Abstract
The psbAI gene of the cyanobacterium Synechococcus elongatus PCC 7942 is one of three psbA genes that encode a critical photosystem II reaction center protein, D1. Regulation of the gene family in response to changes in the light environment is complex, occurs at transcriptional and posttranscriptional levels, and results in an interchange of two different forms of D1 in the membrane. Expression of psbAI is downregulated under high-intensity light (high light) in contrast to induction of the other two family members. We show that, in addition to a known accelerated degradation of the psbAI message, promoter activity decreases upon exposure to high light. Unlike the other psbA genes, additional sequences upstream of the psbAI −35 element are required for expression. Mutagenizing the atypical psbAI −10 element from TCTCCT to TATAAT increased the magnitude of expression from both psbAI::lacZ and psbAI::luxAB fusions but did not affect downregulation under high light. Inactivation of group 2 sigma factor genes rpoD2 and sigC, in both wild-type and −10-element mutagenized backgrounds, resulted in elevated psbAI::luxAB expression but did not alter the response to high light. The results are consistent with redundancy of promoter recognition among cyanobacterial group 2 sigma factors. Electrophoretic mobility shift assays showed that the DNA sequence corresponding to the untranslated leader of the psbAI message binds one or more proteins from an S. elongatus extract. The corresponding region of psbAII efficiently competed for this binding activity, suggesting a shared regulatory factor among these disparately regulated genes.
Cyanobacteria are photosynthetic prokaryotes that carry out oxygenic photosynthesis like the process in the chloroplasts of plants and algae (15). This requires the function of two reaction centers linked in series, of which photosystem II is the site of water splitting and oxygen evolution. Critical to the photosystem II complex are two proteins, D1 and D2, which coordinate the cofactors of light-driven charge separation. In Synechococcus elongatus PCC 7942, small gene families consisting of three psbA and two psbD genes, respectively, encode D1 and D2 (9). The three psbA genes are regulated at both transcriptional and posttranscriptional levels by light intensity and quality (4, 36). Under low light conditions (125 μE m−2 s−1) over 80% of psbA transcripts are from psbAI; however, within 15 min after a shift to high-intensity light conditions (referred to here as “high light”; 750 μE m−2 s−1), psbAI messages decrease by more than 70%, whereas psbAII and psbAIII message levels increase (4). This results in an interchange of two forms of the D1 protein (31), because the product of psbAII and psbAIII differs from that of psbAI by 25 residues. The substitution of one form of D1 for the other is important for cell fitness in a changing light environment (21).
Previous studies have shown that psbAII and psbAIII respond to the shift to high light by transcriptional induction, while transcripts from both psbAI and psbAIII are actively destabilized (23). These responses can be triggered by changes in light fluence or quality and are independent of photosynthetic electron flow, invoking a genuine response to light rather than to redox changes (32, 36). Information that targets these messages, but not that of psbAII, for accelerated degradation at high light resides within their untranslated leaders (22). The apparent half-life of loss of the psbAI transcript at high light is approximately equivalent to the half-life of the message in the presence of a transcription inhibitor, which implies that no new transcription contributes to the psbAI message pool under these conditions. However, there has been no direct investigation of regulation of the psbAI promoter.
Transcriptional fusions with lacZ previously facilitated the dissection of the light-responsive psbAII and psbAIII promoters (25). The minimal promoter elements that drive constitutive expression correspond to consensus Escherichia coli ς70 promoters, residing between −39 and +12 for psbAII and positions −38 and −1 for psbAIII (8, 25). Extension of the right ends of the promoter elements to include the transcribed, untranslated leader regions of the transcripts enhances and confers light-responsive expression. One or more S. elongatus proteins recognizes this region of the DNA, between the transcription start sites and initiation codons of both psbAII and psbAIII. Competition experiments suggest that the same protein(s) recognizes the two genes. Upstream of the basal promoters are negative elements that depress expression of the corresponding gene.
It was not practical to measure a possible high-light-regulated decrease in psbAI transcription with the stable β-galactosidase reporter enzyme that was used to analyze induction of the other psbA promoters. A psbAI::lacZ fusion shows only a slight decrease β-galactosidase activity after 2 h in high light (25; U. Nair and S. S. Golden, unpublished data). Better characterization of the psbAI promoter was needed, not only to complete the analysis of light-dependent regulation of the gene family but also because this promoter figures prominently in the study of cyanobacterial circadian rhythms (1, 16, 19, 20, 35).
The features of the psbAI promoter that account for its strength and organism specificity were of special interest. Extensive assays of promoters fused to luxAB in S. elongatus circadian studies suggest that the psbAI promoter is among the strongest in this organism (1, 27; T. Kondo, personal communication; Nair and Golden, unpublished). However, unlike the other psbA genes and the psbD genes, the promoter for psbAI is entirely silent in E. coli, to the extent that a psbAI fusion to lacZ or luxAB produces no detectable reporter enzyme in that organism (31; unpublished data). The promoters of all three psbA genes have appropriately spaced −35 elements characteristic of E. coli ς70 promoters; however, the psbAI promoter has an atypical −10 element, TCTCCT (10).
Little is known about the recognition of promoters by the multiple group 2 sigma factors characteristically present in the genomes of cyanobacteria (2, 13, 34). Disrupted expression of rpoD2, a group 2 sigma factor gene, causes altered circadian expression of psbAI in S. elongatus, such that the amplitude of oscillation is low: not through decreased expression, but because of elevated expression during a time of day that normally represents the circadian trough (35). Elevated expression of psbAI in the absence of RpoD2 suggested either the unmasking of a competition among the multiple group 2 sigma factors or the loss of an rpoD2-dependent trans-acting factor that controls the temporally regulated strength of psbAI expression (35).
We have defined here the sequences required for psbAI promoter activity and shown that the smallest fragment sufficient for psbAI::lacZ expression extends from −54 to +1. Additional upstream sequences enhance expression but are not required for light-responsive regulation. A psbAI::luxAB fusion that lacks any psbA sequences in the reporter transcript still shows a marked drop in luciferase activity, indicating that the psbAI promoter is downregulated, under high light. One or more proteins in an S. elongatus extract specifically bound to the psbAI upstream region (+1 to +43), as previously shown for psbAII, and the untranslated leader region of psbAII (+1 to +41) could compete efficiently for this binding activity. Mutagenizing the −10 element of the psbAI promoter did not alter its regulation but increased the promoter strength. Inactivation of the group 2 sigma factor genes rpoD2 and sigC in both wild-type and −10 mutagenized backgrounds resulted in elevated psbAI::luxAB expression but did not alter the response to high light.
MATERIALS AND METHODS
Construction of lacZ reporter strains and β-galactosidase assays.
All strains are described in Tables 1 and 2. S. elongatus PCC 7942 has been reported previously without a specific name as Synechococcus sp. strain PCC 7942. However, as a close relative of PCC 6301 (11, 37), which has been proposed as the living neotype of S. elongatus (28, 29), PCC 7942 is assigned to this species name. A pending update to Bergey's Manual of Determinative Bacteriology will include this nomenclature (R. Rippka, personal communication).
TABLE 1.
Reporter strains
| AMC no. | Reporter description
|
Integration site | Antibiotic resistancea | Other information | Source or reference | |
|---|---|---|---|---|---|---|
| Promoter, location | Reporter gene | |||||
| AMC181 | None | lacZ | NSI | Spr | 24 | |
| AMC182 | psbAI, −1291 to +113 | lacZ | NSI | Spr | 24 | |
| AMC213 | psbAIII, −45 to +39 | lacZ | NSI | Spr | 24 | |
| AMC393 | psbAI, −185 to +224 | luxAB | NSI | Spr | 18 | |
| AMC397 | None | luxAB | NSII | Cmr | psbAI::luxCDE | Laboratory collection |
| AMC408 | purF | luxAB | NSII | Cmr | psbAI::luxCDE | 18 |
| AMC437 | psbAI, −183 to +43 | lacZ | NSI | Spr | This study | |
| AMC438 | psbAI, −43 to +43 | lacZ | NSI | Spr | This study | |
| AMC439 | psbAI, −115 to +18 | lacZ | NSI | Spr | This study | |
| AMC440 | psbAI, −54 to +43 | lacZ | NSI | Spr | This study | |
| AMC537 | psbAIII, −38 to +39 | luxAB | NSI | Spr | psbAI::luxCDE | 18 |
| AMC771 | psbAI, −115 to +1 | lacZ | NSI | Spr | This study | |
| AMC773 | psbAI, −54 to +1 | lacZ | NSI | Spr | This study | |
| AMC774 | psbAI, −43 to +113 | lacZ | NSI | Spr | This study | |
| AMC775 | psbAI, −43 to +43 | lacZ | NSI | Spr | −10 element mutagenized from TCTCCT to TATAAT | This study |
| AMC776 | psbAI, −115 to +43 | luxAB | NSII | Spr | psbAI::luxCDE | This study |
| AMC777 | psbAI, −54 to +43 | luxAB | NSII | Cmr | psbAI::luxCDE | This study |
| AMC778 | psbAI, −54 to +43 | luxAB | NSII | Cmr | −10 element mutagenized from TCTCCT to TATAAT, psbAI::luxCDE | This study |
| AMC780 | psbAI, −115 to +18 | luxAB | NSII | Cmr | psbAI::luxCDE | This study |
| AMC781 | psbAI, −115 to +1 | luxAB | NSII | Cmr | psbAI::luxCDE | This study |
Spr, spectinomycin resistance; Cmr, chloramphenicol resistance.
TABLE 2.
Sigma factor inactivation strains
| AMC strain no. | Parent AMC strain | Gene inactivation description (locus [antibiotic marker])a |
|---|---|---|
| AMC791 | AMC777 | rpoD2 (Kmr) |
| AMC792 | AMC777 | sigC (Gmr) |
| AMC793 | AMC777 | rpoD3 (Gmr) |
| AMC794 | AMC777 | rpoD4 (Gmr) |
| AMC795 | AMC777 | rpoD2 (Kmr) and sigC (Gmr) |
| AMC796 | AMC777 | rpoD2 (Kmr) and rpoD3 (Gmr) |
| AMC797 | AMC777 | rpoD3 (Kmr) and rpoD4 (Gmr) |
| AMC798 | AMC777 | rpoD2 (Kmr) and rpoD4 (Gmr) |
| AMC799 | AMC777 | rpoD3 (Kmr) and sigC (Gmr) |
| AMC800 | AMC777 | rpoD4 (Kmr) and sigC (Gmr) |
| AMC825 | AMC778 | rpoD2 (Kmr) |
| AMC826 | AMC778 | sigC (Gmr) |
| AMC827 | AMC778 | rpoD3 (Gmr) |
| AMC828 | AMC778 | rpoD4 (Gmr) |
| AMC829 | AMC778 | rpoD2 (Kmr) and sigC (Gmr) |
| AMC830 | AMC778 | rpoD3 (Kmr) and sigC (Gmr) |
| AMC831 | AMC778 | rpoD4 (Kmr) and sigC (Gmr) |
| AMC832 | AMC778 | rpoD2 (Kmr) and rpoD3 (Gmr) |
| AMC833 | AMC778 | rpoD2 (Kmr) and rpoD4 (Gmr) |
| AMC834 | AMC778 | rpoD3 (Kmr) and rpoD4 (Gmr) |
Kmr, kanamycin resistance; Gmr, gentamicin resistance.
Promoter fragments generated by PCR were cloned into the unique SmaI site of pAM990 to produce transcriptional fusions with a promoterless lacZ gene (25). The nucleotide sequence of each was verified using the cycle sequencing method (dye terminator cycle sequencing ready reaction, ABI PRISM; PE Applied Biosystems, Foster City, Calif.). Wild-type S. elongatus was transformed with the pAM990 derivatives, and transformants were selected and propagated in solid and liquid modified BG-11 (BG-11M) (3) with spectinomycin (20 μg/ml). β-Galactosidase specific activities from cyanobacterial reporter strains under low (125 μE m−2 s−1)- and high (750 μE m−2 s−1)-light conditions were determined as previously described (25). β-Galactosidase activity produced by the promoterless lacZ strain AMC181 (9–12 units) was subtracted from all other values.
Construction of luxAB reporter strains and in vivo luciferase measurements.
psbAI promoter fragments generated by PCR were transcriptionally fused to the promoterless luxAB genes from of Vibrio harveyi in the neutral site II (NSII) targeting vector pAM1580 (http://acs.tamu.edu/∼ssg7231/ns2.html) (1). Promoter fragments were sequenced as described above. Strain AMC395, carrying psbAI driven luxCDE genes to provide aldehyde substrate for luciferase and a spectinomycin-streptomycin resistance marker in neutral site I (NSI), was transformed with the pAM1580 derivatives. The luxAB fusions integrated at NSII, conferring resistance to chloramphenicol and resulting in autonomous bioluminescence. Reporter strains were propagated in spectinomycin (20 μg/ml) and chloramphenicol (7.5 μg/ml). In vivo luciferase activity was measured from cells grown to an optical density at 750 nm (OD750) of 0.4 as described previously (32). A background bioluminescence of 15,000 to 20,000 U produced by the promoterless luxAB strain AMC397 was subtracted from all other values.
Preparation of protein extract.
DNA-binding proteins were isolated using a procedure optimized for psbAII-binding factor purification. Synechococcus cells with an OD750 of 0.15 to 0.20 were grown in 750-ml flat culture flasks containing BG-11M under low light conditions (100 μE m−2 s−1). When the OD750 reached 0.35 to 0.50, cultures were exposed to high light (350 μE m−2 s−1) for 30 min. Cells were collected by centrifugation at 4,400 × g for 5 min, and the pellets were stored at −85°C. The extract was prepared from a 4.2-liter culture of cells. Pellets were resuspended in a total volume of 90 ml of homogenization buffer (50 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.5% Triton X-100, 2 mM dithiothreitol [DTT], 2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 1 μM pepstatin, 10% glycerol). Cells were broken by passage through a French pressure cell twice at approximately 14,000 lb/in2. The extract was centrifuged at 27,000 × g for 15 min to remove most of the cell debris. NaCl was added to the supernatant fraction to a final concentration of 500 mM to dissociate DNA-binding proteins from chromosomal DNA. After 15 min, the extract was clarified by centrifugation at 149,000 × g for 1 h.
Solid (NH4)2SO4 was added to the extract to 30% saturation. After centrifugation at 10,000 × g for 10 min, the supernatant fraction was collected and cleared by passage through a 0.45-μm-pore-size filter. Solid (NH4)2SO4 was added to the clarified supernatant fraction to 60% saturation. After a second centrifugation step, the supernatant was discarded. The pellet was resuspended in dialysis buffer (50 mM Tris-HCl [pH 7.5], 0.1 mM EDTA, 10% glycerol) and dialyzed against the same to remove residual salt. Prior to chromatography, NaCl and DTT were added to the extract to final concentrations of 100 and 2 mM, respectively, and the extract was passed through a 0.45-μm-pore filter. Proteins in the 30 to 60% ammonium sulfate fraction were separated on a 1.7-ml heparin-POROS column (PE Biosystems) equilibrated with buffer A (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.1 mM EDTA, 2 mM DTT, 10% glycerol) using the BioCAD SPRINT chromatography system (PE Biosystems). A protein sample (5 mg) was loaded, the column was washed with two column volumes of buffer A, and proteins were eluted over a 15 column volume gradient from 100% buffer A to 100% buffer B [50 mM Tris-HCl (pH 7.5), 1 M (NH4)2SO4, 0.1 mM EDTA, 2 mM DTT, 10% glycerol]. Fractions with peak psbAII-binding activity were combined, dialyzed, adjusted to 100 mM NaCl and 2 mM DTT, and concentrated on a single heparin column run.
Preparation of DNA fragments for electrophoretic mobility shift assays.
The −54 to +43 and −54 to +1 psbAI fragments were released from pAM990 by digestion with BamHI and BglII and end labeled as described earlier (24). For competition assays the +1 to +41 psbAII fragment and the −54 to +43 and −54 to +1 psbAI fragments were amplified from plasmid templates (pAM1325 and pAM1468) using Pwo polymerase (Roche Molecular Biochemicals, Indianapolis, Ind.).
Electrophoretic mobility shift assays.
DNA-binding assays were performed as described earlier (24), with the following modifications: the binding buffer did not contain KCl, and gels were run at room temperature for 20 min at 200 V. After drying, gels were read by a Fujix BAS 2000 phosphorimager.
Mutagenesis of the −10 region of the psbAI promoter.
The −10 element was mutagenized by PCR with mutant forward primers corresponding to the −54 and −43 endpoints, respectively, and extending through +43: GCTAAAAATTAA GGGTTTTTTACACCTTTTTGACAGTTAATATAATAGCCTAAAAAG and AGGGTTTTTTACACCTTTTTGACAGTTAATATAATAGCCTAAAAAG, respectively. The reverse primer in the construction of both mutant fragments was GAGGTTGTAAAGGGGCAAG (+43 right endpoint of PCR fragments).
Inactivation of sigma factor genes.
A 1.93-kb PvuII fragment from pDAH346 (a gift from D. Hodgson) containing a Gmr gene or a 2.0-kb HincII fragment from pKS101 (33) carrying a Kmr gene was inserted into BclI digested and blunted pAM1519 to generate a Gmr (pAM2332) or Kmr (pAM2413) rpoD4 null allele, respectively (12). The rpoD3 gene in pAM1520 was disrupted by the same fragments after being digested with PstI and blunted (12), resulting in pAM2414 (Kmr) and pAM2333 (Gmr). pAM2330 was digested with BclI and BstEII and blunted, and the Gmr PvuII fragment was inserted to create a sigC null allele (pAM2331). Inactivation of the rpoD2 gene (with pAM1344) was described previously (35).
Single or pairwise inactivations of sigma factor genes were made in strains AMC777 and AMC778. Transformants were selected on BG-11M agar with kanamycin (20 μg/ml) and/or gentamicin (2 μg/ml), as appropriate. They were later grown in BG-11M liquid with spectinomycin (20 μg/ml), chloremphenicol (7.5 μg/ml), and either kanamycin or gentamicin as appropriate.
Assay of bioluminescence in 96-well microtiter plates.
Liquid cultures of AMC777, AMC778, and their sigma-inactivated derivatives were diluted to an OD750 of 0.4. Samples (40 μl) of each were inoculated onto 280-μl BG-11M agar pads in 96-well plates and incubated under continuous light for 12 h. The bioluminescence was measured using a Packard TopCount luminometer (1).
Nucleotide sequence accession number.
The sigC gene sequence was entered into the GenBank database (accession no. AF288784).
RESULTS
Sequences required for basal expression of the psbAI gene.
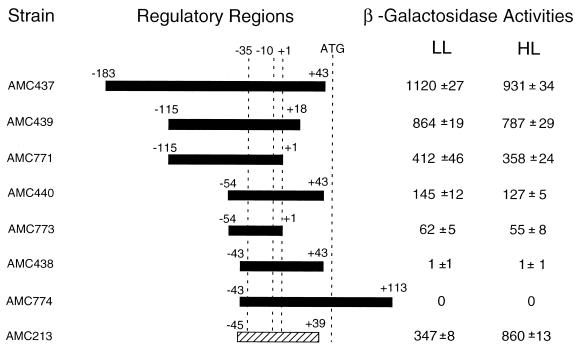
In order to define the promoter elements of the psbAI gene, we constructed transcriptional fusions between different psbAI upstream fragments and a promoterless E. coli lacZ gene in a recombinational vector that targets the reporter gene to a specific locus in the S. elongatus genome. (25). The in vivo expression of each lacZ gene fusion was determined by β-galactosidase assay from strains during growth under low light (125 μE m−2 s−1) and after exposure to high light (750 μE m−2 s−1) (Fig. 1). Control strains AMC181, containing a promoterless lacZ gene, and AMC213, in which the well-characterized light-induced psbAIII promoter is fused to lacZ, were assayed simultaneously with the psbAI fusions. AMC213 showed a 2.5-fold induction under high light, as reported earlier (25).
FIG. 1.
β-Galactosidase activities from psbAI::lacZ fusions at low and high light intensities. Individual psbAI fragments having the indicated endpoints (relative to the transcriptional start site at position +1) were fused to the lacZ gene in pAM990. Wild-type S. elongatus was transformed with the resulting plasmids to generate reporter strains that are identified by AMC culture collection numbers. The values shown are means and standard deviations from three independent experiments to determine the β-galactosidase specific activities at low light and 2 h after a shift to high light; they were corrected by subtracting the background activity produced by the promoterless lacZ strain AMC181, which was assayed in parallel for each experiment. A high-light-inducible psbAIII::lacZ fusion strain, AMC213, was assayed as a positive control. The −35 and −10 indicate the positions of E. coli consensus promoter elements. The translational start codon of the psbAI ORF begins at +53.
The promoter fragment that drives lacZ in AMC182, which extends from positions −1291 to +113 (relative to the transcription start site), was used to initiate analysis of the psbAI promoter and regulatory regions (25). This strain had β-galactosidase activity of 690 U under low light, which dropped ∼15% by 2 h after a shift to high light (data not shown). When the −1291 to +113 fragment was fused to lacZ in the opposite orientation, the β-galactosidase activities were approximately at background levels at both light intensities (data not shown). The shortest fragment assayed that yielded the same pattern and similar strength of expression as AMC182 contained the promoter sequence between positions −115 and +18 (AMC439; Fig. 1). The smallest fragment that allowed expression of the reporter extends from positions −54 to +1 (AMC773); neither AMC438 nor AMC774, whose reporters have upstream promoter endpoints at −43, showed a β-galactosidase activity higher than that of the promoterless control strain. This is in contrast to the promoters of psbAII and psbAIII, which require no additional sequences upstream of the −35 element (i.e., −39 and −38, respectively, are sufficient) (25). The β-galactosidase activity of AMC771 was ∼6-fold higher than that of AMC773 under both low and high light. Because their reporters differ only with respect to the left end points of the promoter region, this difference in β-galactosidase activity is consistent with a positive element located between positions −115 and −54. The β-galactosidase activities of AMC439 and AMC440 were about twice those of AMC771 and AMC773, respectively; the pairs of strains differ in the presence and absence, respectively, of endpoints that extend beyond +1. Therefore, sequences downstream of +1 seem to influence the strength of the psbAI promoter, as was reported previously for psbAII and psbAIII.
psbAI promoter activity decreases under high light.
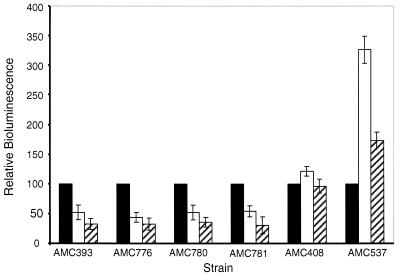
The psbAI transcript rapidly decreases in abundance when cells are shifted to high light, with an apparent half-life of about 10 to 12 min (22, 23). This is, at least in part, attributable to destabilization of the transcript, which depends on the 52-nucleotide untranslated leader (22). Previous reporter gene experiments did not determine whether promoter activity also decreases under high light conditions. The 15% decrease in β-galactosidase activity from a psbAI::lacZ reporter is too small to use as a convincing indicator of decreased transcription, even though it is reproducible and no such decrease is observed with a fusion of a constitutive E. coli promoter to lacZ (25). We expected that, if there is a negative transcriptional response upon exposure to high light, it would be readily detected with the more dynamic luciferase enzyme encoded by luxAB. We constructed psbAI::luxAB fusion strains that lacked the untranslated leader region (AMC781), included the first 18 bp (AMC780) or 43 bp (AMC776) of the untranslated leader region, or had the full untranslated leader and part of the coding region (AMC393). We measured in vivo bioluminescence from whole cells at low light and at 2 and 3 h after a shift to high light.
For all psbAI::luxAB fusion strains, irrespective of the presence or the length of the untranslated leader region, the bioluminescence dropped after a shift to high light to ∼55% (2 h) and to ∼40% (3 h) of the low-light value (Fig. 2). In contrast, the luciferase activity from AMC537, which contains positions −38 to +39 of the light-induced psbAIII promoter fused to luxAB, increased to ∼330 and ∼180% after 2 and 3 h, respectively. AMC408, which carries luxAB fused to the upstream region of purF (a gene involved in purine biosynthesis which is not regulated by light), showed a slight increase in luciferase activity after 2 h and was equivalent to the reference value after 3 h. We concluded that, in addition to the accelerated degradation of the psbAI mRNA mediated by its untranslated leader region, expression of the psbAI promoter decreases after a shift to high light.
FIG. 2.
Expression of psbAI::luxAB fusions assayed by bioluminescence in counts per minute from samples grown under low light and after a shift to high light intensity. Bioluminescence measured at low light for each construct was used as the reference value (100%, black bars). The measurements at 2 h (white bars) and 3 h (hatched bars) after the high-light shift have been normalized to the reference value. Values are the means and standard deviations from three independent experiments and were corrected by subtracting the background luciferase activity produced by the promoterless luxAB strain AMC397.
Specific binding of protein(s) to the untranslated leader region of psbAI.
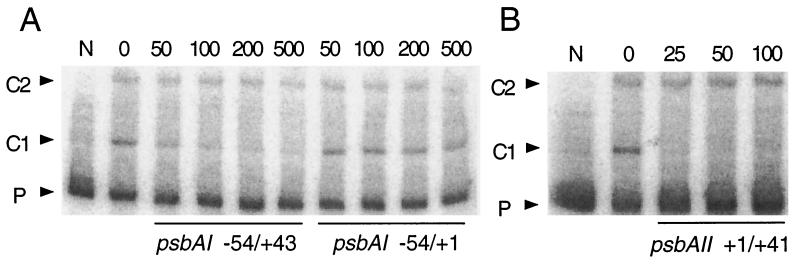
To detect the binding of putative regulatory proteins to the upstream region of psbAI, we performed electrophoretic mobility shift assays with a radiolabeled psbAI probe extending from positions −54 to +43. When partially purified protein extract from high-light-shifted S. elongatus cells was incubated with the probe, two shifted bands, C1 and C2, were formed (Fig. 3A). Addition of a 50-fold molar excess of unlabeled probe fragment greatly reduced formation of the C1 complex. In contrast, the −54 to +1 psbAI competitor fragment did not inhibit formation of C1, even when added at a 500-fold molar excess. Neither competitor fragment affected formation of C2, indicating that it is probably a nonspecific protein-DNA complex. These results suggest that at least one protein binds specifically to the −54 to +43 region of psbAI and that sequences within the +1 to +43 region are required for stable protein binding.
FIG. 3.
Electrophoretic mobility shift assays of a psbAI probe (from positions −54 to +43) with S. elongatus protein extract. Partially purified protein (1.0 μg) prepared from high-light-shifted cells was incubated with 0.01 to 0.02 ng of radiolabeled probe in the presence or absence of the indicated unlabeled psbAI (A) or psbAII (B) competitor fragments as described in Materials and Methods. The molar excess of competitor fragment added is indicated above each lane. Lane N contains no protein. Arrowheads indicate the migration of unbound probe (P) and two protein-DNA complexes (C1 and C2).
The psbAI-binding factor also binds to the untranslated leader region of psbAII.
Previous work has shown that a putative regulatory factor binds to the +1 to +41 region of psbAII and that fragments from the untranslated leader regions of psbAIII and psbDII compete for binding to a psbAII probe (24). To determine whether the psbAI-binding factor may be the same one that binds to the untranslated leader region of psbAII, we used an unlabeled +1 to +41 psbAII fragment to compete for binding to the −54 to +43 psbAI probe (Fig. 3B). A 25-fold molar excess of the psbAII fragment eliminated formation of the C1 complex, suggesting that psbAI and psbAII share a regulatory factor.
Testing the role of an unusual −10 element in the expression properties of psbAI.
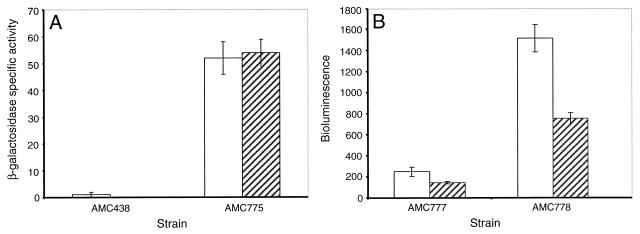
The psbAI promoter, in spite of a “good” −35 element, is silent in E. coli, as evidenced from the lack of expression of both lacZ and luxAB fusions in that organism (31; unpublished data). Its −10 element differs from E. coli ς70 consensus promoters and the psbAII and psbAIII promoters by the substitution of C in place of A residues. To determine whether the unusual −10 region of psbAI accounts for the dependence on upstream sequences and/or negative regulation by high light, we mutated the −10 element of the psbAI fragment in AMC438 from TCTCCT to TATAAT, generating AMC775. Unlike the native psbAI promoter, the mutated promoter drives lacZ expression in E. coli. The β-galactosidase activities from AMC775 were ∼50 U at both light intensities. Thus, alteration of the −10 element allowed expression in S. elongatus in the absence of psbAI sequences upstream of −43. The strength of this artificially activated promoter was approximately equivalent to that of the minimal basal promoter (AMC773), and expression was not influenced by light intensity (Fig. 4A).
FIG. 4.
Effect of mutagenizing the −10 element of the psbAI promoter from TCTCCT to TATAAT on a promoter fragment that, when wild type, is insufficient to drive lacZ (A) and a functional promoter fragment driving luxAB (B). Bars indicate reporter activities for all strains at low light (white bars) and at either 2 h (A) or 3 h (B) after a shift to high light intensity (hatched bars). (A) β-Galactosidase activity was determined as described in the legend for Fig. 1. (B) Bioluminescence was measured by a Packard TopCount luminometer as described in Materials and Methods. The values are averages and standard deviations computed from three independent experiments (A) and eight replicate samples (B).
We also mutagenized the −10 element in a reporter that is normally expressed, a luxAB fusion that carries the −54 to +43 promoter fragment (AMC777). Although the resulting strain AMC778 showed an ∼6-fold-higher bioluminescence than did the wild-type AMC777, expression dropped similarly from both under high light (Fig. 4B). Thus, the atypical −10 element of the psbAI gene is not responsible for reduced expression at high light, and the sequence between −54 and −43 is required.
Inactivating the group 2 sigma factor genes, rpoD2 and sigC, affects psbAI promoter strength but not its regulation.
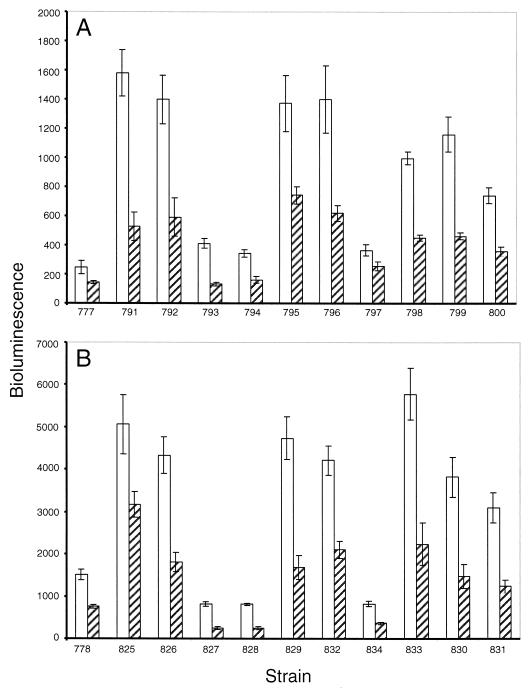
S. elongatus has at least four group 2 sigma factors in addition to the ς70 homolog, RpoD1 (34) (Nair and Golden, unpublished). Recognition of the psbAI promoter may be accomplished by one of these factors, or redundantly by two or more. For example, the principal sigma factor RpoD1 may have a greater affinity for the mutant −10 element of AMC775 and AMC778 than for the wild type, thus explaining the elevated β-galactosidase activity in those strains. Another possibility is that the altered promoter may be transcribed by a group 2 sigma factor that was previously unable to recognize and transcribe the wild-type promoter. In order to determine whether either the wild-type or E. coli consensus mutant promoter is dependent on a specific group 2 sigma factor, we inactivated four group 2 sigma factor genes, rpoD2, rpoD3, rpoD4, or sigC (GenBank accession nos. AB006910, AB024709, AB024710, and AF288784, respectively) singly and pairwise, in strains AMC777 and AMC778.
When rpoD2, rpoD3, rpoD4, or sigC was inactivated in the AMC777 background, bioluminescence increased (Fig. 5A). The most striking increases were those of AMC791 (rpoD2) and AMC792 (sigC) (Fig. 5A). The bioluminescence from these strains at low light was ∼7-fold higher than from AMC777 at the same light intensity. Inactivating the rpoD2 and sigC pair (AMC795) and inactivating either rpoD2 or sigC in combination with rpoD3 (AMC796 and AMC799, respectively) or rpoD4 (AMC798 and AMC800, respectively) also resulted in elevated luciferase activity. All strains showed the drop of ∼50% in bioluminescence at high light as is characteristic of a psbAI::luxAB fusion strain. Although mutagenizing the −10 region of the psbAI promoter (AMC778 background) caused an ∼6-fold increase in bioluminescence relative to the wild-type promoter construct in AMC777 (Fig. 5B), the dependence patterns for various group 2 sigma factors were not altered by the promoter mutation.
FIG. 5.
Effect of inactivating group 2 sigma factor genes singly or in combinations on psbAI::luxAB activity. Sigma factor genes were inactivated in a wild-type psbAI::luxAB reporter background (A) or a mutagenized background in which the −10 region of the psbAI::luxAB reporter has been changed to TATAAT. Bars show bioluminescence measured in counts per second at low light (white bars) and at 3 h after a shift to high light (hatched bars). The values are averages and standard deviations computed from eight replicate samples.
DISCUSSION
The psbAI promoter response to high light is a decrease in expression that is not readily monitored by persistent reporter enzymes such as β-galactosidase. Luciferase as a reporter allowed us to uncouple psbAI transcriptional and posttranscriptional events by providing a clear phenotype that could be assayed from constructs that included or lacked portions of the psbAI transcript in the reporter message. The decrease in expression from these reporter genes upon exposure to high light indicated that the psbAI promoter, as well as its message, is negatively responsive to increased light. In addition, these experiments confirmed that the known posttranscriptional regulation of psbAI does not influence transcriptional reporting by luxAB or lacZ. This was predicted, because both reporter messages are much less stable than the native psbAI transcript and not likely to preserve posttranscriptional regulatory information (26, 30).
The psbAI promoter is different from typical ς70 promoters in that it requires sequences upstream of the −35 element for activity. Analysis of the psbAII and psbAIII promoters revealed previously that they are composed of three elements: a basal ς70-type promoter that is not light responsive, a negative element of unknown length upstream of the promoter, and a light-responsive element downstream of the promoter (8, 25). The basal promoter elements of the two genes correspond to consensus promoters in E. coli, with a left end of −39 or −38 relative to the transcription start site and right ends of +12 for psbAII and −1 for psbAIII. In contrast, the smallest fragment required for the basal expression of a psbAI::lacZ reporter extends from position −54 to +1. The psbAI promoter also differs from the psbAII and psbAIII promoters in that sequences upstream of the minimal promoter stimulate, rather than inhibit, transcription. The segment located between positions −54 and −43 also seems to be required for decreased expression after exposure to high light: the fragment from −43 to +43, artificially activated by changing the −10 element to ς70 consensus, did not show light-responsive expression, whereas the same mutation in the context of sequences up to position −54 allowed a wild-type pattern of decreased expression under high light. Thus, a segment of approximately 20 bp upstream of the consensus −35 element is implicated in both promoter activation per se and light-responsive regulation of this gene. There are no obvious features in this region, other than that it is AT-rich.
Despite differences between the psbAI promoter and those of psbAII and psbAIII, the three genes seem to bind the same factor in the regions that correspond to the untranslated leaders of their transcripts. DNA mobility shift assays previously showed that protein(s) from high light-shifted S. elongatus cells bind specifically to the untranslated leader region of psbAII and that the binding site extends from positions +1 to +41 relative to the transcription start site (24). Fragments containing the upstream regions of the light-regulated psbAIII or psbDII gene compete efficiently for binding to a −70 to +110 psbAII probe, but a fragment containing the equivalent region of the constitutive gene psbDI does not. These results suggest that psbAII, psbAIII, and psbDII share at least one regulatory factor (24). Here we show that at least one protein binds specifically to the region from positions −54 to +43 of psbAI, that sequences between +1 and +43 are required for stable protein binding, and that a psbAII fragment from positions +1 to +41 competes efficiently for this binding, suggesting that psbAI also shares a regulatory factor with psbAII. The untranslated leader regions of the three psbA genes share little similarity beyond a degenerate consensus, TAANANT, that could be involved in binding a regulatory factor. We suggest that binding this factor increases the magnitude of expression of all three psbA genes. Higher β-galactosidase activities were observed under low light for AMC440 (positions −54 to +43 of psbAI), AMC206 (−39 to +41 of psbAII), and AMC221 (−38 to +39 of psbAIII) than for AMC773 (−54 to +1 of psbAI), AMC204 (−39 to +18 of psbAII), and AMC220 (−38 to +6 of psbAIII), respectively (25). The role of this uncharacterized protein in high-light-specific transcriptional regulation of psbAII and psbAIII is uncertain. It is not a repressor of the psbAI promoter, because sequences downstream of position +1 are not required for decreased expression under high light. Determining differences in abundance or activity of the factor between low- and high-light conditions awaits its purification, which is under way (C. Thomas and S. S. Golden, unpublished data).
Cyanobacteria contain a number of group 2 sigma factor genes that are closely related to each other (2, 5–7, 13, 14, 17, 34, 35). In vitro transcription experiments with purified S. elongatus core RNA polymerase reconstituted with RpoD1, RpoD3, or RpoD4 show that both group 1 and group 2 sigma factors recognize and transcribe eubacterial consensus promoters, suggesting a redundancy in sigma factor specificity (12). Work in our lab has shown that all four known group 2 sigma factors are expressed in S. elongatus cells under standard laboratory conditions (35; U. Nair, J. Ditty, and S. S. Golden, unpublished data). In the present study we have shown that strains in which rpoD2, rpoD3, rpoD4, and sigC are inactivated singly or in pairs still express psbAI::luxAB at wild-type or elevated levels. The principal sigma factor and each group 2 sigma factor may be capable of recognizing the psbAI promoter but perhaps with different affinities for the promoter. Our results suggest that, in the absence of RpoD2 or SigC, the psbAI promoter is transcribed much more efficiently by another sigma factor(s). This was previously proposed as an explanation for the loss of the nighttime circadian trough of psbAI expression in an rpoD2 mutant (35). The alternate hypothesis held that RpoD2 transcribes a gene(s) for a repressor(s) of psbAI. However, inactivation of sigC also causes elevated expression from a generic E. coli promoter, conII (Nair and Golden, unpublished); these data argue against a specific repressor accounting for increased expression when a group 2 sigma factor is eliminated. The similarity of phenotypes of rpoD2 and sigC mutants is more consistent with redundancy of promoter recognition by sigma factors with differing affinities for individual promoters, which compete for association with core polymerase.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant GM37040 from the National Institute of General Medical Sciences.
We thank Kan Tanaka for plasmids and sequences used to inactivate rpoD3 and rpoD4.
REFERENCES
- 1.Andersson C A, Tsinoremas N F, Shelton J, Lebedeva N V, Yarrow J, Min H, Golden S S. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods Enzymol. 2000;305:527–542. doi: 10.1016/s0076-6879(00)05511-7. [DOI] [PubMed] [Google Scholar]
- 2.Brahamsha B, Haselkorn R. Identification of multiple RNA polymerase sigma factor homologs in the cyanobacterium Anabaena sp. strain PCC 7120: cloning, expression, and inactivation of the sigB and sigC genes. J Bacteriol. 1992;174:7273–7282. doi: 10.1128/jb.174.22.7273-7282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustos S A, Golden S S. Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J Bacteriol. 1991;173:7525–7533. doi: 10.1128/jb.173.23.7525-7533.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustos S A, Schaefer M R, Golden S S. Different and rapid responses of four cyanobacterial psbA transcripts to changes in light intensity. J Bacteriol. 1990;172:1998–2004. doi: 10.1128/jb.172.4.1998-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell E L, Brahamsha B, Meeks J C. Mutation of an alternative sigma factor in the cyanobacterium Nostoc punctiforme results in increased infection of its symbiotic plant partner, Anthoceros punctatus. J Bacteriol. 1998;180:4938–4941. doi: 10.1128/jb.180.18.4938-4941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caslake L F, Bryant D A. The sigA gene encoding the major sigma factor of RNA polymerase from the marine cyanobacterium Synechococcus sp. strain PCC 7002: cloning and characterization. Microbiology. 1996;142:347–357. doi: 10.1099/13500872-142-2-347. [DOI] [PubMed] [Google Scholar]
- 7.Caslake L F, Gruber T M, Bryant D A. Expression of two alternative sigma factors of Synechococcus sp. strain PCC 7002 is modulated by carbon and nitrogen stress. Microbiology. 1997;143:3807–3318. doi: 10.1099/00221287-143-12-3807. [DOI] [PubMed] [Google Scholar]
- 8.Golden S S. Light-responsive gene expression and the biochemistry of the photosystem II reaction center. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 693–714. [Google Scholar]
- 9.Golden S S. Light-responsive gene expression in cyanobacteria. J Bacteriol. 1995;177:1651–1654. doi: 10.1128/jb.177.7.1651-1654.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden S S, Brusslan J, Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986;5:2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden S S, Nalty M S, Cho D-C. Genetic relationship of two highly studied Synechococcus strains designated Anacystis nidulans. J Bacteriol. 1989;171:4707–4713. doi: 10.1128/jb.171.1.24-29.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto-Seki A, Shirokane M, Masuda S, Tanaka K, Takahashi H. Specificity crosstalk among group 1 and group 2 sigma factors in the cyanobacterium Synechococcus sp. PCC7942: in vitro specificity and a phylogenetic analysis. Mol Microbiol. 1999;34:473–484. doi: 10.1046/j.1365-2958.1999.01608.x. [DOI] [PubMed] [Google Scholar]
- 13.Gruber T M, Bryant D A. Characterization of the alternative sigma-factors SigD and SigE in Synechococcus sp. strain PCC 7002. SigE is implicated in transcription of post-exponential-phase-specific genes. Arch Microbiol. 1998;169:211–219. doi: 10.1007/s002030050563. [DOI] [PubMed] [Google Scholar]
- 14.Gruber T M, Bryant D A. Characterization of the group 1 and group 2 sigma factors of the green sulfur bacterium Chlorobium tepidum and the green non-sulfur bacterium Chloroflexus aurantiacus. Arch Microbiol. 1998;170:285–296. doi: 10.1007/s002030050644. [DOI] [PubMed] [Google Scholar]
- 15.Ho K K, Krogmann D W. Photosynthesis. In: Carr N G, Whitton B A, editors. the biology of cyanobacteria. Vol. 19. Berkeley, Calif: University of California Press; 1982. pp. 191–214. [Google Scholar]
- 16.Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson C R, Tanabe A, Golden S S, Johnson C H, Kondo T. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science. 1998;281:1519–1523. doi: 10.1126/science.281.5382.1519. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A Y, Yasuda M, M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 18.Katayama M, Tsinoremas N F, Kondo T, Golden S S. cpmA, a gene involved in an output pathway of the cyanobacterial circadian system. J Bacteriol. 1999;181:3516–3524. doi: 10.1128/jb.181.11.3516-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo T, Strayer C A, Kulkarni R D, Taylor W, Ishiura M, Golden S S, Johnson C H. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci USA. 1993;90:5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo T, Tsinoremas N F, Golden S S, Johnson C H, Kutsuna S, Ishiura M. Circadian clock mutants of cyanobacteria. Science. 1994;266:1233–1236. doi: 10.1126/science.7973706. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni R D, Golden S S. Form II of D1 is important during transition from standard to high light intensity in Synechococcus sp. strain PCC 7942. Photosyn Res. 1995;46:435–443. doi: 10.1007/BF00032298. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni R D, Golden S S. mRNA stability is regulated by a coding-region element and the unique 5′ untranslated leader sequences of the three Synechococcus psbA transcripts. Mol Microbiol. 1997;24:1131–1142. doi: 10.1046/j.1365-2958.1997.4201768.x. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni R D, Schaefer M R, Golden S S. Transcriptional and posttranscriptional components of psbA response to high light intensity in Synechococcus sp. strain PCC 7942. J Bacteriol. 1992;174:3775–3781. doi: 10.1128/jb.174.11.3775-3781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R, Dickerson N S, Mueller U W, Golden S S. Specific binding of Synechococcus sp. strain PCC 7942 proteins to the enhancer element of psbAII required for high-light-induced expression. J Bacteriol. 1995;177:508–516. doi: 10.1128/jb.177.3.508-516.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Golden S S. Enhancer activity of light-responsive regulatory elements in the untranslated leader regions of cyanobacterial psbA genes. Proc Natl Acad Sci USA. 1993;90:11678–11682. doi: 10.1073/pnas.90.24.11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Golden S S, Kondo T, Ishiura M, Johnson C H. Bacterial luciferase as a reporter of circadian gene expression in cyanobacteria. J Bacteriol. 1995;177:2080–2086. doi: 10.1128/jb.177.8.2080-2086.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Tsinoremas N F, Johnson C H, Lebedeva N V, Golden S S, Ishiura M, Kondo T. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 1995;9:1469–1478. doi: 10.1101/gad.9.12.1469. [DOI] [PubMed] [Google Scholar]
- 28.Rippka R, Cohen-Bazire G. The cyanobacteriales: a legitimate order based on the type strain Cyanobacterium stanieri? Ann Microbiol (Paris) 1983;134B:21–36. doi: 10.1016/s0769-2609(83)80094-5. [DOI] [PubMed] [Google Scholar]
- 29.Rippka R, Herdman M. Pasteur culture collection of cyanobacteria. Catalogue and taxonomic handbook.: catalogue of strains. Paris, France: Institut Pasteur; 1992. [Google Scholar]
- 30.Schaefer M R, Golden S S. Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol. 1989;171:3973–3981. doi: 10.1128/jb.171.7.3973-3981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefer M R, Golden S S. Light availability influences the ratio of two forms of D1 in cyanobacterial thylakoids. J Biol Chem. 1989;264:7412–7417. [PubMed] [Google Scholar]
- 32.Schmitz O, Tsinoremas N F, Anandan S, Golden S S. General effect of photosynthetic electron inhibitors on translation precludes their use for investigating regulation of D1 biosynthesis in Synechococcus sp. strain PCC 7942. Photosynthesis Res. 1999;62:261–271. [Google Scholar]
- 33.Shapira S K, Chou J, Richaud F V, Casadaban M J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of β-galactosidase. Gene. 1983;25:71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Masuda S, Takahashi H. Multiple rpoD-related genes of cyanobacteria. Biosci Biotechnol Biochem. 1992;56:1113–1117. doi: 10.1271/bbb.56.1113. [DOI] [PubMed] [Google Scholar]
- 35.Tsinoremas N F, Ishiura M, Kondo T, Andersson C R, Tanaka K, Takahashi H, Johnson C H, Golden S S. A sigma factor that modifies the circadian expression of a subset of genes in cyanobacteria. EMBO J. 1996;15:2488–2495. [PMC free article] [PubMed] [Google Scholar]
- 36.Tsinoremas N F, Schaefer M R, Golden S S. Blue and red light reversibly control psbA expression in the cyanobacterium Synechococcus sp. strain PCC 7942. J Biol Chem. 1994;269:16143–16147. [PubMed] [Google Scholar]
- 37.Wilmotte A M R, Stam W T. Genetic relationships among cyanobacterial strains originally designated as ‘Anacystis nidulans’ and some other Synechococcus strains. J Gen Microbiol. 1984;130:2737–2740. [Google Scholar]