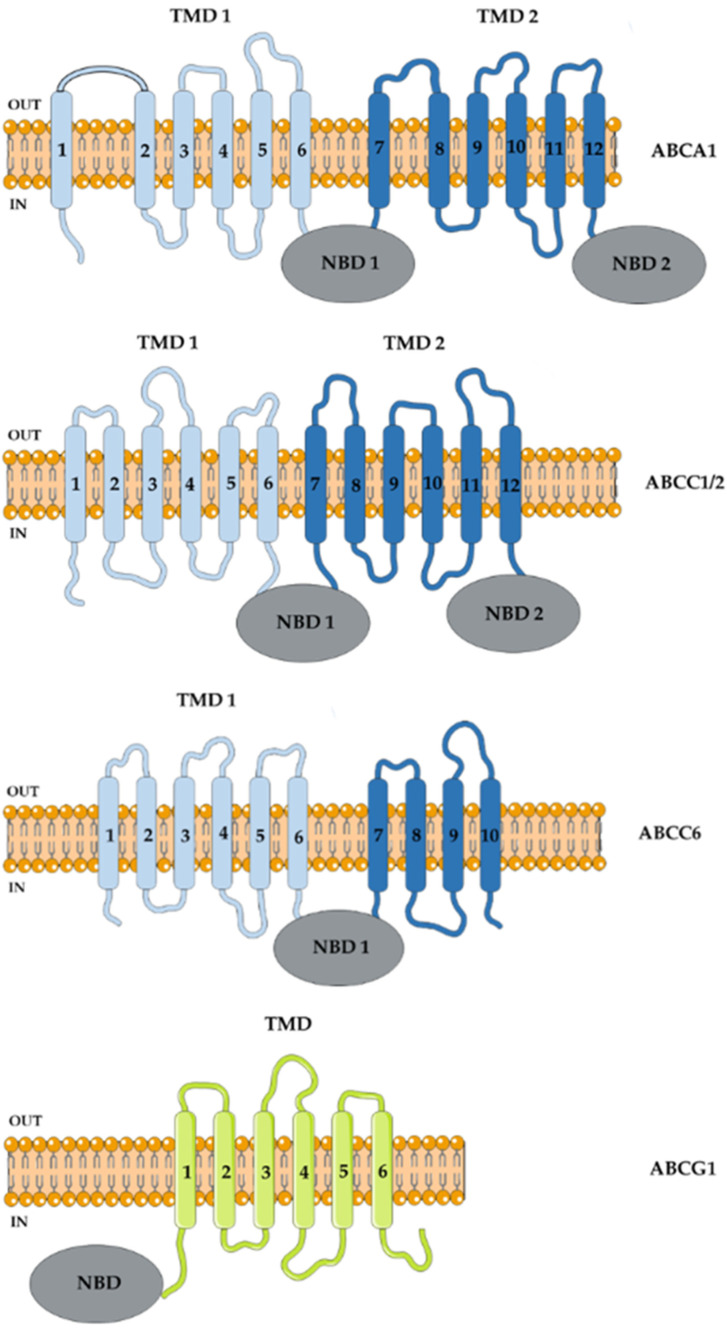
T. cruzi ABC proteins. ABC proteins share a nucleotide-binding domain (NBD), displaying conserved sequences among organisms. A typical full-size ABC transporter presents, in addition to the two NBDs, two transmembrane domains, which could be rearranged in direct (TMD–NBD) or reverse (NBD–TMD) order. Half-size transporters have only a TMD and an NBD, assuming that they form dimers or oligomers to transport substrates across membranes. The standardized names of ABC proteins in subfamilies according to HUGO nomenclature are represented by the forth letter, followed by number. For example ABCA1 is subfamily A, member 1.
Subsequently, Torres et al. (1999) examined the sequence of the TcPgp1 gene [90]. It has an ORF of 3105 bp that encodes a polypeptide of 1,305 amino acids with an estimated molecular mass of 115,252 Da. The primary structure of the first half of the TcPgp1 gene is very similar to that of other ABC transporters (Figure 1). Nonetheless, in the second half of the polypeptide chain the 11th and 12th transmembrane helices and the second NBD are absent owing to the insertion of a non-long terminal repeat retrotransposon cDNA with 99% homology to L1Tc, already described in T. cruzi [91]. In epimastigotes, the presence of truncated TcPgp1 transcripts were observed [90], suggesting that they may be functional as homo- or heterodimers, as seen with ABCG2, but this hypothesis has not yet been confirmed.
In an attempt to find a molecular marker associated with benznidazole resistance in T. cruzi, Murta et al. (1998) identified polymorphisms in TcPgp genes in chemotherapy-sensitive strains [92]. On the other hand, the same group did not observe overexpression or amplification of the TcPgp1 and TcPgp2 genes in benznidazole-resistant strains [93], corroborating the data from Torres et al. (1999) [90]. With the completion of Leishmania genome sequencing, Leprohon et al. (2006) analyzed and classified 42 ORFs from ABC genes found in the genome of L. major and L. infantum, according to the HUGO nomenclature [94]. Orthologous ABC genes were found in other trypanosomatids: 27 ORFs in the T. cruzi genome and 22 ORFs in T. brucei. The gene analysis of TcPgp1 and TcPgp2 showed great homology with the ABCC6 and ABCC2 genes of L. major and T. brucei, respectively.
In 2004, Torres and colleagues identified and sequenced a 5253 bp ORF encoding a 1750 amino acid protein with a predicted molecular weight of approximately 200 kDa [95]. The gene was named TcABC1 and appears to be an ABCA-like transporter, as it shares 33% and 38% amino acid sequence identity with human ABCA1 and ABCA3, and 41% and 47% with LtrABC1.1 and LtrABC1.2 of the ABCA subfamily of L. tropica, respectively (Figure 1). This transporter was found to be expressed in the plasma membrane, in the flagellar pocket and in vesicles in the epimastigote and amastigote replicative forms, and it was absent in the trypomastigote infective form. Many members of the ABCA subfamily participate in the trafficking and transport of lipids, including cholesterol that is an important component of cell membranes, regulating fluidity and functionality [96]. Similarly, ABCA from T. cruzi appears to be involved in endocytic and exocytic pathways [95].
Heme is an important cofactor necessary for many cellular processes, although trypanosomatids partially or totally lack the enzymes of its biosynthesis pathway [97]. Using the fluorescent heme analog metalloporphyrin, Lara et al. (2007) observed a non-endocytic mechanism of heme accumulation in reservosomes of the Y and Dm28c strains of T. cruzi, suggesting specific transmembrane transport [98]. Since ABC transporters are involved with iron transport in bacteria [99] and mammals [100], the authors examined the transport of this analogue in the presence of cyclosporin A (csA) (for inhibition of ABCB1) and indomethacin (for inhibition of ABCC1). The results showed that heme analogue transport was inhibited by csA but not by indomethacin, suggesting that an ABCB1 transporter homologue may be involved [98]. CsA is a cyclic, neutral, lipophilic undecapeptide with immunosuppressive activity produced by a variety of fungi, notably Tolypocladium inflatum [101]. Resistance-reversing activity was described by Slater et al. (1986), who showed that csA completely reversed cross-resistance to vinca alkaloids in leukemia strains [102]. Subsequently, Tamai and Safa demonstrated that this resistance reversal was due to the competitive inhibition of csA in the ABCB1 transporter [103]. Indeed, csA is a broad-spectrum modulator because it is able to inhibit the efflux, not only of ABCB1, but also of ABCC1 and ABCG2 [104,105]. Indomethacin is a non-steroidal anti-inflammatory drug, a non-selective inhibitor of cyclooxygenase that catalyze the formation of prostaglandins [106] and an organic anion transport modulator. Therefore, it is able to inhibit ABCC subfamily members [107], ABCB1 [20,108] and other transporters not belonging to the ABC superfamily, such as the organic anion transporter 2 (OAT2) from the solute carrier subfamily [109].
CsA inhibits the proliferation of epimastigote forms of T. cruzi in vitro, but increases parasitemia and accelerates mortality in infected mice due to its immunosuppressive effects [110]. Non-immunosuppressive derivatives of csA showed an antiparasitic response in vivo and in vitro [111]. Bua et al. (2008) associated this trypanocidal effect as a result of csA binding to two possible targets: (i) cyclophilins, whose enzymatic activity was sensitive to csA and its derivatives, and (ii) ABCB1, since csA and its derivatives inhibited rho 123 efflux in T. cruzi [112]. The authors demonstrated that csA and its derivative F-7-62 reduced the amount of fluorescence in the supernatant of parasites incubated with rho 123, suggesting inhibition of the efflux. The results were similar to controls performed with VP and heat-killed parasites.
Campos and collaborators (2013) induced resistance to the drug benznidazole or to the synthetic compound derived from thiosemicarbazone after 15 passages of the Y strain in the presence of the drugs [113]. The latter was used because of its potent cytotoxic activity in T. cruzi [114]. The acquisition of the resistant phenotype coincided with increased levels of transcripts for the TcPgp1 and TcPgp2 genes and with cross-resistance to the drugs vinblastine, paclitaxel and daunorubicin, transported by both ABCB1 and ABCC1 [113]. The authors demonstrated the reversal of the MDR phenotype when parasites were treated with the selection drug in the presence of VP or csA, suggesting the participation of an ABC transporter. These results were corroborated by the higher consumption of basal ATP in the presence of selection drugs in resistant strains in relation to parental strains. An efflux of rho 123 inhibited by VP or csA was identified and the authors suggest the participation of an ABCB1 transporter. However, rho 123 can also be transported by ABCG2, and the VP and csA inhibitors exhibit a broad spectrum.
In 2015, a 1998 bp ABC gene with a sequence of 665 amino acids was discovered in the CL Brener hybrid strain [115]. The predicted secondary structure showed that it was a half-size transporter with an NBD–TMD arrangement typical of the ABCG subfamily, then called ABCG1 (Figure 1), with 29% similarity to human ABCG2 and more than 50% with ABCG1 and ABCG2 from Leishmania [116]. Naturally resistant strains to benznidazole overexpressed ABCG1 transcripts and protein compared to sensitive ones. The transfection of the CL Brener strain with a vector containing the TcABCG1 gene from the naturally resistant strains YuYu and Silvio X10 clone 11 increased the TcABCG1 transcript and protein level and resulted in an increase in the IC50 value for benznidazole in relation to the wild type or vector-transfected strains [115]. The results suggest that the ABCG1 transporter is related to natural resistance to benznidazole in T. cruzi strains. Several single nucleotide polymorphisms in the gene sequence [115] and of amino acid variations in the predicted protein sequence [116] were identified in the TcABCG1 gene, some of which were specific DTUs. Conversely, these differences could not be associated with drug resistance. In an attempt to unravel the mechanisms involved in benznidazole resistance, the same group mapped the ABCG1 gene in 33 species from seven genera of the Trypanosomatidae family [117]. The authors demonstrated that most benznidazole-resistant species had two copies while Trypanosoma species had one copy of the gene per haploid genome. On the other hand, functional analyses were not performed to corroborate the participation of this transporter in drug resistance.
Analysis of the predicted protein sequence for the TcPgp1 and Tcpgp2 genes showed high identity and similarity with the ABCC6 and ABCC1/2 genes of T. cruzi, identified by Leprohon and collaborators by homology to ABC transporter genes of Leishmania. Our group identified an ABCC1-like efflux in the Y strain of T. cruzi, that was higher in epimastigote than trypomastigote forms. CFDA efflux was shown to be sensitive to: depletion of ATP levels, performed by treatment with sodium azide or iodoacetic acid; depletion of thiol levels, performed by treatment with BSO or NEM; the removal of glucose from the medium during the assay; and also temperature changes [44]. In addition, CFDA efflux was inhibited by MK-571, specific for the ABCC subfamily, and also by probenecid and indomethacin, inhibitors of organic anion transporters in general. GSH, GSSG, ceramide and hemin porphyrin were identified as possible endogenous substrates [118], as well as GSH-conjugated compounds [44], suggesting the participation of this transporter in cellular stress and detoxification pathways. ABCC1-like transporters do not appear to directly transport benznidazole and do not appear to be involved in natural benznidazole resistance, as strains that are naturally sensitive to the drug (CL Brener and Berenice) showed more activity than naturally resistant strains (Y and Colombian) [118]. Da Costa et al. (2021) induced resistance by prolonged exposure to benznidazole, which resulted in a 10-fold increase in the IC50 value in the Y strain. This induction of resistance promoted an increase in the ABCC1-like activity, suggesting the participation of the transporter in drug metabolism. The reversal of benznidazole resistance was dependent on the GSH pathway, suggesting that a GSH-conjugated benznidazole compound would be transported. It is noteworthy that there was no ABCB1-like efflux in any of the analyzed T. cruzi strains, neither in the epimastigote or trypomastigote forms. Rho 123 efflux was not inhibited, even in the presence of VP, csA and trifluoperazine, three inhibitors capable of inhibiting ABCB1, nor by ATP depletion [44]. T. cruzi had a half-size ABCB1 gene identified in its genome, although the results suggest that there is no functional transport for it. The ABC proteins from T. cruzi are listed in Table 2 and displayed in Figure 1.
Table 2.
T. cruzi ABC proteins.
| Nomenclature | T. cruzi Gene ID | NCBI Reference Sequences | Location | Putative Protein Length | T. cruzi Strain | Alias |
|---|---|---|---|---|---|---|
| ABCA1 | TcCLB.504881.50 * | XP_809857 | TcChr27 | 1750 | CL Brener Esmeraldo-like | |
| TcCLB.510045.20 * | XP_806887 | TcChr27 | 967 | CL Brener Non-Esmeraldo-like | ||
| ABCA2/4 | TcCLB.507099.80 (a) | XP_817325 | TcChr14 | 1836 | CL Brener Esmeraldo-like | |
| ABCA3/5 | TcCLB.504149.20 * (b) | XP_818098 | TcChr27 | 1750 | CL Brener Esmeraldo-like | ABC1 [94] |
| TcCLB.503573.9 * (c) | XP_803907 | TcChr27 | 967 | CL Brener Non-Esmeraldo-like | ||
| ABCA10 | TcCLB.510149.80 * | XP_813909 | TcChr36 | 1865 | CL Brener Esmeraldo-like | |
| TcCLB.506989.30 * | XP_818638 | TcChr36 | 1866 | CL Brener Non-Esmeraldo-like | ||
| ABCA11 | TcCLB.511725.80 | XP_818719 | TcChr35 | 2260 | CL Brener Non-Esmeraldo-like | |
| ABCB1 | TcCLB.507093.260 | XP_820554 | TcChr39 | 661 | CL Brener Esmeraldo-like | |
| ABCB3 | TcCLB.511537.8 * |
XP_806158 XP_809384 |
TcChr35 | 537 | CL Brener Esmeraldo-like | |
| TcCLB.511021.70 * | XP_811319 | TcChr35 | 735 | CL Brener Non-Esmeraldo-like | ||
| ABCC1/2 | TcCLB.506417.10 (d) pseudogene |
- | ? | 1577 | CL Brener | Tcpgp2 [86] |
| ABCC6 | TcCLB.509007.99 pseudogene |
- | TcChr31 | 1425 | CL Brener Esmeraldo-like | |
| TcCLB.507079.30 * | XP_805658 | TcChr31 | 388 | CL Brener Esmeraldo-like | ||
| TcCLB.457101.30 * | XP_805394 | ? | 253 | CL Brener | ||
| TcCLB.508965.14 * | XP_815145 | TcChr31 | 765 | CL Brener Non-Esmeraldo-like | Tcpgp1 [86] | |
| ABCC9 | TcCLB.510231.29 * | XP_805357 | TcChr34 | 854 | CL Brener Esmeraldo-like | |
| TcCLB.447255.29 * | XP_803480 | TcChr34 | 220 | CL Brener Esmeraldo-like | ||
| TcCLB.506559.100 * | XP_821792 | TcChr34 | 1472 | CL Brener Non-Esmeraldo-like | ||
| ABCD1 | TcCLB.506925.530 | XP_821597 | TcChr39 | 664 | CL Brener Esmeraldo-like | |
| ABCD2 | TcCLB.508927.20 * | XP_804559 | TcChr31 | 674 | CL Brener Esmeraldo-like | |
| TcCLB.509237.30 * | XP_814630 | TcChr31 | 674 | CL Brener Non-Esmeraldo-like | ||
| ABCD3 | TcCLB.510431.150 | XP_819234 | TcChr39 | 635 | CL Brener Esmeraldo-like | |
| ABCE1 | TcCLB.508637.150 * | XP_815243 | TcChr10 | 647 | CL Brener Non-Esmeraldo-like | |
| TcCLB.511913.9 pseudogene |
- | TcChr10 | 418 | CL Brener Non-Esmeraldo-like | ||
| TcCLB.464879.9 * | XP_802148 | TcChr10 | 339 | CL Brener Non-Esmeraldo-like | ||
| ABCF1 | TcCLB.504867.20 * | XP_812776 | TcChr36 | 723 | CL Brener Esmeraldo-like | |
| TcCLB.510943.80 * | XP_817081 | TcChr36 | 723 | CL Brener Non-Esmeraldo-like | ||
| ABCF2 | TcCLB.508897.30 | XP_810886 | TcChr40 | 594 | CL Brener Non-Esmeraldo-like | |
| ABCF3 | TcCLB.509105.130 | XP_814891 | TcChr37 | 673 | CL Brener Non-Esmeraldo-like | |
| ABCG1/2 | TcCLB.506249.70 * (e) | XP_806666 | TcChr37 | 665 | CL Brener Esmeraldo-like | |
| TcCLB.508231.190 * (f) | XP_818614 | TcChr37 | 665 | CL Brener Non-Esmeraldo-like | ABCG1 [114] | |
| ABCG3 | TcCLB.506249.70 * (e) | XP_806666 | TcChr37 | 665 | CL Brener Esmeraldo-like | |
| TcCLB.508231.190 * (f) | XP_818614 | TcChr37 | 665 | CL Brener Non-Esmeraldo-like | ||
| ABCG4 | TcCLB.506579.10 * | XP_811527 | TcChr7 | 700 | CL Brener Esmeraldo-like | |
| TcCLB.507241.39 * | XP_806410 | TcChr7 | 290 | CL Brener Non-Esmeraldo-like | ||
| ABCG5 | TcCLB.504425.70 * | XP_813191 | TcChr22 | 1171 | CL Brener Esmeraldo-like | |
| TcCLB.509331.200 * | XP_816786 | TcChr22 | 1170 | CL Brener Non-Esmeraldo-like | ||
| ABCG6 | TcCLB.507681.100 | XP_818599 | TcChr4 | 682 | CL Brener Non-Esmeraldo-like | |
| ABCH1 | TcCLB.510381.20 * | XP_807302 | TcChr27 | 303 | CL Brener Esmeraldo-like | |
| TcCLB.506905.40 * | XP_806924 | TcChr27 | 303 | CL Brener Non-Esmeraldo-like | ||
| ABCH2 | TcCLB.509669.30 * | XP_816112 | TcChr36 | 318 | CL Brener Esmeraldo-like | |
| TcCLB.509617.80 * | XP_809836 | TcChr36 | 318 | CL Brener Non-Esmeraldo-like | ||
| ABCH3 | TcCLB.511753.100 * | XP_812902 | TcChr32 | 496 | CL Brener Esmeraldo-like | |
| TcCLB.511501.30 * | XP_805965 | TcChr32 | 502 | CL Brener Non-Esmeraldo-like | ||
| OTHERS | TcCLB.506529.160 * | XP_821943 | TcChr6 | 937 | CL Brener Esmeraldo-like | |
| TcCLB.510885.70 * | XP_816152 | TcChr6 | 937 | CL Brener Non-Esmeraldo-like | ||
| OTHERS | TcCLB.508809.30 * | XP_809200 | TcChr23 | 1241 | CL Brener Esmeraldo-like | |
| TcCLB.506619.90 * | XP_812627 | TcChr23 | 1241 | CL Brener Non-Esmeraldo-like | ||
| OTHERS | TcCLB.507105.70 * | XP_811423 | TcChr35 | 1027 | CL Brener Esmeraldo-like | |
| TcCLB.506817.20 * | XP_809803 | TcChr35 | 999 | CL Brener Non-Esmeraldo-like |
Subfamily names follow the HUGO nomenclature, according to Leprohon et al. (2006) [93]. Gene accession numbers and other information was retrieved from TriTrypDB (tritrypdb.org) and GeneDB database (http://www.genedb.org/Homepage accessed on 10 July 2022). Letters after gene accession numbers indicate genes that present more than one nomenclature due to similar phylogeny to different L. major and T. brucei genes. Asterisks represent gene fragments of alleles that represent the same T. cruzi ORF. (-) represents that the gene ID does not have a protein sequence registered in the NCBI. (?) represents that the gene ID does not have a T. cruzi chromosomal location in the TriTrypDB.
3.2. T. brucei
Human African trypanosomiasis or sleeping sickness is caused by two subspecies of the protozoan T. brucei. In 1902, Joseph Everett Dutton identified and named T. b. gambiense as the cause of chronic sleeping sickness or the Gambian form [119]. In 1910, John W.W. Stephens and Harold Fanthan identified T. b. rhodesiense as the cause of acute sleeping sickness or the Rhodesian form [120]. The disease is unique to sub-Saharan Africa: the Gambian form is found in 24 countries in Central and West Africa, while the Rhodesian form is found in 13 countries in East Africa. Uganda is the only country to have both forms of the disease with non-overlapping regions [121]. The Gambian form accounted for 85% of reported cases in 2020, with the majority occurring in the Democratic Republic of Congo. As a result of disease control and surveillance measures in endemic areas, less than 1000 new cases were reported annually to the WHO since 2018 [122]. WHO data from 2015 estimated that 50–70 thousand people had sleeping sickness [5], with an estimated five million people living in areas of moderate to high risk for sleeping sickness [123]. Currently, sleeping sickness is considered under control by the WHO, which intends to interrupt its transmission by 2030 [5]. On the other hand, it must be considered that the Rhodesian form is a zoonosis, whose main reservoir is cattle [124], thus being difficult to eliminate. In addition, cases of atypical human trypanosomiasis, caused by species not infective to humans, have been reported, indicating an adaptability of species of Trypanosoma to new hosts [125].
The incubation period for the disease can range from days to weeks for the Rhodesian form and months to years for the Gambian form. The first stage of the disease is hemolymphatic, in which parasitemia is mainly associated with lymphadenopathy and recurrent episodes of fever. The second stage is meningoencephalitis, in which parasites cross the blood–brain barrier, compromising the central nervous system and resulting in neuropsychiatric disorders, the most characteristic clinical manifestation being dysregulation of the circadian cycle and sleepiness and/or insomnia [126]. T. b. gambiense infection is milder and may be asymptomatic or with mild symptoms in the first stage. Patients may live asymptomatically for years or heal spontaneously [127,128]; however, in the vast majority of cases, if left untreated, the patient will die within one to two years after entering the second stage [129]. T. b. rhodesiense infection is sporadic, although more severe and with a rapid evolution, leading patients to death within six months [130]. Previously, the oldest trypanocidal drugs suramin, discovered in 1920, and pentamidine, discovered in 1940, were used for the Rhodesian and Gambian forms in the first stage, respectively. In 2019, the nitroimidazole fexinidazole was made available orally as the first-line treatment of both forms in the hemolymphatic stage of the disease and for the meningoencephalitic stage in the absence of severe clinical manifestations [124]. In the second stage, the melaminophenyl arsenical called melarsoprol, discovered in 1949, was used for both forms of disease, although the drug is highly toxic and causes death-associated reactive encephalopathy in up to 10% of patients [131]. For that reason, the ornithine analog eflornithine was introduced in 1990 for the second-stage treatment, being effective only against the Gambian form. In 2009, the nifurtimox–eflornithine combination therapy proved to be more effective, with a lower fatality rate than monotherapy [132].
T. brucei is subdivided into five subspecies: T. b. gambiense and T. b. rhodesiense, which cause human African trypanosomiasis; T. b. brucei, which causes animal African trypanosomiasis or nagana; T. b. evansi, Surra; and T. b. equiperdum, which causes dourine. The subspecies are morphologically similar and the last three do not generally infect humans [124]. Man and cattle are the main reservoirs of T. b. gambiense and T b. rhodesiense, respectively. The main form of transmission is vectorial and alternative transmission routes are rare. The T. brucei life cycle alternates between a hematophagous dipteran vector of the genus Glossina, commonly known as tsetse flies, and a mammalian host [126]. Briefly, T. brucei has an extracellular life cycle divided into five evolutionary forms. In the vector, the proliferative pro-cyclic forms are found in the intestine; the proliferative epimastigote form and the infective metacyclic trypomastigote form are found in the salivary glands. In mammals, the proliferative slender forms and the non-proliferative stumpy forms are found in the bloodstream. With the exception of the epimastigote forms, the other forms are similar to the trypomastigote forms [133,134].
A stock of T. b. brucei isolated from cattle in Somalia showed complete resistance to the trypanocidal drugs diminazene aceturate and quinapyramine sulfate, in addition to reduced sensitivity to homidium chloride, isometamidium, Mel B, and pentamidine isethionate [135]. With the discovery of ABCB1 and its participation in the MDR phenotype, Kaminsky and Zweygarth (1991) tested the effect of VP alone and in combination with trypanocidal drugs on this resistant parasite [136]. Nevertheless, the authors were unable to reverse the resistance phenotype, suggesting that resistance mechanisms in T. brucei are not mediated by ABCB1.
Maser and Kaminsky (1998) were the first to identify three first ABC gene sequences, named TbABC1, TbABC2 and TbABC3 in T. b. brucei and T. b. rhodesiense [137]. Alignment of the predicted TbABC1 protein sequence showed 65% of residues identical to PGPA from L. tarentolae; TbABC2 showed a 57% identity with PGP from L. donovani; and TbABC3 showed no homology. The mRNA for the three genes were expressed in pro-cyclic and bloodstream forms of drug-sensitive and resistant parasites. Later, Shahi et al. (2002) demonstrated two predicted proteins of 1581 and 1759 amino acids with genealogy close to LtPGPA and LtPGPE, belonging to the ABCC subfamily that transports GSH and GSH-conjugated compounds [138]. For that reason, they were called TbMRPA and TbMRPE, the first containing TbABC1 discovered by Maser and Kominsky [137]. The authors demonstrated that overexpression of TbMRPA or TbMRPE in T. b. brucei (927 strain) increased resistance to melarsoprol (by 10-fold IC50 value) or to suramin (by three-fold IC50 value), respectively. TbMRPA was detected by immunofluorescence expressed in the plasma membrane and TbMRPE in the vesicles between the nucleus and the flagellar pocket in the bloodstream forms [138]. In 2006, Lusher and colleagues evaluated resistance to melarsoprol in T. b. brucei with MRPA overexpression and/or loss of function of the P2/AT1 aminopurine influx transporter [139]. MRPA+ mutants (IC50 = 37.2 nM) were more resistant to melarsen oxide, a melarsoprol metabolite, than P2/AT1− mutants (IC50 = 11.9 nM), with the highest degree of resistance achieved by the double mutant (IC50 = 66.1 nM). Phenylarsine oxide is a reducing agent with a high affinity for the thiol radical; it enters the cell by passive diffusion and would be extruded by MRPA [140]. MRPA+ mutants (IC50 = 5.6 nM) also showed an increase in drug resistance compared to the parental strain (IC50 = 3 nM) and the loss of the P2/AT1 function did not interfere in the process. In contrast, diminazine aceturate, used for the treatment of nagana, enters the cell via the P2/AT1 transporter. Its loss of function raised the IC50 value for the drug from 33 nM to 168 nM. MRPA overexpression increased the IC50 value to 58 nM, though the resistance effect was more pronounced in the double mutant, in which the IC50 value reached 264 nM [139]. In the same year, it was demonstrated that overexpression of MRPA did not cause resistance in infected mice treated with melarsoprol. As well, gene knockdown by RNAi caused moderate hypersensitivity to melarsoprol and its metabolite melarsen oxide in vitro. In addition, overexpression of MRPA was not detected in melarsoprol-resistant clinical samples from sleeping sickness patients [141]. In accordance with the HUGO nomenclature, the ABC genes MRPA and MRPE are now recognized as ABCC2 and ABCC6 (Figure 2) [7].
Figure 2.
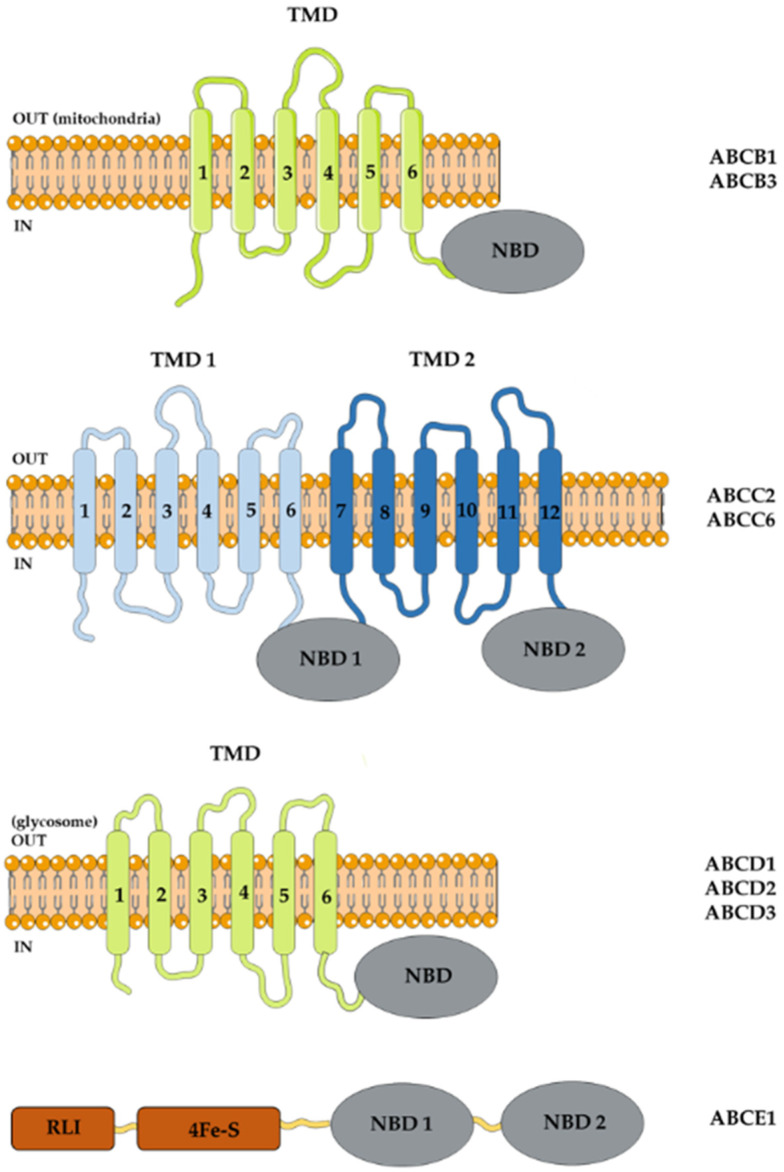
T. brucei ABC proteins. ABC proteins share a nucleotide-binding domain (NBD), displaying conserved sequences among organisms. A typical full-size ABC transporter presents, in addition to the two NBDs, two transmembrane domains, which could be rearranged in direct (TMD–NBD) or reverse (NBD–TMD) order. Half-size transporter has only a TMD and an NBD, assuming that they form dimers or oligomers to transport substrates across membranes. The standardized names of ABC members in subfamilies according to the HUGO nomenclature are represented by forth letter, followed by number. For example, ABCB1 is subfamily B, member 1. RLI, L ribonuclease inhibitor domain. 4Fe-S, iron-sulfur cluster binding domain.
Besides the transporters, Estevez et al. (2004) found the ribonuclease L inhibitor (RLI) protein, now recognized as ABCE1 in T. brucei [142]. The ABCE1 gene encodes a protein with 631 amino acids and 71 kDa with an RLI metal binding domain, a 4Fe-4S binding domain and two NBDs, and a predicted cytosolic localization (Figure 2). It appears to be involved in protein synthesis, as protein depletion via RNAi resulted in increased riboendonuclease activity and in mRNA levels, in addition to the growth arrest of pro-cyclic and bloodstream forms. Protein overexpression had the opposite effect on riboendonuclease activity and mRNA levels, with a moderate inhibitory effect on cell growth only on bloodstream forms.
In 2006, Yernaux and collaborators discovered three glycosomal ABC transporter (GAT) sequences, named GAT1-3 in pro-cyclic forms of T. b. brucei [143]. Glycosomes are organelles similar to peroxisomes [144]. GAT1-3 encodes half-size transporters with a TMD–NBD arrangement (Figure 2) with low identity with ABC transporters from mammalian peroxisomes (about 25%) and with colocalization with T. brucei glycosomal hexokinase [143]. According to new nomenclature, GAT1-3 are actually the transporters ABCD2, ABCD3 and ABCD1, respectively [7]. ABCD2 and ABCD1 are expressed in both pro-cyclic and bloodstream forms, while ABCD3 is expressed exclusively in bloodstream forms. The results suggest that ABCD2 transports oleoyl-CoA, and possibly other fatty acids, from the cytosol to the glycosomal lumen, as gene silencing results in the accumulation of cellular linoleate, probably due to the presence of an active oleate desaturase [145].
Using the sequence of mitochondrial ABC transporter 1 (Atm1) and multidrug resistance-like (Mdl) genes from S. cerevisiae as the query, Harakova and colleagues (2015) identified homologous genes in the T. brucei genome, which were designated TbAtm and TbMdl [146]. TbAtm encodes a protein of approximately 79 kDa and shares about a 40% amino acid identity with the homolog genes Atm1 from S. cerevisiae, Atm3 from A. thaliana and human ABCB7. TbMdl encodes a protein of a predicted molecular weight of 76 kDa that shows almost a 40% amino acid sequence similarity to the homolog genes Mdl1 and Mdl2 of S. cerevisiae and human ABCB10. Both are mitochondrial ABC transporters: Atm is involved in the Fe-S cluster in yeast [147] and in the molybdenum cofactor arrangement in the cytosol in plants [148] while Mdl interacts with Fe-S-containing ferrochelatase, an enzyme of the heme biosynthesis pathway in erythroid cells [149]. The sequences TbAtm and TbMdl correspond to TbABCB3 and TbABCB1 (Figure 2), respectively [7], with the TbABCB3 transporter colocalizing with a mitochondrial marker in T. brucei. The authors did not propose a precise function of these transporters in the parasite, but the downregulation of TbABCB3 via RNAi causes a slight reduction in parasite growth, accompanied by a reduction in tRNA thiolation and in the activity of enzymes involved in the Fe-S cluster in the cytosol. Downregulation of TbABCB1 decreased the mitochondrial heme content, suggesting participation in heme metabolism [146]. The ABC proteins from T. brucei are listed in Table 3 displayed in Figure 2.
Table 3.
The ABC proteins from T. brucei.
| Nomenclature | T. brucei Gene ID | NCBI Reference Sequences | Location | Putative Protein Length | T. Brucei Strain | Alias |
|---|---|---|---|---|---|---|
| ABCA3 | Tb927.11.6120 | XP_828686.1 | TbChr11 | 1738 | brucei TREU927 | |
| ABCA10 | Tb927.3.3730 | XP_843965.1 | TbChr03 | 1845 | brucei TREU927 | |
| ABCB1 | Tb927.11.540.2 | XP_828146.1 | TbChr11 | 665 | brucei TREU927 | TbMdl [145] |
| ABCB3 | Tb927.11.16930 | XP_829749.1 | TbChr11 | 719 | brucei TREU927 | TbAtm [145] |
| ABCC1/2 | Tb927.8.2160 | XP_847049.1 | TbChr08 | 1581 | brucei TREU927 | TbMRPA [137] |
| ABCC6 | Tb927.4.4490 | XP_844598.1 | TbChr04 | 1759 | brucei TREU927 | TbMRPE [137] |
| ABCC9 | Tb927.4.2510 | XP_844401.1 | TbChr04 | 1503 | brucei TREU927 | |
| ABCD1 | Tb927.11.1070 | XP_828200.1 | TbChr11 | 684 | brucei TREU927 | TbGAT3 [142] |
| ABCD2 | Tb927.4.4050 | XP_844555.1 | TbChr04 | 683 | brucei TREU927 | TbGAT1 [142] |
| ABCD3 | Tb927.11.3130 | XP_828395.1 | TbChr11 | 641 | brucei TREU927 | TbGAT2 [142] |
| ABCE1 | Tb927.10.1630 | XP_822420.1 | TbChr10 | 631 | brucei TREU927 | TbRLI [141] |
| ABCF1 | Tb927.10.3170 | XP_822569.1 | TbChr10 | 723 | brucei TREU927 | |
| ABCF2 | Tb927.10.15530 | XP_828024.1 | TbChr10 | 602 | brucei TREU927 | |
| ABCF3 | Tb927.10.10880 | XP_823306.1 | TbChr10 | 684 | brucei TREU927 | |
| ABCG1/2/3 | Tb927.10.7700 | XP_823004.1 | TbChr10 | 668 | brucei TREU927 | |
| ABCG4 | Tb927.9.6310 | XP_011776526.1 | TbChr09 | 646 | brucei TREU927 | |
| ABCG5 | Tb927.8.2380 | XP_847071.1 | TbChr08 | 1158 | brucei TREU927 | |
| ABCG6 | Tb927.10.7360 | XP_822971.1 | TbChr10 | 646 | brucei TREU927 | |
| ABCH3 | Tb927.6.2810 | XP_845402.1 | TbChr06 | 524 | brucei TREU927 | |
| OTHERS | Tb927.1.4420 | XP_001219135.1 | TbChr01 | 945 | brucei TREU927 | |
| OTHERS | Tb927.2.5410 | XP_951709.1 | TbChr02 | 1281 | brucei TREU927 | |
| OTHERS | Tb927.2.6130 | XP_951745.1 | TbChr02 | 1006 | brucei TREU927 |
Subfamily names follow the HUGO nomenclature, according to Leprohon et al. (2006) [93]. Gene accession numbers and other information was retrieved from TriTrypDB (tritrypdb.org) and GeneDB database (http://www.genedb.org/Homepage accessed on 10 July 2022).
The MDR phenotype is characterized by cross-resistance to chemically and structurally unrelated drugs. This phenotype is multifactorial, involving the pathways of (i) drug entry, (ii) activation, (iii) metabolism and (iv) efflux. Among the factors involved, the overexpression of ABC transporters is directly related to increased drug efflux and reduced toxic effects within cells. Several protozoan parasites exhibit an MDR phenotype, causing a reduction in the efficacy of chemotherapy. T. cruzi and T. brucei are not exceptions to the rule, since not only natural but also acquired resistance through prolonged treatment are reported in the literature. ABC transporters were discovered in the context of the MDR phenotype; however, they play important physiological roles, such as the control of cell detoxification, cellular stress and apoptosis. The first ABC genes described in trypanosomatids were in Leishmania species in the early 1990s, in parallel with the description of ABCB1/PGP transporter functionality in humans. This comparison led some authors to name the first transporters as PGPs, when in fact their amino acid sequence had more similarity and identity with the ABCC1/MRP transporter. This fact led to some naming confusion, which was standardized with the identification of 42 ABC genes in Leishmania, with 27 homologous ABC genes identified in T. cruzi and 22 ABC genes in T. brucei. At this time, few ABC genes have been characterized in the Trypanosoma genus and some are related to chemotherapy resistance. The understanding of how these transporters work in the physiology of the parasite and, consequently, in the acquisition of the resistant phenotype can contribute to the design of new drugs, having these transporters as therapeutic targets, resulting in the improvement of current chemotherapy.
Nomenclature
| ABC | ATP-binding cassette |
| ABCA1 | Subfamily A, member 1 |
| ABCA3 | Subfamily A, member 3 |
| ABCB1 | Subfamily B, member 1 |
| ABCB10 | Subfamily B, member 10 |
| ABCB3 | Subfamily B, member 3 |
| ABCB7 | Subfamily B, member 7 |
| ABCC1 | Subfamily C, member 1 |
| ABCC2 | Subfamily C, member 2 |
| ABCC6 | Subfamily C, member 6 |
| ABCD1 | Subfamily D, member 1 |
| ABCD2 | Subfamily D, member 2 |
| ABCD3 | Subfamily D, member 3 |
| ABCE1 | Subfamily E, member 1 |
| ABCG1 | Subfamily G, member 1 |
| ABCG2 | Subfamily G, member 2 |
| ABCP | ABC placental protein |
| Atm1 | Mitochondrial ABC transporter 1 |
| ATP | Adenosine triphosphate |
| BCRP | Breast cancer resistance protein |
| BSO | Butionine sulfoximine |
| CCCP | Carbonyl cyanide m-chlorophenyl hydrazine |
| CFDA | 5(6)-carboxyfluorescein diacetate |
| CsA | Cyclosporin A |
| DTU | Discrete typing unit |
| FTC | Fumitremorgin C |
| GAT | Glycosomal ABC transporter (GAT) |
| GPG | P-glycoprotein |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| HUGO | Human Genome Organization |
| IC50 | Half maximal inhibitory concentration |
| Ko143 | (3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1′,2′1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester |
| LTC4 | Leukotriene C4 |
| Mdl | Multidrug resistance-like |
| MDR | Multidrug resistance |
| MDR1 | Multidrug resistance protein 1 (MDR1) |
| MK-571 | L-660711, 5-(3-(2-(7-Chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid |
| mRNA | Messenger RNA |
| MRP1 | Multidrug resistance protein 1 |
| MXR | Mitoxantrone resistance protein |
| NBD | Nucleotide-binding domain |
| NEM | N-ethylmaleimide |
| OAT | Organic anion transporter |
| ORF | Open reading frame |
| Rho 123 | Rhodamine 123 |
| RLI | Ribonuclease L inhibitor |
| RNAi | RNA interference |
| T(SH)2 | Trypanothione |
| TMD | Transmembrane domain |
| tRNA | Transporter RNA |
| VP | Verapamil |
| WHO | World Health Organization |
Author Contributions
K.M.C. searched the bibliographic materials, reviewed the existing literature, and wrote the article. R.C.V. and L.M.F. also wrote the article. L.F.L., J.O.P. and L.M.P. reviewed the manuscript and supervised the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnolόgico (CNPq), and Instituto Nacional de Ciência e Tecnologia de Vacinas (INCT-V).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stuart K., Brun R., Croft S., Fairlamb A., Gurtler R.E., McKerrow J., Reed S., Tarleton R. Kinetoplastids: Related protozoan pathogens, different diseases. J. Clin. Investig. 2008;118:1301–1310. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuma A.A., Dos Santos Barrias E., de Souza W. Basic Biology of Trypanosoma cruzi. Curr. Pharm. Des. 2021;27:1671–1732. doi: 10.2174/1381612826999201203213527. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson R., Stevens J.R. Barcoding in trypanosomes. Parasitology. 2018;145:563–573. doi: 10.1017/S0031182017002049. [DOI] [PubMed] [Google Scholar]
- 4.WHO Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis. World Health Organ. Tech. Rep. Ser. 2012;975:1–100. [PubMed] [Google Scholar]
- 5.WHO . Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Diseases 2015. World Health Organization; Geneva, Switzerland: 2015. pp. 1–211. [Google Scholar]
- 6.Bernardes L.S., Zani C.L., Carvalho I. Trypanosomatidae diseases: From the current therapy to the efficacious role of trypanothione reductase in drug discovery. Curr. Med. Chem. 2013;20:2673–2696. doi: 10.2174/0929867311320210005. [DOI] [PubMed] [Google Scholar]
- 7.Sauvage V., Aubert D., Escotte-Binet S., Villena I. The role of ATP-binding cassette (ABC) proteins in protozoan parasites. Mol. Biochem. Parasitol. 2009;167:81–94. doi: 10.1016/j.molbiopara.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Dassa E. Natural history of ABC systems: Not only transporters. Essays Biochem. 2011;50:19–42. doi: 10.1042/bse0500019. [DOI] [PubMed] [Google Scholar]
- 9.ter Beek J., Guskov A., Slotboom D.J. Structural diversity of ABC transporters. J. Gen. Physiol. 2014;143:419–435. doi: 10.1085/jgp.201411164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean M., Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genom. Hum. Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 11.Theodoulou F.L., Kerr I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015;43:1033–1040. doi: 10.1042/BST20150139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dassa E., Bouige P. The ABC of ABCS: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001;152:211–229. doi: 10.1016/S0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- 13.Locher K.P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 2016;23:487–493. doi: 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- 14.Dean M., Rzhetsky A., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 15.Verrier P.J., Bird D., Burla B., Dassa E., Forestier C., Geisler M., Klein M., Kolukisaoglu U., Lee Y., Martinoia E., et al. Plant ABC proteins--a unified nomenclature and updated inventory. Trends Plant Sci. 2008;13:151–159. doi: 10.1016/j.tplants.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Thomas C., Aller S.G., Beis K., Carpenter E.P., Chang G., Chen L., Dassa E., Dean M., Duong Van Hoa F., Ekiert D., et al. Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett. 2020;594:3767–3775. doi: 10.1002/1873-3468.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juliano R.L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Et Biophys. Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 18.Thiebaut F., Tsuruo T., Hamada H., Gottesman M.M., Pastan I., Willingham M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie E.M., Deeley R.G., Cole S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Silva R., Vilas-Boas V., Carmo H., Dinis-Oliveira R.J., Carvalho F., de Lourdes Bastos M., Remiao F. Modulation of P-glycoprotein efflux pump: Induction and activation as a therapeutic strategy. Pharmacol. Ther. 2015;149:1–123. doi: 10.1016/j.pharmthera.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Ambudkar S.V., Kim I.W., Sauna Z.E. The power of the pump: Mechanisms of action of P-glycoprotein (ABCB1) Eur. J. Pharm. Sci. 2006;27:392–400. doi: 10.1016/j.ejps.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Loo T.W., Clarke D.M. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J. Membr. Biol. 2005;206:173–185. doi: 10.1007/s00232-005-0792-1. [DOI] [PubMed] [Google Scholar]
- 23.Cole S.P., Bhardwaj G., Gerlach J.H., Mackie J.E., Grant C.E., Almquist K.C., Stewart A.J., Kurz E.U., Duncan A.M., Deeley R.G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 24.Couture L., Nash J.A., Turgeon J. The ATP-binding cassette transporters and their implication in drug disposition: A special look at the heart. Pharmacol. Rev. 2006;58:244–258. doi: 10.1124/pr.58.2.7. [DOI] [PubMed] [Google Scholar]
- 25.Slot A.J., Molinski S.V., Cole S.P. Mammalian multidrug-resistance proteins (MRPs) Essays Biochem. 2011;50:179–207. doi: 10.1042/bse0500179. [DOI] [PubMed] [Google Scholar]
- 26.Yang A.K., Zhou Z.W., Wei M.Q., Liu J.P., Zhou S.F. Modulators of multidrug resistance associated proteins in the management of anticancer and antimicrobial drug resistance and the treatment of inflammatory diseases. Curr. Top. Med. Chem. 2010;10:1732–1756. doi: 10.2174/156802610792928040. [DOI] [PubMed] [Google Scholar]
- 27.Cole S.P. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharmacol. Toxicol. 2014;54:95–117. doi: 10.1146/annurev-pharmtox-011613-135959. [DOI] [PubMed] [Google Scholar]
- 28.Hanssen K.M., Haber M., Fletcher J.I. Targeting multidrug resistance-associated protein 1 (MRP1)-expressing cancers: Beyond pharmacological inhibition. Drug Resist. Updat. 2021;59:100795. doi: 10.1016/j.drup.2021.100795. [DOI] [PubMed] [Google Scholar]
- 29.Circu M.L., Aw T.Y. Glutathione and apoptosis. Free. Radic. Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole S.P., Deeley R.G. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 2006;27:438–446. doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Doyle L.A., Yang W., Abruzzo L.V., Krogmann T., Gao Y., Rishi A.K., Ross D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allikmets R., Schriml L.M., Hutchinson A., Romano-Spica V., Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 33.Miyake K., Mickley L., Litman T., Zhan Z., Robey R., Cristensen B., Brangi M., Greenberger L., Dean M., Fojo T., et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 34.Litman T., Brangi M., Hudson E., Fetsch P., Abati A., Ross D.D., Miyake K., Resau J.H., Bates S.E. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J. Cell Sci. 2000;113:2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 35.Bhatia A., Schafer H.J., Hrycyna C.A. Oligomerization of the human ABC transporter ABCG2: Evaluation of the native protein and chimeric dimers. Biochemistry. 2005;44:10893–10904. doi: 10.1021/bi0503807. [DOI] [PubMed] [Google Scholar]
- 36.Wong K., Briddon S.J., Holliday N.D., Kerr I.D. Plasma membrane dynamics and tetrameric organisation of ABCG2 transporters in mammalian cells revealed by single particle imaging techniques. Biochim. Biophys. Acta. 2016;1863:19–29. doi: 10.1016/j.bbamcr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Pena-Solorzano D., Stark S.A., Konig B., Sierra C.A., Ochoa-Puentes C. ABCG2/BCRP: Specific and Nonspecific Modulators. Med. Res. Rev. 2017;37:987–1050. doi: 10.1002/med.21428. [DOI] [PubMed] [Google Scholar]
- 38.Staud F., Pavek P. Breast cancer resistance protein (BCRP/ABCG2) Int. J. Biochem. Cell Biol. 2005;37:720–725. doi: 10.1016/j.biocel.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Zhou S., Schuetz J.D., Bunting K.D., Colapietro A.M., Sampath J., Morris J.J., Lagutina I., Grosveld G.C., Osawa M., Nakauchi H., et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamurthy P., Ross D.D., Nakanishi T., Bailey-Dell K., Zhou S., Mercer K.E., Sarkadi B., Sorrentino B.P., Schuetz J.D. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 41.Kukal S., Guin D., Rawat C., Bora S., Mishra M.K., Sharma P., Paul P.R., Kanojia N., Grewal G.K., Kukreti S., et al. Multidrug efflux transporter ABCG2: Expression and regulation. Cell. Mol. Life Sci. 2021;78:6887–6939. doi: 10.1007/s00018-021-03901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litman T., Druley T.E., Stein W.D., Bates S.E. From MDR to MXR: New understanding of multidrug resistance systems, their properties and clinical significance. Cell. Mol. Life Sci. 2001;58:931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbatsch I.L., Sankaran B., Weber J., Senior A.E. P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J. Biol. Chem. 1995;270:19383–19390. doi: 10.1074/jbc.270.33.19383. [DOI] [PubMed] [Google Scholar]
- 44.da Costa K.M., Valente R.C., Salustiano E.J., Gentile L.B., Freire-de-Lima L., Mendonca-Previato L., Previato J.O. Functional Characterization of ABCC Proteins from Trypanosoma cruzi and Their Involvement with Thiol Transport. Front. Microbiol. 2018;9:205. doi: 10.3389/fmicb.2018.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–1972. [PubMed] [Google Scholar]
- 46.Shapiro A.B., Ling V. ATPase activity of purified and reconstituted P-glycoprotein from Chinese hamster ovary cells. J. Biol. Chem. 1994;269:3745–3754. doi: 10.1016/S0021-9258(17)41923-5. [DOI] [PubMed] [Google Scholar]
- 47.Ledwitch K.V., Gibbs M.E., Barnes R.W., Roberts A.G. Cooperativity between verapamil and ATP bound to the efflux transporter P-glycoprotein. Biochem. Pharmacol. 2016;118:96–108. doi: 10.1016/j.bcp.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perrotton T., Trompier D., Chang X.B., Di Pietro A., Baubichon-Cortay H. (R)- and (S)-verapamil differentially modulate the multidrug-resistant protein MRP1. J. Biol. Chem. 2007;282:31542–31548. doi: 10.1074/jbc.M703964200. [DOI] [PubMed] [Google Scholar]
- 49.Loe D.W., Deeley R.G., Cole S.P. Verapamil stimulates glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1) J. Pharmacol. Exp. Ther. 2000;293:530–538. [PubMed] [Google Scholar]
- 50.Dantzig A.H., Shepard R.L., Cao J., Law K.L., Ehlhardt W.J., Baughman T.M., Bumol T.F., Starling J.J. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996;56:4171–4179. [PubMed] [Google Scholar]
- 51.Shepard R.L., Cao J., Starling J.J., Dantzig A.H. Modulation of P-glycoprotein but not MRP1- or BCRP-mediated drug resistance by LY335979. Int. J. Cancer. 2003;103:121–125. doi: 10.1002/ijc.10792. [DOI] [PubMed] [Google Scholar]
- 52.Dantzig A.H., Law K.L., Cao J., Starling J.J. Reversal of multidrug resistance by the P-glycoprotein modulator, LY335979, from the bench to the clinic. Curr. Med. Chem. 2001;8:39–50. doi: 10.2174/0929867013373903. [DOI] [PubMed] [Google Scholar]
- 53.Neyfakh A.A. Use of fluorescent dyes as molecular probes for the study of multidrug resistance. Exp. Cell Res. 1988;174:168–176. doi: 10.1016/0014-4827(88)90152-8. [DOI] [PubMed] [Google Scholar]
- 54.Dogan A.L., Legrand O., Faussat A.M., Perrot J.Y., Marie J.P. Evaluation and comparison of MRP1 activity with three fluorescent dyes and three modulators in leukemic cell lines. Leuk. Res. 2004;28:619–622. doi: 10.1016/j.leukres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Jones T.R., Zamboni R., Belley M., Champion E., Charette L., Ford-Hutchinson A.W., Frenette R., Gauthier J.Y., Leger S., Masson P., et al. Pharmacology of L-660,711 (MK-571): A novel potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 1989;67:17–28. doi: 10.1139/y89-004. [DOI] [PubMed] [Google Scholar]
- 56.Gekeler V., Ise W., Sanders K.H., Ulrich W.R., Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995;208:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- 57.Wang J.Q., Yang Y., Cai C.Y., Teng Q.X., Cui Q., Lin J., Assaraf Y.G., Chen Z.S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updat. 2021;54:100743. doi: 10.1016/j.drup.2021.100743. [DOI] [PubMed] [Google Scholar]
- 58.Benderra Z., Morjani H., Trussardi A., Manfait M. Evidence for functional discrimination between leukemic cells overexpressing multidrug-resistance associated protein and P-glycoprotein. Adv. Exp. Med. Biol. 1999;457:151–160. doi: 10.1007/978-1-4615-4811-9_17. [DOI] [PubMed] [Google Scholar]
- 59.Berger W., Hauptmann E., Elbling L., Vetterlein M., Kokoschka E.M., Micksche M. Possible role of the multidrug resistance-associated protein (MRP) in chemoresistance of human melanoma cells. Int. J. Cancer. 1997;71:108–115. doi: 10.1002/(SICI)1097-0215(19970328)71:1<108::AID-IJC18>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 60.Zhou S.F., Wang L.L., Di Y.M., Xue C.C., Duan W., Li C.G., Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Med. Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 61.Robey R.W., Honjo Y., van de Laar A., Miyake K., Regis J.T., Litman T., Bates S.E. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochim. Biophys. Acta. 2001;1512:171–182. doi: 10.1016/S0005-2736(01)00308-X. [DOI] [PubMed] [Google Scholar]
- 62.Honjo Y., Hrycyna C.A., Yan Q.W., Medina-Perez W.Y., Robey R.W., van de Laar A., Litman T., Dean M., Bates S.E. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–6639. [PubMed] [Google Scholar]
- 63.Rabindran S.K., He H., Singh M., Brown E., Collins K.I., Annable T., Greenberger L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58:5850–5858. [PubMed] [Google Scholar]
- 64.Rabindran S.K., Ross D.D., Doyle L.A., Yang W., Greenberger L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 65.Nishiyama M., Kuga T. Central effects of the neurotropic mycotoxin fumitremorgin A in the rabbit (I). Effects on the spinal cord. Jpn. J. Pharmacol. 1989;50:167–173. doi: 10.1016/S0021-5198(19)42469-4. [DOI] [PubMed] [Google Scholar]
- 66.Yamazaki M., Suzuki S., Ozaki N. Biochemical investigation on the abnormal behaviors induced by fumitremorgin A, a tremorgenic mycotoxin to mice. J. Pharm.-Dyn. 1983;6:748–751. doi: 10.1248/bpb1978.6.748. [DOI] [PubMed] [Google Scholar]
- 67.Allen J.D., van Loevezijn A., Lakhai J.M., van der Valk M., van Tellingen O., Reid G., Schellens J.H., Koomen G.J., Schinkel A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 68.Weidner L.D., Zoghbi S.S., Lu S., Shukla S., Ambudkar S.V., Pike V.W., Mulder J., Gottesman M.M., Innis R.B., Hall M.D. The Inhibitor Ko143 Is Not Specific for ABCG2. J. Pharmacol. Exp. Ther. 2015;354:384–393. doi: 10.1124/jpet.115.225482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szafraniec M.J., Szczygiel M., Urbanska K., Fiedor L. Determinants of the activity and substrate recognition of breast cancer resistance protein (ABCG2) Drug Metab. Rev. 2014;46:459–474. doi: 10.3109/03602532.2014.942037. [DOI] [PubMed] [Google Scholar]
- 70.Chagas C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz. 1909;1:159–218. doi: 10.1590/S0074-02761909000200008. [DOI] [Google Scholar]
- 71.Chagas C. Nova entidade morbida do homem: Rezumo geral de estudos etiolojicos e clinicos. Mem. Inst. Oswaldo Cruz. 1911;3:219–275. doi: 10.1590/S0074-02761911000200003. [DOI] [Google Scholar]
- 72.Bern C., Messenger L.A., Whitman J.D., Maguire J.H. Chagas Disease in the United States: A Public Health Approach. Clin. Microbiol. Rev. 2019;33:e00023–e0002319. doi: 10.1128/CMR.00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guarner J. Chagas disease as example of a reemerging parasite. Semin. Diagn. Pathol. 2019;36:164–169. doi: 10.1053/j.semdp.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Coura J.R. Chagas disease: What is known and what is needed--a background article. Mem. Do Inst. Oswaldo Cruz. 2007;102((Suppl. S1)):113–122. doi: 10.1590/S0074-02762007000900018. [DOI] [PubMed] [Google Scholar]
- 75.Bern C. Chagas’ Disease. N. Engl. J. Med. 2015;373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 76.Oliveira M.D.F., Nagao-Dias A.T., De Pontes V.M.O., Júnior A.S.D.S., Coelho H.L.L., Coelho I.C.B. Tratamento etiológico da doença de Chagas no Brasil. Rev. Patol. Trop. 2008;37:209–228. doi: 10.5216/rpt.v37i3.5063. [DOI] [Google Scholar]
- 77.Le Loup G., Pialoux G., Lescure F.X. Update in treatment of Chagas disease. Curr. Opin. Infect. Dis. 2011;24:428–434. doi: 10.1097/QCO.0b013e32834a667f. [DOI] [PubMed] [Google Scholar]
- 78.Rassi A., Jr., Rassi A., Marcondes de Rezende J. American trypanosomiasis (Chagas disease) Infect. Dis. Clin. N. Am. 2012;26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Manne-Goehler J., Umeh C.A., Montgomery S.P., Wirtz V.J. Estimating the Burden of Chagas Disease in the United States. PLoS Negl. Trop. Dis. 2016;10:e0005033. doi: 10.1371/journal.pntd.0005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herreros-Cabello A., Callejas-Hernandez F., Girones N., Fresno M. Trypanosoma Cruzi Genome: Organization, Multi-Gene Families, Transcription, and Biological Implications. Genes. 2020;11:1196. doi: 10.3390/genes11101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis M.D., Llewellyn M.S., Gaunt M.W., Yeo M., Carrasco H.J., Miles M.A. Flow cytometric analysis and microsatellite genotyping reveal extensive DNA content variation in Trypanosoma cruzi populations and expose contrasts between natural and experimental hybrids. Int. J. Parasitol. 2009;39:1305–1317. doi: 10.1016/j.ijpara.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zingales B., Andrade S.G., Briones M.R., Campbell D.A., Chiari E., Fernandes O., Guhl F., Lages-Silva E., Macedo A.M., Machado C.R., et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/S0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 83.Breniere S.F., Waleckx E., Barnabe C. Over Six Thousand Trypanosoma cruzi Strains Classified into Discrete Typing Units (DTUs): Attempt at an Inventory. PLoS Negl. Trop. Dis. 2016;10:e0004792. doi: 10.1371/journal.pntd.0004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Filardi L.S., Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987;81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 85.Neal R.A., van Bueren J., McCoy N.G., Iwobi M. Reversal of drug resistance in Trypanosoma cruzi and Leishmania donovani by verapamil. Trans. R. Soc. Trop. Med. Hyg. 1989;83:197–198. doi: 10.1016/0035-9203(89)90642-1. [DOI] [PubMed] [Google Scholar]
- 86.Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990;9:1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dallagiovanna B., Castanys S., Gamarro F. Trypanosoma cruzi: Sequence of the ATP-binding site of a P-glycoprotein gene. Exp. Parasitol. 1994;79:63–67. doi: 10.1006/expr.1994.1061. [DOI] [PubMed] [Google Scholar]
- 88.Dallagiovanna B., Gamarro F., Castanys S. Molecular characterization of a P-glycoprotein-related tcpgp2 gene in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1996;75:145–157. doi: 10.1016/0166-6851(95)02519-7. [DOI] [PubMed] [Google Scholar]
- 89.Irigoin F., Cibils L., Comini M.A., Wilkinson S.R., Flohe L., Radi R. Insights into the redox biology of Trypanosoma cruzi: Trypanothione metabolism and oxidant detoxification. Free Radic. Biol. Med. 2008;45:733–742. doi: 10.1016/j.freeradbiomed.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 90.Torres C., Barreiro L., Dallagiovanna B., Gamarro F., Castanys S. Characterization of a new ATP-binding cassette transporter in Trypanosoma cruzi associated to a L1Tc retrotransposon. Biochim. Biophys. Acta. 1999;1489:428–432. doi: 10.1016/S0167-4781(99)00195-5. [DOI] [PubMed] [Google Scholar]
- 91.Martin F., Maranon C., Olivares M., Alonso C., Lopez M.C. Characterization of a non-long terminal repeat retrotransposon cDNA (L1Tc) from Trypanosoma cruzi: Homology of the first ORF with the ape family of DNA repair enzymes. J. Mol. Biol. 1995;247:49–59. doi: 10.1006/jmbi.1994.0121. [DOI] [PubMed] [Google Scholar]
- 92.Murta S.M., Gazzinelli R.T., Brener Z., Romanha A.J. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol. Biochem. Parasitol. 1998;93:203–214. doi: 10.1016/S0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 93.Murta S.M., dos Santos W.G., Anacleto C., Nirde P., Moreira E.S., Romanha A.J. Drug resistance in Trypanosoma cruzi is not associated with amplification or overexpression of P-glycoprotein (PGP) genes. Mol. Biochem. Parasitol. 2001;117:223–228. doi: 10.1016/S0166-6851(01)00350-4. [DOI] [PubMed] [Google Scholar]
- 94.Leprohon P., Legare D., Girard I., Papadopoulou B., Ouellette M. Modulation of Leishmania ABC protein gene expression through life stages and among drug-resistant parasites. Eukaryot. Cell. 2006;5:1713–1725. doi: 10.1128/EC.00152-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torres C., Perez-Victoria F.J., Parodi-Talice A., Castanys S., Gamarro F. Characterization of an ABCA-like transporter involved in vesicular trafficking in the protozoan parasite Trypanosoma cruzi. Mol. Microbiol. 2004;54:632–646. doi: 10.1111/j.1365-2958.2004.04304.x. [DOI] [PubMed] [Google Scholar]
- 96.Pasello M., Giudice A.M., Scotlandi K. The ABC subfamily A transporters: Multifaceted players with incipient potentialities in cancer. Semin. Cancer Biol. 2020;60:57–71. doi: 10.1016/j.semcancer.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Tripodi K.E., Menendez Bravo S.M., Cricco J.A. Role of heme and heme-proteins in trypanosomatid essential metabolic pathways. Enzym. Res. 2011;2011:873230. doi: 10.4061/2011/873230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lara F.A., Sant’anna C., Lemos D., Laranja G.A., Coelho M.G., Reis Salles I., Michel A., Oliveira P.L., Cunha E.S.N., Salmon D., et al. Heme requirement and intracellular trafficking in Trypanosoma cruzi epimastigotes. Biochem. Biophys. Res. Commun. 2007;355:16–22. doi: 10.1016/j.bbrc.2006.12.238. [DOI] [PubMed] [Google Scholar]
- 99.Koster W. ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res. Microbiol. 2001;152:291–301. doi: 10.1016/S0923-2508(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 100.Krishnamurthy P.C., Du G., Fukuda Y., Sun D., Sampath J., Mercer K.E., Wang J., Sosa-Pineda B., Murti K.G., Schuetz J.D. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 101.Borel J.F., Feurer C., Gubler H.U., Stahelin H. Biological effects of cyclosporin A: A new antilymphocytic agent. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 102.Slater L.M., Sweet P., Stupecky M., Gupta S. Cyclosporin A reverses vincristine and daunorubicin resistance in acute lymphatic leukemia in vitro. J. Clin. Investig. 1986;77:1405–1408. doi: 10.1172/JCI112450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tamai I., Safa A.R. Competitive interaction of cyclosporins with the Vinca alkaloid-binding site of P-glycoprotein in multidrug-resistant cells. J. Biol. Chem. 1990;265:16509–16513. doi: 10.1016/S0021-9258(17)46252-1. [DOI] [PubMed] [Google Scholar]
- 104.Pawarode A., Shukla S., Minderman H., Fricke S.M., Pinder E.M., O’Loughlin K.L., Ambudkar S.V., Baer M.R. Differential effects of the immunosuppressive agents cyclosporin A, tacrolimus and sirolimus on drug transport by multidrug resistance proteins. Cancer Chemother. Pharmacol. 2007;60:179–188. doi: 10.1007/s00280-006-0357-8. [DOI] [PubMed] [Google Scholar]
- 105.Qadir M., O’Loughlin K.L., Fricke S.M., Williamson N.A., Greco W.R., Minderman H., Baer M.R. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin. Cancer Res. 2005;11:2320–2326. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 106.Fitzpatrick F.A. Cyclooxygenase enzymes: Regulation and function. Curr. Pharm. Des. 2004;10:577–588. doi: 10.2174/1381612043453144. [DOI] [PubMed] [Google Scholar]
- 107.Reid G., Wielinga P., Zelcer N., van der Heijden I., Kuil A., de Haas M., Wijnholds J., Borst P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA. 2003;100:9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parasrampuria D.A., Lantz M.V., Benet L.Z. A human lymphocyte based ex vivo assay to study the effect of drugs on P-glycoprotein (P-gp) function. Pharm. Res. 2001;18:39–44. doi: 10.1023/A:1011070509191. [DOI] [PubMed] [Google Scholar]
- 109.Shen H., Lai Y., Rodrigues A.D. Organic Anion Transporter 2: An Enigmatic Human Solute Carrier. Drug Metab. Dispos. Biol. Fate Chem. 2017;45:228–236. doi: 10.1124/dmd.116.072264. [DOI] [PubMed] [Google Scholar]
- 110.McCabe R.E., Remington J.S., Araujo F.G. In vivo and in vitro effects of cyclosporin A on Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 1985;34:861–865. doi: 10.4269/ajtmh.1985.34.861. [DOI] [PubMed] [Google Scholar]
- 111.Bua J., Ruiz A.M., Potenza M., Fichera L.E. In vitro anti-parasitic activity of Cyclosporin A analogs on Trypanosoma cruzi. Bioorganic Med. Chem. Lett. 2004;14:4633–4637. doi: 10.1016/j.bmcl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 112.Bua J., Fichera L.E., Fuchs A.G., Potenza M., Dubin M., Wenger R.O., Moretti G., Scabone C.M., Ruiz A.M. Anti-Trypanosoma cruzi effects of cyclosporin A derivatives: Possible role of a P-glycoprotein and parasite cyclophilins. Parasitology. 2008;135:217–228. doi: 10.1017/S003118200700371X. [DOI] [PubMed] [Google Scholar]
- 113.Campos M.C., Castro-Pinto D.B., Ribeiro G.A., Berredo-Pinho M.M., Gomes L.H., da Silva Bellieny M.S., Goulart C.M., Echevarria A., Leon L.L. P-glycoprotein efflux pump plays an important role in Trypanosoma cruzi drug resistance. Parasitol. Res. 2013;112:2341–2351. doi: 10.1007/s00436-013-3398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soares R.O., Echevarria A., Bellieny M.S., Pinho R.T., de Leo R.M., Seguins W.S., Machado G.M., Canto-Cavalheiro M.M., Leon L.L. Evaluation of thiosemicarbazones and semicarbazones as potential agents anti-Trypanosoma cruzi. Exp. Parasitol. 2011;129:381–387. doi: 10.1016/j.exppara.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 115.Zingales B., Araujo R.G., Moreno M., Franco J., Aguiar P.H., Nunes S.L., Silva M.N., Ienne S., Machado C.R., Brandao A. A novel ABCG-like transporter of Trypanosoma cruzi is involved in natural resistance to benznidazole. Mem. Inst. Oswaldo Cruz. 2015;110:433–444. doi: 10.1590/0074-02760140407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Franco J., Ferreira R.C., Ienne S., Zingales B. ABCG-like transporter of Trypanosoma cruzi involved in benznidazole resistance: Gene polymorphisms disclose inter-strain intragenic recombination in hybrid isolates. Infect. Genet. Evol. 2015;31:198–208. doi: 10.1016/j.meegid.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 117.Petravicius P.O., Costa-Martins A.G., Silva M.N., Reis-Cunha J.L., Bartholomeu D.C., Teixeira M.M.G., Zingales B. Mapping benznidazole resistance in trypanosomatids and exploring evolutionary histories of nitroreductases and ABCG transporter protein sequences. Acta Trop. 2019;200:105161. doi: 10.1016/j.actatropica.2019.105161. [DOI] [PubMed] [Google Scholar]
- 118.da Costa K.M., Salustiano E.J., Valente R.D.C., Freire-de-Lima L., Mendonca-Previato L., Previato J.O. Intrinsic and Chemotherapeutic Stressors Modulate ABCC-Like Transport in Trypanosoma cruzi. Molecules. 2021;26:3510. doi: 10.3390/molecules26123510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dutton J.E. Preliminary Note upon A Trypanosome Occurring in the Blood of Man. Liverpool University Press; Liverpool, UK: 1902. pp. 455–468. [Google Scholar]
- 120.Stephens J.W.W., Fantham H. On the Peculiar Morphology of a Trypanosome From a Case of Sleeping Sickness and the Possibility of Its Being a New Species (T. Rhodesiense) Ann. Trop. Med. Parasitol. 1910;4:343–350. doi: 10.1080/00034983.1910.11685723. [DOI] [Google Scholar]
- 121.Bottieau E., Clerinx J. Human African Trypanosomiasis: Progress and Stagnation. Infect. Dis. Clin. N. Am. 2019;33:61–77. doi: 10.1016/j.idc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 122.WHO Number of New Reported Cases of Human African Trypanosomiasis. [(accessed on 21 June 2022)]; Available online: https://www.who.int/data/gho/data/themes/topics/human-african-trypanosomiasis.
- 123.Franco J.R., Cecchi G., Priotto G., Paone M., Diarra A., Grout L., Simarro P.P., Zhao W., Argaw D. Monitoring the elimination of human African trypanosomiasis at continental and country level: Update to 2018. PLoS Negl. Trop. Dis. 2020;14:e0008261. doi: 10.1371/journal.pntd.0008261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kasozi K.I., MacLeod E.T., Ntulume I., Welburn S.C. An Update on African Trypanocide Pharmaceutics and Resistance. Front. Vet. Sci. 2022;9:828111. doi: 10.3389/fvets.2022.828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumar R., Gupta S., Bhutia W.D., Vaid R.K., Kumar S. Atypical human trypanosomosis: Potentially emerging disease with lack of understanding. Zoonoses Public Health. 2022;69:259–276. doi: 10.1111/zph.12945. [DOI] [PubMed] [Google Scholar]
- 126.Buscher P., Cecchi G., Jamonneau V., Priotto G. Human African trypanosomiasis. Lancet. 2017;390:2397–2409. doi: 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- 127.Sudarshi D., Lawrence S., Pickrell W.O., Eligar V., Walters R., Quaderi S., Walker A., Capewell P., Clucas C., Vincent A., et al. Human African trypanosomiasis presenting at least 29 years after infection—What can this teach us about the pathogenesis and control of this neglected tropical disease? PLoS Negl. Trop. Dis. 2014;8:e3349. doi: 10.1371/journal.pntd.0003349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jamonneau V., Ilboudo H., Kabore J., Kaba D., Koffi M., Solano P., Garcia A., Courtin D., Laveissiere C., Lingue K., et al. Untreated human infections by Trypanosoma brucei gambiense are not 100% fatal. PLoS Negl. Trop. Dis. 2012;6:e1691. doi: 10.1371/journal.pntd.0001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Checchi F., Funk S., Chandramohan D., Haydon D.T., Chappuis F. Updated estimate of the duration of the meningo-encephalitic stage in gambiense human African trypanosomiasis. BMC Res. Notes. 2015;8:292. doi: 10.1186/s13104-015-1244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Franco J.R., Simarro P.P., Diarra A., Jannin J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014;6:257–275. doi: 10.2147/CLEP.S39728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chappuis F., Udayraj N., Stietenroth K., Meussen A., Bovier P.A. Eflornithine is safer than melarsoprol for the treatment of second-stage Trypanosoma brucei gambiense human African trypanosomiasis. Clin. Infect. Dis. 2005;41:748–751. doi: 10.1086/432576. [DOI] [PubMed] [Google Scholar]
- 132.Hidalgo J., Ortiz J.F., Fabara S.P., Eissa-Garces A., Reddy D., Collins K.D., Tirupathi R. Efficacy and Toxicity of Fexinidazole and Nifurtimox Plus Eflornithine in the Treatment of African Trypanosomiasis: A Systematic Review. Cureus. 2021;13:e16881. doi: 10.7759/cureus.16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wheeler R.J., Gull K., Sunter J.D. Coordination of the Cell Cycle in Trypanosomes. Annu. Rev. Microbiol. 2019;73:133–154. doi: 10.1146/annurev-micro-020518-115617. [DOI] [PubMed] [Google Scholar]
- 134.Quintana J.F., Zoltner M., Field M.C. Evolving Differentiation in African Trypanosomes. Trends Parasitol. 2021;37:296–303. doi: 10.1016/j.pt.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 135.Zweygarth E., Rottcher D. Efficacy of experimental trypanocidal compounds against a multiple drug-resistant Trypanosoma brucei brucei stock in mice. Parasitol. Res. 1989;75:178–182. doi: 10.1007/BF00931271. [DOI] [PubMed] [Google Scholar]
- 136.Kaminsky R., Zweygarth E. The effect of verapamil alone and in combination with trypanocides on multidrug-resistant Trypanosoma brucei brucei. Acta Trop. 1991;49:215–225. doi: 10.1016/0001-706X(91)90040-Q. [DOI] [PubMed] [Google Scholar]
- 137.Maser P., Kaminsky R. Identification of three ABC transporter genes in Trypanosoma brucei spp. Parasitol. Res. 1998;84:106–111. doi: 10.1007/s004360050365. [DOI] [PubMed] [Google Scholar]
- 138.Shahi S.K., Krauth-Siegel R.L., Clayton C.E. Overexpression of the putative thiol conjugate transporter TbMRPA causes melarsoprol resistance in Trypanosoma brucei. Mol. Microbiol. 2002;43:1129–1138. doi: 10.1046/j.1365-2958.2002.02831.x. [DOI] [PubMed] [Google Scholar]
- 139.Luscher A., Nerima B., Maser P. Combined contribution of TbAT1 and TbMRPA to drug resistance in Trypanosoma brucei. Mol. Biochem. Parasitol. 2006;150:364–366. doi: 10.1016/j.molbiopara.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 140.Quentmeier A., Klein H., Unthan-Fechner K., Probst I. Attenuation of insulin actions in primary rat hepatocyte cultures by phenylarsine oxide. Biol. Chem. Hoppe-Seyler. 1993;374:965–971. doi: 10.1515/bchm3.1993.374.7-12.965. [DOI] [PubMed] [Google Scholar]
- 141.Alibu V.P., Richter C., Voncken F., Marti G., Shahi S., Renggli C.K., Seebeck T., Brun R., Clayton C. The role of Trypanosoma brucei MRPA in melarsoprol susceptibility. Mol. Biochem. Parasitol. 2006;146:38–44. doi: 10.1016/j.molbiopara.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 142.Estevez A.M., Haile S., Steinbuchel M., Quijada L., Clayton C. Effects of depletion and overexpression of the Trypanosoma brucei ribonuclease L inhibitor homologue. Mol. Biochem. Parasitol. 2004;133:137–141. doi: 10.1016/j.molbiopara.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 143.Yernaux C., Fransen M., Brees C., Lorenzen S., Michels P.A. Trypanosoma brucei glycosomal ABC transporters: Identification and membrane targeting. Mol. Membr. Biol. 2006;23:157–172. doi: 10.1080/09687860500460124. [DOI] [PubMed] [Google Scholar]
- 144.Moyersoen J., Choe J., Fan E., Hol W.G., Michels P.A. Biogenesis of peroxisomes and glycosomes: Trypanosomatid glycosome assembly is a promising new drug target. FEMS Microbiol. Rev. 2004;28:603–643. doi: 10.1016/j.femsre.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 145.Igoillo-Esteve M., Mazet M., Deumer G., Wallemacq P., Michels P.A. Glycosomal ABC transporters of Trypanosoma brucei: Characterisation of their expression, topology and substrate specificity. Int. J. Parasitol. 2011;41:429–438. doi: 10.1016/j.ijpara.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 146.Horakova E., Changmai P., Paris Z., Salmon D., Lukes J. Simultaneous depletion of Atm and Mdl rebalances cytosolic Fe-S cluster assembly but not heme import into the mitochondrion of Trypanosoma brucei. FEBS J. 2015;282:4157–4175. doi: 10.1111/febs.13411. [DOI] [PubMed] [Google Scholar]
- 147.Kispal G., Csere P., Prohl C., Lill R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999;18:3981–3989. doi: 10.1093/emboj/18.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Teschner J., Lachmann N., Schulze J., Geisler M., Selbach K., Santamaria-Araujo J., Balk J., Mendel R.R., Bittner F. A novel role for Arabidopsis mitochondrial ABC transporter ATM3 in molybdenum cofactor biosynthesis. Plant Cell. 2010;22:468–480. doi: 10.1105/tpc.109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Taketani S., Kakimoto K., Ueta H., Masaki R., Furukawa T. Involvement of ABC7 in the biosynthesis of heme in erythroid cells: Interaction of ABC7 with ferrochelatase. Blood. 2003;101:3274–3280. doi: 10.1182/blood-2002-04-1212. [DOI] [PubMed] [Google Scholar]