Abstract
Shiga toxin (stx) is the principal virulence factor of the foodborne pathogen, Shiga toxin-producing Escherichia coli (STEC) O157:H7 and is associated with various lambdoid bacterio (phages). A comparative genomic analysis was performed on STEC O157 isolates from cattle (n = 125) and clinical (n = 127) samples to characterize virulence genes, stx-phage insertion sites and antimicrobial resistance genes that may segregate strains circulating in the same geographic region. In silico analyses revealed that O157 isolates harboured the toxin subtypes stx1a and stx2a. Most cattle (76.0%) and clinical (76.4%) isolates carried the virulence gene combination of stx1, stx2, eae and hlyA. Characterization of stx1 and stx2-carrying phages in assembled contigs revealed that they were associated with mlrA and wrbA insertion sites, respectively. In cattle isolates, mlrA and wrbA insertion sites were occupied more often (77% and 79% isolates respectively) than in clinical isolates (38% and 1.6% isolates, respectively). Profiling of antimicrobial resistance genes (ARGs) in the assembled contigs revealed that 8.8% of cattle (11/125) and 8.7% of clinical (11/127) isolates harboured ARGs. Eight antimicrobial resistance genes cassettes (ARCs) were identified in 14 isolates (cattle, n = 8 and clinical, n = 6) with streptomycin (aadA1, aadA2, ant(3’’)-Ia and aph(3’’)-Ib) being the most prevalent gene in ARCs. The profound disparity between the cattle and clinical strains in occupancy of the wrbA locus suggests that this trait may serve to differentiate cattle from human clinical STEC O157:H7. These findings are important for stx screening and stx-phage insertion site genotyping as well as monitoring ARGs in isolates from cattle and clinical samples.
Keywords: Escherichia coli O157, Shiga toxins, stx-carrying phages, insertion sites, antimicrobial resistance
1. Introduction
Shiga toxin-producing Escherichia coli (STEC), especially O157:H7, is an important food and waterborne pathogen. Cattle are considered asymptomatic carriers of STEC O157:H7 and food products and surface/ground water contaminated with cattle feces can serve as vehicles of transmission to humans and other animals. STEC O157:H7 outbreaks associated with beef [1] and pork products [2] have been reported in Alberta. This corroborates the evidence that people living in areas with large numbers of cattle are at higher risk of STEC infections [3,4]. One possible explanation for this phenomenon is that particular strains persist in cattle and their environment, occasionally crossing over to humans and causing small, local outbreaks and sporadic infections.
Shiga toxin (Stx) is STEC’s principal virulence factor, encoded by stx1 and stx2 genes and is often associated with lambda bacteriophages [5] which insert into the bacterial chromosome at specific sites. Some of these insertion sites include wrbA, yecE, sbcB, argW, Z2755, torS/T, ynfH, yciD, potC, serU, yjbM and mlrA [6,7]. Occupation of these insertion sites by different prophages contributes to diversity within STEC [8,9] as well as virulence [10]. For instance, stx1- and stx2-carrying prophages are inserted at mlrA and wrbA insertion sites, respectively, in E. coli O157:H7 Sakai [5], a strain associated with an outbreak from contaminated radish sprouts in Japan [11]. The stx-carrying φPOC-J13 phages can infect clinical strains such as Shigella flexneri [12,13] and S. sonnei [14]. These stx-carrying φPOC-J13 phages contribute to the genetic heterogeneity of the stx-carrying phage pool in humans and cattle. Thus, a differential diagnostic method which combines stool culture and stx detection is necessary to distinguish between STEC- and non-STEC-related infections [15] for outbreak surveillance purposes.
Stx can bind to globotriosylceramide (Gb3) receptor cells [16] which are located in the human intestinal tract, kidney and brain, causing damage to these organs usually characterized by bloody diarrhoea, thrombocytopenia, haemorrhagic colitis and hemolytic uremic syndrome (HUS) [17]. Symptoms may vary depending on the virulent nature of the strain, immunological condition of the patient and age [15]. Stx are usually transported in blood to these target organs through polymorphonuclear leukocytes and have a half-life of about 5 days in blood [18], an important etiologic factor for diagnosis and treatment as these toxins are undetectable in stools after 5 days [18]. Supportive therapy with hydration to maintain electrolyte balance is the most reliable method of treating STEC infections in humans. Antibiotic treatment of STEC O157 is not usually recommended in humans due to concerns that it may increase toxin production [19,20,21]. Some DNA-based vaccines that are capable of neutralizing stx activity [22] and STEC shedding [23] in mice are a promising strategy but have yet to undergo human trials. Polyphenolic compounds such as condensed tannins and phlorotannins, which can inhibit STEC growth [24] could be promising therapeutic agents in humans that can help reduce STEC growth and subsequent attachment in the intestinal tract. Furthermore, Weissella confuse, a probiotic which has shown activity against STEC in a zebrafish model [25] and monoclonal antibodies that can clear stx2 in the bloodstream of mice [26] all have potential as therapeutic agents in humans, if clinical efficacy can be confirmed.
STEC strains are mosaic in their virulence profiles locally and globally, however, certain lineages may persist in the environment and circulate between animals and/or humans. For example, STEC O157 sequence type eleven (ST11) is a predominant clone [27,28] circulating in humans and cattle that has been isolated from human, bovine and porcine samples in Alberta [29,30,31]. STEC O157 infections in Alberta have been associated with HUS, primarily in children <10-years-old and the elderly >60-years-old [32].
In addition to stx genes, other virulence factors such as eae (intimin), which are essential to the locus of enterocyte effacement (LEE), translocated intimin receptor gene (tir), esc cluster genes that encode for components of the type III secretion system responsible for secretion of Esp proteins (coded by espA, espB and espD) and hemolysin (hylA) are associated with intestinal and kidney disease in humans [33].
Antimicrobial agents that are used to control or treat infections in humans, livestock, and crops can promote carriage of antimicrobial resistance (AMR)/resistance genes (ARGs) in bacteria. Antimicrobial resistant-bacteria are considered adulterants in food producing animals and pose a threat to global public health, food security and economic growth [34]. STEC O157 strains resistant to streptomycin, sulfisoxazole, and tetracyclines are commonly associated with isolates from commercial feedlots [35] and clinical [36,37] samples in Canada and abroad [38,39]. Mobile DNA elements such as transposons and plasmids are vehicles and major distributors of ARGs through horizontal gene transfer within or across bacterial species.
Whole genome sequencing (WGS) is increasingly being used by the Centers for Disease Control and Prevention, the Food and Drug Administration, the United States Department of Agriculture’s Food Safety and Inspection Service [40] and the Public Health Agency of Canada [41] for surveillance and to discriminate closely related STEC from outbreak events. STEC are prevalent and highly diverse in cattle [42], while strains that cause severe human disease are less diverse and infrequent [43]. Furthermore, there is growing evidence of secondary transmission of STEC O157:H7 [44,45], which suggest that humans can act as carriers. Therefore, to understand STEC O157 strains circulating within the same geographic region in cattle and humans in Alberta, we sequenced STEC O157 for a comparative genomic analysis of clinical isolates collected in hospitals and isolates collected from feedlot cattle from 2007 to 2015.
2. Results
2.1. In Silico Serotyping and Multi-Locus Sequence Typing (MLST) Analysis
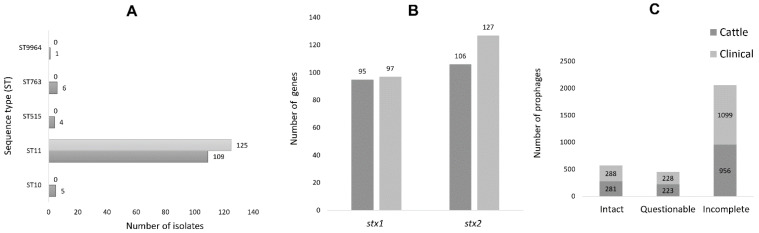
Two hundred and fifty-two bacterial isolates from cattle (n = 125) and humans (n = 127) were sequenced. In silico serotyping revealed that all clinical STEC O157 isolates possessed the H7 antigen determinant, whereas 79.2%, 4.0%, 4.8% and 4.0% of cattle isolates had H7 (n = 99), H12 (n = 5), H19 (n = 6) and H29 (n = 5), respectively (Table S3). These O157:non-H7 (n = 16) were detected only in cattle samples. MLST analyses revealed five different sequence types (STs). Most cattle (87.2%) and clinical isolates (98.4%) belonged to ST11. Cattle isolates also included ST10 (n = 5), ST515 (n = 4), ST763 (n = 6) and ST9964 (n = 1). The ST of two clinical isolates could not be identified. Overall, 98.4% (125/127) of clinical and 87.2 (109/125) of cattle isolates were ST11 (Figure 1A).
Figure 1.
(A) Genetic relatedness of O157 STEC using MLST scheme with seven housekeeping genes: adk, fumC, gyrB, icd, mdh, purA, and recA, (B) the number of identified stx genes and (C) prophages in cattle and clinical O157 STEC isolates.
2.2. Distribution of Stx Genes, Prophages, and Stx-Phage Insertion Sites
The majority of cattle and clinical isolates carried stx1/2 loci in tandem that encoded A and B toxin subunits that were identical to the stx1/2 in E. coli O157:H7 strain Sakai (NC002695.2) [5] and EDL933 (CP008957.1) [46]. In E. coli O157 genomes of both cattle and clinical origin, only stx1a and stx2a gene subtypes (referred in this study as stx1 and stx2) were identified. These genes showed 100% nucleotide sequence similarity across cattle and the clinical isolates. The stx1 and stx2 genes were common in both cattle (76.0%; n = 95, 84.8%; n = 106) and clinical (76.4%; n = 97, 100%; n = 127) isolates, with the majority of cattle (76.0%) and clinical (76.4 %) isolates possessing both stx1 and stx2 (Figure 1b). The O157:non-H7 (n = 16) lacked stx1/stx2.
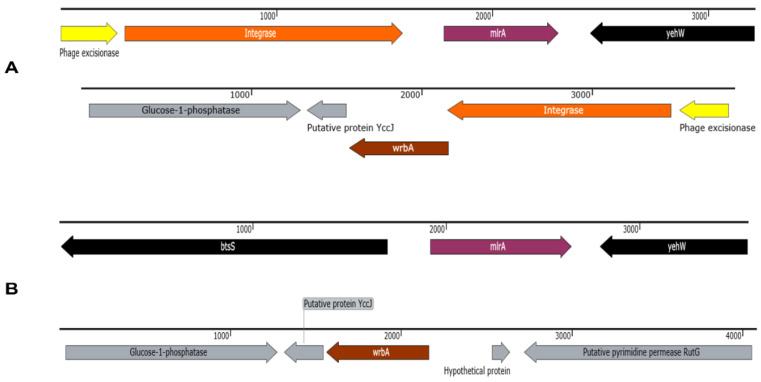
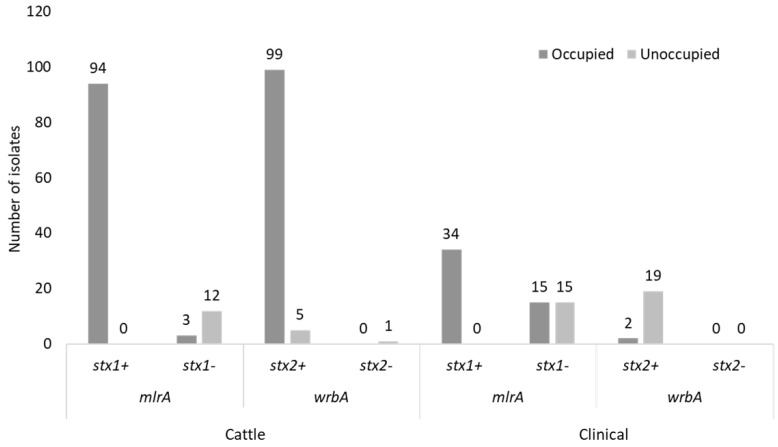
Identified prophages were distributed between cattle (intact; n = 278, questionable; n = 223 and incomplete n = 951) and clinical (intact; n = 287, questionable; n = 228 and incomplete n = 1096) isolates, with incomplete prophages being the most prevalent (Figure 1C). Manual screening of assembled contigs for stx1 and stx2-carrying phages revealed that the integrase (int) gene was present at mlrA or wrbA insertion sites (Figure 2A) and indicative of phage occupation whereas these regions were unoccupied (absence of phage int) in some isolates (Figure 2B). The O157:non-H7 (n = 16) cattle isolates that lacked both stx1/stx2 were not considered for insertion site comparative analysis. Insertion sites were more occupied in cattle (mlrA, n = 97 and wrbA, n = 99) than clinical (mlrA, n = 49 and wrbA, n = 2) isolates (Figure 3). Of the 49 clinical isolates having occupied mlrA loci, 30.6% (15/49) lacked stx1 in their genome compared to 3.1% (3/97) in cattle (Figure 3). Overall, all the unoccupied mlrA regions in cattle (n = 12) and clinical (n = 15) isolates lacked stx1 (Figure 3) and int in their genomes. Contrarily, 100% (19/19) of clinical isolates with unoccupied wrbA loci, possessed stx2, whereas 83.3% (5/6) of cattle isolates had stx2 (Figure 3). With the exception of argW, no other stx2-insertion site such as sbcB or yecE were occupied by prophages (data not shown). Those prophages identified within the argW insertion site were not stx-phages.
Figure 2.
(A) Presence of stx-carrying prophage integrase flanking mlrA and wrbA in cattle and clinical O157 STEC and (B) absence of stx-carrying prophage integrase at mlrA and wrbA in cattle and clinical O157 STEC. Arrow color indicates gene direction.
Figure 3.
Distribution of stx genes in cattle and clinical O157 STEC isolates with occupied and unoccupied insertion (mlrA and wrbA) regions.
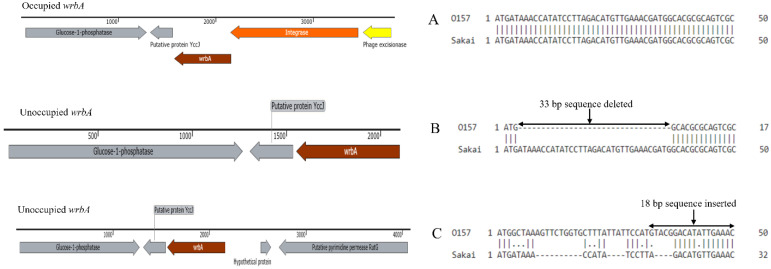
Sequence polymorphism (33 bp nucleotide deletions and 18 bp nucleotide insertions) were identified when wrbA within unoccupied clinical (83.5%, 106/127 and 14.9%, 19/127) and cattle isolate (3.2%, 4/125 and 4.8%, 6/125) sites were aligned against that of E. coli O157:H7 strain Sakai (Figure 4). The 18 bp insertions in wrbA did not correspond to phage sequence, but rather to an intergenic sequence that flanked wrbA within isolates with occupied stx2 prophages.
Figure 4.
Sequence alignment for occupied and unoccupied wrbA gene of O157 isolates with that of E. coli O157 strain Sakai. Only the first 50 bp are represented (A) no polymorphism, (B) 33 bp deleted in O157 and (C) 18 bp inserted in O157, compared to Sakai strain.
2.3. Additional Identified Virulence Factors
Additional virulence genes identified included Type II secretion protein (EtpD), essential genes of the locus of enterocyte effacement (LEE) intimin (eae), translocated intimin receptor gene (tir), esc cluster genes that encode for the type III secretion system responsible for secretion of Esp proteins (coded by espA, espB and espF), prophage-encoded type III secretion system effector (espJ), extracellular serine protease plasmid-encoded (espP), hemolysin (hylA), EAST-1 heat-stable toxin (astA), colicin encoded virulence factor (cib, cia and cba), glutamate decarboxylase (gad), plasmid-encoded catalase peroxidase (katP), Toxin B (toxB), increased serum survival (iss), outer membrane hemin receptor (chuA), Non-LEE encoded effector (NleA, NleB and NleC), outer membrane protease (OmpT), outer membrane complement resistance protein (TraT), tellurium ion resistance protein (TerC) and adherence protein (Iha) (Table S4). In both cattle and clinical isolates carrying either stxt1+ /stx2+ or stx1−/stx2+, additional virulence genesincluding eae, hylA, tir, espA, espB, espP, espJ, katP, toxB, nleABC and chuA were always present, followed by iha (clinical n = 127 and cattle n = 110), espS (clinical n = 110 and cattle n = 39), cia (clinical n = 4 and cattle n = 9), cba (clinical n = 2 and cattle n = 5) and cib (cattle n = 3). Sixteen cattle isolates with the stx1−/stx2− profile lacked eae, hylA, tir, espA, espB, espP, katP, toxB, nleABC and chuA, with terC being the only virulence gene present in all O157 isolates.
2.4. Predicted Antimicrobial Resistance/Resistance Cassette Genes and Plasmids
ResFinder revealed that 8.8% of cattle (11/125) and 8.7% of clinical (11/127) isolates harboured ARGs. Among ARG-carrying isolates, 19 isolates (clinical, n = 10 and cattle, n = 9) harboured between 2 to 10 ARGs (Table 1). Six of the 11 cattle isolates were non-STEC (lacked both stx1/stx2) whereas all 11 clinical isolates had either stx2 or both stx1/stx2. The predicted phenotypes included resistance against ampicillin, streptomycin, kanamycin/neomycin, chloramphenicol, trimethoprim, sulfisoxazole and tetracycline. The most prevalent multidrug resistance (MDR) gene combinations included ant(3’’)-Ia, sul1, tet(A), (n = 5 isolates) associated with 4 cattle and 1 clinical isolate, followed by aph(6)-Id, aph(3’’)-Ib, sul2, tet(B), which were associated with 3 clinical isolates. Collectively, aminoglycoside resistance genes were most prevalent (n = 28), followed by tetracycline (n = 26), sulfonamide (n = 17), phenicol (n = 5), beta-lactam (4), and trimethoprim (n = 2). Beta-lactam resistance genes were only present in clinical isolates. Eleven clinical isolates with ARGs carried virulence gene combination (stx1+/stx2+ and stx1−/stx2+) compared to five cattle isolates (stx1+/stx2+ and stx1−/stx2+) (Table 1).
Table 1.
Predicted ARGs profiles and virulence genes for cattle and clinical isolates.
| Number of Isolates | Source | Year | stx1 | stx2 | Resistance Genotype |
|---|---|---|---|---|---|
| 4 | Cattle | 2014 | − | + | ant(3’’)-Ia, sul1, tet(A) |
| 1 | Clinical | 2009 | + | + | |
| 1 | Clinical | 2008 | + | + | blaTEM-1B |
| 3 | Clinical | 2014 | − | + | aph(6)-Id, aph(3’’)-Ib, sul2, tet(B) |
| 1 | Clinical | 2015 | + | + | blaTEM-1B, tet(A) |
| 1 | Clinical | 2007 | − | + | aadA1, aadA2, cmlA1, sul3 |
| 1 | Clinical | 2014 | − | + | aadA2, ant(3’’)-Ia, cmlA1, sul3, tet(A) |
| 1 | Clinical | 2009 | + | + | aph(3’’)-Ib, aph(6)-Id, blaTEM-1C, sul2 |
| 1 | Clinical | 2009 | + | + | aph(3’’)-Ib, aph(6)-Id, blaTEM-1B, sul2, tet(B) |
| 1 | Clinical | 2015 | − | + | aph(3’’)-Ib, aph(6)-Id, dfrA14, floR, sul2, tet(A) |
| 1 | Cattle | 2014 | − | − | aadA1, aadA2, aph(3’)-IIa, aph(6)-Ic, cmlA1, dfrA12, sul3, tet(A), tet(B), tet(M) |
| 1 | Cattle | 2013 | + | + | aph(3’’)-Ib, aph(6)-Id, floR, sul2, tet(A) |
| 1 | Cattle | 2009 | − | − | tet(M) |
| 3 | Cattle | 2015 | − | − | tet(A), tet(M) |
| 1 | Cattle | 2015 | − | − | tet(A) |
Streptomycin; (aadA1, aadA2, ant(3’’)-Ia, aph(6)-Ic, aph(3’’)-Ib), Kanamycin; (aph(3′)-IIa, aph(6)-Id), Trimethoprim; (dfrA14), Chloramphenicol; (cmlA1, floR), Sulfisoxazole; (sul2), Tetracycline; (tetA, tetE, tetM,), Ampicillin; (blaTEM-1B). +; gene present, −; gene absent.
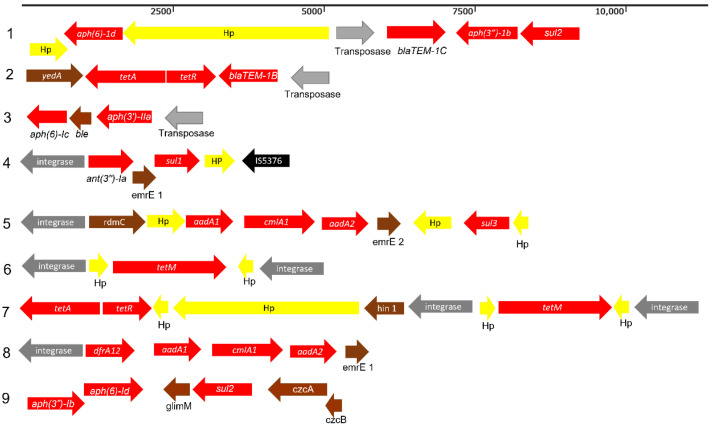
Eight antimicrobial resistance genes cassettes (ARCs) were associated with transposase and integron-integrase in 14 isolates (cattle, n = 9 and clinical, n = 5) and streptomycin resistance (aadA1, aadA2, ant(3’’)-Ia and aph(3’’)-Ib) was most prevalent in different ARCs followed by the sulfonamide resistance (sul2) (Figure 5). Two clinical isolates with beta-lactam resistance genes (blaTEM-1C and blaTEM-1B) were flanked by transposase as well as one cattle isolate (Figure 5; ARC1, 2 and 3). Meanwhile, three clinical isolates (Figure 5; ARC4, n = 1 and ARC5, n = 2) and nine cattle isolates (Figure 5; ARC4, n = 4, ARC6, n = 1, ARC7, n = 3 and ARC8, n = 1) were flanked by an integron-integrase gene (class 1). ARC4 was the only ARC profile detected in both clinical (n = 1) and cattle (n = 4) isolates. ARC9, a non-integrated ARC was detected in three clinical isolates.
Figure 5.
Identified antibiotic resistance cassettes (ARCs). Arrow color indicates gene direction. Gray; mobile gene element, Red; resistance gene, Yellow; hypothetical protein, Black; insertion sequence, Brown; other gene.
Twenty-seven different types of small plasmid replicons <1 kb (63–885 bp) were detected in both cattle and clinical isolates (Table S5). The most prevalent was IncFII (n = 245), followed by IncFIB(AP001918) (n = 242), IncFIA (n = 16), IncI1 (n = 16), pEC4115 (n = 13) and IncI2 (n = 8). Plasmid replicons, Col(BS512) (n = 1), ColRNAI (n = 2), IncA/C2 (n = 1), IncFIA(HI1) (n = 1), IncFIB(K) (n = 1), IncFIC(FII) (n = 6), IncHI2 (n = 1), IncHI2A (n = 1), IncX4 (n = 4) and IncY (n = 4) were unique to cattle isolates, whereas Col440I (n = 2), ColpVC (n =1), IncB/O/K/Z (n = 2), IncFII(pCoo) (n = 1) IncN (n = 1), p0111 (n = 1) and pXuzhou21 (n = 1) were associated with clinical isolates. Overall, the Inc (n = 19) types were the most prevalent followed by colicinogenic (n = 5) plasmids (Table S5).
3. Discussion
This study conducted a comparative analyses of STEC O157 from cattle and clinical samples in the same geographic region. Our findings revealed evidence of similar and dissimilar virulence profiles in STEC O157 strains based on MLST, stx and stx-phage insertion site genotyping. In addition, mobile DNA elements such as transposons and integrons, which are major contributors to genetic variation and drivers of antimicrobial resistance among bacteria, were identified. In silico serotyping revealed that clinical (n = 127) and cattle (n = 99) isolates with O- and H-antigen determinant genes also have a stx1+, stx2+, eae+ and hly+ profile confirming their pathogenicity potential as O157:H7. However, sixteen O157:non-H7 cattle isolates H19; (n = 6), H29; (n = 5) and H12; (n =5) were considered non-STEC and may not be pathogenic as they all lacked stx1−, stx2−, eae− and hly−. Based on whole genome (wg) MLST, the most prevalent O157:H7 STEC clonal lineage circulating in cattle and humans belong to the same sequence type, ST11 as the O157:H7 pathogen type [27] and corroborates previous E. coli O157:H7 studies in Alberta which found that ST11 was the predominant clone among cattle isolates [30,31]. Most ST11 isolates in both cattle and clinical samples possessed a similar virulence gene profile (stx1+, stx2+, eae+ and hly+), suggesting that wgMLST is a good predictor of isolates from cattle that may cause clinical disease in humans. Similarly, ST10, a non pathogen based on O157:H7 sequence typing [27] and other STs 515; (n = 4), 763; (n = 6) and 9964; (n = 1) in cattle isolates, had similar non-pathogenic (stx1−, stx2−, eae− and hly−) profiles. Although wgMLST can distinguish potential pathogenic and non-pathogenic strains and may have a high discriminatory power compared to phenotypic typing methods, the discriminatory power of this method should be interpreted with caution or used in conjunction with other virulence profiling methods as it failed to predict the ST for two clinical isolates (stx1−/stx2+) in this study. These could be new, uncharacterized STs that possess a different genetic repertoire compared to those STs that are reported in the E. coli MLST [27] database.
The stx1/2 including subtypes are classified based on difference in protein sequence and biological activity [47]. Toxicity and cell receptor binding affinity of the different stx types play a major role in clinical outcomes [48] with stx2a and stx2c more potent than stx1, stx1c, sx2d and stx2e in humans [49,50,51,52]. The stx1a/2a subtypes identified in this study have previously been reported in cattle [31] and clinical [29] isolates from Alberta and suggest these are the main subtypes circulating in this region. This indicates a possible risk of E. coli O157:H7 circulating between cattle and humans given that human to human transmission should be less common in high-resource countries due to more robust sanitation practices. A combination of stx2a and eae genes in O157:H7 maybe associated with hemolytic uremic syndrome [29]. The majority of cattle and clinical isolates in this study possessed both stx2a and eae genes which could be indicative of their virulence potential in humans.
The stx1 and stx2 genes are carried by two different prophages which can infect the same bacterial strain. In this study, fragmented assemblies failed to reveal intact stx1/2-carrying prophages in O157:H7 genomes. Therefore, two Sakai phages (Sp) Sp5 and Sp15 from E. coli O157:H7 Sakai which carry stx2 and stx1, respectively, were used as reference stx-carrying phages to search for corresponding gene sequences in O157:H7 isolates. The int gene, which catalyses phage integration in Sakai phage had 100% sequence identity to that in O157:H7 from Alberta, illustrating the conservation of this phage within this serotype.
Stx-carrying phages in O157 E. coli are important in the dissemination of stx genes and genetic diversity of the bacterial host. Although most cattle and clinical isolates showed a similar genotypic profile by wgMLST and stx typing, this was not the case for stx1/2-carrying-phage insertion genotyping. There was a substantial disparity in occupied/unoccupied wrbA insertion site between cattle and clinical isolates, which raises the question as to why this site was occupied by phage in cattle, but not in clinical isolates. According to Serra-Moreno et al. [53], insertion site occupancy by stx-carrying phage is host strain and locus specific. Fourteen cattle isolates with stx1−/stx2− profile expectedly lacked both mlrA and wrbA insertion sites, whereas most clinical isolates (n = 95) unexpectedly had unoccupied wrbA. Differential occupation of stx2-phages in clinical isolates compared to cattle isolates suggests that O157:H7 strains circulating in humans and cattle differ, even though cattle are seen as the main reservoir of this pathogen. This may serve as an important epidemiologic trait which could differentiate these strains during outbreak events. This finding is supported by that of Shaikh and Tarr [9] who found that O157:H7 isolates from several sources including hamburgers had unoccupied wrbA locus. However, most isolates in the study of Shaikh and Tarr [9] were stx1−/stx2+, contrary to our findings as most O157:H7 clinical isolates (n = 95) were stx1+/stx2+.
To further understand the disparity in the unoccupied wrbA locus in clinical isolates, we looked for sequence polymorphisms between the unoccupied O157:H7 isolates’ wrbA locus and that of E. coli O157:H7 Sakai. A 33 bp sequence deletion or an 18 bp insertion in the wrbA gene were identified, modifications that may be responsible for the lack of occupancy in most clinical as compared to cattle isolates. An altered and unoccupied locus, indicates that selection of secondary insertion site is not only limited to absence of primary site [53], but also possibly to modification of the insertion sequence. Therefore, we hypothesized that the presence of stx2-carrying phages in O157:H7 with an unoccupied and a defective wrbA locus may be an adaptive response by phages to overcome a possible host-associated defense mechanism through integration at a different site in the O157:H7 chromosome. Fragmented assemblies failed to reveal flanking genes (insertion site) of the phage integrase of these isolates with unoccupied and or a defective (33 bp deletion/18 bp insertion) wrbA site. Further, the phage int gene in stx2-carrying phages in isolates with defective wrbA locus was 100% identical to that of Sp15, ruling out the possibility that changes in the sequence of int were responsible for the lack of integration at this site.
Other stx2-carrying phage integration sites in O157:H7 such as argW, sbcB, and yecE have been reported [6,54] and phage may prefer these insertion sites when the primary wrbA site is absent [53]. Except for argW, these integration sites were unoccupied in this study. However, the int at the argW site was identical to integrase of Sp16, a non stx-carrying phage in E. coli O157:H7 strain Sakai. The fact that a 33 bp sequence deletion is limited to most (n = 95) stx2-carrying clinical isolates with the stx1+/stx2+ profile, suggests they are a common clone that circulates in humans and may be genotypically different from those in cattle (n = 90) with the same stx (stx1+/stx2+) profile. Only 4 cattle isolates with unoccupied defective wrbA had the same stx (stx1+/stx2+) profile as those of clinical isolates, possibly a reflection of a similar lineage that circulates in both cattle and humans.
Few (clinical, n = 15/19 and cattle, n = 3/6) isolates with 18 bp sequence insertion in the unoccupied wrbA locus harboured stx1−/stx2+ in their genome. In the study of Shaikh and Tarr [9], these stx1−/stx2+ isolates lacked the duplicated GACATATTGAAAC intergenic sequences that flanked the wrbA insertion site. However, we found that, the terminal repeat sequence GACATATTGAAAC and a 5 bp (GTACG) sequence was unexpectedly fused (18 bp insert) within the wrbA gene of unoccupied O157:H7 strains (Figure 4). Thus, the O157:H7 STEC in this study with altered wrbA locus (18 bp insert) are genotypically different to unoccupied O157:H7 STEC with a 33 bp deletion as well as an occupied intact wrbA site. Shaikh and Tarr [9], suggest that O157:H7 STEC with stx1−/stx2+ profile might be the remnants of O157:H7 that gave rise to stx1+/stx2+ O157:H7 or were previously stx1+/stx2+ O157:H7 that lost their stx1 gene.
Interestingly, we also observed that clinical, 30.6% (15/49) and cattle, 4.1% (4/98) isolates with an occupied mlrA loci, lacked stx1 in their genome. A possible explanation for this is the partial loss of the stx1-carrying phage segment. For example, the remnants of integrated phage int gene were still present in the occupied site in agreement with Shaikh and Tarr [9] who reported the absence of stx1 in most O157:H7 strains with an occupied mlrA. Shiga toxin loss is an observation reported in O157:H7 [30,55] and is strain- and stx type-related [56]. However, the absence of stx1 was more prevalent in clinical isolates than cattle and suggest it could be host-associated. It has been shown that the remnants of stx1-carrying phage segments in O157 have the propensity to recombine with other mobile genetic elements or acquire and disseminate the stx1 gene [57]. Consequently, defective stx1-carrying phages in O157:H7 strains in this study may reflect a cycle of loss and gain of these elements.
Other than stx genes, most cattle and clinical isolates with stx1+/stx2+ or stx1−/stx2+ profile possessed a similar virulence gene profile which included Type III secretion LEE encoded Esp effector proteins, tir receptor protein, intimin protein, toxin B and associated adherence protein factors common to STEC O157 [58,59]. These factors, especially eae, detected in all stx1+/stx2+ or stx1−/stx2+ are important for STEC adherence to intestinal epithelia cells during infection in humans.
The number of isolates with antimicrobial resistance genes (ARGs) were equally distributed (11 each) in cattle and clinical samples with different resistance/multidrug profiles, possible reflective of differences in antimicrobial use in humans vs. cattle. Although ARGs were detected in low numbers in cattle (8.8%; 11/125) and clinical (8.7%; 11/127) isolates, most genes were associated with antimicrobials of clinical importance, including category II antimicrobials such as aminoglycosides and penicillins [60].This category was also more prevalent in clinical isolates with stx1+/stx2+ or stx1−/stx2+ profiles than cattle. Additionally, ARGs were flanked by transposase and integron-intergrase genes, which are mobile elements that can facilitate the dissemination and prevalence of ARGs horizontally and vertically within O157:H7 or other strains. The integron-integrase detected in this study was a class 1 integron common to the Enterobacteriaceae [61,62]. The streptomycin resistance gene (aadA1, aadA2, ant(3’’)-Ia and aph(3’’)-Ib) was the most abundant within the different antimicrobial resistance genes cassettes (ARCs) a finding that aligns with previous studies within Alberta [31,63].
Two ARCs that carried beta lactam gene with profiles, aph(3’’)-Ib/ blaTEM-1C/ sul2 and blaTEM-1B/ tet(R)/tet(A) were associated with transposons which are a common category of mobile DNA element in E. coli and Salmonella spp [64]. ARC4, detected in a single clinical and four cattle isolates with the ant(3’’)-Ia (streptomycin) and sul1 (sulfisoxazole) is indicative of an ARC of both cattle and human origin. ARC9, a non-integrated ARC aph(3’’)-Ib, aph(6)-Id and sul2 in three clinical isolates may have the propensity to become integrated with an integron or transposon which can facilitate the dissemination of ARGs. Like prophages, fragmented assemblies failed to be annotated if transposons and integrons were carried on plasmids, as plasmid replicons were <1 kb. However, the majority of plasmid replicons detected in this study, were of the Inc plasmid type, especially IncF which are common plasmid replicons described in E. coli from animal and human sources [65]. Amongst the IncF plasmids, IncFII (clinical, n = 126 and cattle, n = 116), and IncFIB (AP001918) (clinical, n = 134 and cattle, n = 111) were equally distributed between clinical and cattle isolates, although some were unique to clinical (IncFII(pCoo), n = 1) and cattle (IncFIA(HI1) (n = 1), IncFIB(K) (n = 1) and IncFIC(FII) (n = 6)) isolates. Similar/different types of plasmid replicon in the bacterial strains of different origin may be useful in plasmid characterization and spread of ARGs, since certain plasmid types such as IncF, IncI, IncA/C and IncL are mostly associated with ARGs in Enterobacteriaceae [65].
4. Conclusions
This study analysed O157 STEC from cattle and clinical sources in the same geographic region using in silico typing methods. MLST and stx typing revealed that most O157:H7 isolates from cattle and clinical samples had a similar ST11 lineage and stx1+/stx2+ profile. Furthermore, a common profile for additional virulence genes (eae, hylA, tir, espA, espB, espP, espJ, katP, toxB, nleABC and chuA,) was observed between cattle and clinical isolates. Beta lactam resistance genes were only detected in four clinical isolates. The majority of clinical isolates with stx2+ profile, had a defective (nucleotide sequence deletion or insertion) wrbA loci which was unoccupied by stx2-carrying phage. There is considerable interest in understanding why there was a huge disparity in stx2-carrying phage occupancy in O157:H7 STEC from clinical and cattle samples in the same geographic region, which may highlight the knowledge gap between stx2-phage occupancy and dissemination of stx2. We intend to explore long read sequencing in combination with short reads to generate hybrid assemblies to further define unique features that have the potential to differentiate cattle from clinical O157:H7 isolates. If this mosaic in stx2-carrying phage occupancy between the clinical and cattle isolates is a stable trait, it could be employed for food safety screening and monitoring of STEC O157:H7 in both farm and clinical environments.
5. Materials and Methods
5.1. Sample Collection and Bacterial Isolation
Cattle faecal samples were collected from 2007 to 2015 from commercial feedlot pens or from the floors of transport trucks. At feedlots, E. coli were isolated from rectal grab samples, hide swabs and pooled fecal pats from the pen floor (Table S1). Cattle isolates were collected from nine locations in southern Alberta, Canada.
For cattle isolates, presumptive O157 E. coli [66,67] were retrieved from storage at −80 °C, thawed and 1 mL incubated in 4 mL EC broth at 37 °C for 24 h. A 1 mL aliquot of the enriched culture was then centrifuged at 8000× g for 10 min before extraction of DNA from the pellet using the NucleoSpin Tissue Kit (Macherey-Nagel, Islington, ON, Canada). Extracted genomic DNA was used to confirm O157 serogroup and the presence of stx1, stx2 and eae using primers and PCR as described by Conrad et al. [68].
Human clinical O157:H7 isolates (322) were isolated from 2007 to 2015 from stools cultures from patients experiencing gastroenteritis from Alberta and O157 was confirmed by antiserum agglutination [69,70,71]. A random subset (n = 127) were selected and sequenced for the same sampling years as the cattle isolates. DNA was extracted using the MagaZorb DNA mini-prep kit (Promega Corporation, Madison, WI, USA). The University of Calgary Conjoint Health Research Ethics Board approved the study, REB19–0510.
5.2. Bacterial Whole Genome Sequencing and Data Analysis
DNA was extracted from 252 bacterial isolates collected from cattle (n = 125) and humans (n = 127) and were subjected to whole genome sequencing on an Illumina NovaSeq 6000 at Génome Québec (Montreal, QC, Canada) to generate 250 bp paired-end reads. Sequence data were downloaded as Fastq files and assessed for quality using the FASTQC tool. Trimmomatic v0.36.5 was used to remove adapter sequences as well as low quality sequences based on a Phred Q score of <30 (99.9%). Reads that met quality standards were de novo assembled into contigs using the Unicycler pipeline [72] and annotated using Prokka [73]. Using an average genome size of 5.5 Mb for E. coli O157, the average sequencing coverage was estimated at 158x. Assemblies of all 252 isolates generated, on average, 276 contigs per genome with an average of 117 contigs being ≥1kb size, and N50 contig length of 154,553 bases (Table S2). The average genome size of the sequenced isolates was 5,304,568 bp (5.3 Mb). The assembled contigs were used for in silico analysis as described. The draft whole genome sequence assemblies of the 252 bacterial isolates have been deposited in GenBank under BioProject ID PRJNA870153.
5.3. In Silico Serotyping and Multi-Locus Sequence Typing (MLST) Analysis
The ECTyper tool for Escherichia coli serotyping, version 1.0. [74] was used to confirm the serotype of isolates as O157 using default parameters: O antigen minimum ≥ 90% identity/coverage and H antigen minimum ≥ 90% identity and ≥50% coverage. Genetic relatedness of cattle and clinical O157 STEC strains were determined using an in silico E. coli MLST scheme. Seven housekeeping genes loci (adk, fumC, gyrB, icd, mdh, purA, and recA), previously described for E. coli [27] were used in MLST. The E. coli MLST database was used to assign a number to each locus and a sequence type (ST) for each unique combination of loci.
5.4. Identification of Stx Genes, Prophages, and Stx-Carrying Phage and Associated-Insertion Sites
Chromosomal sequences of O157 isolates were verified for stx genes and subtypes by searching genomes using the stx1 and stx2 primers [68], followed by subtype (stx1a-stx1d and stx2a-stx2f) primers [75] using Geneious 10.2.6. FASTA files of O157 genomes were queried for intact, questionable, and incomplete prophages harbored within the strains using Phage Search Tool Enhanced Release [76]. Hits with a query > 70% were considered as incomplete, 70 to 90% as questionable and >90 as intact prophages [76]. The stx-carrying contigs and flanking genes were manually screened for prophage carrying stx with Geneious 10.2.6 using E. coli O157:H7 Sakai phage (Sp5 and Sp15) as a reference. The sequences for wrbA, mlrA, argW, sbcB, and yecE from E. coli O157:H7 strain Sakai were manually curated against assembled contigs of O157 isolates to identify stx insertion sites with possible occupancy based on the presence of the stx-phage integrase (int) using Geneious. To understand the variability between the presence of stx-associated phage and unoccupied wrbA or mlrA insertion sites, we aligned the sequence of the wrbA and mlrA region in cattle and clinical isolates against wrbA and mlrA DNA sequence from O157 strain Sakai using a pairwise sequence alignment tool, in the EMBL-EBI search and sequence analysis tools [77].
5.5. In Silico Determination of Virulence Factors, Antimicrobial Resistance, and Mobile Element Genes
VirulenceFinder 2.0 was used for the identification of virulence genes in each of the assembled contigs with default settings: coverage ≥ 60% and identity ≥ 90% (https://cge.cbs.dtu.dk/services/VirulenceFinder, accessed on 2 February 2022) [78]. Assembled genomes were screened against ResFinder antimicrobial resistance database (https://bitbucket.org/genomicepidemiology/resfinder_db.git, accessed on 15 December 2021) to determine the presence of antimicrobial resistance genes (ARGs) and PlasmidFinder; (https://bitbucket.org/genomicepidemiology/plasmidfinder_db.git, accessed on 15 December 2021) to detect plasmids. ARGs and/or antimicrobial resistance genes cassettes (ARCs; identical resistance/flanking gene profile in different STEC isolates) flanked by mobile element gene (Integron/transposon) were manually identified using Geneious.
Acknowledgments
The authors would like to thank Cheyenne Sargeant for technical assistance. Support for bioinformatics resources leveraged from Genomics Research and Development Initiative (GRDI) of the Government of Canada project is also acknowledged.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins14090603/s1, Table S1: List of cattle isolates from different regions in Southern Alberta; Table S2: Combined assembly statistics for sequenced O157 STEC isolates, Table S3: In silico serotyping of E. coil strains, Table S4: Predicted additional virulence factors in cattle and clinical O157 STEC isolates, Table S5: Plasmid replicon profile in cattle and clinical O157 STEC isolates.
Author Contributions
Conceptualization, T.A.M., R.Z., L.C., G.A.M.T. and K.S.; methodology, T.A.M., K.S., E.W.B. and R.Z.; software, R.Z., E.W.B. and T.A.M.; validation, T.A.M., R.Z., K.S. and E.W.B.; formal analysis, E.W.B. and R.Z.; investigation, E.W.B. and R.Z.; resources, T.A.M., L.C., G.A.M.T. and K.S.; writing—original draft preparation, E.W.B.; writing—review and editing, T.A.M., E.W.B., R.Z., K.S., L.C., G.A.M.T., L.L.G., C.L. and D.N.; supervision, T.A.M., R.Z., K.S. and D.N.; project administration, T.A.M., R.Z., L.C., G.A.M.T. and K.S.; funding acquisition, T.A.M., G.A.M.T. and D.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Conjoint Health Research Ethics Board (pro-tocol code 20190408, 1.0, 8 April 2019).
Informed Consent Statement
No patient information was released and no identification was attached to the isolates being used in this study.
Data Availability Statement
Data of the 252 bacterial isolates generated in this study have been deposited in GenBank under BioProject ID PRJNA870153.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
The huge disparity in stx2-carrying phage occupancy in the O157:H7 STEC from clinical and cattle samples in the same geographic region suggest that different STEC O157:H7 strains could be circulating in humans and cattle, even though cattle are thought to be the main reservoir. This may serve as an important epidemiologic trait which can be used to segregate these strains during outbreak events.
Funding Statement
This work was supported by Agriculture Funding Consortium, project #2019R048R and the Beef Cattle Research Council (BCRC) #FOS.01.18.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Currie A., Honish L., Cutler J., Locas A., Lavoie M.-C., Gaulin C., Galanis E., Tschetter L., Chui L., Taylor M. Outbreak of Escherichia coli O157: H7 infections linked to mechanically tenderized beef and the largest beef recall in Canada, 2012. J. Food Prot. 2019;82:1532–1538. doi: 10.4315/0362-028X.JFP-19-005. [DOI] [PubMed] [Google Scholar]
- 2.Honish L., Punja N., Nunn S., Nelson D., Hislop N., Gosselin G., Stashko N., Dittrich D. Enteric Disease Outbreaks: Escherichia coli O157: H7 Infections associated with contaminated pork products—Alberta, Canada, July–October 2014. Can. Commun. Dis. Rep. 2017;43:21. doi: 10.14745/ccdr.v43i01a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elson R., Grace K., Vivancos R., Jenkins C., Adak G.K., O’Brien S.J., Lake I.R. A spatial and temporal analysis of risk factors associated with sporadic Shiga toxin-producing Escherichia coli O157 infection in England between 2009 and 2015. Epidemiol. Infect. 2018;146:1928–1939. doi: 10.1017/S095026881800256X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulder A., Van de Kassteele J., Heederik D., Pijnacker R., Mughini-Gras L., Franz E. Spatial effects of livestock farming on human infections with shiga toxin-producing Escherichia coli o157 in small but densely populated regions: The case of the netherlands. GeoHealth. 2020;4:e2020GH000276. doi: 10.1029/2020GH000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi T., Makino K., Ohnishi M., Kurokawa K., Ishii K., Yokoyama K., Han C.-G., Ohtsubo E., Nakayama K., Murata T. Complete genome sequence of enterohemorrhagic Eschelichia coli O157: H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001;8:11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Scheutz F. Enterohemorrhagic Escherichia coli and Other Shiga Toxin-Producing E. coli. American Society for Microbiology; Washington, DC, USA: 2015. Taxonomy meets public health: The case of Shiga toxin-producing Escherichia coli; pp. 15–36. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Rubio L., Haarmann N., Schwidder M., Muniesa M., Schmidt H. Bacteriophages of Shiga toxin-producing Escherichia coli and their contribution to pathogenicity. Pathogens. 2021;10:404. doi: 10.3390/pathogens10040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohnishi M., Terajima J., Kurokawa K., Nakayama K., Murata T., Tamura K., Ogura Y., Watanabe H., Hayashi T. Genomic diversity of enterohemorrhagic Escherichia coli O157 revealed by whole genome PCR scanning. Proc. Natl. Acad. Sci. USA. 2002;99:17043–17048. doi: 10.1073/pnas.262441699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaikh N., Tarr P.I. Escherichia coli O157: H7 Shiga toxin-encoding bacteriophages: Integrations, excisions, truncations, and evolutionary implications. J. Bacteriol. 2003;185:3596–3605. doi: 10.1128/JB.185.12.3596-3605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies E.V., Winstanley C., Fothergill J.L., James C.E. The role of temperate bacteriophages in bacterial infection. FEMS Microbiol. Lett. 2016;363:fnw015. doi: 10.1093/femsle/fnw015. [DOI] [PubMed] [Google Scholar]
- 11.Michino H., Araki K., Minami S., Takaya S., Sakai N., Miyazaki M., Ono A., Yanagawa H. Massive outbreak of Escherichia coli O157: H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 1999;150:787–796. doi: 10.1093/oxfordjournals.aje.a010082. [DOI] [PubMed] [Google Scholar]
- 12.Fogolari M., Mavian C., Angeletti S., Salemi M., Lampel K.A., Maurelli A.T. Distribution and characterization of Shiga toxin converting temperate phages carried by Shigella flexneri in Hispaniola. Infect. Genet. Evol. 2018;65:321–328. doi: 10.1016/j.meegid.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhi S., Parsons B.D., Szelewicki J., Yuen Y.T., Fach P., Delannoy S., Li V., Ferrato C., Freedman S.B., Lee B.E. Identification of Shiga-Toxin-Producing Shigella Infections in Travel and Non-Travel Related Cases in Alberta, Canada. Toxins. 2021;13:755. doi: 10.3390/toxins13110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray M.D., Leonard S.R., Lacher D.W., Lampel K.A., Alam M.T., Morris J.G., Ali A., LaBreck P.T., Maurelli A.T. Stx-producing Shigella species from patients in Haiti: An emerging pathogen with the potential for global spread. Open Forum Infect. Dis. 2015;2:4. doi: 10.1093/ofid/ofv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph A., Cointe A., Mariani Kurkdjian P., Rafat C., Hertig A. Shiga toxin-associated hemolytic uremic syndrome: A narrative review. Toxins. 2020;12:67. doi: 10.3390/toxins12020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingwood C.A. Methods Molecular Medicine. Humana Press; Totowa, NJ, USA: 2003. Shiga toxin receptor glycolipid binding; pp. 165–186. [DOI] [PubMed] [Google Scholar]
- 17.Tarr P., Gordon C., Chandler W. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 18.Brigotti M., Caprioli A., Tozzi A.E., Tazzari P.L., Ricci F., Conte R., Carnicelli D., Procaccino M.A., Minelli F., Ferretti A.V. Shiga toxins present in the gut and in the polymorphonuclear leukocytes circulating in the blood of children with hemolytic-uremic syndrome. J. Clin. Microbiol. 2006;44:313–317. doi: 10.1128/JCM.44.2.313-317.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith K.E., Wilker P.R., Reiter P.L., Hedican E.B., Bender J.B., Hedberg C.W. Antibiotic treatment of Escherichia coli O157 infection and the risk of hemolytic uremic syndrome, Minnesota. Pediatr. Infect. Dis. J. 2012;31:37–41. doi: 10.1097/INF.0b013e31823096a8. [DOI] [PubMed] [Google Scholar]
- 20.Freedman S.B., Xie J., Neufeld M.S., Hamilton W.L., Hartling L., Tarr P.I., Team A.P.P.E.I., Nettel-Aguirre A., Chuck A., Lee B. Shiga toxin–producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: A meta-analysis. Clin. Infect. Dis. 2016;62:1251–1258. doi: 10.1093/cid/ciw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong C.S., Mooney J.C., Brandt J.R., Staples A.O., Jelacic S., Boster D.R., Watkins S.L., Tarr P.I. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157: H7: A multivariable analysis. Clin. Infect. Dis. 2012;55:33–41. doi: 10.1093/cid/cis299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues-Jesus M., Fotoran W., Cardoso R., Araki K., Wunderlich G., Ferreira L. Nano-multilamellar lipid vesicles (NMVs) enhance protective antibody responses against Shiga toxin (Stx2a) produced by enterohemorrhagic Escherichia coli strains (EHEC) Braz. J. Microbiol. 2019;50:67–77. doi: 10.1007/s42770-018-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeshvaghani F.S., Amani J., Kazemi R., Rahjerdi A.K., Jafari M., Abbasi S., Salmanian A.H. Oral immunization with a plant-derived chimeric protein in mice: Toward the development of a multipotent edible vaccine against E. coli O157: H7 and ETEC. Immunobiology. 2019;224:262–269. doi: 10.1016/j.imbio.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Bumunang E.W., Ateba C.N., Stanford K., Niu Y.D., Wang Y., McAllister T.A. Activity of bacteriophage and complex tannins against biofilm-forming shiga toxin-producing Escherichia coli from Canada and South Africa. Antibiotics. 2020;9:257. doi: 10.3390/antibiotics9050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dey D.K., Kang S.C. Weissella confusa DD_A7 pre-treatment to zebrafish larvae ameliorates the inflammation response against Escherichia coli O157: H7. Microbiol. Res. 2020;237:126489. doi: 10.1016/j.micres.2020.126489. [DOI] [PubMed] [Google Scholar]
- 26.Cheng L.W., Henderson T.D., Patfield S., Stanker L.H., He X. Mouse in vivo neutralization of Escherichia coli Shiga toxin 2 with monoclonal antibodies. Toxins. 2013;5:1845–1858. doi: 10.3390/toxins5101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirth T., Falush D., Lan R., Colles F., Mensa P., Wieler L.H., Karch H., Reeves P.R., Maiden M.C., Ochman H. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006;60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barth S.A., Menge C., Eichhorn I., Semmler T., Wieler L.H., Pickard D., Belka A., Berens C., Geue L. The accessory genome of Shiga toxin-producing Escherichia coli defines a persistent colonization type in cattle. Appl. Environ. Microbiol. 2016;82:5455–5464. doi: 10.1128/AEM.00909-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chui L., Li V., Fach P., Delannoy S., Malejczyk K., Patterson-Fortin L., Poon A., King R., Simmonds K., Scott A.N. Molecular profiling of Escherichia coli O157: H7 and non-O157 strains isolated from humans and cattle in Alberta, Canada. J. Clin. Microbiol. 2015;53:986–990. doi: 10.1128/JCM.03321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro V.S., Ortega Polo R., Figueiredo E.E.d.S., Bumunange E.W., McAllister T., King R., Conte-Junior C.A., Stanford K. Inconsistent PCR detection of Shiga toxin-producing Escherichia coli: Insights from whole genome sequence analyses. PLoS ONE. 2021;16:e0257168. doi: 10.1371/journal.pone.0257168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P., Essendoubi S., Keenliside J., Reuter T., Stanford K., King R., Lu P., Yang X. Genomic analysis of Shiga toxin-producing Escherichia coli O157: H7 from cattle and pork-production related environments. NPJ Sci. Food. 2021;5:1–12. doi: 10.1038/s41538-021-00097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisboa L.F., Szelewicki J., Lin A., Latonas S., Li V., Zhi S., Parsons B.D., Berenger B., Fathima S., Chui L. Epidemiology of Shiga Toxin-Producing Escherichia coli O157 in the Province of Alberta, Canada, 2009–2016. Toxins. 2019;11:613. doi: 10.3390/toxins11100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abu-Ali G.S., Ouellette L.M., Henderson S.T., Lacher D.W., Riordan J.T., Whittam T.S., Manning S.D. Increased adherence and expression of virulence genes in a lineage of Escherichia coli O157: H7 commonly associated with human infections. PLoS ONE. 2010;5:e10167. doi: 10.1371/journal.pone.0010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Global Antimicrobial Resistance Surveillance System ( GLASS) Report: Early Implementation 2017–2018. [(accessed on 3 April 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/279656/9789241515061-eng.pdf.
- 35.Aslam M., Stanford K., McAllister T.A. Characterization of antimicrobial resistance and seasonal prevalence of Escherichia coli O157: H7 recovered from commercial feedlots in Alberta, Canada. Lett. Appl. Microbiol. 2010;50:320–326. doi: 10.1111/j.1472-765X.2010.02798.x. [DOI] [PubMed] [Google Scholar]
- 36.Vidovic S., Tsoi S., Medihala P., Liu J., Wylie J.L., Levett P.N., Korber D.R. Molecular and antimicrobial susceptibility analyses distinguish clinical from bovine Escherichia coli O157 strains. J. Clin. Microbiol. 2013;51:2082–2088. doi: 10.1128/JCM.00307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen K.J., Laing C.R., Cancarevic A., Zhang Y., Mesak L.R., Xu H., Paccagnella A., Gannon V.P., Hoang L. Characteristics of clinical Shiga toxin-producing Escherichia coli isolated from British Columbia. BioMed Res. Int. 2013;2013:878956. doi: 10.1155/2013/878956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder C.M., Zhao C., DebRoy C., Torcolini J., Zhao S., White D.G., Wagner D.D., McDermott P.F., Walker R.D., Meng J. Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle, swine, and food. Appl. Environ. Microbiol. 2002;68:576–581. doi: 10.1128/AEM.68.2.576-581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amézquita-López B.A., Quiñones B., Soto-Beltrán M., Lee B.G., Yambao J.C., Lugo-Melchor O.Y., Chaidez C. Antimicrobial resistance profiles of Shiga toxin-producing Escherichia coli O157 and Non-O157 recovered from domestic farm animals in rural communities in Northwestern Mexico. Antimicrob. Resist. Infect. Control. 2016;5:1. doi: 10.1186/s13756-015-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown E., Dessai U., McGarry S., Gerner-Smidt P. Use of whole-genome sequencing for food safety and public health in the United States. Foodborne Pathog. Dis. 2019;16:441–450. doi: 10.1089/fpd.2019.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Health Canada Evaluation of the Public Health Agency of Canada’s Food-Borne and Water-Borne Enteric Illness Activities 2012–2017. [(accessed on 25 April 2022)]; Available online: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/report-evaluation-food-borne-water-borne-enteric-illness-activities-2012-2017.html.
- 42.Stanford K., Reuter T., Bach S.J., Chui L., Ma A., Conrad C.C., Tostes R., McAllister T.A. Effect of severe weather events on the shedding of Shiga toxigenic Escherichia coli in slaughter cattle and phenotype of serogroup O157 isolates. FEMS Microbiol. Ecol. 2017;93:fix098. doi: 10.1093/femsec/fix098. [DOI] [PubMed] [Google Scholar]
- 43.Fratamico P.M., DebRoy C., Liu Y., Needleman D.S., Baranzoni G.M., Feng P. Advances in molecular serotyping and subtyping of Escherichia coli. Front. Microbiol. 2016;7:644. doi: 10.3389/fmicb.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snedeker K.G., Shaw D.J., Locking M.E., Prescott R.J. Primary and secondary cases in Escherichia coliO157 outbreaks: A statistical analysis. BMC Infect. Dis. 2009;9:144. doi: 10.1186/1471-2334-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morita-Ishihara T., Iyoda S., Iguchi A., Ohnishi M. Secondary Shiga Toxin–Producing Escherichia coli Infection, Japan, 2010–2012. Emerg. Infect. Dis. 2016;22:2181. doi: 10.3201/eid2212.160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perna N.T., Plunkett G., Burland V., Mau B., Glasner J.D., Rose D.J., Mayhew G.F., Evans P.S., Gregor J., Kirkpatrick H.A. Genome sequence of enterohaemorrhagic Escherichia coli O157: H7. Nature. 2001;409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 47.Scheutz F., Teel L., Beutin L., Piérard D., Buvens G., Karch H., Mellmann A., Caprioli A., Tozzoli R., Morabito S. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012;50:2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menge C. Molecular biology of Escherichia coli Shiga toxins’ effects on mammalian cells. Toxins. 2020;12:345. doi: 10.3390/toxins12050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orth D., Grif K., Khan A.B., Naim A., Dierich M.P., Würzner R. The Shiga toxin genotype rather than the amount of Shiga toxin or the cytotoxicity of Shiga toxin in vitro correlates with the appearance of the hemolytic uremic syndrome. Diagn. Microbiol. Infect. Dis. 2007;59:235–242. doi: 10.1016/j.diagmicrobio.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Fuller C.A., Pellino C.A., Flagler M.J., Strasser J.E., Weiss A.A. Shiga toxin subtypes display dramatic differences in potency. Infect. Immun. 2011;79:1329–1337. doi: 10.1128/IAI.01182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krüger A., Lucchesi P.M. Shiga toxins and stx phages: Highly diverse entities. Microbiology. 2015;161:451–462. doi: 10.1099/mic.0.000003. [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald S.F., Beckett A.E., Palarea-Albaladejo J., McAteer S., Shaaban S., Morgan J., Ahmad N.I., Young R., Mabbott N.A., Morrison L. Shiga toxin sub-type 2a increases the efficiency of Escherichia coli O157 transmission between animals and restricts epithelial regeneration in bovine enteroids. PLoS Pathog. 2019;15:e1008003. doi: 10.1371/journal.ppat.1008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serra-Moreno R., Jofre J., Muniesa M. Insertion site occupancy by stx 2 bacteriophages depends on the locus availability of the host strain chromosome. J. Bacteriol. 2007;189:6645–6654. doi: 10.1128/JB.00466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yara D.A., Greig D.R., Gally D.L., Dallman T.J., Jenkins C. Comparison of Shiga toxin-encoding bacteriophages in highly pathogenic strains of Shiga toxin-producing Escherichia coli O157: H7 in the UK. Microb. Genom. 2020;6:e000334. doi: 10.1099/mgen.0.000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanford K., Reuter T., Hallewell J., Tostes R., Alexander T.W., McAllister T.A. Variability in Characterizing Escherichia coli from Cattle Feces: A Cautionary Tale. Microorganisms. 2018;6:74. doi: 10.3390/microorganisms6030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senthakumaran T., Brandal L.T., Lindstedt B.-A., Jørgensen S.B., Charnock C., Tunsjø H.S. Implications of stx loss for clinical diagnostics of Shiga toxin-producing Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:2361–2370. doi: 10.1007/s10096-018-3384-6. [DOI] [PubMed] [Google Scholar]
- 57.Asadulghani M., Ogura Y., Ooka T., Itoh T., Sawaguchi A., Iguchi A., Nakayama K., Hayashi T. The defective prophage pool of Escherichia coli O157: Prophage–prophage interactions potentiate horizontal transfer of virulence determinants. PLoS Pathog. 2009;5:e1000408. doi: 10.1371/journal.ppat.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeBlanc J.J. Implication of virulence factors in Escherichia coli O157: H7 pathogenesis. Crit. Rev. Microbiol. 2003;29:277–296. doi: 10.1080/748638745. [DOI] [PubMed] [Google Scholar]
- 59.Bai X., Zhang J., Hua Y., Jernberg C., Xiong Y., French N., Löfgren S., Hedenström I., Ambikan A., Mernelius S. Genomic Insights Into Clinical Shiga Toxin-Producing Escherichia coli Strains: A 15-Year Period Survey in Jönköping, Sweden. Front. Microbiol. 2021;12:627861. doi: 10.3389/fmicb.2021.627861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Health Canada Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. [(accessed on 11 May 2022)]; Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html.
- 61.Brown H.J., Stokes H., Hall R.M. The integrons In0, In2, and In5 are defective transposon derivatives. J. Bacteriol. 1996;178:4429–4437. doi: 10.1128/jb.178.15.4429-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X., Li X., Wang W., Qi J., Wang D., Xu L., Liu Y., Zhang Y., Guo K. Diverse Gene Cassette Arrays Prevail in Commensal Escherichia coli From Intensive Farming Swine in Four Provinces of China. Front. Microbiol. 2020;11:565349. doi: 10.3389/fmicb.2020.565349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benedict K.M., Gow S.P., McAllister T.A., Booker C.W., Hannon S.J., Checkley S.L., Noyes N.R., Morley P.S. Antimicrobial resistance in Escherichia coli recovered from feedlot cattle and associations with antimicrobial use. PLoS ONE. 2015;10:e0143995. doi: 10.1371/journal.pone.0143995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X.-Z., Mehrotra M., Ghimire S., Adewoye L. β-Lactam resistance and β-lactamases in bacteria of animal origin. Vet. Microbiol. 2007;121:197–214. doi: 10.1016/j.vetmic.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 65.Rozwandowicz M., Brouwer M., Fischer J., Wagenaar J., Gonzalez-Zorn B., Guerra B., Mevius D., Hordijk J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018;73:1121–1137. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- 66.Stanford K., Gibb D., McAllister T. Evaluation of a shelf-stable direct-fed microbial for control of Escherichia coli O157 in commercial feedlot cattle. Can. J. Anim. Sci. 2013;93:535–542. doi: 10.4141/cjas2013-100. [DOI] [Google Scholar]
- 67.Stanford K., Johnson R.P., Alexander T.W., McAllister T.A., Reuter T. Influence of season and feedlot location on prevalence and virulence factors of seven serogroups of Escherichia coli in feces of western-Canadian slaughter cattle. PLoS ONE. 2016;11:e0159866. doi: 10.1371/journal.pone.0159866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conrad C.C., Stanford K., McAllister T.A., Thomas J., Reuter T. Further development of sample preparation and detection methods for O157 and the top 6 non-O157 STEC serogroups in cattle feces. J. Microbiol. Methods. 2014;105:22–30. doi: 10.1016/j.mimet.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 69.Couturier M.R., Lee B., Zelyas N., Chui L. Shiga-toxigenic Escherichia coli detection in stool samples screened for viral gastroenteritis in Alberta, Canada. J. Clin. Microbiol. 2011;49:574–578. doi: 10.1128/JCM.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chui L., Lee M.-C., Malejczyk K., Lim L., Fok D., Kwong P. Prevalence of Shiga toxin-producing Escherichia coli as detected by enzyme-linked immunoassays and real-time PCR during the summer months in northern Alberta, Canada. J. Clin. Microbiol. 2011;49:4307–4310. doi: 10.1128/JCM.05211-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chui L., Lee M.-C., Allen R., Bryks A., Haines L., Boras V. Comparison between ImmunoCard STAT!® and real-time PCR as screening tools for both O157: H7 and non-O157 Shiga toxin-producing Escherichia coli in Southern Alberta, Canada. Diagn. Microbiol. Infect. Dis. 2013;77:8–13. doi: 10.1016/j.diagmicrobio.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 72.Wick R.R., Judd L.M., Gorrie C.L., Holt K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 74.Bessonov K., Laing C., Robertson J., Yong I., Ziebell K., Gannon V.P., Nichani A., Arya G., Nash J.H., Christianson S. ECTyper: In silico Escherichia coli serotype and species prediction from raw and assembled whole-genome sequence data. Microb. Genom. 2021;7:000728. doi: 10.1099/mgen.0.000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He X., Quiñones B., McMahon S., Mandrell R.E. A single-step purification and molecular characterization of functional Shiga toxin 2 variants from pathogenic Escherichia coli. Toxins. 2012;4:487–504. doi: 10.3390/toxins4070487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arndt D., Grant J.R., Marcu A., Sajed T., Pon A., Liang Y., Wishart D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madeira F., Park Y.M., Lee J., Buso N., Gur T., Madhusoodanan N., Basutkar P., Tivey A.R., Potter S.C., Finn R.D. The EMBL-EBI search and sequence analysis tools APIs in 2022. Nucleic Acids Res. 2022;47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malberg Tetzschner A.M., Johnson J.R., Johnston B.D., Lund O., Scheutz F. In silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. J. Clin. Microbiol. 2020;58:e01269-20. doi: 10.1128/JCM.01269-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of the 252 bacterial isolates generated in this study have been deposited in GenBank under BioProject ID PRJNA870153.