Abstract
Microcin J25 is a 2,107-Da, plasmid-encoded, cyclopeptide antibiotic produced by Escherichia coli. We have isolated lacZ fusions to mcjA (encoding the 58-amino-acid microcin precursor) and mcjB and mcjC (which are required for microcin maturation), and the regulation of these fusions was used to identify factors that control the expression of these genes. The mcjA gene was found to be dramatically induced as cells entered the stationary phase. Expression of mcjA could be induced by resuspending uninduced exponential-phase cells in spent supernatant obtained from an early-stationary-phase culture. Induction of mcjA expression was not dependent on high cell density, pH changes, anaerobiosis, or the buildup of some inducer. A starvation for carbon and inorganic phosphate induced mcjA expression, while under nitrogen limitation there was no induction at all. These results taken together suggest that stationary-phase induction of mcjA is triggered by nutrient depletion. The mcjB and mcjC genes were also regulated by the growth phase of the culture, but in contrast to mcjA, they showed substantial expression already during exponential growth. Induction of the microcin genes was demonstrated to be independent of RpoS, the cyclic AMP-Crp complex, OmpR, and H-NS. Instead, we found that the growth-phase-dependent expression of mcjA, mcjB, and mcjC may be explained by the concerted action of the positively acting transition state regulators ppGpp, Lrp, and integration host factor. Measurements of microcin J25 production by strains defective in these global regulators showed a good correlation with the reduced expression of the fusions in such mutant backgrounds.
Microcin J25 (MccJ25) is a plasmid-encoded, 2,107-Da cyclopeptide antibiotic of 21 unmodified amino acids, produced and excreted into the culture medium by the Escherichia coli strain AY25, isolated from human feces (5, 39). As with other microcins but unlike colicins, production of MccJ25 is neither lethal nor stimulated by DNA-damaging treatments that activate the SOS response (3, 38). MccJ25 is primarily active on gram-negative bacteria related to the producer strain, inducing cell filamentation in an SOS-independent way (39). Thus, in addition to its interest as an antibacterial compound, MccJ25 holds promise as a tool for cell division studies. Some pathogenic bacteria, including Salmonella and Shigella species, are hypersensitive to MccJ25 (39). MccJ25 uptake into E. coli is dependent on the cell envelope proteins FhuA, TonB, ExbB (and possibly ExbD), and SbmA (40, 41). We have reported the molecular characterization of the four plasmid genes, mcjABCD, involved in microcin synthesis and immunity (45, 46). While active MccJ25 may be extracted from cells expressing the three genes mcjABC, no active peptide was detected in cells bearing plasmids that expressed only two genes, mcjA and mcjB or mcjA and mcjC (45). Therefore, McjB and McjC must take part in MccJ25 maturation, which would imply the removal of an N-terminal leader of 37 amino acids from the 58-residue precursor McjA, followed by the head-tail cyclization of the 21-residue C-terminal propeptide (5). The microcin immunity protein, McjD, which is highly similar to many ATP-binding cassette exporters, was found to be required for MccJ25 secretion (45). Thus, the immunity conferred by McjD could be mediated by active efflux of the peptide, which would keep its intracellular concentration below a critical level. Also, we have found that the E. coli outer membrane protein TolC may be implicated in the secretion of MccJ25, possibly by forming an export complex with McjD (12).
We previously noted that production of MccJ25 increased when cells reached the stationary phase (39). To understand the molecular basis of this growth-phase-dependent regulation we have constructed fusions between lacZ and the genes mcjA, -B, and -C, involved in production of the antibiotic. This report consists of a study of the physiological and genetic factors affecting the expression of these fusions. Our results suggest that the stationary-phase increase is triggered by nutrient depletion. Induction of the microcin genes was shown to be independent of RpoS, the cyclic AMP (cAMP)-cAMP receptor protein (C/RP) complex, OmpR, and H-NS. By examining the effect of mutations in a number of other regulatory loci on expression of mcjABC we have found that ppGpp and the proteins Lrp and integration host factor (IHF) are required for the growth phase induction. In addition, the histone-like protein H-NS acts as a positive regulator of mcjB but has a negative effect on the expression of mcjC.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacteria and plasmids used in this study are listed in Table 1. All strains were E. coli K-12 derivatives. The rpoS::Tn10, ΔrelA251::kan, ΔspoT207::cat, and ΔhimA82-Tn10 alleles were introduced into MC4100 by P1 transduction (31), using strains ZK1171, RO98, and RO71 as donors. The presence of the rpoS::Tn10 allele was determined by the reduced oxygen evolution when colonies were flooded with hydrogen peroxide. The standard Luria-Bertani (LB) broth and M63 minimal medium have been previously described (31). M63 medium was supplemented with glucose (0.2%) and vitamin B1 (1 μg/ml). Solid media contained 1.5% agar. When specified, the media were supplemented with ampicillin (50 μg/ml), kanamycin (50 μg/ml), tetracycline (15 μg/ml), chloramphenicol (30 μg/ml), or 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (40 μg/ml). All cultures were incubated at 37°C. Growth was monitored by measuring optical density at 600 nm (OD600). Aerobic cultures for β-galactosidase assays (50-ml volume in 250-ml Erlenmeyer flasks) were inoculated with overnight cultures grown in the same medium and incubated with vigorous shaking. To ensure the dilution of products from previous induction during stationary phase, cells from overnight cultures were diluted 1:100 in LB broth or M63-glucose and grown to early log phase. Samples were then taken and used to inoculate (1:100) LB or M63 cultures. For anaerobic growth, liquid cultures were grown without agitation in filled tubes with the medium overlaid with mineral oil or in Oxoid anaerobic jars with an H2-CO2 atmosphere generated by using Oxoid gas generating kits.
TABLE 1.
Bacteria and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| MC4100 | araD139 Δ(argF-lac)205 λ−flbB5301 ptsF25 relA1 rpsL150 deoC1 | CGSCa |
| RYC1000 | MC4100 Δrib-7 recA56 gyrA | F. Moreno |
| POI1681TR | F− Mu dI1681 (Kmr) ara::(Mu cts)3 Δ(proAB-argF-lacIPOZYA) XIII rpsL recA56 srl::Tn10 (Tcr) | 8 |
| ZK1171 | W3110 ΔlacU169 tna-2 rpoS::Tn10 | R. Kolter |
| RO71 | MC4100 φ (csi-5::lacZ) (λplacMu55) ΔhimA82-Tn10 | R. Hengge-Aronis |
| RO64 | MC4100 lrp-201::Tn10 | R. Hengge-Aronis |
| RH74 | MC4100 Δcya851 ilv::Tn10 | R. Hengge-Aronis |
| ZK213 | Hfr rpsL | R. Kolter |
| ZK216 | ZK213 Δcrp45 | R. Kolter |
| RO98 | MC4100 ΔrelA251::kan ΔspoT207::cat | R. Hengge-Aronis |
| MJ150 | MC4100 ΔhimA82-Tn10 | This study |
| MJ152 | MC4100 ΔspoT207::cat | This study |
| MJ153 | MC4100 rpoS::Tn10 | This study |
| RYC514 | MC4100 ompR101 | F. Moreno |
| CU284 | MC4100 hns::neo | C. Ueguchi |
| CC118 | F− Δ(ara-leu)7697 araD139 ΔlacX74 galE galK ΔphoA20 thi rpsE rpoB argE(Am) recA1 appR1 | 28 |
| CC170 | As CC118, with a chromosomal insertion of TnlacZ | 28 |
| pop3001.6 | MC4100 Mu cts | F. Moreno |
| Plasmids | ||
| pTUC202 | pACYC184 with 6-kb BamHI-SalI fragment encompassing mcjABCD genes | 45 |
| pTUC341 | pBR322 with 5.2-kb HindIII-SalI fragment encompassing mcjABCD genes | 45 |
| pMcjA-LacZ | pTUC202 carrying mcjA::lacZ gene fusion | This study |
| pMcjB-LacZ | pTUC202 carrying mcjB::lacZ gene fusion | This study |
| pMcjC-LacZ | pTUC202 carrying mcjC::lacZ gene fusion | This study |
| pMcjA-LacZ(op) | pTUC202 carrying mcjA::lacZ operon fusion | This study |
CGSC, E. coli Genetic Stock Center.
To make conditioned LB medium, an overnight culture of MC4100(pMcjA-LacZ) was diluted 1:100 into 50 ml of fresh LB broth and left to grow aerobically for 7 h (OD600, 2.3) until the same state at which expression of the fusion had been observed to be induced. After growth, the cells were spun down twice and the medium was filter sterilized and used no later than 24 h after it had been prepared. Starvation by depletion of glucose or phosphate was obtained by growing cultures in M63 medium with 0.02% glucose or 0.5 mM KH2PO4, respectively. In the latter case, 40 mM MOPS (morpholinepropanesulfonic acid; pH 7) was used to provide buffering capacity of the low-phosphate medium. A medium limited in nitrogen was obtained by replacing ammonium sulfate in M63 with an equimolar concentration of potassium sulfate and by adding 1.5 mM ammonium chloride as the sole source of nitrogen.
Assay of antibiotic activity.
MccJ25 activity in supernatants was determined by the critical dilution method as described previously (39). The microcin titer is reported as the reciprocal of the last dilution which gave a clear spot.
Isolation and characterization of lacZ fusions to microcin genes.
Translational lacZ fusions to mcjABC were constructed by TnlacZ insertion mutagenesis of pTUC202, essentially as described previously (28). Plasmid pTUC202 was transformed into strain CC170, which carries the TnlacZ transposable element on the chromosome. Independently mutagenized cultures were plated on LB medium containing 300 μg of kanamycin per ml in addition to chloramphenicol to select TnlacZ transpositions onto the multicopy plasmid. Resistant colonies were pooled, and plasmid DNA was extracted and used to transform the ΔlacX74 strain CC118 with selection for Cmr Kmr on LB agar containing X-Gal. Transformants that produced blue colonies were purified, and those that had lost MccJ25 production were retained for further analysis. To identify the gene inactivated by the insertions we did a complementation analysis using strain RYC1000 (recA), which was transformed with all possible combinations of pTUC202::TnlacZ MccJ25− derivatives and a set of pTUC341::Tn5 compatible mutant plasmids with well-characterized insertions in mcjA, mcjB, or mcjC (45). Selection was done on LB medium containing chloramphicol, kanamycin, and ampicillin. Complementation was considered positive when the two plasmids together restored the MccJ25 production phenotype. Three plasmids bearing fusions to the mcjA, -B, and -C genes, designated pMcjA-LacZ, pMcjB-LacZ, and pMcjC-LacZ, respectively, were selected for further study. The DNA sequences of the fusion joints were determined by the chain termination method (43). Sequencing reactions were primed with a synthetic oligonucleotide primer that hybridized to the N-terminal segment of the lacZ gene present in the transposon.
Operon lacZ fusions to mcjA were obtained by Mu dI1681 insertion mutagenesis (8). Strain POI1681TR(pTUC202) was used to produce transducing particles, which were used to infect pop3001.6. The transduction mixture was incubated at 30°C for 2 h to allow expression of antibiotic resistance and then was plated on LB medium containing kanamycin, chloramphenicol, and X-Gal. Blue colonies were tested for MccJ25 production. Nonproducing plasmid DNAs were isolated, and inactivation of mcjA was tested by complementation analysis, as outlined above for TnlacZ insertions. The position of the Mu dI1681 element was confirmed by restriction enzyme mapping. To circumvent the problems that Mu dI1681 is transposition proficient and the fusion strain is temperature sensitive for growth, a selected fusion was stabilized by deleting DNA between a HindIII site within Mu dI1681 and the unique HindIII site in pTUC202 (see Fig. 6A). The resulting plasmid, designated pMcjA-LacZ(op), was retained for further study.
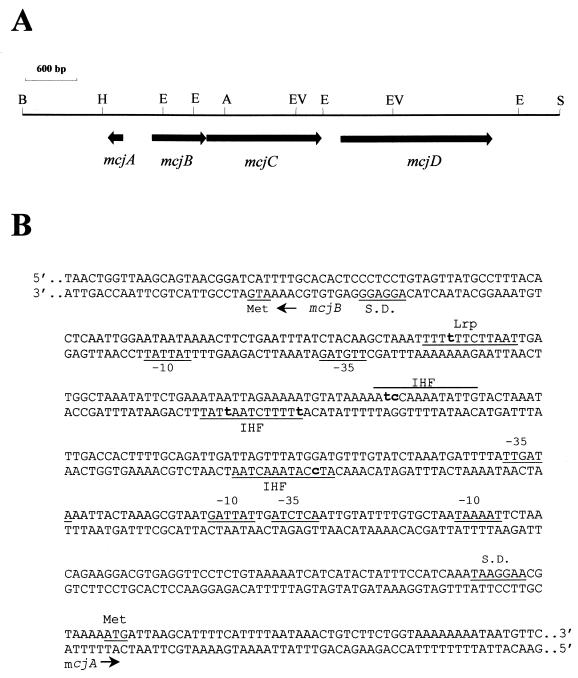
FIG. 6.
(A) Physical and genetic map of the DNA fragment (6.2 kb) cloned in pTUC202. Horizontal arrows indicate the mcjABCD genes and the direction of transcription. (B) Nucleotide sequence of the mcjA and mcjB promoter regions, including the beginning of the genes. Note that mcjA and mcjB are transcribed from the opposite DNA strands (46). Predicted ribosome binding sites (S.D.) and promoter sequences as well as start codons for McjA and McjB translation are shown. Note that two tentative promoter regions (−35 and −10) are shown for mcjA. The regions corresponding to possible IHF and Lrp recognition sequences are indicated. Boldface lowercase letters represent the nucleotides which are different from the canonical ones. The sequence of the entire MccJ25 operon has been deposited in the GenBank database under accession no. AF061787.
Other genetic and DNA techniques.
Plasmid DNA minipreps were prepared by using the Wizard kit of Promega. Phage P1 vir was used for routine transduction of genetic markers (31). E. coli strains were transformed with plasmid DNA by the CaCl2 procedure (42).
β-Galactosidase assays.
β-Galactosidase activity was measured as described by Miller (31), using cells permeabilized with sodium dodecyl sulfate and chloroform, and is reported in Miller units. The assays were repeated at least twice for each sample.
RESULTS
Growth-phase-dependent production of MccJ25.
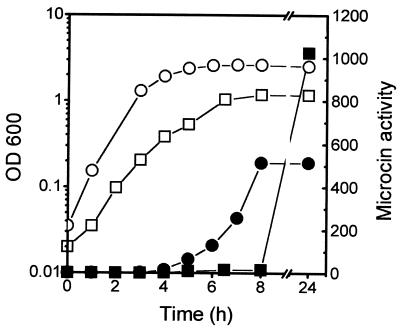
We have shown previously that MccJ25 production by cells carrying the low-copy-number, natural plasmid pTUC100 is virtually undetectable during early and mid-exponential growth; the antibiotic appears when cells approach the stationary phase (39). The MccJ25 genes from pTUC100 were then cloned into a low-copy-number vector to yield pTUC202, a pACYC184 derivative which carries a 6.2-kb BamHI-SalI fragment containing the mcjABCD genes (see Fig. 6A) (45). To examine whether MccJ25 produced from this plasmid showed the same kinetics of synthesis as that produced by the wild-type plasmid, the antibiotic activity of cultures of strain MC4100(pTUC202) in LB medium and M63-glucose minimal medium was measured during both exponential growth and the stationary phase. No assayable or very low amounts of the antibiotic could be detected in supernatants from cells in the exponential phase of growth in both media (Fig. 1). As with the wild-type plasmid, the activity sharply rose in the early stationary phase. Thus, pTUC202 mimics the growth phase regulation of the wild-type system.
FIG. 1.
Growth phase regulation of microcin activity. Strain MC4100(pTUC202) was grown in LB (circles) and M63 (squares) media. The OD600 (open symbols) and microcin activity (closed symbols) in the two cultures were determined. Microcin activity is expressed as the reciprocal of the last dilution which gave a clear spot (see Materials and Methods).
Growth-phase-dependent expression of mcjA, mcjB, and mcjC.
In order to obtain quantitative data on the expression of microcin genes by a convenient assay, we used pTUC202 to construct translational fusions between mcjA, -B, and -C and lacZ (as indicated in Materials and Methods). Three representative mutant plasmids, designated pMcjA-LacZ, pMcjB-LacZ, and pMcjC-LacZ, with TnlacZ insertions in mcjA, mcjB, and mcjC, respectively, were selected and characterized in detail. The fusion joints from the three plasmids were determined by DNA sequencing, showing that codons 43, 11, and 122 of mcjA, mcjB, and mcjC, respectively, were connected to the ′lacZ coding region in proper reading frame. In all three plasmids, TnlacZ insertion completely blocked MccJ25 production, as shown by the absence of inhibition zones on a lawn of indicator cells, even when a deferred-antagonism test was employed to increase the sensitivity of detection.
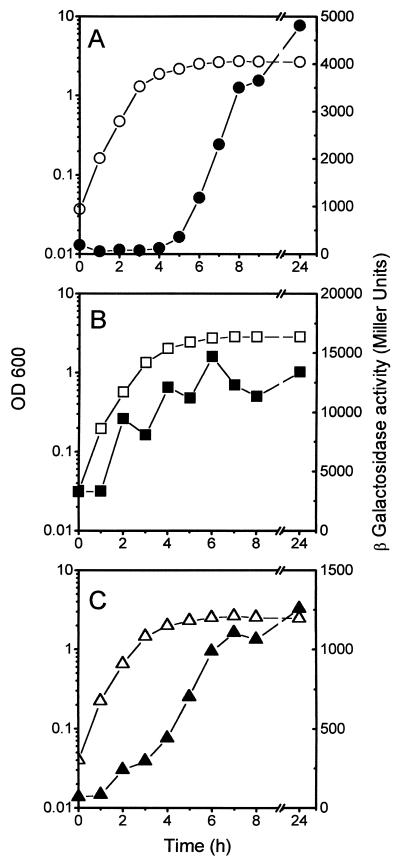
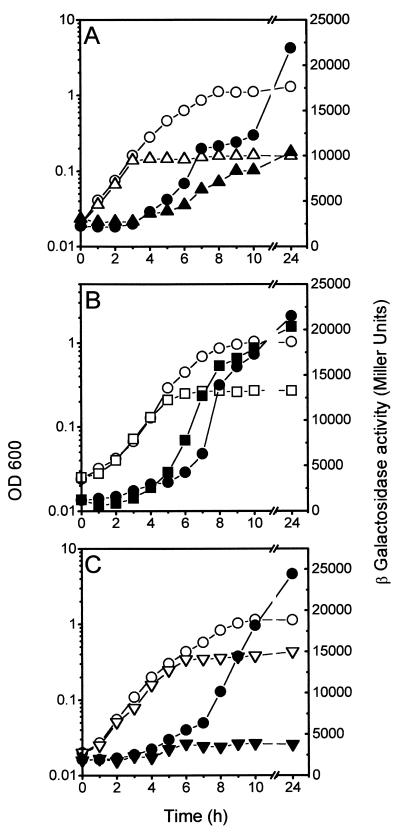
The effect of the growth phase of cells on the expression of mcjA, the gene encoding the microcin precursor, was measured with the mcjA::lacZ fusion in plasmid pMcjA-LacZ. In LB medium, β-galactosidase activity was low (about 60 U) throughout the exponential phase but began to increase concomitantly with growth transition from exponential to stationary phase (Fig. 2A). Twenty-four hours after inoculation 4,800 U of β-galactosidase was detected, an 80-fold increase with respect to early log phase. Expression of the fusion was similarly induced in the late exponential phase in M63 minimal medium, except that the levels observed in stationary phase (20,000 U in an overnight culture) were higher than those in LB medium. This is consistent with the higher activities of MccJ25 found in supernatants of cultures in minimal medium of MC4100 harboring plasmid pTUC100, compared with those in LB medium (39). As shown in Fig. 2B and C, mcjB and mcjC are also regulated by the growth phase of the culture. However, in contrast to mcjA, there was substantial expression from these genes during exponential growth. The mcjC::lacZ fusion expresses relatively low levels of β-galactosidase compared to mcjB::lacZ. Although mcjB and mcjC have been proposed to form an operon (46), we suspect that the concentration of McjC in the cells may be low, possibly due to a reduced translation efficiency. This presumption would be supported by our repeated failure to visualize the McjC polypeptide in maxicells, while McjB was readily identified (J. O. Solbiati, R. N. Farías, and R. A. Salomón, unpublished data).
FIG. 2.
Growth-phase-dependent expression of MccJ25 genes. Strains MC4100(pMcjA-LacZ), MC4100(pMcjB-LacZ), and MC4100(pMcjC-LacZ) were grown in LB medium. Open and closed symbols show OD600 and β-galactosidase activities, respectively. (A) Expression of mcjA::lacZ; (B) expression of mcjB::lacZ; (C) expression of mcjC::lacZ.
Influence of environmental factors on mcjA::lacZ expression.
Various factors could be responsible for the enhanced expression of mcjA in stationary phase, including the accumulation of compounds excreted by the cells, nutrient depletion, pH changes, reduced oxygen availability, or high cell density. The following experiments addressed the identity of the factor(s) responsible for triggering the induction at the transition from exponential to stationary phase.
(i) mcjA::lacZ expression in conditioned LB.
We studied the induction kinetics of mcjA::lacZ in LB medium that had been used for cell growth (conditioned LB medium [see Materials and Methods]). Exponentially growing cells of strain MC4100 (pMcjA-LacZ) were suspended in culture supernatant recovered from early-stationary-phase cells. mcjA was induced immediately in cells exposed to spent medium (Fig. 3), while in fresh LB medium induction did not start until the onset of stationary phase (Fig. 2A). To test whether this behavior was dependent on either accumulation or depletion of a compound, 1/15 volume of 5× fresh LB medium was added to the conditioned medium (addition of concentrated fresh medium avoided excessive dilution of a putative inducer). As observed in fresh medium, induction occurred in the supplemented medium only upon the onset of the stationary phase (Fig. 3). Thus, the stationary-phase induction was not due to an inducer released by the cells used to deplete the medium.
FIG. 3.
Expression of the mcjA::lacZ fusion in plasmid pMcjA-lacZ in conditioned LB medium and in conditioned LB medium supplemented with fresh medium. MC4100(pMcjA-lacZ) was grown in LB medium that had been conditioned as indicated in Materials and Methods (triangles) and in conditioned LB medium supplemented with 1/15 volume of 5× fresh LB medium (circles). Open and closed symbols show OD600 and β-galactosidase activities, respectively.
(ii) Effect of oxygen limitation.
The fact that induction in conditioned medium was observed in cells growing at low density in the presence of oxygen suggested that a factor(s) other than a decrease in the oxygen partial pressure or cell density was responsible for the effect. We tested the effect of anoxia on the increase in β-galactosidase activity from mcjA::lacZ. It was found that anaerobic conditions imposed during early exponential phase reduced expression of the fusion. Late-stationary-phase cells from anaerobic cultures contained β-galactosidase levels sevenfold lower than those of stationary-phase cells from aerobic cultures (700 versus 4,800 U). This is consistent with the observation that production of microcin is greater at high aeration (data not shown).
(iii) Effect of pH.
The pH of the LB medium when the experiments were started was 7, but cultures grown in LB medium become alkaline in stationary phase. To test whether this increase in pH was responsible for the induction of MccJ25 expression, 40 mM MOPS was added to buffer the LB medium. In another experiment, the β-galactosidase activity of the mcjA::lacZ fusion was tested in fresh LB medium adjusted to pH 8 with NaOH before inoculation. Cultures grown in these media showed induction kinetics and levels of expression comparable to those of controls (data not shown), demonstrating that an increase in pH in stationary-phase cultures did not affect mcjA expression.
(iv) Effect of carbon, phosphorus, and nitrogen limitations.
Limitation for some nutrient might be the signal for increasing microcin levels late in exponential phase. To determine whether the fusion was induced upon starvation for different macronutrients, MC4100 (pMcjA-LacZ) was grown in M63 medium which was limited for either glucose, phosphate, or ammonia (see Materials and Methods). A halt of the growth provoked by a 10-fold reduction in glucose availability was followed by an increase in β-galactosidase activity (Fig. 4A). Yet the induction kinetics was slower and the absolute level of expression was reduced compared with those observed in standard M63-glucose medium. Phosphate depletion strongly stimulated mcjA expression (Fig. 4B). In contrast, no induction was seen under conditions of ammonia starvation (Fig. 4C).
FIG. 4.
Effects of starvation for carbon, phosphorus, or nitrogen on the expression of mcjA::lacZ. Strain MC4100(pMcjA-LacZ) was grown in minimal M63 medium with limiting concentrations of glucose (A) (triangles), phosphate (B) (squares), or ammonium (C) (inverted triangles). The control grown in standard M63 medium (circles) is included in all three panels. At the time intervals indicated, OD600 (open symbols) and β-galactosidase activities (filled symbols) were measured.
Stationary-phase induction of mcjA expression does not require rpoS, cya, crp, ompR, or hns function.
The increase in mcjA expression as cell growth slows, especially during growth in rich medium, is characteristic of genes regulated by the rpoS-encoded sigma factor ςS (27). We measured β-galactosidase activity at different growth phases with ZK1171 (rpoS::Tn10) cells harboring plasmid pMcjA-LacZ. The mutation decreased late-stationary-phase levels of β-galactosidase by 40%, but an induction ratio of 60 was still observed, indicating that the growth phase response of the mcjA gene does not require the presence of an intact rpoS gene, thus resembling many stationary-phase-induced genes which are independent of ςS regulation (21, 34).
It is well known that the growth-dependent expression of many genes requires cAMP and the cAMP receptor protein (CRP) (44). We therefore tested the expression in LB medium of mcjA::lacZ in cya (strain RH74) and crp (strain ZK216) mutants, along with their isogenic parents MC4100 and ZK213, respectively. The final β-galactosidase levels in the cya and crp strains were reproducibly decreased by 20 and 43% with respect to those observed for the wild-type controls. However, the mutant strains still showed the induction in stationary phase. It is interesting that microcin levels in stationary-phase cultures of the natural producer E. coli AY25 were twofold higher on glycerol or lactose than on glucose, suggesting carbon catabolite repression of microcin synthesis (39).
It has been shown that stationary-phase levels of MccB17 are greatly enhanced by the regulatory protein OmpR (6, 22), which also controls expression of the outer membrane porins OmpC and OmpF (37). This appears not to be the case for MccJ25, since synthesis of β-galactosidase from the mcjA::lacZ fusion in ompR101 cells (strain RYC514) was found not to be significantly affected (data not shown).
H-NS is a global modulator of gene expression affecting the synthesis, both positively and negatively, of more than 50 E. coli proteins (26). Both exponential- and stationary-phase levels of expression from mcjA::lacZ were reduced almost threefold in the hns mutant CU284 (1,700 versus 4,800 U for MC4100, in overnight cultures), yet the fusion still showed at least 60-fold induction.
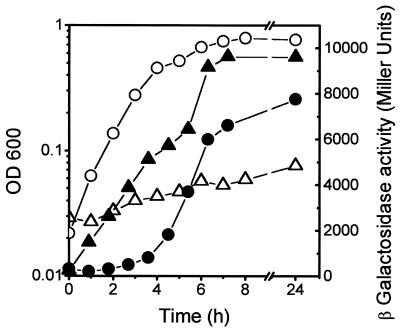
Mutations in himA, lrp, and spoT affect mcjA induction.
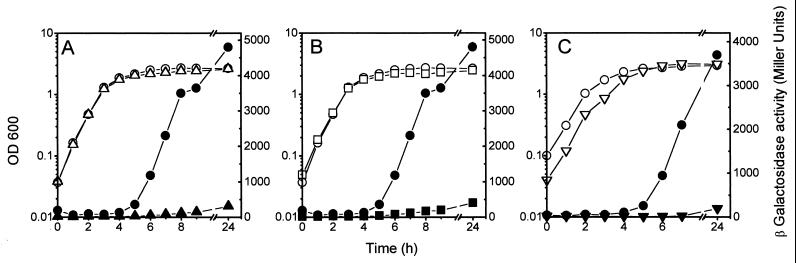
IHF is a heterodimer made up of two subunits, IHFα and IHFβ, encoded by the genes himA and hip (or himD), respectively (15). IHF has a direct positive or negative role in the expression of a number of genes in E. coli (20). As shown in Fig. 5A, the himA mutation in strain MJ150 drastically reduced the β-galactosidase levels from mcjA::lacZ, indicating that IHF positively affects the expression of mcjA. In support of this observation we have detected several candidate IHF-binding sites in the putative promoter region of the mcjA gene (Fig. 6B) that have high identity with the 13-bp consensus sequence WATCAANNNNTTR (W = A or T; R = A or G) (10).
FIG. 5.
Effect of lrp, himA, and spoT mutations on growth-phase-dependent mcjA::lacZ expression. Strain MC4100(pMcjA-LacZ) (circles) and its himA (triangles) (A), lrp (squares) (B), and spoT (inverted triangles) (C) mutant derivatives were grown in LB medium. Optical densities (open symbols) and β-galactosidase activities (closed symbols) were determined along the growth curves.
The leucine-responsive regulatory protein (Lrp) can be either an activator or repressor of many genes in E. coli (33). To test the effect of Lrp on the expression of mcjA we transformed RO64 (lrp201::Tn10) with plasmid pMcjA-LacZ and examined the kinetics of mcjA-lacZ expression by measuring β-galactosidase activity in cells grown in LB medium. The lrp mutation decreased the expression of mcjA to an extent comparable to that of the himA mutant (Fig. 5B), indicating that mcjA is also under positive control of Lrp. A potential Lrp target consensus sequence was detected upstream from the presumptive mcjA promoter region (Fig. 6B). This 12-bp sequence deviates by only one nucleotide from the consensus (TTTATTCtNaAT, where the less-conserved nucleotides are in lower case) defined by Rex et al. (36).
After entry into stationary phase, the level of (p)ppGpp is known to increase (7). Intracellular concentrations of (p)ppGpp are controlled by the relA gene, encoding the ribosome-dependent (p)ppGpp synthetase I (or stringent factor), and the spoT gene, encoding the ribosome-independent (p)ppGpp 3′-pyrophosphohydrolase–(p)ppGpp synthetase II. The RelA enzyme is required for the rapid increase in (p)ppGpp synthesis following amino acid starvation. The bifunctional SpoT enzyme is required for maintenance of basal levels of (p)ppGpp and is responsible for the RelA-independent (p)ppGpp accumulation following nutrient limitations that do not involve amino acid starvation, such as carbon and energy source exhaustion (7, 17). To examine the effect of (p)ppGpp deficiency on mcjA expression, we transformed pMcjA-LacZ into strain MJ152 (relA1 ΔspoT) and measured β-galactosidase activity in LB medium (Fig. 5C). No induction of mcjA::lacZ was seen, indicating that mcjA expression is positively regulated by (p)ppGpp. Strain MC4100, which has been employed throughout the present study as a host for fusion plasmids, harbors a relA1 mutation (30). However, the activity of the mcjA::lacZ fusion was very similar in strain W3110, which carries the wild-type relA allele (data not shown). Also, no significant difference in β-galactosidase activity from mcjA::lacZ was noted when the relA-null allele from strain RO98 was substituted for the relA1 mutation of MC4100. Altogether, these data indicate that induction of mcjA is dependent on spoT but is relA independent. The relA1 allele apparently has a weak residual (p)ppGpp synthetic activity, which could lead to low basal levels of (p)ppGpp when the nucleotide degradation is severely compromised, as in relA1 ΔspoT mutants (30). We could not examine the effect of a complete (p)ppGpp depletion on the expression of the fusion, since the available double mutant RO98 (ΔrelA251::kan ΔspoT207::cat) was already resistant to kanamycin and chloramphenicol, making it impossible to select for acquisition of the fusion plasmid, which has the same antibiotic markers. Instead, we transduced both null mutations to MC4100 harboring pTUC341 (Apr), a pBR322 derivative carrying the MccJ25 genetic system, and measured MccJ25 production. No antibiotic activity was detected in the supernatant of an overnight culture of the double mutant grown in LB medium, while the control culture showed a titer of 512.
Regulation of expression of a mcjA::lacZ operon fusion.
The activity of the mcjA::lacZ gene fusion did not permit a distinction between transcriptional or translational controls. In all cases studied so far, IHF, Lrp, and (p)ppGpp function as transcriptional regulators, and it is to be expected that they play a similar role in the expression of mcjA. To verify whether the expression of mcjA is regulated transcriptionally, we constructed the plasmid pMcjA-LacZ(op), harboring an mcjA::lacZ transcriptional fusion, as described in Materials and Methods. This plasmid was transformed into strain MC4100 and its mutant derivatives MJ150 (himA), RO64 (lrp), and MJ152 (spoT). As shown in Table 2, β-galactosidase activity from the transcriptional fusion of parent cells increased at the end of the exponential phase but no induction was seen in the mutant cells. Thus, the expression pattern of the transcriptional fusion was similar to that of the translational one, indicating that regulation of mcjA is under transcriptional control. However, note that the translational fusion showed lower levels of β-galactosidase and a higher factor of activation than the transcriptional fusion. This difference could possibly be explained by a low stability of the fusion protein. Alternatively, mcjA expression may also be subjected to posttranscriptional control.
TABLE 2.
Effects of lrp, himA, and spoT mutations on expression of an mcjA::lacZ transcriptional fusion
| Straina | Relevant genotype | β-Galactosidase activity (Miller units) during:
|
||
|---|---|---|---|---|
| Early exponential phaseb | Late exponential phasec | Stationary phased | ||
| MC4100 | Wild type | 1,100 | 24,300 | 28,000 |
| RO64 | lrp::Tn10 | 811 | 1,390 | 2,595 |
| MJ150 | ΔhimA | 506 | 820 | 1,066 |
| MJ152 | relA ΔspoT | 956 | 1,012 | 1,650 |
All strains harbor the pMcjA-LacZ(op) plasmid.
OD600, approximately 0.3.
OD600, approximately 2.
OD600, approximately 3.
Regulation of expression of the mcjB and mcjC genes.
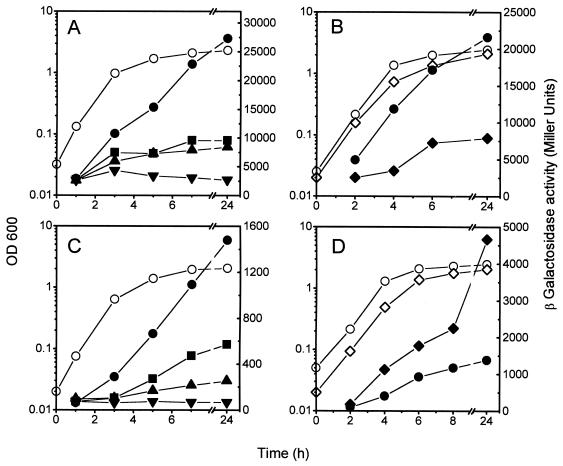
To determine the effect of global regulators on expression of mcjBC, the β-galactosidase activity of mcjB::lacZ and mcjC::lacZ translational fusions was assayed in the same mutant hosts used for mcjA. We found that cya, crp, rpoS, and ompR mutants showed the growth phase increase in expression of these genes (data not shown). Similar to what had been found for mcjA, the final β-galactosidase levels of mcjB::lacZ in the cya strain was decreased by 53% with respect to that observed for the wild type. Also, late-stationary-phase levels of β-galactosidase for mcjB::lacZ and mcjC::lacZ in the rpoS mutant were reduced by 40 and 50%, respectively, with respect to the control. The most remarkable effects were seen with the Lrp-, IHF-, (p)ppGpp-, and H-NS-defective strains. As can be seen in Fig. 7A and C, in the absence of Lrp or IHF, the fusions showed a residual level of expression which was still subject to growth phase regulation. (p)ppGpp deficiency, on the other hand, had a more severe effect, since expression of mcjB::lacZ and mcjC::lacZ was low and constant throughout the growth cycle. This indicates that (p)ppGpp is the principal factor modulating the expression of mcjBC genes and that Lrp and IHF are important for maximum levels of expression from mcjBC during stationary phase. To test for H-NS-dependent effects on mcjB::lacZ and mcjC::lacZ, β-galactosidase activity from the fusions was measured in the isogenic strains MC4100 and CU284 (hns). While stationary-phase expression of mcjB was reduced threefold (Fig. 7B), we consistently detected a threefold stimulation of the induced level of expression from mcjC in the hns mutant (Fig. 7D). Thus, these genes were affected in an opposite way by the same regulator.
FIG. 7.
Effect of global regulators on growth-phase-dependent expression of mcjB::lacZ and mcjC::lacZ fusions. β-Galactosidase activity (solid symbols) and cell growth (open symbols) were measured in LB medium at the indicated times. (A) MC4100(pMcjB-LacZ) (circles) and its isogenic lrp (squares), himA (triangles), and spoT (inverted triangles) mutants; (B) MC4100(pMcjB-LacZ) (circles) and its hns mutant (diamonds); (C) MC4100(pMcjC-lacZ) and its isogenic lrp (squares), himA (triangles), and spoT (inverted triangles) mutants; (D) MC4100(pMcjC-lacZ) (circles) and its hns mutant (diamonds). For clarity, only the growth curve of the control strain has been represented in panels A and C, as those of the mutants were nearly identical.
The reduced activity of microcin genes in mutant backgrounds is paralleled by a decrease in MccJ25 production.
To verify in a different manner the reduced expression of fusions to microcin genes in lrp, himA, and spoT mutant backgrounds, we measured the effect of these mutations on the production of biologically active MccJ25. Microcin titers in overnight LB cultures of RO64 (lrp), and MJ150 (himA) harboring plasmid pTUC202 were 256- and 512-fold lower, respectively, than that of the control (titer, 512). No activity was detected in supernatants of MJ152 (relA1ΔspoT) transformed with pTUC202.
DISCUSSION
In this study, the environmental and genetic factors governing the expression of MccJ25 genes were analyzed. We demonstrated that expression of mcjA, the gene encoding the MccJ25 precursor, is induced upon transition from exponential to stationary phase in both LB and M63-glucose media. This increase is not dependent on cell density, pH changes, anaerobiosis (which in fact was found to repress the mcjA gene), or the buildup of some inducer, suggesting that the stationary- phase induction may result from the depletion of one or more nutrients. In fact, we have shown that carbon and inorganic phosphate starvation were able to promote the growth phase response, while no induction occurred under nitrogen limitation. By using strains deficient in the E. coli regulatory proteins RpoS, cAMP-CRP, OmpR, and H-NS, we demonstrated that these regulators are not involved in the growth-phase-dependent expression of mcjA.
The principal finding of the present study is that expression of mcjA is positively regulated by a complex network of global regulators, including at least Lrp, IHF, and (p)ppGpp. Taking into account that these regulators themselves are growth phase responsive, we propose a model describing their interplay in the regulation of mcjA expression. During exponential phase in LB medium the low level of mcjA expression may be explained by the low concentrations of (p)ppGpp, Lrp, and IHF (7, 14, 21, 25). In the decelerating phase, the intracellular concentration of (p)ppGpp would start to increase via the SpoT mechanism, which specifically responds to carbon and energy source deprivation. On the other hand, it has been shown that Lrp and IHF levels also display a maximum at the phase transition of cell growth (2, 14, 25). The concerted action of (p)ppGpp, Lrp, and IHF would stimulate the expression of mcjA at the onset of stationary phase. Since (p)ppGpp is required for substantial Lrp and IHF expression (1, 25), it is possible that (p)ppGpp deficiency acts not only directly but also indirectly via its role in the control of Lrp and IHF. If this were so, (p)ppGpp would be the main positive effector of mcjA expression. If optimal induction of mcjA resulted from the additive effects of the three regulators, one should expect that in the absence of one of them a certain level of induction would remain, promoted by the presence of the other two. However, mcjA induction is practically abolished in strains deficient in either (p)ppGpp, Lrp, or IHF (Fig. 5). It seems, therefore, that induction requires the combined action of these factors. Basically, the same considerations may be invoked to explain the increased activity of the mcjB and mcjC genes upon entering stationary phase, since they are also positively modulated by IHF, Lrp, and ppGpp.
At present, we cannot decide whether Lrp and IHF directly bind to the mcjA regulatory region. However, this seems highly likely since potential binding sites with strong homology to the consensus can be found (Fig. 6B). IHF has been found to directly stimulate transcription from the Pe promoter of bacteriophage Mu (19, 23), the pL1 promoter of bacteriophage lambda (18), and the PG2 promoter of the ilvGMEDA operon of E. coli (35). In all three cases, IHF binds to a site located just upstream from the promoter. Note that the putative Lrp- and IHF-binding sites indicated in Fig. 6B not only are close to the mcjA promoter region but are also in close proximity to the mcjBC promoter region. Therefore, these sites might also participate in the control of the two latter genes.
Another interesting finding of this work is that the histone-like protein H-NS exerts a negative effect on mcjC expression, while mcjB, in contrast, appears to be positively regulated by this factor. H-NS influences the transcription of a number of genes and, in nearly all cases studied so far, it inhibits their expression. Since mcjB and mcjC are closely linked (in fact, the end of mcjB overlaps the beginning of mcjC) (46), it is likely that both genes are directed by the presumptive promoter we have located upstream of mcjB (Fig. 6B). It is difficult to imagine how an alteration in the DNA topology mediated by the regulator could differentially affect the expression of two genes expressed from the same promoter (we could not find any putative mcjC promoter within the coding region of mcjB). The possibility of a posttranscriptional control of mcjC by H-NS should be considered. In fact, Yamashino et al. (47) and Barth et al. (4) showed that H-NS inhibits the expression of rpoS during the exponential phase by a mechanism acting at the posttranscriptional level and that relief of repression by H-NS plays a role in rpoS induction upon entry into stationary phase. In light of these findings, we propose that H-NS could also repress mcjC and that the higher levels of mcjC in hns mutants may result from an elevated translational efficiency of mcjC mRNA. Clearly, the function of H-NS in the regulation of mcjBC warrants further study.
It is worth noting that a correlation between the activity of fusions to microcin genes and MccJ25 synthesis was demonstrated by measuring the production of biologically active MccJ25 in stationary-phase supernatants of cultures of Lrp-, IHF-, and (p)ppGpp-deficient strains. In all cases, the reduced expression of mcjABC in the mutant backgrounds was paralleled by a corresponding decrease in MccJ25 activity.
Like most antibiotics, synthesis of microcins is induced when cultures cease exponential growth (3, 29). The regulatory mechanisms underlying the production of microcins have been studied in detail only for MccB17 and MccC7. Expression of the MccB17 operon is induced when cells stop growing because of exhaustion of glucose, ammonia, or phosphate and also when cells enter stationary phase in rich medium (9). Stationary-phase induction of MccB17 does not require ςs (6), cAMP (9), or (p)ppGpp (9). The expression of MccB17, on the other hand, is activated by OmpR (6, 22) and IHF (32), while Emr (also known as MprA) negatively regulates transcription from the major promoter of the operon (11). Expression of MccC7 appears to be activated not by OmpR and IHF but by the products of the chromosomal genes crp and appR (13, 32). The expression of colicins E1 and K is also growth phase dependent. Eraso et al. (16) demonstrated that expression of the colicin E1 structural gene, cea, is stimulated when cells reach the stationary phase. This increase in expression is due to depletion of nutrients and was found to be independent of the SOS response, anaerobiosis, and catabolite repression as well as the regulators IHF, RpoS, and Lrp. Nutrient depletion also induces the expression of the colicin K structural gene cka through an increase in ppGpp (24). This induction is independent of the cAMP-CRP complex, RpoS, Lrp, and H-NS. From a comparison of the regulatory patterns of these colicins and microcins with that of MccJ25 several conclusions can be drawn. First, nutrient depletion is the common signal for stationary-phase induction of all of them. Second, neither RpoS nor the cAMP-CRP complex is involved in the growth-phase response (except for MccC7, whose production is undetectable in cya or crp strains). Third, although these compounds share some regulatory factors, induction of individual microcins and colicins is further influenced by specific regulators.
ACKNOWLEDGMENTS
We thank Regine Hengge-Aronis, Barbara Bachman, Roberto Kolter, Felipe Moreno, and Chiharu Ueguchi for gifts of bacterial strains.
This work was funded by FONCYT (grant 01-00132-02291), CABBIO (grant 11Ar/12Br), and CIUNT (grant 26/D114). M.J.C. and M.A.D. were supported by fellowships from CONICET, and R.N.F. was a career investigator of CONICET.
REFERENCES
- 1.Aviv M, Giladi H, Schreiber G, Oppenheim A B, Glaser G. Expression of the genes coding for the Escherichia coli integration host factor are controlled by growth phase, rpoS, ppGpp and by autoregulation. Mol Microbiol. 1994;14:1021–1031. doi: 10.1111/j.1365-2958.1994.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 2.Azam T A, Iwata A, Nishimura A, Ueda S, Ishihama A. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol. 1999;181:6361–6370. doi: 10.1128/jb.181.20.6361-6370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baquero F, Moreno F. The microcins. FEMS Microbiol Lett. 1984;23:117–124. [Google Scholar]
- 4.Barth M, Marschall C, Muffler A, Fischer D, Hengge-Aronis R. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of ςs and many ςs-dependent genes in Escherichia coli. J Bacteriol. 1995;177:3455–3464. doi: 10.1128/jb.177.12.3455-3464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blond A, Péduzzi J, Goulard C, Chiuchiolo M J, Barthélémy M, Prigent Y, Salomón R A, Farías R N, Moreno F, Rebuffat S. The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Eur J Biochem. 1999;259:747–755. doi: 10.1046/j.1432-1327.1999.00085.x. [DOI] [PubMed] [Google Scholar]
- 6.Bohannon D E, Connell N, Keener J, Tormo A, Espinosa-Urgel M, Zambrano M M, Kolter R. Stationary-phase-inducible “gearbox promoters”: differential effects of katF mutations and role of ς70. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashel M, Gentry D R, Hernandez V J, Vinella D. The stringent response. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 1458–1496. [Google Scholar]
- 8.Castilho B A, Olfson P, Casadaban M J. Plasmid insertion mutagenesis and lac gene fusion with mini-Mu bacteriophage transposons. J Bacteriol. 1984;158:488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell N, Han Z, Moreno F, Kolter R. An E. coli promoter induced by the cessation of growth. Mol Microbiol. 1987;1:195–201. doi: 10.1111/j.1365-2958.1987.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 10.Craig N L, Nash H A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984;39:707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- 11.del Castillo I, Gómez J M, Moreno F. mprA, an Escherichia coli gene that reduces growth-phase-dependent synthesis of microcins B17 and C7 and blocks osmoinduction of proU when cloned on a high-copy-number plasmid. J Bacteriol. 1990;172:437–445. doi: 10.1128/jb.172.1.437-445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado M A, Solbiati J O, Chiuchiolo M J, Farías R N, Salomón R A. Escherichia coli outer membrane protein TolC is involved in production of the peptide antibiotic microcin J25. J Bacteriol. 1999;181:1968–1970. doi: 10.1128/jb.181.6.1968-1970.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Díaz-Guerra L, Moreno F, San Millán J L. appR gene product activates transcription of microcin C7 plasmid genes. J Bacteriol. 1989;171:2906–2908. doi: 10.1128/jb.171.5.2906-2908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ditto M D, Roberts D, Weisberg R A. Growth phase variation of integration host factor level in Escherichia coli. J Bacteriol. 1994;176:3738–3748. doi: 10.1128/jb.176.12.3738-3748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drlica K, Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987;51:301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eraso J M, Chidambaram M, Weinstock G M. Increased production of colicin E1 in stationary phase. J Bacteriol. 1996;178:1928–1935. doi: 10.1128/jb.178.7.1928-1935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallant J, Margason G, Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972;247:6055–6058. [PubMed] [Google Scholar]
- 18.Giladi H, Gottesman M, Oppenheim A B. Integration host factor stimulates the phage lambda pL promoter. J Mol Biol. 1990;213:109–121. doi: 10.1016/S0022-2836(05)80124-X. [DOI] [PubMed] [Google Scholar]
- 19.Goosen N, van de Putte P. Regulation of Mu transposition. I. Localization of the presumed recognition sites for HimD and Ner functions controlling bacteriophage Mu transcription. Gene. 1984;30:41–46. doi: 10.1016/0378-1119(84)90103-3. [DOI] [PubMed] [Google Scholar]
- 20.Goosen N, van de Putte P. The regulation of transcription initiation by integration host factor. Mol Microbiol. 1995;16:1–7. doi: 10.1111/j.1365-2958.1995.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 21.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 1497–1512. [Google Scholar]
- 22.Hernández-Chico C, San Millán J L, Kolter R, Moreno F. Growth phase and OmpR regulation of transcription of microcin B17 genes. J Bacteriol. 1986;167:1058–1065. doi: 10.1128/jb.167.3.1058-1065.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krause H M, Higgins N P. Positive and negative regulation of the Mu operator by Mu repressor and Escherichia coli integration host factor. J Biol Chem. 1986;261:3744–3752. [PubMed] [Google Scholar]
- 24.Kuhar I, Zgur-Bertok D. Transcription regulation of the colicin K cka gene reveals induction of colicin synthesis by differential responses to environmental signals. J Bacteriol. 1999;181:7373–7380. doi: 10.1128/jb.181.23.7373-7380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landgraf J R, Wu J, Calvo J M. Effects of nutrition and growth rate on Lrp levels in Escherichia coli. J Bacteriol. 1996;178:6930–6936. doi: 10.1128/jb.178.23.6930-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurent-Winter C, Ngo S, Danchin A, Bertin P. Role of Escherichia coli histone-like nucleoid-structuring protein in bacterial metabolism and stress response—identification of targets by two-dimensional electrophoresis. Eur J Biochem. 1997;244:767–773. doi: 10.1111/j.1432-1033.1997.00767.x. [DOI] [PubMed] [Google Scholar]
- 27.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςs (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 28.Manoil C. Analysis of protein localization by use of gene fusions with complementary properties. J Bacteriol. 1990;172:1035–1042. doi: 10.1128/jb.172.2.1035-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin J F, Demain A L. Control of antibiotic biosynthesis. Microbiol Rev. 1980;44:230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzger S, Schreiber G, Aizenman E, Cashel M, Glaser G. Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J Biol Chem. 1989;264:21146–21152. [PubMed] [Google Scholar]
- 31.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. [Google Scholar]
- 32.Moreno F, San Millán J L, Del Castillo I, Gómez J M, Rodríguez-Sáinz M C, González-Pastor J E, Díaz-Guerra L. Escherichia coli genes regulating the production of microcins MccB17 and MccC7. In: James R, Lazdunski C, Pattus F, editors. Bacteriocins, microcins and lantibiotics. Berlin, Germany: Springer-Verlag; 1992. pp. 3–13. [Google Scholar]
- 33.Newman E B, Lin R T, D'Ari R. The leucine/Lrp regulon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 1513–1525. [Google Scholar]
- 34.Nyström T, Neidhardt F C. Cloning, mapping, and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli. Mol Microbiol. 1992;6:3187–3198. doi: 10.1111/j.1365-2958.1992.tb01774.x. [DOI] [PubMed] [Google Scholar]
- 35.Pagel J M, Hatfield G W. Integration host factor-mediated expression of the ilvGMEDA operon of Escherichia coli. J Biol Chem. 1991;266:1985–1996. [PubMed] [Google Scholar]
- 36.Rex J H, Aronson B D, Somerville R L. The tdh and serA operons of Escherichia coli: mutational analysis of the regulatory elements of leucine-responsive genes. J Bacteriol. 1991;173:5944–5953. doi: 10.1128/jb.173.19.5944-5953.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo F D, Silhavy T J. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J Mol Biol. 1991;222:567–580. doi: 10.1016/0022-2836(91)90497-t. [DOI] [PubMed] [Google Scholar]
- 38.Salomón R A. Doctoral thesis. Tucumán, Argentina: Universidad Nacional de Tucumán; 1995. [Google Scholar]
- 39.Salomón R A, Farías R N. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J Bacteriol. 1992;174:7428–7435. doi: 10.1128/jb.174.22.7428-7435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomón R A, Farías R N. The FhuA protein is involved in microcin 25 uptake. J Bacteriol. 1993;175:7741–7742. doi: 10.1128/jb.175.23.7741-7742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salomón R A, Farías R N. The peptide antibiotic microcin 25 is imported through the TonB pathway and the SbmA protein. J Bacteriol. 1995;177:3323–3325. doi: 10.1128/jb.177.11.3323-3325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 43.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz J E, Latter G I, Matin A. Differential regulation by cyclic AMP of starvation protein synthesis in Escherichia coli. J Bacteriol. 1988;170:3903–3909. doi: 10.1128/jb.170.9.3903-3909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solbiati J O, Ciaccio M, Farías R N, Salomón R A. Genetic analysis of plasmid determinants for microcin 25 production and immunity J. Bacteriol. 1996;178:3661–3663. doi: 10.1128/jb.178.12.3661-3663.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solbiati J O, Ciaccio M, Farías R N, González-Pastor E, Moreno F, Salomón R A. Sequence analysis of the four plasmid genes required to produce the circular peptide antibiotic microcin J25. J Bacteriol. 1999;181:2659–2662. doi: 10.1128/jb.181.8.2659-2662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashino T, Ueguchi C, Mizuno T. Quantitative control of the stationary phase-specific sigma factor, ςs, in Escherichia coli: involvement of the nucleoid protein H-NS. EMBO J. 1995;14:594–602. doi: 10.1002/j.1460-2075.1995.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]