Abstract
A new series of luminescent heterometallic europium(III)–lutetium(III) terephthalate metal–organic frameworks, namely (EuxLu1−x)2bdc3·nH2O, was synthesized using a direct reaction in a water solution. At the Eu3+ concentration of 1–40 at %, the MOFs were formed as a binary mixture of the (EuxLu1−x)2bdc3 and (EuxLu1−x)2bdc3·4H2O crystalline phases, where the Ln2bdc3·4H2O crystalline phase was enriched by europium(III) ions. At an Eu3+ concentration of more than 40 at %, only one crystalline phase was formed: (EuxLu1−x)2bdc3·4H2O. All MOFs containing Eu3+ exhibited sensitization of bright Eu3+-centered luminescence upon the 280 nm excitation into a 1ππ* excited state of the terephthalate ion. The fine structure of the emission spectra of Eu3+ 5D0-7FJ (J = 0–4) significantly depended on the Eu3+ concentration. The luminescence quantum yield of Eu3+ was significantly larger for Eu-Lu terephthalates containing a low concentration of Eu3+ due to the absence of Eu-Eu energy migration and the presence of the Ln2bdc3 crystalline phase with a significantly smaller nonradiative decay rate compared to the Ln2bdc3·4H2O.
Keywords: metal–organic framework, luminescence, rare earth, europium, lutetium, phase transition
1. Introduction
In recent decades, rare-earth-element metal–organic frameworks (REE-MOFs) were actively designed and synthetized due to their unique luminescence properties. They are unique platforms for fabricating advanced luminescent materials, which are widely used in various fields of science and technology [1,2,3,4]. The position of the lanthanide ionic luminescence bands strongly depends only on the lanthanide ion, which allows the construction of REE-MOFs with the desired optical properties [5]. Taking this fact into account and considering the high stability, low solubility and toxicity, and highly effective charge transport of Ln-MOFs, they are prospective materials for OLEDs [6,7], luminescent thermometers [8,9], and imaging [10,11,12,13]. Variations in organic linkers in MOFs allow synthetic chemists to form structures with different porosities large surface areas, and high structural flexibility [14,15,16], which allows the use of REE-MOFs as highly selective sensors on organic and inorganic materials [17,18,19,20,21,22,23,24,25]. Typical linkers in REE-MOFs are organic carboxylates due to the simple synthesis of REE-MOFs in undemanding conditions and the unlimited possibilities in MOF design [26,27].
Lanthanide ions possess characteristic luminescence; however, direct UV excitation of them is inefficient because they have very small light absorption coefficients: 4f-4f transitions are forbidden by selection rules. This issue can be resolved using the energy transfer from the excited linker to the lanthanide ion (antenna effect) [28,29]. Aromatic organic molecules such as 1,4-benzenedicarboxylate (bdc) are widely used as antenna linkers due to their effective UV absorbance and pronounced antenna effect [30,31]. Usually, the energy transfer takes place from the lower-level triplet electronic state (T1) of the linker molecule but not from the lowest excited singlet state (S1). In some cases, the heavy lanthanides increase the rate of S1-T1 intersystem crossing [32,33,34,35]. The high concentration of luminescent lanthanide ion in homometallic REE-MOFs could result in concentration quenching through Ln-Ln energy migration and, therefore, the drop in luminescence quantum yield (PLQY) [36]. Utochnikova et al. proposed a solution to this problem by doping of a luminescent europium(III) terephthalate with nonluminescent Gd3+ ions, which not only diluted the luminescent Tb3+ ions, but also increased the probability of intersystem crossing, which increased the PLQY. It was found that Eu-Gd and Eu-Y heterometallic terephthalates were formed in the same crystalline phases. In the current work, we studied a series of luminescent heterometallic europium(III)–lutetium(III) terephthalates MOFs and observed that the substitution of a large amount of Eu(III) for Lu(III) resulted in a crystalline phase change as well as a significant rise in the PLQY.
2. Results and Discussion
2.1. XRD Results and Analysis
The X-ray powder diffraction (XRD) patterns (Figure 1a) were measured for the range of heterometallic europium(III)–lutetium(III) terephthalates (EuxLu1−x)2bdc3·nH2O; bdc = 1,4-benzenedicarboxylate) with a Eu3+ concentration from 0 to 100 at %. An analysis of the XRD patterns demonstrated that in a range of Eu3+ concentration of 6 to 100 at %, the samples were isostructural to the Ln2bdc3·4H2O (Ln = Ce-Yb) [37]. This structure, which is common for the rare-earth terephthalates from Ce to Yb [38], was a three-dimensional metal–organic framework (MOF) in which octacoordinated lanthanide ions were bound to the two water molecules and six terephthalate ions through the oxygen atoms (Figure 1b). The analysis of XRD patterns of Eu-Lu terephthalates with 0–2 at % of Eu3+ showed that the samples were isostructural to Er2bdc3 [38], which is a 3D MOF in which heptacoordinated lanthanide ions are bound to the seven oxygen atoms from the terephthalate ions (Figure 1c). At a Eu3+ concentration in the range of 3–5 at %, both the Ln2bdc3·4H2O and Ln2bdc3 crystalline phases were observed. The XRD peaks for heterometallic europium(III)–lutetium(III) terephthalates in Eu3+ concentration ranged from 6 to 100 at % and were slightly shifted relative to the XRD peaks measured for Ln2bdc3·4H2O reported previously [37]. To compare these Ln2bdc3·4H2O structures, the refinement of unit cell parameters was performed for some samples with a Eu3+ concentration between 6 and 100 at % (Table 1) using UnitCell software [39], which retrieved unit cell parameters from diffraction data using a method of least squares from the 2Θ data of the XRD patterns. Calculation errors also are shown in Table 1. We observed that in the range, the unit cell parameters increased. The observed growth of the unit cell parameters of heterometallic europium(III)–lutetium(III) terephthalates was explained by the smaller ionic radius of the octacoordinated Lu3+ (0.977 Å) compared with the ionic radius of the Eu3+ ion (1.066 Å) [40].
Figure 1.
(a) The XRD patterns of (EuxLu1−x)2bdc3·nH2O in heterometallic europium(III)–lutetium(III) terephthalate powders from 0% Eu3+ to 100% Eu3+) and the simulated XRD pattern of Er2bdc3 and Eu2bdc3·4H2O single-crystals structure taken from refs. [37,38]. (b,c) The generated crystal structures of Eu2bdc3·4H2O and Er2bdc3, respectively.
Table 1.
Unit cell parameters with calculation errors for (EuxLu1−x)2bdc3·nH2O refined for Eu2bdc3·4H2O crystalline phase.
| χEu (%) | a, Å | b, Å | c, Å | α | β | γ | V, Å3 |
|---|---|---|---|---|---|---|---|
| 100 | 6.1904 ±0.0019 |
9.856 ±0.003 |
10.251 ±0.003 |
101.673 ±0.027 |
90.273 ±0.028 |
104.796 ±0.025 |
591.13 ±0.22 |
| 90 | 6.1862 ±0.0019 |
9.845 ±0.003 |
10.236 ±0.003 |
101.583 ±0.027 |
90.300 ±0.028 |
104.727 ±0.025 |
589.63 ±0.22 |
| 60 | 6.1727 ±0.0018 |
9.815 ±0.003 |
10.206 ±0.003 |
101.553 ±0.027 |
90.418 ±0.028 |
104.657 ±0.025 |
585.00 ±0.22 |
| 40 | 6.1635 ±0.0018 |
9.783 ±0.003 |
10.179 ±0.003 |
101.497 ±0.027 |
90.472 ±0.028 |
104.651 ±0.025 |
580.75 ±0.22 |
| 20 | 6.1405 ±0.0018 |
9.738 ±0.003 |
10.145 ±0.003 |
101.570 ±0.027 |
90.562 ±0.028 |
104.617 ±0.025 |
573.90 ±0.21 |
| 10 | 6.1411 ±0.0018 |
9.7178 ±0.003 |
10.1334 ±0.003 |
101.626 ±0.026 |
90.461 ±0.028 |
104.590 ±0.025 |
572.13 ±0.21 |
| 6 | 6.1313 ±0.0018 |
9.714 ±0.003 |
10.130 ±0.003 |
101.582 ±0.026 |
90.474 ±0.027 |
104.608 ±0.025 |
570.784 ±0.21 |
2.2. Thermogravimetric Analysis (TGA)
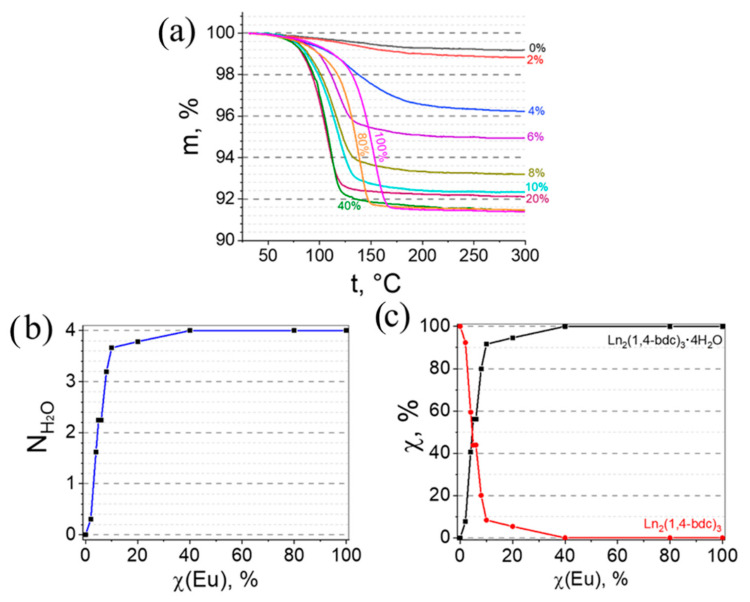
The thermogravimetric analysis (TGA) was carried out for the selected heterometallic europium(III)–lutetium(III) terephthalates in a temperature range of 25–300 °C (Figure 2a). The mass loss was observed at 120–180 °C for all measured samples. As previously reported [38], the mass loss in this temperature range can be assigned to the dehydration of the compounds resulting in the formation of Ln2bdc3. An analysis of the TGA curves allowed us to calculate the average numbers of water molecules in the heterometallic europium(III)–lutetium(III) terephthalates ((EuxLu1−x)2bdc3·nH2O). We observed that this number increased with the increase in the Eu3+ concentration (Figure 2b). An analysis of the XRD patterns demonstrated the presence of two crystalline phases: Ln2bdc3·4H2O and Ln2bdc3. Therefore, we could estimate the molar fraction of each coexisting crystalline phase (Figure 2c). The molar fraction of Ln2bdc3·4H2O increased along with the Eu3+ concentration in a range between 0 and 40 at %. In the Eu3+ concentration range of 40–100%, only Ln2bdc3·4H2O was present.
Figure 2.
(a) Thermogravimetric analysis (TGA) curves showing the mass loss profile of (EuxLu1−x)2bdc3·xH2O during thermal decomposition; (b) the number of water molecules per one formula unit in (EuxLu1−x)2bdc3·xH2O; (c) the molar fraction of Ln2bdc3 and Ln2bdc3·4H2O in heterometallic europium(III)–lutetium(III) terephthalates as a function of Eu concentration.
2.3. Luminescent Properties
The terephthalate ion is a typical linker used in luminescent antenna MOFs [41] due to its intensive UV absorbance [42] followed by efficient energy transfer to the luminescent lanthanide ion. The excitation of (EuxLu1−x)2bdc3·nH2O (λex. = 280 nm) resulted in emission in the visible range corresponding to 5D0-7FJ (J = 0–5) transitions of the Eu3+ ion [5] (Figure 3 and Figure 4). Upon UV excitation, the terephthalate ion was promoted into the Sn(1ππ*) state followed by the fast internal conversion to S1(1ππ*). Due to the heavy atom effect, the S1 state efficiently moved to the T1(3ππ*) triplet electronic excited state [34] via intersystem crossing. The T1 state of the terephthalate ion [34] (≈20,000 cm−1) had a higher energy than the 5D1 energy level of the Eu3+ ion [5] (≈19,000 cm−1) and a significantly lower energy than that of the lower excited state of the Lu3+ ion [43] (80,000 cm−1). Therefore, an efficient energy transfer from the T1 triplet electronic excited state of the terephthalate ion to the 5D1 energy level of the Eu3+ ion occurred. The 5D1 level of the Eu3+ ion then underwent an internal conversion into the 5D0 energy level followed by the emission into the 7FJ (J = 0–4) lower-lying levels.
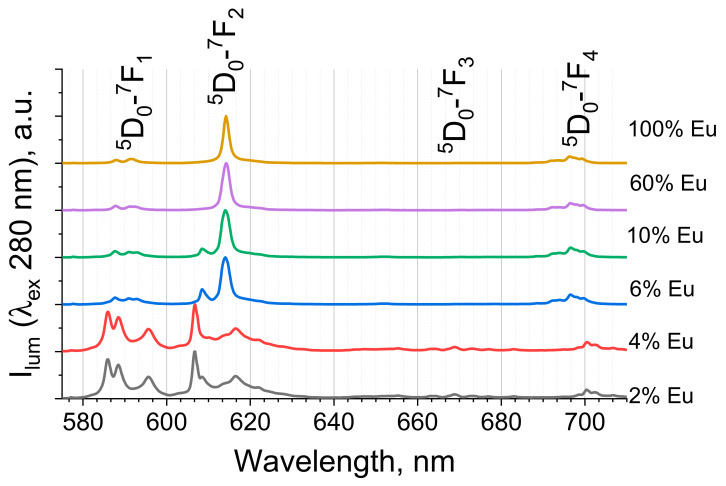
Figure 3.
The normalized emission spectra of (EuxLu1−x)2bdc3·nH2O at selected Eu3+ concentrations (given in legend) upon 280 nm excitation.
Figure 4.
Fine structure of lines in emission spectra of heterometallic europium(III)–lutetium(III) terephthalates normalized at maximum point for (a) 5D0-7F0, (b) 5D0-7F1, and (c) 5D0-7F2 transitions.
We observed that the fine structure of the Eu3+ emission spectra significantly depended on the Eu3+ concentration in the (EuxLu1−x)2bdc3·nH2O (Figure 4). At Eu3+ ion concentrations of more than 6 at %, in which the Ln2bdc3·4H2O phase dominated, the emission spectra were similar to that of Eu2bdc3·4H2O [31] and consisted of narrow bands corresponding to 5D0-7FJ (J = 0–4) transitions of Eu3+: 5D0-7F0 (577.6 nm), 5D0-7F1 (587.9 and 591.5 nm), 5D0-7F2 (614.0 nm), 5D0-7F3 (649.0 nm), and 5D0-7F4 (697.0 nm) (Figure 3). At low Eu3+ concentrations (2 and 4 at % Eu3+), in which the Ln2bdc3 phase dominated, the fine structure of the emission spectra was significantly different. The emission spectra contained 5D0-7F0 (577.2 nm and 577.6 nm), 5D0-7F1 (585.9, 588.4, and 595.6 nm), 5D0-7F2 (606.6, 610.2, 616.6, 619.4 (shoulder), and 621.8 nm), 5D0-7F3 (649.0 nm), and 5D0-7F4 (700.0 nm) Eu3+ narrow emission bands.
5D0-7F0 transition is strictly forbidden by the Judd–Ofelt theory; one can observe this transition only for europium(III) ions in coordination sites with Cn, Cnv, and Cs symmetry. For all measured samples, 5D0-7F0 transitions were observed. The analysis of the fine structure of this transition allowed us to determine the number of Eu3+ coordination sites because the 7F0 level was not degenerate and did not split in the crystal field, hence 5D0-7F0 could present in the emission spectrum as a single line for one type of Eu3+ coordination. The fine structure of the (EuxLu1−x)2bdc3·nH2O emission bands 5D0-7F0 is shown in Figure 4a. In the emission spectra of Eu-Lu terephthalates with a Eu3+ concentration of 6–100 at %, a single line was observed in the 570–585 nm range (5D0-7F0) with a maximum at 577.6 nm, which indicated that Eu3+ ions existed in the single-crystal-phase isostructural Ln2bdc3·4H2O. Meanwhile, by using a TGA, we estimated the molar fractions of Ln2bdc3·4H2O and Ln2bdc3 as equal to 60 and 40%, respectively. Therefore, the single 5D0-7F0 emission band (6–100 at % Eu3+) can be explained by uneven ion distribution between the two phases: the Ln2bdc3·4H2O crystalline phase was enriched by Eu3+ ions. In the Eu-Lu terephthalates with 2–4 at % Eu3+, two emission bands corresponding to the 5D0-7F0 transition were observed to peak at 577.2 and 577.6 nm, indicating the two different coordination sites of the Eu3+ ion. Therefore, in the terephthalates containing 2–4 at % Eu3+, europium(III) ions were distributed between two phases, namely Ln2bdc3·4H2O and Ln2bdc3, which was consistent with the TGA and XRD data.
The fine structure of the 5D0-7FJ emission bands and their relative intensities were very sensitive to the Eu3+ ions’ local symmetry. The degeneracy of each spin–orbit level was 2J+1 [5]. Hence, the maximum amount of crystal-field transitions of the 5D0-7F1 and 5D0-7F2 transitions were 3 and 5, respectively. According to previous studies, lanthanide(III) ions had pseudo-C4 symmetry in Ln2bdc3·4H2O (Ln = Tb, Eu) [38]. For Eu2bdc3·4H2O (100 at % Eu3+), the 5D0-7F1 transition split into two crystal field transitions (587.9 and 591.6 nm), and the 5D0-7F2 transition was presented in the emission spectrum as a single line (614.0 nm). Interestingly, for the Eu-Lu terephthalates containing 6–60 at % Eu3+, different splitting patterns of the 5D0-7FJ transitions in the crystal field were observed. The 5D0-7F1 and 5D0-7F2 transition split into three (587.6, 591.0, and 592.8 nm) and two components (608.5 and 614.0 nm), respectively (Figure 4b,c). The difference between the abovementioned emission spectra can be explained by the distortion of the coordination polyhedron due to the appearance of structural defects caused by the addition of Lu3+ ions, which have a lower ionic radius than europium(III) ions (lanthanide contraction). This resulted in the lowering of the local symmetry of the Eu3+ ion and the larger number of crystal-field transitions of Eu-Lu terephthalates compared to the Eu2bdc3·4H2O. The number of 5D0-7FJ crystal-field transitions indicated that the Eu3+ had symmetry of C2 or lower [44].
In the emission spectra of Ln2bdc3 (2–4 at % Eu3+), the 5D0-7F1 and 5D0-7F2 transitions split into three (585.9, 588.4, and 595.6 nm) and five components (606.6, 610.2, 616.6, 619.4 (shoulder), and 621.8 nm), respectively. In the emission spectra of the Lu-Eu terephthalates containing 2–4 at % Eu3+, we observed the presence of (EuxLu1−x)2bdc3·4H2O emission bands (weak 608.5 and 614.0 nm signals) because the Eu3+ ion was distributed between the Ln2bdc3 and Ln2bdc3·4H2O crystalline phases. A careful analysis of the Ln2bdc3 crystalline structure (Figure 1c) allowed us to conclude that the Ln3+ ion had C1 local symmetry, which was consistent with the number of crystal-field components of the 5D0-7F0, 5D0-7F1, and 5D0-7F2 transitions (1, 3, and 5, respectively) [44].
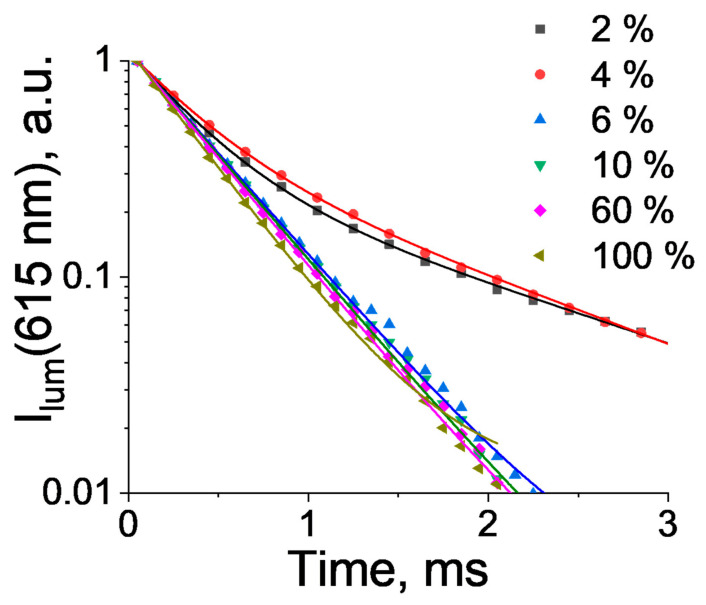
The luminescence decay curves of the (EuxLu1−x)2bdc3·nH2O phosphors monitored at 615 nm (5D0-7F2 transition) are presented in Figure 5 (λex. = 280 nm). At a low Eu3+ concentration (2 and 4 at %), the decay curves were fitted by a double exponential function (1), whereas the decay curves of the Eu-Lu terephthalates containing 6–100 at % Eu3+ were fitted by a single exponential function (2):
| (1) |
| (2) |
where τ1 and τ2 are the observed 5D0 lifetimes (Table 2).
Figure 5.
The 615 nm luminescence decay curves of heterometallic europium(III)–lutetium(III) terephthalates at 2, 4, 6, 10, 60, and 100 at % Eu.
Table 2.
The lifetimes of excitation state 5D0 of Eu3+ in heterometallic europium(III)–lutetium(III) terephthalates at 2, 4, 6, 10, 60, and 100 at % Eu.
| χEu (%) | τ1, ms | τ2, ms | PLQY, % |
|---|---|---|---|
| 100 | 0.390 | 10 ± 1 | |
| 60 | 0.435 | 11 ± 1 | |
| 10 | 0.449 | 12 ± 1 | |
| 6 | 0.459 | 16 ± 1 | |
| 4 | 0.392 | 1.602 | 22 ± 1 |
| 2 | 0.367 | 1.878 | 22 ± 1 |
The Eu-Lu terephthalates containing 6–100 at % Eu3+ had 5D0 lifetimes of 0.390–0.459 ms and luminescence quantum yields of 10–16%. The measured PLQY of the Eu2bdc3·4H2O was comparable with the literature data [31,38,45]. The 5D0 lifetime values and the luminescence quantum yields decreased with an increase in the Eu3+ concentration due to the energy migration between the Eu3+ ions and subsequent quenching by impurities and defects. We demonstrated in this work that Eu3+ ions predominantly existed in the Ln2bdc3·4H2O phase in the europium(III)–lutetium(III) terephthalates containing 6–100 at % Eu3+, in which the presence of a single Eu3+ coordination site resulted in a single 5D0 lifetime. Interestingly, the europium(III)–lutetium(III) terephthalates containing 2–4 at % Eu3+ were characterized by two 5D0 lifetimes. One emission decay component (τ1 = 0.392–0.367 ms) was close to the value observed for the europium(III)–lutetium(III) terephthalates containing 6–100 at % Eu3+, while another component (τ2 = 1.602–1.878 ms) was 4–4.8 times larger. In the Eu-Lu terephthalates containing 2–4 at % Eu3+, the Eu3+ ions were distributed between the Ln2bdc3·4H2O and Ln2bdc3 crystalline phases. Therefore, τ1 and τ2 could be assigned to the Eu3+ ions located in the Ln2bdc3·4H2O and Ln2bdc3, respectively. The water molecules in the Ln2bdc3·4H2O structure were coordinated with the Eu3+ ion and quenched the Eu3+ luminescence due to efficient energy transfer to high-energy O-H stretching vibrational modes of coordinated water molecules [46,47]. In the Ln2bdc3 crystalline phase, the Eu3+ ion was coordinated only with oxygen atoms of carboxylic groups of terephthalate ions. The efficient quenching of Eu3+ ions by water molecules in the Ln2bdc3·4H2O structure resulted in a significant decrease in the Eu3+ ion 5D0 lifetime compared to anhydrous Ln2bdc3.The emission quantum yield of the Eu3+ was significantly larger for the Eu-Lu terephthalates doped with a low Eu3+ concentration. This observation can be explained by two reasons: the absence of efficient Eu-Eu energy migration and the presence of the Ln2bdc3 crystalline phase with a significantly smaller nonradiative decay rate compared to the Ln2bdc3·4H2O.
3. Materials and Methods
Lutetium (III) chloride hexahydrate and europium (III) chloride hexahydrate were purchased from Chemcraft (Kaliningrad, Russia). Benzene-1,4-dicarboxylic (terephthalic, H2bdc) acid (>98%), sodium hydroxide (>99%), nickel(II) chloride hexahydrate (>99%), and EDTA disodium salt (0.1 M aqueous solution) were purchased from Sigma-Aldrich Pty Ltd. (Germany) and used without additional purification. The 0.2M solutions of EuCl3 and LuCl3 were prepared and standardized using complexometric titration with EDTA. A total of 0.6 mole of sodium hydroxide and 0.3 mole of terephthalic acid were dissolved in distilled water to obtain a 1 L solution of a 0.3 M solution of the disodium terephthalate (Na2bdc).
The heterometallic europium(III)–lutetium(III) terephthalates were obtained by mixing 1 mL of 0.2 M EuCl3 and LuCl3 aqueous solutions taken in stoichiometric ratios with 2 mL of 0.3 M Na2bdc water solution (Table 3). White precipitates of heterometallic europium(III)–lutetium(III) terephthalates were separated from the reaction mixture using centrifugation (4000× g) and washed using deionized water 5 times. All samples were driedat 60 °C.
Table 3.
The heterometallic europium(III)–lutetium(III) terephthalates’ synthesis conditions.
| χEu, % | V(0.2M EuCl3), mL | V(0.2M LuCl3), mL |
|---|---|---|
| 0 | 0 | 1.00 |
| 1 | 0.01 | 0.99 |
| 2 | 0.02 | 0.98 |
| 3 | 0.03 | 0.97 |
| 4 | 0.04 | 0.96 |
| 5 | 0.05 | 0.95 |
| 6 | 0.06 | 0.94 |
| 7 | 0.07 | 0.93 |
| 8 | 0.08 | 0.92 |
| 9 | 0.09 | 0.91 |
| 10 | 0.10 | 0.90 |
| 20 | 0.20 | 0.80 |
| 30 | 0.30 | 0.70 |
| 40 | 0.40 | 0.60 |
| 50 | 0.50 | 0.50 |
| 60 | 0.60 | 0.40 |
| 70 | 0.70 | 0.30 |
| 80 | 0.80 | 0.20 |
| 90 | 0.90 | 0.10 |
| 100 | 1.00 | 0 |
The Eu3+/Lu3+ ratios in the heterometallic europium(III)–lutetium(III) terephthalates were confirmed using energy-dispersive X-ray spectroscopy (EDX) (EDX spectrometer EDX-800P, Shimadzu, Japan) (Table 4). The Eu/Lu ratios measured via EDX were consistent with the ratios of Eu3+/Lu3+ taken for the synthesis in the form of EuCl3 and LuCl3 aqueous solutions (Table 3). The X-ray powder diffraction (XRD) measurements were performed on a D2 Phaser (Bruker, USA) X-ray diffractometer using Cu Kα radiation (λ = 1.54056 Å). The thermogravimetry curves were obtained using a TG 209 F1 Libra thermo-microbalance (Netzsch, Germany). The luminescence spectra were recorded with a Fluoromax-4 fluorescence spectrometer (Horiba Jobin Yvon, Japan). Lifetime measurements were performed with the same spectrometer using a pulsed Xe lamp (pulse duration: 3 µs). The absolute values of the photoluminescence quantum yields were recorded using a Fluorolog 3 Quanta-phi device. All measurements were performed at 25 °C.
Table 4.
Eu3+ atomic fraction (relative to the total amount of Eu3+ and Lu3+) in heterometallic europium(III)–lutetium(III) terephthalates taken during synthesis and obtained from EDX data.
| χEu (%), Taken | χEu (%), EDX |
|---|---|
| 0 | 0 |
| 2 | 2.07 ± 0.21 |
| 4 | 3.9 ± 0.4 |
| 5 | 4.7 ± 0.5 |
| 6 | 6.3 ± 0.6 |
| 10 | 10.2 ± 1.0 |
| 20 | 19.3 ± 1.9 |
| 40 | 37 ± 4 |
| 80 | 80 ± 8 |
| 100 | 100 |
4. Conclusions
In this work, we reported on the photoluminescence properties of luminescent antenna MOF—heterometallic europium(III)–lutetium(III) terephthalates. The series of (EuxLu1−x)2bdc3·nH2O (x = 0–1) was synthesized in the aqueous solution. At Eu3+ concentrations of 1–40 at %, the heterometallic europium(III)–lutetium(III) terephthalates were formed as a mixture of the (EuxLu1−x)2bdc3 and (EuxLu1−x)2bdc3·4H2O crystalline phases. At higher Eu3+ concentrations, a single crystalline phase was formed: (EuxLu1−x)2bdc3·4H2O. All the synthesized samples containing Eu3+ demonstrated a bright red emission corresponding to the 5D0-7FJ (J = 0–4) transitions of Eu3+ ions upon 280 nm excitation into the singlet electronic excited state of terephthalate ions. An analysis of the fine structure of the emission spectra allowed us to conclude that the Eu3+ ions were unevenly distributed between the Ln2bdc3 and Ln2bdc3·4H2O phases: the Ln2bdc3·4H2O crystalline phase was enriched by Eu3+ ions. The local symmetry of the Eu3+ ions in the heterometallic Eu-Lu terephthalates was proposed based on a careful analysis of the fine structure of the emission spectra and the structural data. We demonstrated that the 5D0 excited state lifetimes were 4–4.8 times larger for Eu3+ in the Ln2bdc3 crystalline phase than in Ln2bdc3·4H2O due to the absence of luminescence quenching of the Eu3+ by coordinated water molecules. The luminescence quantum yields of terephthalate ions decreased with an increase in the europium concentration from 2 to 100 at % Eu3+ (λex. = 280 nm).
Acknowledgments
The measurements were performed in the Research Park of Saint Petersburg State University (Magnetic Resonance Research Centre, Chemical Analysis and Materials Research Centre, Cryogenic Department, Interdisciplinary Resource Centre for Nanotechnology, Centre for X-ray Diffraction Studies, Centre for Optical and Laser Materials Research, Thermogravimetric and Calorimetric Research Centre, and Centre for Innovative Technologies of Composite Nanomaterials).
Author Contributions
Conceptualization, A.S.M. and V.G.N.; methodology, A.S.M., A.A.V. and V.G.N.; software, A.S.M., M.Y.S. and N.A.B.; validation, M.N.R., A.S.K. and I.E.K.; formal analysis, A.S.M. and V.G.N.; investigation, A.S.M., I.I.T., I.E.K. and V.G.N.; resources, A.S.M., M.Y.S. and N.A.B.; data curation, A.S.M., V.G.N. and A.S.K.; writing—original draft preparation, A.S.M. and V.G.N.; writing—review and editing, A.S.K., M.Y.S., I.E.K., N.A.B., V.G.N. and A.S.M.; visualization, A.S.M., V.G.N. and S.S.K.; supervision, A.S.M.; project administration, A.S.M.; funding acquisition, A.S.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the reported compounds are available from the authors.
Funding Statement
This work was supported by the Russian Science Foundation under grant no. 22-73-10040 (https://rscf.ru/en/project/22-73-10040/, accessed on 1 September 2022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xia T., Zhang J. Our Journey of Developing Dual-emitting Metal-organic Framework-based Fluorescent Sensors. Z. Anorg. Allg. Chem. 2022;648:e202100355. doi: 10.1002/zaac.202100355. [DOI] [Google Scholar]
- 2.Han X., Liu J., Yu K., Lu Y., Xiang W., Zhao D., He Y. Water-Stable Eu 6 -Cluster-Based Fcu-MOF with Exposed Vinyl Groups for Ratiometric and Fluorescent Visual Sensing of Hydrogen Sulfide. Inorg. Chem. 2022;61:5067–5075. doi: 10.1021/acs.inorgchem.2c00019. [DOI] [PubMed] [Google Scholar]
- 3.Zhao D., Yu K., Han X., He Y., Chen B. Recent Progress on Porous MOFs for Process-Efficient Hydrocarbon Separation, Luminescent Sensing, and Information Encryption. Chem. Commun. 2022;58:747–770. doi: 10.1039/D1CC06261A. [DOI] [PubMed] [Google Scholar]
- 4.Bryleva Y.A., Artem’ev A.V., Glinskaya L.A., Rakhmanova M.I., Samsonenko D.G., Komarov V.Y., Rogovoy M.I., Davydova M.P. Bright Photo- and Triboluminescence of Centrosymmetric Eu(Iii) and Tb(Iii) Complexes with Phosphine Oxides Containing Azaheterocycles. New J. Chem. 2021;45:13869–13876. doi: 10.1039/D1NJ02441H. [DOI] [Google Scholar]
- 5.Binnemans K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015;295:1–45. doi: 10.1016/j.ccr.2015.02.015. [DOI] [Google Scholar]
- 6.Kozlov M.I., Aslandukov A.N., Vashchenko A.A., Medvedko A.V., Aleksandrov A.E., Grzibovskis R., Goloveshkin A.S., Lepnev L.S., Tameev A.R., Vembris A., et al. On the Development of a New Approach to the Design of Lanthanide-Based Materials for Solution-Processed OLEDs. Dalton Trans. 2019;48:17298–17309. doi: 10.1039/C9DT03823J. [DOI] [PubMed] [Google Scholar]
- 7.Shurygin A.V., Vovna V.I., Korochentsev V.V., Mirochnik A.G., Kalinovskaya I.V., Sergienko V.I. Optical Properties and Electronic Structure of Eu(III) Complexes with HMPA and TPPO. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;250:119397. doi: 10.1016/j.saa.2020.119397. [DOI] [PubMed] [Google Scholar]
- 8.Khudoleeva V., Tcelykh L., Kovalenko A., Kalyakina A., Goloveshkin A., Lepnev L., Utochnikova V. Terbium-Europium Fluorides Surface Modified with Benzoate and Terephthalate Anions for Temperature Sensing: Does Sensitivity Depend on the Ligand? J. Lumin. 2018;201:500–508. doi: 10.1016/j.jlumin.2018.05.002. [DOI] [Google Scholar]
- 9.Zhou X., Wang H., Jiang S., Xiang G., Tang X., Luo X., Li L., Zhou X. Multifunctional Luminescent Material Eu(III) and Tb(III) Complexes with Pyridine-3,5-Dicarboxylic Acid Linker: Crystal Structures, Tunable Emission, Energy Transfer, and Temperature Sensing. Inorg. Chem. 2019;58:3780–3788. doi: 10.1021/acs.inorgchem.8b03319. [DOI] [PubMed] [Google Scholar]
- 10.Liu D., Lu K., Poon C., Lin W. Metal-Organic Frameworks as Sensory Materials and Imaging Agents. Inorg. Chem. 2014;53:1916–1924. doi: 10.1021/ic402194c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panikar S.S., Ramírez-García G., Vallejo-Cardona A.A., Banu N., Patrón-Soberano O.A., Cialla-May D., Camacho-Villegas T.A., de La Rosa E. Novel Anti-HER2 Peptide-Conjugated Theranostic Nanoliposomes Combining NaYF4:Yb,Er Nanoparticles for NIR-Activated Bioimaging and Chemo-Photodynamic Therapy against Breast Cancer. Nanoscale. 2019;11:20598–20613. doi: 10.1039/C9NR06535K. [DOI] [PubMed] [Google Scholar]
- 12.Liu H., Lu W., Wang H., Rao L., Yi Z., Zeng S., Hao J. Simultaneous Synthesis and Amine-Functionalization of Single-Phase BaYF5:Yb/Er Nanoprobe for Dual-Modal In Vivo Upconversion Fluorescence and Long-Lasting X-ray Computed Tomography Imaging. Nanoscale. 2013;5:6023–6029. doi: 10.1039/c3nr00999h. [DOI] [PubMed] [Google Scholar]
- 13.Shen T., Zhang Y., Kirillov A.M., Hu B., Shan C., Liu W., Tang Y. Versatile Rare-Earth Oxide Nanocomposites: Enhanced Chemo/Photothermal/Photodynamic Anticancer Therapy and Multimodal Imaging. J. Mater. Chem. B. 2016;4:7832–7844. doi: 10.1039/C6TB02244H. [DOI] [PubMed] [Google Scholar]
- 14.Liu W., Yin R., Xu X., Zhang L., Shi W., Cao X. Structural Engineering of Low-Dimensional Metal–Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019;6:1802373. doi: 10.1002/advs.201802373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu U.J., Jee S., Rao P.C., Shin J., Ko C., Yoon M., Park K.S., Choi K.M. Recent Advances in Process Engineering and Upcoming Applications of Metal–Organic Frameworks. Coord. Chem. Rev. 2021;426:213544. doi: 10.1016/j.ccr.2020.213544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook T.R., Zheng Y.R., Stang P.J. Metal-Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal-Organic Materials. Chem. Rev. 2013;113:734–777. doi: 10.1021/cr3002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puglisi R., Pellegrino A.L., Fiorenza R., Scirè S., Malandrino G. A Facile One-Pot Approach to the Synthesis of Gd-Eu Based Metal-Organic Frameworks and Applications to Sensing of Fe3+ and Cr2O72− Ions. Sensors. 2021;21:1679. doi: 10.3390/s21051679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lustig W.P., Mukherjee S., Rudd N.D., Desai A.V., Li J., Ghosh S.K. Metal-Organic Frameworks: Functional Luminescent and Photonic Materials for Sensing Applications. Chem. Soc. Rev. 2017;46:3242–3285. doi: 10.1039/C6CS00930A. [DOI] [PubMed] [Google Scholar]
- 19.Liu D., Dong G., Wang X., Nie F., Li X. A Luminescent Eu Coordination Polymer with Near-Visible Excitation for Sensing and Its Homologues Constructed from 1,4-Benzenedicarboxylate and 1: H-Imidazo[4,5-f] [1,10]-Phenanthroline. CrystEngComm. 2020;22:7877–7887. doi: 10.1039/D0CE01256D. [DOI] [Google Scholar]
- 20.Ding S.B., Wang W., Qiu L.G., Yuan Y.P., Peng F.M., Jiang X., Xie A.J., Shen Y.H., Zhu J.F. Surfactant-Assisted Synthesis of Lanthanide Metal-Organic Framework Nanorods and Their Fluorescence Sensing of Nitroaromatic Explosives. Mater. Lett. 2011;65:1385–1387. doi: 10.1016/j.matlet.2011.02.009. [DOI] [Google Scholar]
- 21.Zhao J.J., Liu P.Y., Dong Z.P., Liu Z.L., Wang Y.Q. Eu(III)-Organic Framework as a Multi-Responsive Photoluminescence Sensor for Efficient Detection of 1-Naphthol, Fe3+ and MnO4− in Water. Inorg. Chim. Acta. 2020;511:119843. doi: 10.1016/j.ica.2020.119843. [DOI] [Google Scholar]
- 22.Han X., Gu C., Ding Y., Yu J., Li K., Zhao D., Chen B. Stable Eu3+/Cu2+-Functionalized Supramolecular Zinc(II) Complexes as Fluorescent Probes for Turn-On and Ratiometric Detection of Hydrogen Sulfide. ACS Appl. Mater. Interfaces. 2021;13:20371–20379. doi: 10.1021/acsami.1c04013. [DOI] [PubMed] [Google Scholar]
- 23.Dong C.L., Li M.F., Yang T., Feng L., Ai Y.W., Ning Z.L., Liu M.J., Lai X., Gao D.J. Controllable Synthesis of Tb-Based Metal–Organic Frameworks as an Efficient Fluorescent Sensor for Cu2+ Detection. Rare Met. 2021;40:505–512. doi: 10.1007/s12598-020-01621-z. [DOI] [Google Scholar]
- 24.Nguyen L.H., Oveissi F., Chandrawati R., Dehghani F., Naficy S. Naked-Eye Detection of Ethylene Using Thiol-Functionalized Polydiacetylene-Based Flexible Sensors. ACS Sens. 2020;5:1921–1928. doi: 10.1021/acssensors.0c00117. [DOI] [PubMed] [Google Scholar]
- 25.Feng L., Dong C., Li M., Li L., Jiang X., Gao R., Wang R., Zhang L., Ning Z., Gao D., et al. Terbium-Based Metal-Organic Frameworks: Highly Selective and Fast Respond Sensor for Styrene Detection and Construction of Molecular Logic Gate. J. Hazard. Mater. 2020;388:121816. doi: 10.1016/j.jhazmat.2019.121816. [DOI] [PubMed] [Google Scholar]
- 26.Janicki R., Mondry A., Starynowicz P. Carboxylates of Rare Earth Elements. Coord. Chem. Rev. 2017;340:98–133. doi: 10.1016/j.ccr.2016.12.001. [DOI] [Google Scholar]
- 27.Utochnikova V.V., Kuzmina N.P. Photoluminescence of Lanthanide Aromatic Carboxylates. Russ. J. Coord. Chem. 2016;42:679–694. doi: 10.1134/S1070328416090074. [DOI] [Google Scholar]
- 28.Cao W., Tang Y., Cui Y., Qian G. Energy Transfer in Metal–Organic Frameworks and Its Applications. Small Struct. 2020;1:2000019. doi: 10.1002/sstr.202000019. [DOI] [Google Scholar]
- 29.Yin H.Q., Wang X.Y., Yin X.B. Rotation Restricted Emission and Antenna Effect in Single Metal-Organic Frameworks. J. Am. Chem. Soc. 2019;141:15166–15173. doi: 10.1021/jacs.9b06755. [DOI] [PubMed] [Google Scholar]
- 30.Do Nascimento J.F.S., Barros B.S., Kulesza J., de Oliveira J.B.L., Pereira Leite A.K., de Oliveira R.S. Influence of Synthesis Time on the Microstructure and Photophysical Properties of Gd-MOFs Doped with Eu3+ Mater. Chem. Phys. 2017;190:166–174. doi: 10.1016/j.matchemphys.2017.01.024. [DOI] [Google Scholar]
- 31.Kolesnik S.S., Nosov V.G., Kolesnikov I.E., Khairullina E.M., Tumkin I.I., Vidyakina A.A., Sysoeva A.A., Ryazantsev M.N., Panov M.S., Khripun V.D., et al. Ultrasound-Assisted Synthesis of Luminescent Micro-and Nanocrystalline Eu-Based Mofs as Luminescent Probes for Heavy Metal Ions. Nanomaterials. 2021;11:2448. doi: 10.3390/nano11092448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuno Y., Cavagnero S. Effect of Heavy Atoms on Photochemically Induced Dynamic Nuclear Polarization in Liquids. J. Magn. Reson. 2018;286:172–187. doi: 10.1016/j.jmr.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui G., Fang W.H. State-Specific Heavy-Atom Effect on Intersystem Crossing Processes in 2-Thiothymine: A Potential Photodynamic Therapy Photosensitizer. J. Chem. Phys. 2013;138:044315. doi: 10.1063/1.4776261. [DOI] [PubMed] [Google Scholar]
- 34.Utochnikova V.V., Grishko A.Y., Koshelev D.S., Averin A.A., Lepnev L.S., Kuzmina N.P. Lanthanide Heterometallic Terephthalates: Concentration Quenching and the Principles of the “Multiphotonic Emission”. Opt. Mater. 2017;74:201–208. doi: 10.1016/j.optmat.2017.02.052. [DOI] [Google Scholar]
- 35.Bryleva Y.A., Ustimenko Y.P., Plyusnin V.F., Mikheilis A.V., Shubin A.A., Glinskaya L.A., Komarov V.Y., Agafontsev A.M., Tkachev A.V. Ln(Iii) Complexes with a Chiral 1H-Pyrazolo[3,4-b]Pyridine Derivative Fused with a (−)-α-Pinene Moiety: Synthesis, Crystal Structure, and Photophysical Studies in Solution and in the Solid State. New J. Chem. 2021;45:2276–2284. doi: 10.1039/D0NJ05277A. [DOI] [Google Scholar]
- 36.Kolesnikov I.E., Vidyakina A.A., Vasileva M.S., Nosov V.G., Bogachev N.A., Sosnovsky V.B., Skripkin M.Y., Tumkin I.I., Lähderanta E., Mereshchenko A.S. The Effect of Eu3+ and Gd3+ co-Doping on the Morphology and Luminescence of NaYF4:Eu3+, Gd3+ phosphors. New J. Chem. 2021;45:10599–10607. doi: 10.1039/D1NJ02193A. [DOI] [Google Scholar]
- 37.Reineke T.M., Eddaoudi M., Fehr M., Kelley D., Yaghi O.M. From Condensed Lanthanide Coordination Solids to Microporous Frameworks Having Accessible Metal Sites. J. Am. Chem. Soc. 1999;121:1651–1657. doi: 10.1021/ja983577d. [DOI] [Google Scholar]
- 38.Daiguebonne C., Kerbellec N., Guillou O., Bünzli J.C., Gumy F., Catala L., Mallah T., Audebrand N., Gérault Y., Bernot K., et al. Structural and Luminescent Properties of Micro- and Nanosized Particles of Lanthanide Terephthalate Coordination Polymers. Inorg. Chem. 2008;47:3700–3708. doi: 10.1021/ic702325m. [DOI] [PubMed] [Google Scholar]
- 39.Holland T.J.B., Redfern S.A.T. Unit Cell Refinement from Powder Diffraction Data: The Use of Regression Diagnostics. Mineral. Mag. 1997;61:65–77. doi: 10.1180/minmag.1997.061.404.07. [DOI] [Google Scholar]
- 40.Shannon R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A. 1976;32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 41.Cui Y., Yue Y., Qian G., Chen B. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012;112:1126–1162. doi: 10.1021/cr200101d. [DOI] [PubMed] [Google Scholar]
- 42.Alammar T., Hlova I.Z., Gupta S., Biswas A., Ma T., Zhou L., Balema V., Pecharsky V.K., Mudring A.V. Mechanochemical Synthesis, Luminescent and Magnetic Properties of Lanthanide Benzene-1,4-Dicarboxylate Coordination Polymers (Ln0.5Gd0.5)2 (1,4-BDC)3(H2O)4; Ln = Sm, Eu, Tb. New J. Chem. 2020;44:1054–1062. doi: 10.1039/C9NJ02583A. [DOI] [Google Scholar]
- 43.Dieke G.H., Crosswhite H.M. The Spectra of the Doubly and Triply Ionized Rare Earths. Appl. Opt. 1963;2:675–686. doi: 10.1364/AO.2.000675. [DOI] [Google Scholar]
- 44.Tanner P.A. Some Misconceptions Concerning the Electronic Spectra of Tri-Positive Europium and Cerium. Chem. Soc. Rev. 2013;42:5090–5101. doi: 10.1039/c3cs60033e. [DOI] [PubMed] [Google Scholar]
- 45.Haquin V., Etienne M., Daiguebonne C., Freslon S., Calvez G., Bernot K., le Pollès L., Ashbrook S.E., Mitchell M.R., Bünzli J.C., et al. Color and Brightness Tuning in Heteronuclear Lanthanide Terephthalate Coordination Polymers. Eur. J. Inorg. Chem. 2013;2013:3464–3476. doi: 10.1002/ejic.201300381. [DOI] [Google Scholar]
- 46.Li M., Zhou Y., Yao Y., Gao T., Yan P., Li H. Designing Water-Quenching Resistant Highly Luminescent Europium Complexes by Regulating the Orthogonal Arrangement of Bis-β-Diketone Ligands. Dalton Trans. 2021;50:9914–9922. doi: 10.1039/D1DT00155H. [DOI] [PubMed] [Google Scholar]
- 47.Ivanova A.A., Gontcharenko V.E., Lunev A.M., Sidoruk A.V., Arkhipov I.A., Taydakov I.V., Belousov Y.A. New Carboxylate Anionic Sm-MOF: Synthesis, Structure and Effect of the Isomorphic Substitution of Sm3+ with Gd3+ and Tb3+ Ions on the Luminescent Properties. Inorganics. 2022;10:104. doi: 10.3390/inorganics10080104. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.