Abstract
The metabolism of Clostridium butyricum was manipulated at pH 6.5 and in phosphate-limited chemostat culture by changing the overall degree of reduction of the substrate using mixtures of glucose and glycerol. Cultures grown on glucose alone produced only acids (acetate, butyrate, and lactate) and a high level of hydrogen. In contrast, when glycerol was metabolized, 1,3-propanediol became the major product, the specific rate of acid formation decreased, and a low level of hydrogen was observed. Glycerol consumption was associated with the induction of (i) a glycerol dehydrogenase and a dihydroxyacetone kinase feeding glycerol into the central metabolism and (ii) an oxygen-sensitive glycerol dehydratase and an NAD-dependent 1,3-propanediol dehydrogenase involved in propanediol formation. The redirection of the electron flow from hydrogen to NADH formation was associated with a sharp decrease in the in vitro hydrogenase activity and the acetyl coenzyme A (CoA)/free CoA ratio that allows the NADH-ferredoxin oxidoreductase bidirectional enzyme to operate so as to reduce NAD in this culture. The decrease in acetate and butyrate formation was not explained by changes in the concentration of phosphotransacylases and acetate and butyrate kinases but by changes in in vivo substrate concentrations, as reflected by the sharp decrease in the acetyl-CoA/free CoA and butyryl-CoA/free CoA ratios and the sharp increase in the ATP/ADP ratio in the culture grown with glucose and glycerol compared with that in the culture grown with glucose alone. As previously reported for Clostridium acetobutylicum (L. Girbal, I. Vasconcelos, and P. Soucaille, J. Bacteriol. 176:6146–6147, 1994), the transmembrane pH of C. butyricum is inverted (more acidic inside) when the in vivo activity of hydrogenase is decreased (cultures grown on glucose-glycerol mixture). For both cultures, the stoichiometry of the H+ ATPase was shown to remain constant and equal to 3 protons exported per molecule of ATP consumed.
By-products of the food industry have great potential for the production of intermediates for the chemical industry; however, until now only a few applications have been found. Glycerol, a by-product with three atoms of carbon, can easily enter the metabolic pathways of several microorganisms to produce a wide range of compounds.
Bioconversion of glycerol to 1,3-propanediol is already known for several bacterial strains, e.g., Lactobacillus brevis and Lactobacillus buchnerii (44, 46), Bacillus welchii (23), Citrobacter freundii and Klebsiella pneumoniae (32, 40, 47), Clostridium acetobutylicum (14), Clostridium pasteurianum (35), and Clostridium butyricum (3, 21, 43). Anaerobic metabolic pathways of glycerol catabolism have, until now, only been completely characterized in the genera Klebsiella (11, 12, 13, 36) and Citrobacter (7, 8, 9, 45). According to the experiments done in these microorganisms, glycerol is metabolized in two simultaneous pathways. In the first, an NAD+-dependent glycerol dehydrogenase catalyzes the oxidation of glycerol to dihydroxyacetone (DHA), which is then phosphorylated to dihydroxyacetone phosphate (DHAP) via a DHA kinase. A triosephosphate isomerase catalyzes the transformation of DHAP to glyceraldehyde-3-phosphate, which enters the glycolytic pathway. According to several authors (31, 41), the glycerol dehydrogenase of K. pneumoniae is inactivated by oxygen, explaining why, under aerobic conditions, another glycerol catabolic pathway is used. In parallel, a second pathway involving a coenzyme B12-dependent glycerol dehydratase catalyzes the transformation of glycerol to 3-hydroxypropionaldehyde, which is reduced to 1,3-propanediol via an NAD+-dependent 1,3-propanediol dehydrogenase. This second metabolic pathway maintains the redox balance of the cell and is necessary while the microorganism is using glycerol as a carbon and energy source.
Although C. butyricum VPI 3266 is probably the best producer of 1,3-propanediol (42), no physiological investigation has been carried out regarding the conversion of glycerol to this diol. In this report, we studied the metabolic flexibility of C. butyricum in response to an increase in NAD(P)H pressure resulting from the use of glycerol. In order to explain the drastic change in energetics and the shift in fermentative metabolism resulting from glycerol cometabolism, the concentrations of enzymes involved in carbon and electron flow, the nucleotide pools, and several physiological parameters were quantified.
MATERIALS AND METHODS
Organism and growth conditions.
The organism used was C. butyricum strain VPI 3266 (Virginia Polytechnic Institute, Blacksburg, Va.) maintained on glycerol synthetic medium (43). The feed medium for continuous culture was phosphate limited and contained (per liter of distilled water) a carbon source, either glucose (1,100 mM C) or a mixture of glucose (110 mM C) and glycerol (990 mM C); KH2PO4 (0.1 g); KCl (0.65 g); MgSO4 · 7H2O (0.2 g); FeSO4 · 7H2O (0.028 g); CoCl2 · 6H2O (0.01 g); NH4Cl (1.5 g); biotin (0.04 mg); p-aminobenzoic acid (8 mg); Structol (antifoam) (0.1 g); and Na2S2O4 (35 mg). Vitamins and sodium dithionite were not autoclaved and were sterilized by filtration. The pH was automatically maintained at 6.5 by the addition of NH4OH (6 M). The dilution rate was 0.05 h−1, and the temperature was controlled at 35°C.
The experiments were carried out in a 0.4-liter bioreactor with a constant volume of 0.3 liter. Details of the chemostat culture conditions can be found in the report of Vasconcelos et al. (51).
Analysis.
Biomass concentration was determined by a cell dry weight method. The fermentation products were quantified by high-pressure liquid chromatography (HPLC). The fermentor gas effluent was measured by a gas flow meter and periodically analyzed by gas chromatography. All the operating conditions of separation were adopted from Vasconcelos et al. (51). Protein concentration was determined by the method of Bradford (5) to prevent interference by thiol reagents.
Preparation of cell extracts.
Extracts were prepared anaerobically by the procedure of Vasconcelos et al. (51) except for glycerol dehydrogenase, glycerol-3-phosphate (G3P) dehydrogenase, and glycerol dehydratase assays. Sixty-milliliter samples were taken from steady-state chemostat cultures (between each sampling, a four-residence-time period was allowed) and centrifuged immediately at 9,000 × g for 5 min at 4°C. The cell pellets were resuspended and washed anaerobically in cell lysis buffer containing either 100 mM potassium bicarbonate (pH 9.0) with 2 mM dithiotreithol (DTT) for glycerol and G3P dehydrogenase or 100 mM potassium phosphate (pH 8.0) with 2 mM DTT for glycerol dehydratase. Cell suspensions were sonicated in an ultrasonic desintegrator (Vibracell 72434; Bioblock, Illkirch, France) at 4°C for four cycles of 30 s with 2-min cooling intervals. Pyrex tubes (6 ml) with a conic bottom were used, since they improved the sonication efficiency. Cell debris was removed by centrifugation at 13,000 × g for 10 min. For glycerol and G3P dehydrogenase and glycerol dehydratase assays, low-molecular-weight compounds were removed by passage through a Sephadex G25 (Pharmacia LKB Biotechnology, Uppsala, Sweden) column equilibrated with the cell lysis buffer used for extract preparations.
Enzyme assays.
All enzyme assays were done in duplicate on two different culture samples (n = 4). Unless otherwise indicated, all enzyme activities were determined in their physiological direction, under strictly anaerobic conditions, at 30°C. The following assays were adopted from Vasconcelos et al. (51): hydrogenase in both the hydrogen uptake and hydrogen evolution directions; ferredoxin NAD(P) reductase; NAD(P)H ferredoxin reductase; phosphotransacetylase and phosphotransbutyrylase; acetate and butyrate kinases (in the nonphysiological direction); pyruvate-ferredoxin oxidoreductase; and glyceraldehyde-3-phosphate dehydrogenase.
Glycerol and G3P dehydrogenase were measured spectrophotometrically by following the glycerol or glycerol-3-phosphate-dependent formation of NADH at 340 nm, by a method adapted from Johnson et al. (25). The assay mixture contained 0.6 mM NAD, 30 mM ammonium sulfate, 100 mM potassium bicarbonate (pH 9.0), and 100 mM glycerol or G3P.
DHA kinase activity was followed in a coupled system in which NADH-dependent reduction of the reaction product (DHA phosphate) to G3P was measured in a modified assay based on that described by Johnson et al. (24). The assay mixture contained 10 mM DHA, 6 mM ATP, 2 mM MnCl2, 1 mM NADH, 1 U of G3P dehydrogenase from rabbit muscle, and 100 mM potassium bicarbonate buffer (pH 9.0) containing 2 mM DTT. Glycerol kinase was measured in the NAD reduction direction.
1,3-Propanediol dehydrogenase activity was measured in the oxidative direction as described by Heyndricks et al. (21). At 340 nm, reduction of NAD was followed in an assay mixture containing 2 mM NAD, 30 mM (NH4)2SO4, 100 mM 1,3-propanediol, and 100 mM potassium bicarbonate (pH 9.0) with 2 mM DTT.
Glycerol dehydratase activity was determined by the 3-methyl-2-benzothiazolinone hydrazone (MBTH) method of Toraya et al. (50) based on the ability of aldehydes to react with MBTH and form the azine derivatives, whose concentration can be determined spectrophotometrically. The assay mixture contained 0.2 mM glycerol, 0.05 mM KCl, 100 mM potassium phosphate buffer (pH 8.0), and, when added, 15 μM coenzyme B12. The reaction was done at 37°C, and samples were taken 0, 1, 2, and 5 min after addition of glycerol. The enzyme reactions were terminated by adding 1 ml of 100 mM potassium citrate buffer (pH 3.6) and 0.5 ml of 0.1% MBTH hydrochloride. After 15 min at 37°C, 1 ml of water was added, and the amount of 3-hydroxypropionaldehyde (3HPA) formed was determined from the absorbance at 305 nm relative to a standard curve.
Determination of nucleotide pools.
Intracellular concentrations of ATP, ADP, NAD(P)+, and NAD(P)H were determined after extraction of a culture broth sample and fluorimetric enzyme assays as reported by Vasconcelos et al. (51).
Determination of free CoA, acetyl-CoA, and butyryl-CoA pools.
Intracellular concentrations of free CoA, acetyl-CoA, and butyryl-CoA were determined by HPLC as described by Boyton et al. (4) after extraction by the method of Takamura and Nomura (48).
Determination of inorganic phosphate pool.
The extraction method used was adapted from Lipman and Tuttle (33). This method avoids the measurement of labile phosphate compounds (such as acetylphosphate and butyrylphosphate) as inorganic phosphate. Samples (1.5 ml) were taken from steady-state chemostat cultures, immediately transferred into a cooled (to −20°C) Eppendorf tube, and centrifuged at 4°C for 1 min at 14,000 × g. The pellet was washed with ice-cold MilliQ water (1.5 ml) and centrifuged again at 4°C for 1 min at 14,000 × g. Ice-cold 5% trichloroacetic acid (0.5 ml) was added to the pellet, and the suspension was vortexed and centrifuged at 4°C for 1 min at 14,000 × g. The acidic extract was then transferred to a chilled test tube containing a drop of thymol blue, and ice-cold neutralization solution (1 ml of concentrated ammonia, 0.4 ml of glacial acetic acid, 7.6 ml of water, and 1 ml of 0.4 M Na2CO3) was added quickly until a pH of 8 was reached. Alcoholic calcium chloride solution (2.5 ml; 3.3% CaCl2 in 33% ethanol) was added dropwise to the neutralized extract, and the calcium precipitate was recovered by centrifugation for 2 min at 14,000 × g. The precipitate was washed with 2 ml of alcoholic calcium chloride solution and dissolved in 0.5 ml of 0.5 N HCl. The phosphate content of the extract was then determined by the method of Ames and Dubin (1).
Determination of ΔpH and ΔΨ.
The ΔpH was assayed by measuring the accumulation of [14C]benzoate (13.6 μM, 16 mCi mmol−1) by the silicone oil technique (29, 34). [3H]polyethylene glycol (PEG) 4000 (38.5 μM, 1.3 mCi g−1) was incorporated to measure the extracellular volume. Membrane-bound [3H]PEG was measured in a parallel procedure; the cell pellet obtained after centrifugation through silicone oil was resuspended in cold PEG solution (500 μl of 38.5 μM) to keep the bound [3H]PEG attached to the cell and to dilute the [3H]PEG present in the interstitial fluid. After centrifugation through silicone oil, the 3H radioactivity content of the second pellet treated with NaOH was determined.
The intracellular volume (Vi) was determined using 22.2 mM 3H2O (0.1 Ci mol−1) and 33.3 μM [14C]PEG 4000 (6 Ci mol−1) to label the total volume of the pellet and the extracellular volume, respectively (34). The [14C]PEG bound to the cell was measured as described above.
The transmembrane electrical gradient (ΔΨ) was assayed by measuring the accumulation of [3H]tetraphenylphosphonium bromide ([3H]TPP+) (23 mCi mmol−1) by the silicone oil technique as described above except that the sample and radioactive probe were incubated for 12 min instead of 5 min. Binding of TPP+ was reported to be dependent on at least its external and internal concentrations (49). Controls using [3H]TPP+ concentrations of up to 20 μM showed that concentrations above 10 μM led to an underestimation of the electrical gradient. The concentration chosen in our procedure was 1 μM. The [3H]TPP+ bound to the cells was measured after treatment of the cellular suspension with 10 μM valinomycin and 200 mM KCl (34).
Chemicals.
Enzymes and coenzymes were purchased from Sigma Chemical Company (St. Louis, Mo.). All gases used (carbon monoxide, hydrogen, argon, and a mixture of nitrogen, carbon dioxide, and hydrogen) were of the highest purity available. All other chemicals were of analytical grade.
RESULTS
Metabolism and energetics of C. butyricum VPI 3266 on glucose and glucose-glycerol at pH 6.5.
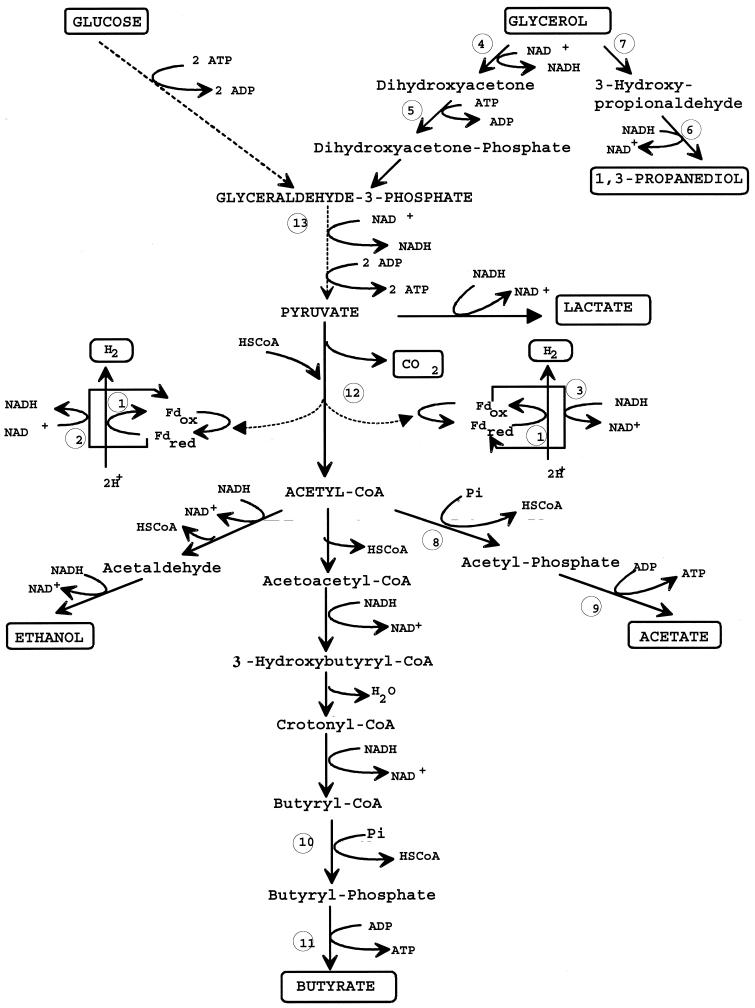
As glycerol is chemically more reduced than glucose and hence generates more NAD(P)H during its catabolism (Fig. 1), it is possible to vary the flow of reductant to the pyridine nucleotide pool by using glucose-glycerol mixtures in the feed medium. The specific rates of substrate consumption and product formation for a constant total amount of carbon (1,100 mM) are shown in Table 1. The glucose-grown culture was acidogenic. The main products were butyrate, acetate, and lactate. Part of the NADH produced during the glycolytic pathway was reoxidized by the NADH-ferredoxin reductase (qNAD(P)H from Fd < 0) to yield reduced ferredoxin, which in turn was reoxidized by the hydrogenase to produce H2. When glycerol was metabolized, 1,3-propanediol became the major product. The specific rate of acid formation decreased, acetate and lactate being the most affected. The NADH produced in the glycolytic pathway was not sufficient for the formation of butyrate and 1,3-propanediol, and part of the reduced ferredoxin produced by the pyruvate ferredoxin oxidoreductase was reoxidized by the ferredoxin-NAD(P) reductase(s) to produce NAD(P)H (qNAD(P)H from Fd > 0). H2 production was thereby significantly decreased, and the CO2/H2 molar ratio was much higher than 1.
FIG. 1.
Metabolic pathway of C. butyricum. 1, Hydrogenase; 2, ferredoxin-NAD(P)+ reductase; 3, NAD(P)H-ferredoxin reductase; 4, glycerol dehydrogenase; 5, DHA kinase; 6, 1,3-propanediol dehydrogenase; 7, glycerol dehydratase; 8, phosphotransacetylase; 9, acetate kinase; 10, phosphotransbutyrylase; 11, butyrate kinase; 12, pyruvate-ferredoxin oxidoreductase; 13, glyceraldehyde-3-phosphate dehydrogenase.
TABLE 1.
Specific rates of production and consumption and other parameters for continuous steady-state cultures of C. butyricum VPI 3266 on glucose and glucose-glycerol mixture at constant pH 6.5 and dilution rate of 0.05 h−1
| Parameter | Glucose | Glucose-glycerol |
|---|---|---|
| Substrate amounts (mmol liter−1) | ||
| Glucose | 183 | 18 |
| Glycerol | 0 | 326 |
| Biomass (g liter−1) | 1.6 | 1.00 |
| YATP (g [dry wt] [mol of ATP]−1) | 2.7 | 4.4 |
| Specific rate of formation or consumption (mmol/h g [dry wt]) | ||
| Glucose | 5.72 | 0.92 |
| Glycerol | 0 | 15.7 |
| Ethanol | 0.29 | 0.1 |
| Acetate | 1.8 | 0.34 |
| Butyrate | 4.00 | 2.87 |
| Lactate | 0.81 | 0.08 |
| 1,3-Propanediol | 0 | 10.8 |
| CO2 | 10.2 | 6.1 |
| H2 | 11.3 | 0.39 |
| NAD(P)H from Fda | −1.17 | 5.98 |
| CO2/H2 ratio | 0.89 | 15.7 |
| Acetate/butyrate ratio | 0.456 | 0.12 |
| Y1,3-propanediol (mol/mol) | 0 | 0.69 |
qNAD(P)H from Fd is the difference between the rate of NAD(P)H consumption and the rate of NAD(P)H formation in the central metabolism. qNAD(P)H from Fd = qpropanediol + 2qbutyrate + 2qethanol + qlactate − 2(qglycerol − qpropanediol) − 2(qglucose − 8.8μ).
Enzymatic activities.
In order to explain these observations, an enzymatic analysis of key metabolic activities was undertaken for both glucose and glucose-glycerol chemostat cultures of C. butyricum.
(i) Enzymes responsible for glycerol metabolism.
The enzymatic activities involved in glycerol assimilation were determined for both cultures (Table 2). The glycerol dehydrogenase and DHA kinase activities were 11- and 10-fold higher, respectively, than in the glucose-glycerol-fed culture. 1,3-Propanediol dehydrogenase was 280-fold higher for this culture. The glycerol dehydratase activity was 100-fold higher in the glucose-glycerol-fed culture. Surprisingly, the activity was not stimulated by addition of coenzyme B12 (although the cell extract was treated by G25 gel filtration) and was very sensitive to oxygen.
TABLE 2.
Enzymatic activities in cell extracts of glucose and glucose-glycerol continuous phosphate-limited steady-state cultures of C. butyricum
| Enzyme types | Reactiona | Enzyme | Mean sp act (μmol/min/mg of protein) (SD) (n = 4)
|
|
|---|---|---|---|---|
| Glucose | Glucose-glycerol | |||
| Hydrogenases and coupling enzymes | 1 | Hydrogenase | ||
| Hydrogen uptake | 4.8 (1.7) | 2.65 (0.9) | ||
| Hydrogen evolution | 0.21 (0.12) | 0.045 (0.03) | ||
| 2 | Ferredoxin-NAD reductase | 0.0045 (0.0018) | 0.03 (0.012) | |
| Ferredoxin-NADP reductase | 0.0035 (0.0015) | 0.0032 (0.002) | ||
| 3 | NADH-ferredoxin reductase | 0.045 (0.009) | 0.328 (0.0045) | |
| NADPH-ferredoxin reductase | 0.0236 (0.008) | 0.021 (0.01) | ||
| Glycerol catabolism enzymes | 4 | Glycerol dehydrogenase | 0.023 (0.008) | 0.260 (0.05) |
| 5 | DHA kinase | 0.0097 (0.002) | 0.092 (0.015) | |
| 6 | 1,3-Propanediol dehydrogenase | 0.002 (0.0005) | 0.565 (0.06) | |
| 7 | Glycerol dehydratase | |||
| −CoB12, −O2 | 0.010 (0.003) | 0.45 (0.15) | ||
| +CoB12, −O2 | 0.009 (0.004) | 0.42 (0.11) | ||
| −CoB12, +O2 | 0 | 0 | ||
| Acidogenic enzymes | 8 | Phosphotransacetylase | 0.14 (0.06) | 0.18 (0.07) |
| 9 | Acetate kinase | 1.22 (0.3) | 1.21 (0.2) | |
| 10 | Phosphotransbutyrylase | 2.5 (0.4) | 3.5 (0.5) | |
| 11 | Butyrate kinase | 2.1 (0.6) | 2.4 (0.7) | |
| Central axis enzymes | 12 | Pyruvate-ferredoxin oxidoreductase | 4.9 (1) | 4 (0.8) |
| 13 | Glyceraldehyde-3-phosphate dehydrogenase | 1.21 (0.2) | 5.8 (1.2) | |
Numbers refer to Fig. 1.
No G3P dehydrogenase or glycerol kinase activity was found in either culture, indicating that only one pathway of glycerol assimilation exists under these growth conditions. The apparent Km values of the glycerol dehydrogenase, glycerol dehydratase, and 1,3-propanediol dehydrogenase were measured on G25-treated crude extract from glucose-glycerol-grown cells. Glycerol dehydrogenase had an apparent Km of 23 mM for glycerol and 1.2 mM for NAD+. Glycerol dehydratase had an apparent Km of 2.5 mM for glycerol. 1,3-Propanediol dehydrogenase had an apparent Km of 0.4 mM for 3HPA and 0.06 mM for NADH in the physiological direction, while in the reverse direction the Km was 3.3 mM for 1,3-propanediol and 0.17 mM for NAD+.
(ii) Enzyme associated with acid formation.
The in vitro activities of acetate kinase (in the nonphysiological direction) and phosphotransacetylase were the same in both cultures and therefore cannot explain the lower acetate production in the glucose-glycerol culture.
The phosphotransbutyrylase and the butyrate kinase activities were 40 and 15% higher, respectively (Table 2), in the glucose-glycerol-fed culture, which also does not explain the lower butyrate production of the culture.
(iii) Hydrogenase and coupling enzymes.
The in vitro hydrogenase activity measured by hydrogen uptake was twofold lower for the culture on glucose-glycerol, while the activity for the opposing direction (H2 production) decreased by a factor of 5 (Table 2). The NADH-ferredoxin reductase and the ferredoxin-NAD reductase were both increased by a factor of 7 when glycerol was metabolized. On the other hand, the in vitro activities of the ferredoxin-NADP reductase and the NADPH-ferredoxin reductase were similar in both cultures.
(iv) Other enzyme activities.
The pyruvate-ferredoxin oxidoreductase activity was similar in both cultures (Table 2), while glyceraldehyde-3-phosphate dehydrogenase presented a fivefold-higher activity for the culture grown on glucose-glycerol.
Intracellular nucleotide, Pi, CoA, acetyl-CoA, and butyryl-CoA pools.
The intracellular concentrations of various metabolites for glucose and glucose-glycerol cultures are presented in Table 3. A twofold increase in NADH was observed for the mixed-substrate culture, while the NAD+ pool was not affected. Although the ATP + ADP pool remained almost constant, a 2.5-fold increase in ATP corresponding to a 5.3-fold increase in the ATP/ADP ratio occurred for the glucose-glycerol culture. A similar 1.5-fold increase in the Pi concentration was also observed for this culture. Regarding acetyl-CoA, butyryl-CoA, and free CoA, a 5.2-fold decrease in the acetyl-CoA/CoA ratio and 2.2-fold decrease in the butyryl-CoA/CoA ratio were obtained for the glucose-glycerol culture compared to the glucose culture.
TABLE 3.
Nucleotide and CoA intermediate concentrations for cells obtained from continuous, phosphate-limited, steady-state cultures of C. butyricum with glucose and a glucose-glycerol mixture as feed substrates
| Metabolite | Mean intracellular concn (μmol/g [dry wt]) (SD) (n = 4)
|
|
|---|---|---|
| Glucose | Glucose-glycerol | |
| ATP | 2.67 (0.17) | 5.85 (0.33) |
| ADP | 7.02 (0.8) | 2.88 (0.47) |
| Pi | 14.8 (2.3) | 22.7 (4.5) |
| NAD+ | 14.6 (1.2) | 15 (1.14) |
| NADH | 4.85 (0.47) | 7.7 (0.4) |
| NADP+ | 2.83 (0.1) | 2.63 (0.22) |
| NADPH | 1.63 (0.28) | 1.9 (0.46) |
| Acetyl-CoA | 0.54 (0.02) | 0.36 (0.03) |
| Butyryl-CoA | 2.09 (0.12) | 3.36 (0.17) |
| Free CoA | 0.5 (0.01) | 1.73 (0.09) |
PMF.
ΔpH and ΔΨ, the two components of the proton motive force (PMF), were measured for both continuous cultures of C. butyricum VPI 3266 (Table 4). An inversion of the ΔpH (more acidic inside) was observed when a glucose-glycerol mixture was used as the carbon and energy source, but this was more than compensated for by the more negative ΔΨ, and a 25 mV decrease in the PMF was recorded for this culture compared to the glucose-grown continuous culture. The phosphorylation potential and the stoichiometry of the ATPase were evaluated. For both cultures, the stoichiometry of the ATPase was close to 3 protons exported for 1 molecule of ATP used (Table 4).
TABLE 4.
PMF in C. butyricum grown in phosphate-limited chemostat cultures at pH 6.5 on either glucose or a glucose-glycerol mixture
| Culture component | Glucose | Glucose-glycerol |
|---|---|---|
| Chemical component (−61 · ΔpH) (mV) | −2.5 | +12 |
| Electrical component ΔΨ (mV) | −132 | −171 |
| PMFa (mV) | −134.5 | −159 |
| Phosphorylation potentialb ΔG′p (mV) | −425 | −449 |
| ATPase stoichiometry (n) | 3.16 | 2.82 |
The PMF was the sum of the chemical and electrical components.
The phosphorylating potential ΔG′p was calculated using a value of 30 kJ/mol for the ΔG0′.
DISCUSSION
The growth of C. butyricum at pH 6.5 on glucose-glycerol mixtures induced the formation of 1,3-propanediol and decreased the production of butyrate, acetate, lactate, and hydrogen, although ethanol production remained constant. This unusual fermentation pattern has already been described for other Clostridium species (14, 21). Glycerol is a more reduced substrate than glucose; for the same amount of carbon, glycerol generates twice as much NADH as glucose. The reducing equivalent excess provided by the conversion of glycerol to pyruvate must be oxidized through the NADH uptake pathways. Surprisingly, this was not accomplished by stimulating H2 production. On the contrary, most of the reduced ferredoxin produced by the pyruvate ferredoxin oxidoreductase was used to generate NADH [qNAD(P)H from Fd > 0], leading to low hydrogen production. Similar results were obtained with C. acetobutylicum grown on glucose-glycerol mixtures (51) and were associated with decreased activity of the in vitro NADH-ferredoxin reductase and an increase in the activity of the in vitro ferredoxin NAD-reductase, while in vitro hydrogenase activity was slightly increased (19). The present study shows that the results are completely different for C. butyricum, since the ratio of the in vitro NADH-ferredoxin reductase activity to the in vitro ferredoxin-NAD reductase activity remained unchanged and was equal to 10 for both cultures, and the in vitro hydrogenase activity decreased sharply for the glucose-glycerol culture. These data suggest that only one NADH-ferredoxin oxidoreductase was present in this microorganism. In view of the estimated flux through this enzyme under the two conditions studied here, this enzyme appeared to be bidirectional and to catalyze both production and oxidation of NADH, as in C. tyrobutyricum and C. pasteurianum (39). In vitro experiments have shown that the activity of this enzyme is regulated by the acetyl-CoA/CoA ratio in Clostridium species; at a high ratio, the enzyme reduced ferredoxin using NADH as an electron donor, while at a low ratio, reduced ferredoxin is used to reduce NAD+ (27). The in vitro hydrogenase activity in the direction for hydrogen production was decreased fivefold in the glucose-glycerol culture. This decrease probably (reduced ferredoxin concentration was not experimentally accessible) led to an increased concentration of reduced ferredoxin. A higher concentration of reduced ferredoxin associated with the low measured acetyl-CoA/CoA ratio explains why the NADH ferredoxin oxidoreductase is functioning to reduce NAD+ in the glucose-glycerol culture. As both glycerol catabolism and NADH-ferredoxin oxidoreductase lead to NADH formation, NAD regeneration must proceed through the pathways of 1,3-propanediol and butyrate formation. The production of 1,3-propanediol was associated with the induction of a glycerol dehydratase and an NADH-dependent 1,3-propanediol dehydrogenase. The glycerol dehydratase activity of a G25-treated cell extract is not stimulated by coenzyme B12 and is very oxygen sensitive. Similar results have been shown for the diol dehydratase of Clostridium glycolycum, a B12-independent radical enzyme (20). More biochemical data will be needed to clearly demonstrate that the C. butyricum glycerol dehydratase is of the same type. The apparent Km of this enzyme for glycerol was 2.5 mM, a value higher than that observed for the B12-dependent enzyme from K. pneumoniae (0.73 mM) (12). The 1,3-propanediol dehydrogenase exhibited Michaelis-Menten kinetics, with an apparent Km of 0.4 mM for 3HPA and 0.06 mM for NADH in the physiological direction and 3.3 mM for 1,3-propanediol and 0.17 mM for NAD+ in the reverse direction. These latter values are lower than those obtained for the enzyme of K. pneumoniae (18 mM for 1,3-propanediol and 0.31 mM for NAD+) (26).
Lin (30) showed that only two chemical modes exist by which glycerol is dissimilated in K. pneumoniae. In the first pathway, glycerol utilization is initiated by phosphorylation followed by oxidation of G3P to DHAP by an aerobic or anaerobic G3P dehydrogenase. This metabolic system closely resembles the glp regulon of Escherichia coli K-12. The alternative pathway involves an NAD+-dependent glycerol dehydrogenase that converts glycerol into DHA, which is then phosphorylated to DHAP by DHA kinase. In this study it has been demonstrated that only the glycerol dehydrogenase and the DHA kinase activities were present, indicating that C. butyricum VPI 3266 uses this second pathway to dissimilate glycerol. The glucose-glycerol cultures showed 10-fold-higher activities for these two enzymes, indicating better expression of the corresponding genes. Glycerol dehydrogenase measured in the presence of 15 mM (NH4)2SO4 has an apparent Km of 23 mM for glycerol and 1.18 mM for NAD+. It has been reported that the Km for glycerol of the Klebsiella oxytoca enzyme is affected by monovalent cations (22), but the value obtained for the same NH4+ concentration is comparable.
In the present chemostat cultures, metabolic fluxes leading to the production of organic acids were not regulated at the genetic level, since in vitro enzyme activities did not correlate with the corresponding specific production rates. The drastic 5.3-fold decrease in the specific production rate of acetate in the glucose-glycerol culture corresponds to the same in vitro activity of phosphotransacetylase and acetate kinase. The reactions catalyzed by these two enzymes are reversible, and the sharp decrease in the acetyl-CoA/CoA and increase in the ATP/ADP ratios in the glucose-glycerol culture show that acetate production is regulated at the enzyme level by substrate concentrations. This explanation is in agreement with that reported for C. kluyveri (10), in which increased flux through the glycolytic pathway resulted in an increase in the concentration of acetyl-CoA, with a concomitant increased rate of acetate production.
Similarly, the 1.5-fold decrease in the specific rate of butyrate production was associated with an increase in the phosphotransbutyrylase and butyrate kinase activities. As the butyryl-CoA/CoA ratio is also lower in the glucose-glycerol culture, the explanation given for the acetate pathway also applies here. These data are different from those observed in C. acetobutylicum, in which the highest activities of phosphotransacetylase, phosphotransbutyrylase, acetate kinase, and butyrate kinase were found in acid-producing cells compared to alcohol-producing ones (2).
One question that remains unsolved is the nature of the signal for induction of the 1,3-propanediol formation pathway. Meyer and Papoutsakis (37) reported several relationships between ATP and NADH concentrations and solvent production in different continuous cultures of C. acetobutylicum. An acidogenic metabolism is associated with low ATP and NADH concentrations, whereas high concentrations of both were reported in different solventogenic cultures. On the glucose-glycerol mixture, induction of the 1,3-propanediol pathway in C. butyricum and the induction of alcohol production in C. acetobutylicum (15, 16, 17) were both associated with higher ATP and NADH concentrations.
It is noteworth that the ΔpH of the glucose-glycerol culture was inverted (more acidic inside). Similar results have been obtained for C. acetobutylicum during growth on a glucose-glycerol mixture (18) and shown to be linked to a low in vivo activity of the hydrogenase, an enzyme that participates in alkalization of the cytoplasm. As the specific rate of H2 formation was decreased 28-fold in the glucose-glycerol culture, a similar explanation might apply for C. butyricum.
The increase in the chemical component of the PMF is more than compensated for by a decrease in the electrical component, resulting in a 25-mV lower PMF in the glucose-glycerol culture. This lower PMF value corresponds, in agreement with the chemiosmotic principle (38), to a more negative value of the phosphorylation potential ΔGp′. The stoichiometry of the ATPase was calculated to be close to 3 protons exported for 1 molecule of ATP consumed for both culture conditions. Similar values were obtained for E. coli grown under anaerobic conditions (28).
C. butyricum is probably the best producer of 1,3-propanediol. However, the competition, at the level of NADH consumption, between the butyrate and the 1,3-propanediol pathways reduces the yield of glycerol conversion to 1,3-propanediol. If the butyrate pathway could be deleted, the glycolytic pathway would then be used only for acetate, ATP, and reducing equivalent production, the 1,3-propanediol pathway being the only path for the regeneration of NAD+. To develop a better strain for the conversion of glycerol to 1,3-propanediol, we are currently working on cloning the phosphotransbutyrylase and butyrate kinase genes, which have been shown to be associated in an operon in C. acetobutylicum (6, 52), which we will later use to delete the butyrate pathway.
ACKNOWLEDGMENTS
This research was supported by BEGHIN-SAY firm (Gruppo Ferruzi) and the French Agency of Environment and Energy (ADEME).
We thank G. Goma and P. Perlot for their continuous interest in this work and G. Whited for critical reading of the manuscript.
REFERENCES
- 1.Ames B N, Dubin D T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;253:769–775. [PubMed] [Google Scholar]
- 2.Andersch W, Bahl H, Gottschalk G. Level of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum. Eur J Appl Microbiol Biotechnol. 1983;18:327–332. [Google Scholar]
- 3.Biebl H, Marten S, Hippe H, Decker W D. Glycerol conversion to 1,3-propanediol by newly isolated clostridia. Appl Microbiol Biotechnol. 1992;36:592–597. [Google Scholar]
- 4.Boynton Z L, Bennett G N, Rudolf F B. Intracellular concentration of coenzyme A and its derivative from Clostridium acetobutylicum and their role in enzyme regulation. Appl Environ Microbiol. 1994;60:39–44. doi: 10.1128/aem.60.1.39-44.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Cary J W, Peterson D J, Papoutsakis E T, Bennett G N. Cloning and expression of Clostridium acetobutylicum phosphotransbutyrylase and butyrate kinase genes in Escherichia coli. J Bacteriol. 1988;170:4613–4618. doi: 10.1128/jb.170.10.4613-4618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel R, Boenigk R, Gottschalk G. Purification of the 1,3-propanediol dehydrogenase from Citrobacter freundii and cloning, sequencing, and overexperession of the corresponding gene in Escherichia coli. J Bacteriol. 1995;177:2151–2156. doi: 10.1128/jb.177.8.2151-2156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel R, Gottschalk G. Growth temperature-dependent activity of glycerol dehydratase in Escherichia coli expressing the Citrobacter freundii dha regulon. FEMS Microbiol Lett. 1992;100:281–286. doi: 10.1111/j.1574-6968.1992.tb14053.x. [DOI] [PubMed] [Google Scholar]
- 9.Daniel R, Stuertz K, Gottschalk G. Biochemical and molecular characterization of the oxidative branch of glycerol utilization by Citrobacter freundii. J Bacteriol. 1995;177:4392–4401. doi: 10.1128/jb.177.15.4392-4401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decker K, Rossle O, Kreuch J. The role of nucleotides in the regulation of the energy metabolism of C. kluyveri, p 75–83. In: Schlegel H G, Gottschalk G, Pfenning N, editors. Proceedings of the Symposium on Microbial Production and Utilization of Gases (H2, CH4, CO). Gottingen, Federal Republic of Germany: Goltze; 1976. [Google Scholar]
- 11.Doelle H W. Bacterial metabolism. 2nd ed. New York, N.Y: Academic Press, Inc.; 1975. [Google Scholar]
- 12.Forage R G, Foster M A. Glycerol fermentation in Klebsiella pneumoniae: functions of the coenzyme B12-dependent glycerol and diol dehydratases. J Bacteriol. 1982;149:413–419. doi: 10.1128/jb.149.2.413-419.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forage R G, Lin E C C. dha system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB 418. J Bacteriol. 1982;151:591–599. doi: 10.1128/jb.151.2.591-599.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg C W. Production of 1,3-propanediol from glycerol by Clostridium acetobutylicum and other Clostridium species. Appl Environ Microbiol. 1987;53:639–643. doi: 10.1128/aem.53.4.639-643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girbal L, Croux C, Vasconcelos I, Soucaille P. Metabolic shifts in Clostridium acetobutylicum. FEMS Microbiol Rev. 1995;17:287–297. [Google Scholar]
- 16.Girbal L, Soucaille P. Regulation of Clostridium acetobutylicum metabolism as revealed by mixed-substrate steady-state continuous culture: role of NADH/NAD ratio and ATP pool. J Bacteriol. 1994;176:6433–6438. doi: 10.1128/jb.176.21.6433-6438.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girbal L, Soucaille P. Regulation of solvent production in Clostridium acetobutylicum. Trends Biotechnol. 1998;16:11–16. [Google Scholar]
- 18.Girbal L, Vasconcelos I, Soucaille P. Transmembrane pH of Clostridium acetobutylicum is inverted (more acidic inside) when the in vivo activity of hydrogenase is decreased. J Bacteriol. 1994;176:6146–6147. doi: 10.1128/jb.176.19.6146-6147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorwa M F, Croux C, Soucaille P. Molecular characterization and transcriptional analysis of the putative hydrogenase gene of Clostridium acetobutylicum ATCC 824. J Bacteriol. 1996;178:2668–2675. doi: 10.1128/jb.178.9.2668-2675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmanis M G N, Stadtman T C. Solubilization of a membrane-bound diol dehydratase with retention of EPR g=2,02 signal by using 2-(N-cyclohexylamino)ethanesulfonic acid buffer. Proc Natl Acad Sci USA. 1987;84:76–79. doi: 10.1073/pnas.84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyndricks M, De Vos P, Vancanneyt M, De Ley J. The fermentation of glycerol by Clostridium butyricum LMG 1212t2 and 1213t1 and C. pasteurianum LMG 3285. Appl Microbiol Biotechnol. 1991;34:637–642. [Google Scholar]
- 22.Homann T, Tag C, Biebl H, Decker W D, Shink B. Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains. Appl Microbiol Biotechnol. 1990;33:121–126. [Google Scholar]
- 23.Humphreys F B. Formation of acrolein by Bacillus welchii. Infect Dis. 1924;35:282–290. [Google Scholar]
- 24.Johnson E A, Burke S K, Forage R G, Lin E C C. Purification and properties of dihydroxyacetone kinase from Klebsiella pneumoniae. J Bacteriol. 1984;160:55–60. doi: 10.1128/jb.160.1.55-60.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson E A, Levine R L, Lin E C C. Inactivation of glycerol dehydrogenase of Klebsiella pneumoniae and the role of divalent cations. J Bacteriol. 1985;164:479–483. doi: 10.1128/jb.164.1.479-483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson E A, Lin E C C. Klebsiella pneumoniae 1,3-propanediol: NAD+ oxidoreductase. J Bacteriol. 1987;169:2050–2054. doi: 10.1128/jb.169.5.2050-2054.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jungermann K, Rupprecht E, Ohrloff C, Thauer R, Decker K. Regulation of the reduced nicotinamide adenine dinucleotide-ferredoxin reductase system in Clostridium kluyveri. J Biol Chem. 1971;246:960–963. [PubMed] [Google Scholar]
- 28.Kashket E R. Stoichiometry of the H+ATPase of Escherichia coli cells during anaerobic growth. FEBS Lett. 1983;154:343–346. doi: 10.1016/0014-5793(83)80179-3. [DOI] [PubMed] [Google Scholar]
- 29.Kashket E R. The proton motive force in bacteria: a critical assesment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- 30.Lin E C C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- 31.Lin E C C, Levin A P, Magasanik B. The effect of aerobic metabolism on the inducible glycerol dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1960;235:1824–1829. [PubMed] [Google Scholar]
- 32.Lin E C C, Magasanik B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960;235:1820–1823. [PubMed] [Google Scholar]
- 33.Lipmann F, Tuttle L C. Acetyl-phosphate: chemistry, determination and synthesis. J Biol Chem. 1944;153:571–578. [Google Scholar]
- 34.Loubiere P, Salou P, Leroy M J, Lindley N D, Pareilleux A. Electrogenic malate uptake and improved growth energetics of the malolactic bacterium Leuconostoc oenos grown on glucose-malate mixtures. J Bacteriol. 1992;174:5302–5308. doi: 10.1128/jb.174.16.5302-5308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luers F, Seyfried M, Daniel R, Gottschalk G. Glycerol conversion to 1,3-propanediol by Clostridium pasteurianum: cloning and expression of the gene encoding 1,3-propanediol dehydrogenase. FEMS Microbiol Lett. 1997;154:337–345. doi: 10.1111/j.1574-6968.1997.tb12665.x. [DOI] [PubMed] [Google Scholar]
- 36.Magasanik B M, Brooke M S, Karibian D. Metabolic pathways of glycerol dissimilation: a comparative study of two strains of Aerobacter aerogenes. J Bacteriol. 1953;66:611–619. doi: 10.1128/jb.66.5.611-619.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer C L, Papoutsakis E T. Increased levels of ATP and NADH are associated with increased solvent production in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol. 1989;30:450–459. [Google Scholar]
- 38.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 39.Petitdemange H, Cherrier C, Raval G, Gay R. Regulation of NADH and NADPH-ferredoxin oxidoreductases in clostridia of the butyric group. Biochim Biophys Acta. 1976;421:334–347. doi: 10.1016/0304-4165(76)90300-7. [DOI] [PubMed] [Google Scholar]
- 40.Ruch D, Karibian D, Karnovsky M, Magasanik B. Pathways of glycerol dissimilation in two strains of Aerobacter aerogenes: enzymatic and tracer studies. J Biol Chem. 1957;226:891–899. [PubMed] [Google Scholar]
- 41.Ruch F E, Lin E C C, Kowit J D, Tang C T, Goldberg A L. In vivo inactivation of glycerol dehydrogenase in Klebsiella aerogenes. J Bacteriol. 1980;141:1077–1085. doi: 10.1128/jb.141.3.1077-1085.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saint-Amans S, Perlot P, Goma G, Soucaille P. High production of 1,3-propanediol from glycerol by Clostridium butyricum in a simply controlled fed-batch system. Biotechnol Lett. 1995;16:831–836. [Google Scholar]
- 43.Saint-Amans S, Soucaille P. Carbon and electron flow in Clostridium butyricum VPI 3266 grown in chemostat culture on glucose-glycerol mixtures. Biotechnol Lett. 1995;17:211–216. doi: 10.1128/JB.183.5.1748-1754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schütz H, Radler F. Anaerobic reduction of glycerol to propanediol-1,3 by Lactobacillus brevis and Lactobacillus buchneri. Syst Appl Microbiol. 1984;5:169–178. [Google Scholar]
- 45.Seyfried M, Daniel R, Gottschalk G. Cloning, sequencing, and overexpression of the genes encoding coenzyme B12-dependent glycerol dehydrase of Citrobacter freundii. J Bacteriol. 1996;178:5793–5796. doi: 10.1128/jb.178.19.5793-5796.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soboloy M, Smiley K L. Metabolism of glycerol by an acrolein-forming lactobacillus. J Bacteriol. 1959;79:261–266. doi: 10.1128/jb.79.2.261-266.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Streekstra H, Teixeira de Mattos M J, Neijssel O M, Tempest D W. Overflow metabolism during anaerobic growth of Klebsiella aerogenes NCTC 418 on glycerol and dihydroxyacetone in chemostat culture. Arch Microbiol. 1987;147:268–275. [Google Scholar]
- 48.Takamura Y, Nomura G. Changes in the intacellular concentration of acetyl-CoA and malonyl-CoA in relation to the carbon and energy metabolism of Escherichia coli K12. J Gen Microbiol. 1988;134:2249–2253. doi: 10.1099/00221287-134-8-2249. [DOI] [PubMed] [Google Scholar]
- 49.Ten Brink N, Lolkema J S, Hellingwerf K J, Konings W N. Variable stoichiometry of proton:lactose symport in Escherichia coli cells. FEMS Microbiol Lett. 1981;12:237–240. [Google Scholar]
- 50.Toraya T, Kuno S, Fukui S. Distribution of coenzyme B12-dependent diol dehydratase and glycerol dehydratase in selected genera of Enterobacteriaceae and Propionibacteriaceae. J Bacteriol. 1980;141:1439–1442. doi: 10.1128/jb.141.3.1439-1442.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasconcelos I, Girbal L, Soucaille P. Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J Bacteriol. 1994;176:1443–1450. doi: 10.1128/jb.176.5.1443-1450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walters K A, Nair R V, Cary J W, Bennett G N, Papoutsakis E T. Sequence and arrangement of the genes encoding enzymes of the butyrate formation pathway of Clostridium acetobutylicum ATCC 824. Gene. 1994;134:107–111. doi: 10.1016/0378-1119(93)90182-3. [DOI] [PubMed] [Google Scholar]