Abstract
The Gibberellic Acid Stimulated Arabidopsis/Gibberellin Stimulated Transcript (GASA/GAST) gene family is a group of plant-specific genes encoding cysteine-rich peptides essential to plant growth, development, and stress responses. Although GASA family genes have been identified in various plant species, their functional roles in Prunus mume are still unknown. In this study, a total of 16 PmGASA genes were identified via a genome-wide scan in Prunus mume and were grouped into three major gene clades based on the phylogenetic tree. All PmGASA proteins possessed the conserved GASA domain, consisting of 12-cysteine residues, but varied slightly in protein physiochemical properties and motif composition. With evolutionary analysis, we observed that duplications and purifying selection are major forces driving PmGASA family gene evolution. By analyzing PmGASA promoters, we detected a number of hormonal-response related cis-elements and constructed a putative transcriptional regulatory network for PmGASAs. To further understand the functional role of PmGASA genes, we analyzed the expression patterns of PmGASAs across different organs and during various biological processes. The expression analysis revealed the functional implication of PmGASA gene members in gibberellic acid-, abscisic acid-, and auxin-signaling, and during the progression of floral bud break in P. mume. To summarize, these findings provide a comprehensive understanding of GASA family genes in P. mume and offer a theoretical basis for future research on the functional characterization of GASA genes in other woody perennials.
Keywords: GASA gene family, Prunus mume, evolutionary analysis, gene family analysis, gibberellin-responsive genes
1. Introduction
The Gibberellic Acid Stimulated Arabidopsis/Gibberellin Stimulated Transcript (GASA/GAST) is a plant-specific gene family widely present in gymnosperms, angiosperms, and pteridophytes [1]. GASA family genes encode cysteine-rich peptides (CRPs) consisting of a secretory peptide signal at the N-terminal, a highly hydrophilic middle part, and the GASA domain at the C-terminal [2]. The GASA domain is a conserved 60 amino acid domain containing 12 featured cysteine residues arranged in a repetitive pattern [3,4]. In previous studies, proteins lacking a complete GASA domain were found to be non-functional [5]. GASA family proteins were first reported in tomato (Lycopersicon esculentum) [6] but, over the past decades, have then characterized in many other plant species, including Arabidopsis [7], rice (Oryza sativa) [8], potato (Solanum tuberosum) [9], and poplar (Populus trichocarpa) [10]. However, the GASA gene family was absent in charophytes or bryophytes [3,11].
GASA family genes play a vital role in plant development and reproduction [12]. In Arabidopsis, AtGASA4 is reported to regulate floral meristem identity and positively influence seed development [12]. AtGASA5, on the other hand, is involved in the gibberellin-promoted flowering in Arabidopsis [13]. The over-expression of AtGASA5 leads to delayed flowering time through enhancing the transcription of the flowering repressor gene, FLC (FLOWERING LOCUS C) and repressing FT (FLOWERING LOCUS T) and LFY (LEAFY) [13]. AtGASA10 encodes a cell wall protein that functions in promoting cell elongation in developing anthers and seeds [14]. FaGAST1 and FaGAST2, two GAST-like proteins isolated from strawberry, act together to determine cell size during fruit development and ripening [15]. In Gerbera hybrida, GhGEG, the Gerbera ortholog of tomato GAST1, is reported to inhibit cell elongation during ray petal development [16,17]. For perennial trees, GASA gene members are reported to potentially regulate floral induction in apple (Malus domestica) [18] and bud dormancy cycling in pear (Pyrus pyrifolia) [19].
GASA family genes are also involved in hormone signaling transduction and biotic/abiotic stress tolerance [10,20]. Most GASA genes are part of the gibberellin pathways. For example, six out of fifteen GASA genes (AtGASA1, AtGASA4, AtGASA6, AtGASA7, AtGASA8, and AtGASA13) are responsive to the exogeneous application of gibberellin in Arabidopsis [21]. In Glycine max, GmGASA32 is up-regulated by gibberellin and promotes plant height by interacting with GmCDC25 (cell cycle-associated protein) [22]. GASA family genes are also responsive to other phytohormones. In rice, OsGSR1, a GASA family gene, mediates the crosstalk between brassinosteroid (BR) and GA signaling pathways [2]. Acting as the integrator of gibberellin, abscisic acid (ABA), and glucose signaling, AtGASA6 regulate seed germination and hypocotyl elongation through connecting the AtRGL2 (RGA-LIKE 2) and AtEXPA1 (EXPANSIN A1) gene in Arabidopsis [23]. The constitutive expression of AtGASA14 in transgenic Arabidopsis exhibited an elevated Reactive Oxygen Species (ROS) level and increased tolerance to salt stress [24]. In addition, GASA family proteins are linked with resistance to bacterial and fungal pathogens. In Hevea brasiliensis, HbGASA genes are also found to be involved in regulating innate immunity through modulating ROS accumulation to repressing fungal pathogens [25].
Prunus mume, commonly known as Japanese apricot, is an important ornamental and fruit tree species widely cultivated in East Asia [26]. The fruits of Prunus mume can be processed into preserved delicacies and wine, or applied for medical purposes [27]. Prunus mume is a deciduous tree species with more than 1500 years cultivation history and is highly prized for its delicate blossoms and pleasant fragrance [28]. Therefore, Prunus mume is also considered as an asset to landscape and gardening [29]. GASA family genes are implicated in many important plant biological processes. However, their functional roles are still uncharacterized in Prunus mume. In this study, we identified GASA family genes and analyzed their gene structure, phylogenetic relationships, protein chemical properties, and conserved motif, as well as their putative regulatory networks in Prunus mume. With genome synteny and selection analysis, we clarified the evolutionary trajectory of GASA family genes in Prunus mume. Furthermore, we performed spatial and temporal expression analysis to investigate the functional role of GASA family genes during floral development and hormonal response processes in Prunus mume. Our findings provide comprehensive insights into the evolution and functional importance of GASA family genes in Prunus mume.
2. Results
2.1. Genome-Wide Identification of GASA Genes across Six Plant Species
Previous studies reported a total of fifteen GASA genes in A. thaliana [21]. To identify GASA family genes in other plant species, we employed two approaches, combining Hmmer and BLASTP search. With genome-wide scans, we identified a total of 11 GASA genes for Asian rice (Oryza sativa), 28 from apple (Malus domestica), 16 from Prunus mume, 19 from poplar (Populus tricocarpa), and 17 from peach (Prunus persica) (Table 1). All 106 GASA family genes were manually verified using conserved domain search tools (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi; accessed on 24 June 2022) and the Pfam database containing GASA domains (Table 1 and Table S2). The GASA family proteins were further blasted against the NCBI non-redundant protein sequence database, with their closest gene accession returned (Table 1 and Table S2). The GASA gene family members from each species are renamed by their sequential order across chromosomes. All detailed information of the GASA genes analyzed in this study were listed (Table 1 and Table S2).
Table 1.
Detailed information of GASA family genes identified in P. mume. For each gene, their Gene_ID (gene identifier in the published genome of P. mume), NCBI accession (closest gene accession name returned from NCBI BLASTP), the start and end coordinate of GASA domain, HMM accession ID, domain name, and e-value for each PmGASA protein query in the Pfam database are listed.
| Gene_ID | Gene_Name | NCBI Accesion | Start | End | HMM Accession | HMM Name | E-Value (Pfam) |
|---|---|---|---|---|---|---|---|
| Pm005363 | PmGASA1 | XP_007223838.1 | 29 | 88 | PF02704 | GASA | 4.29 × 10−26 |
| Pm006215 | PmGASA2 | CAB4265575.1 | 50 | 109 | PF02704 | GASA | 3.41 × 10−27 |
| Pm009495 | PmGASA3 | XP_008224510.1 | 35 | 92 | PF02704 | GASA | 1.44 × 10−26 |
| Pm009496 | PmGASA4 | XP_008224511.1 | 36 | 95 | PF02704 | GASA | 8.24 × 10−25 |
| Pm014337 | PmGASA5 | XP_007215190.1 | 35 | 94 | PF02704 | GASA | 1.19 × 10−24 |
| Pm015762 | PmGASA6 | ONI19434.1 | 159 | 219 | PF02704 | GASA | 1.76 × 10−26 |
| Pm015883 | PmGASA7 | PQQ10068.1 | 49 | 108 | PF02704 | GASA | 2.24 × 10−18 |
| Pm021248 | PmGASA8 | KAH0977881.1 | 9 | 62 | PF02704 | GASA | 1.88 × 10−13 |
| Pm021249 | PmGASA9 | CAB4290623.1 | 13 | 60 | PF02704 | GASA | 2.52 × 10−26 |
| Pm022352 | PmGASA10 | XP_008237395.1 | 54 | 113 | PF02704 | GASA | 1.05 × 10−26 |
| Pm024681 | PmGASA11 | XP_008239716.1 | 62 | 122 | PF02704 | GASA | 3.03 × 10−27 |
| Pm024909 | PmGASA12 | XP_008239947.1 | 48 | 107 | PF02704 | GASA | 6.36 × 10−26 |
| Pm025746 | PmGASA13 | XP_008240742.1 | 57 | 116 | PF02704 | GASA | 5.82 × 10−31 |
| Pm026754 | PmGASA14 | XP_016651693.1 | 49 | 108 | PF02704 | GASA | 8.88 × 10−24 |
| Pm029238 | PmGASA15 | XP_008244953.1 | 53 | 112 | PF02704 | GASA | 2.15 × 10−30 |
| Pm030034 | PmGASA16 | XP_008245155.1 | 46 | 106 | PF02704 | GASA | 2.75 × 10−23 |
2.2. Gene Structure and Protein Motif Analysis of PmGASAs
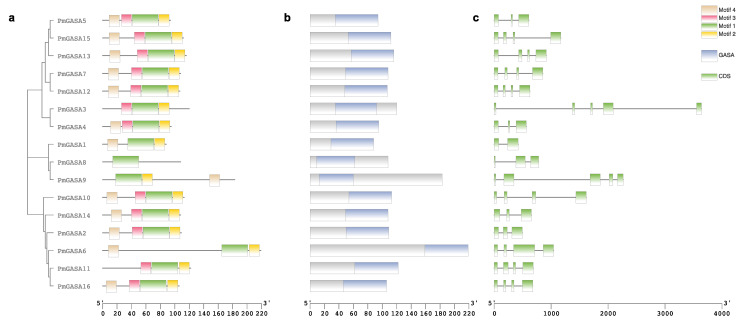
The GASA family genes were distributed unevenly across eight chromosomes in P. mume (Figure S1). Out of 16 genes, four (PmGASA1–4) were mapped to chromosome 2 (Figure S1). Gene clusters PmGASA5–7 and PmGASA8–10 were mapped to chromosome 4 and chromosome 6, respectively (Figure S1). PmGASA11–12 and PmGASA13–14 were located to chromosome 7 and chromosome 8, respectively, and the other two genes were present in the form of scaffolds (Figure S1). All PmGASA proteins contained the GASA domain and can be grouped into three sub-clusters based on the polygenetic tree (Figure 1). Protein motif analysis revealed four featured motifs, including RRCSLTSRKKPCMRFCGKCCEKCLCVPPGTYGNKEEC (motif 1), PCYNNWKTKGGGPKC (motif 2), GPGSLRPIECGSACT (motif 3), and FLLLALLLLSMVAEV (motif 4) (Figure S2). Among the four motifs, motif 1 was universally present on every member of the PmGASA family proteins (Figure 1a). PmGASA3, PmGASA8, and PmGASA11 lack protein motif 4, while only PmGASA8 lacks motif 2 (Figure 1a).
Figure 1.
Protein motif and gene structure analysis of GASA family genes identified in P. mume. (a) Phylogenetic tree of PmGASA protein sequences with conserved protein motifs colored differently. (b) Conserved protein domain identified for PmGASA proteins using the NCBI CDD tool. The blue box indicates the conserved GASA domain identified. (c) Exon–intron distribution analysis of PmGASA genes. The green boxes represent exons and the black lines represent intron positions, respectively.
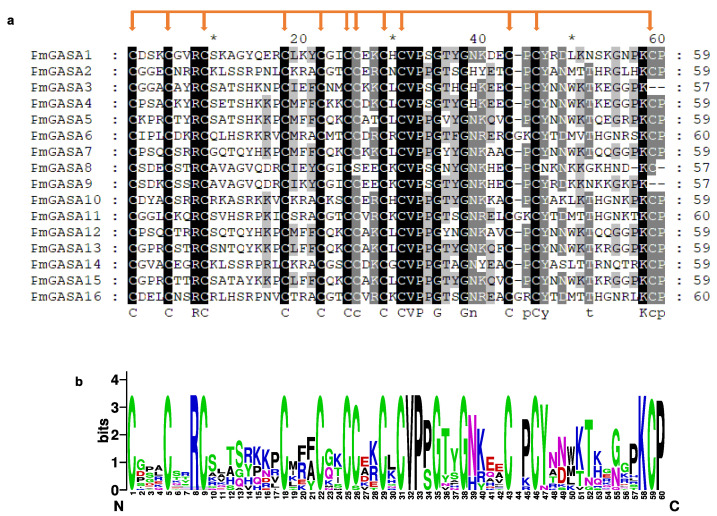
The exon/intron distribution of PmGASA genes were analyzed based on the gene annotation of the P. mume genome (Figure 1c). The exon number of PmGASA genes ranged from two to five. The largest numbers of exons were present in PmGASA3 and PmGASA9 (five exons), while PmGASA1 contained only two exons. The exon/intron size was generally conserved among PmGASA genes, except for PmGASA3, PmGASA9, PmGASA10, and PmGASA15, with relatively long introns of more than 500 bp (Figure 1c). To further examine the conservation of the GASA domain, we extracted the GASA domain sequence of PmGASA proteins and performed multiple alignment. All PmGASA proteins were shown to possess the 12 conserved cysteines within the GASA domain region, except for PmGASA3 and PmGASA9, which both lacked the 12th featured cysteine, which is likely attributed to amino acid mutations (Figure 2).
Figure 2.
Amino acid alignment of GASA domain from PmGASA proteins. (a) Multiple alignment of the PmGASA protein sequences within the GASA domain. The alignment was visualized in GeneDoc, with grey shading indicating the amino acid identity. The featured cysteines within the GASA domain are highlighted with red arrows. The 10th, 30th, 50th amino acid residues were labeled with *. (b) Sequence logo analysis of the conserved GASA domains performed with WebLogo platform.
2.3. Physiochemical Analysis of PmGASA Proteins
Protein sequences of PmGASAs ranged from 88 to 219 amino acids, with a molecular weight between 9613.29 and 23,383.3 Da (Table 2). By assessing the physiochemical properties of PmGASA proteins, we determined the isoelectric point (pI), instability index, and the grand average of hydropathy (GRAVY) using the ExPASy tool. Most PmGASA family proteins shared relatively high pI values, among which fifteen had pI value > 8.0 (Table 2). The instability index of most PmGASA proteins are higher than 40, except for PmGASA1, PmGASA12, and PmGASA15 (Table 2). The aliphatic index of PmGASAs ranged from 28.67 to 87.72, which is approximately equivalent to that of GASA proteins in other plant species [3]. The GRAVY values of all PmGASAs are below −0.018, indicating that all PmGASA proteins are hydrophilic (Table 2). The subcellular localization of PmGASA genes was predicted to be in the cellular nucleus and golgiosome (Table 2). In the 3D structure model of PmGASA proteins, they are predicted to possess random coils. However, we observed no transmembrane helix on PmGASA proteins.
Table 2.
Protein physiochemical analysis of GASA family genes identified in P. mume.
| Gene_Name | Protein Length (aa) | MW (Da) | pI | Instability Index | Aliphatic Index | GRAVY | Subcellular Prediction |
|---|---|---|---|---|---|---|---|
| PmGASA1 | 88 | 9613.29 | 8.58 | 35.77 | 59.77 | −0.064 | Golgi; Nucleus |
| PmGASA2 | 109 | 11,962.01 | 8.28 | 83.81 | 65.23 | −0.353 | Nucleus |
| PmGASA3 | 120 | 12,321.77 | 8.9 | 50.46 | 28.67 | −0.911 | Nucleus |
| PmGASA4 | 95 | 10,646.52 | 8.61 | 56.36 | 56.53 | −0.28 | Golgi |
| PmGASA5 | 94 | 10,595.56 | 9.09 | 43.35 | 61.17 | −0.181 | Golgi |
| PmGASA6 | 219 | 23,383.3 | 10 | 72.24 | 87.72 | −0.058 | Nucleus |
| PmGASA7 | 108 | 11,732.75 | 9.26 | 42.49 | 47.13 | −0.276 | Golgi |
| PmGASA8 | 108 | 12,081.8 | 8.12 | 76.86 | 44.26 | −0.867 | Golgi; Nucleus |
| PmGASA9 | 183 | 20,356.95 | 6.06 | 46.45 | 62.4 | −0.426 | Nucleus |
| PmGASA10 | 113 | 12,489.76 | 9.58 | 57.15 | 62.3 | −0.358 | Golgi |
| PmGASA11 | 122 | 13,454.97 | 9.16 | 43.61 | 79.02 | −0.018 | Nucleus |
| PmGASA12 | 107 | 11,856.05 | 9.11 | 39.51 | 57.48 | −0.176 | Golgi |
| PmGASA13 | 116 | 12,872.11 | 9.11 | 40.8 | 55.52 | −0.342 | Nucleus |
| PmGASA14 | 108 | 11,611.52 | 8.92 | 61.23 | 53.43 | −0.188 | Golgi |
| PmGASA15 | 112 | 12,392.59 | 9.02 | 39.73 | 58.39 | −0.178 | Golgi |
| PmGASA16 | 106 | 11,785.77 | 8.89 | 44.91 | 71.7 | −0.163 | Golgi; Nucleus |
2.4. Phylogenetic Analysis of GASA Family Genes
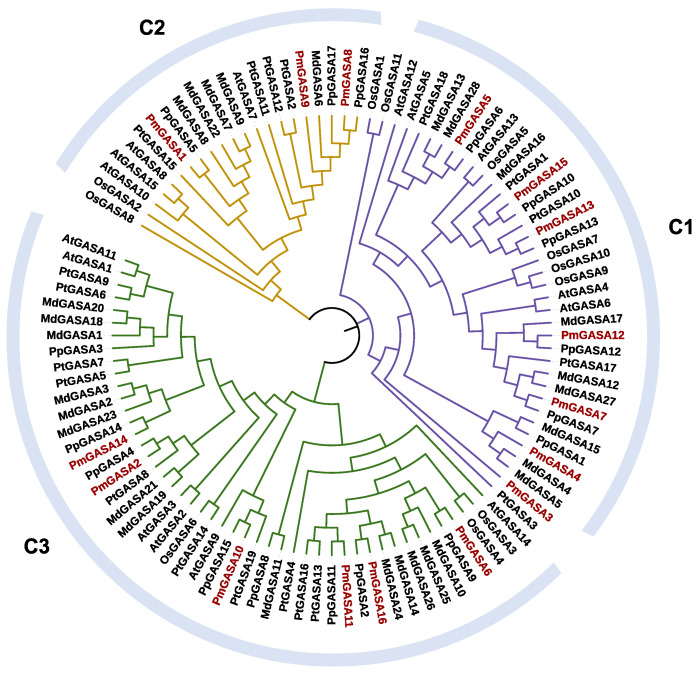
To study the phylogeny of GASA family genes in P. mume, we constructed the phylogenetic tree for 106 GASA proteins from A. thaliana, O. sativa, M. domestica, P. mume, P. persica, and Populus tricocarpa (Figure 3). The GASA family genes can be divided into three major clades, with Clade 1 containing 38 genes, Clade 2 containing 21 genes, and Clade 3 containing 47 genes (Figure 3). All three clades have genes from six species. Among 16 PmGASA genes, PmGASA3, PmGASA4, PmGASA5, PmGASA7, PmGASA12, PmGASA13, and PmGASA15 were clustered with AtGASA4, AtGASA5, AtGASA6, AtGASA12, AtGASA13 into Clade 1 (Figure 3). Three PmGASA genes (PmGASA1, PmGASA8, and PmGASA9) fell into Clade 2 with AtGASA7, AtGASA8, AtGASA10, and AtGASA15 (Figure 3). The remaining six PmGASA genes were clustered into Clade 3 with Arabidopsis orthologs AtGASA1, AtGASA2, AtGASA3, AtGASA9, AtGASA11, and AtGASA14 (Figure 3). Within each subgroup, orthologous genes of P. mume were first clustered with P. persica, then with M. domestica, and with Populus tricocarpa, Arabidopsis, and finally with O. sativa (Figure 3). OsGASA proteins were clearly separated from their GASA orthologs in other dicotyledonous species (Figure 3). Among all species, the number of GASA family genes in M. domestica is the highest within each clade, which is almost twice that in Arabidopsis and in P. mume.
Figure 3.
Phylogenetic analysis of 106 GASA family genes from six plant species based on their protein alignment. The phylogenetic tree was constructed using the NJ method, with 1000 bootstrap replicates in MEGA. The GASA genes were clustered into three clades (C1, C2, C3), each one colored differently.
2.5. Evolutionary Analysis of GASA Family Genes in P. mume and Other Species
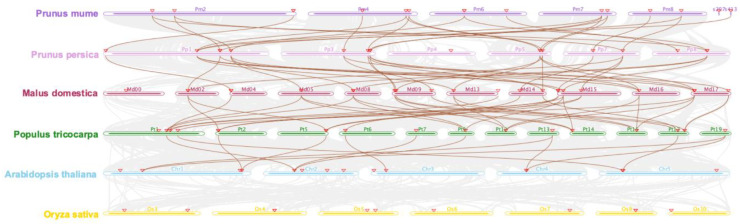
To characterize the evolutionary mechanism of GASA family genes, we conducted all-against-all BLASTP and identified collinearity blocks across the genomes of six plant species. In general, we observed extensive genome synteny between P. persica and P. mume, P. persica and M. domestica, and Populus tricocarpa and A.thaliana (Figure 4). However, the synteny is less abundant between genomes of A. thaliana and O. sativa. In our study, we identified 13 PmGASA genes in collinearity with 13 PpGASAs, which are located within the syntenic regions with 18 orthologs from M. domestica (Figure 4). In A. thaliana, only seven GASAs have orthologous gene pairs in Populus tricocarpa (Figure 4). However, we detected no corresponding collinear GASA orthologs between A. thaliana and O. sativa.
Figure 4.
Synteny analysis of GASA genes between Arabidopsis thaliana, Malus domestica, Prunus mume, Prunus persica, Populus tricocarpa, and Oryza sativa. Red triangle boxes highlight the GASA family genes. The grey shade highlights syntenic regions between genomes, and the colored lines represent colinear GASA gene pairs.
We also analyzed the duplication events of GASA family genes among six species (Table S3). Dispersed duplications, tandem duplications, and WGD/segmental duplications are three gene duplication modes mainly involved in the evolution of the GASA family genes among dicotyledonous plant species (Table S3). Proximal duplications were only reported in MdGASA26, MdGASA9, and PpGASA17 (Table S3). In O. sativa, dispersed and singleton duplications are two major types of duplications responsible for GASA gene family expansion (Table S3). In P. mume, a tandem duplication event should have generated the gene cluster containing PmGASA3 and PmGASA4 on chromosome 2 (Table S3). On the other hand, WGD or segmental duplications were detected across different chromosomes, generating four gene pairs including PmGASA1–PmGASA8, PmGASA2–PmGASA14, PmGASA6–PmGASA11, and PmGASA7–PmGASA12 (Figure S3 and Table S3). KaKs tests were conducted on these five duplicated PmGASA gene pairs (Table 3). All five gene pairs have Ka/Ks ratios of less than 1, which suggests that the duplicated PmGASA gene pairs have undergone strong purifying selection in the course of evolution (Table 3). To understand the adaptive selection pressure on PmGASA proteins, we also performed selection tests on three gene clades separately using software Selecton. The Clade 1 and Clade 3 genes all showed strong signs of purifying selection among most amino acid sites, indicating the conserved protein evolution (Figure S4). In contrast, genes within PmGASA Clade 3 revealed a signature of positive selection on 41 residues, while the rest of the amino acids were under purifying selection (with PmGASA9 as reference gene) (Figure S4).
Table 3.
The Ka/Ks ratios of paralogous gene pairs of PmGASA gene family.
| Gene 1 | Gene 2 | Ka | Ks | Ka/Ks | Selection Pressure | Gene Duplications |
|---|---|---|---|---|---|---|
| PmGASA3 | PmGASA4 | 0.36 | 1.17 | 0.31 | Purifying | Tandem |
| PmGASA1 | PmGASA8 | 0.39 | 8.17 | 0.05 | Purifying | WGD or Segmental |
| PmGASA2 | PmGASA14 | 0.41 | 1.67 | 0.24 | Purifying | WGD or Segmental |
| PmGASA6 | PmGASA11 | 0.43 | 3.39 | 0.13 | Purifying | WGD or Segmental |
| PmGASA7 | PmGASA12 | 0.16 | 0.92 | 0.18 | Purifying | WGD or Segmental |
2.6. Promoter Analysis of PmGASA Genes and Their Possible Activators
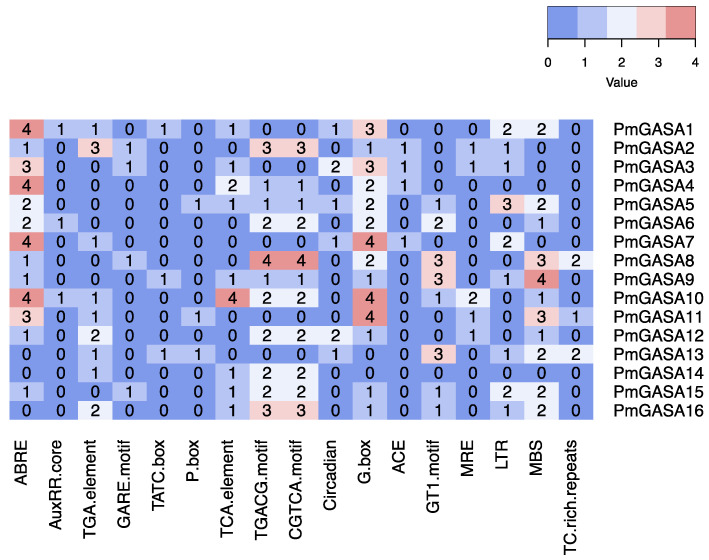
We first analyzed the cis-regulatory elements within the promoter regions of PmGASA genes (Figure 5 and Table S4). We detected a number of hormone responsive elements. For example, the ABA (abscisic acid) responsive element ABRE, the auxin responsive elements (AuxRR-core and TGA-element), the gibberellin responsive elements (GARE-motif, TATC-box, and P-box), the salicylic acid (SA) responsive TCA-element, and the Methyl Jasmona (MeJA) responsive elements (TGACG-motif and CGTCA-motif) were identified (Figure 5 and Table S4). Among the PmGASAs, the gibberellin responsive elements were absent from PmGASA4, PmGASA6, PmGASA7, PmGASA10, PmGASA12, PmGASA14, and PmGASA16 promoters (Figure 5). The ABRE element was abundantly detected within most PmGASA promoters, except for PmGASA13, PmGASA14, and PmGASA16 (Figure 5). Moreover, we identified abiotic-stress related elements, such as light responsive cis-elements (G-box, ACE motif, GT1-motif, and MRE motif), LTR (low-temperature responsiveness), MBS (drought-inducibility), and TC-rich repeats (defense and stress responsiveness) (Figure 5 and Table S4).
Figure 5.
Analysis of cis-regulatory elements within PmGASA gene promoters. The numerical values and colors represent the number of cis-elements identified within each PmGASA gene.
We also inferred the possible transcriptional regulatory network of PmGASAs using the online tool PlantRegMap (Figure S5). The predicted transcription factor (TFs) acting upstream of PmGASA genes included the AREB (ABA-RESPONSIVE ELEMENT BINDING PROTEIN) transcription factors, the ARF (AUXIN RESPONSE FACTOR) family transcription factors, the bZIP (BASIC LEUCINE-ZIPPER) transcription factors, and the MYB family transcription factors (Figure S5). Among the TFs, the bZIP transcription factors, such as bZIP16, bZIP42, bZIP44, bZIP53, and bZIP63 are putative activators of PmGASA12. Additionally, a MADS-box transcription factor SOC1 (SUPPRESSOR OF OVEREXPRESSION OF CO 1) is predicted to target PmGASA7, PmGASA9, PmGASA13, PmGASA14, PmGASA15, and PmGASA16 in P. mume (Figure S5). We also detected a few hormone responsive transcription factors. For example, RGA1 (REPRESSOR OF GA1), a transcriptional regulator acting on upstream PmGASA1, PmGASA13, PmGASA14, PmGASA15, and PmGASA16 represses gibberellin responses (Figure S5). The ABA induced transcription factors, including ABF2 (ABSCISIC ACID RESPONSIVE ELEMENTS-BINDING FACTOR 2), ABI5 (ABA INSENSITIVE 5), and AREB3 (ABSCISIC ACID RESPONSIVE ELEMENTS-BINDING POTEIN 3), are predicted to bind the ABRE elements within the promoters of PmGASA7 and PmGASA12 (Figure S5). In addition, DOF (DNA BINDING WITH ONE FINGER), OBP (OBF-binding protein), and BPCs (BASIC PENTACYSTEINE) family TFs were also predicted to be the transcriptional activators for PmGASA3, PmGASA7, PmGASA9, PmGASA13, PmGASA14, and PmGASA15.
2.7. Tissue-Specific Expression Patterns of PmGASA Genes
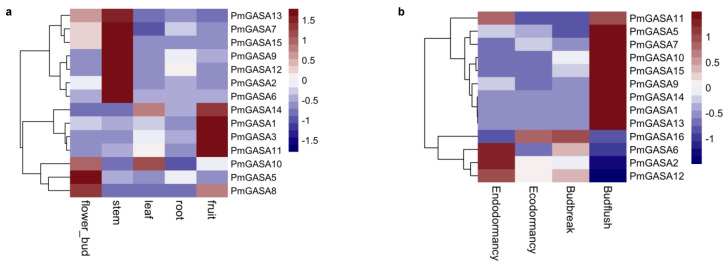
We investigated the expression patterns of PmGASA genes across different organ tissues of P. mume (Figure 6a). Among all genes, PmGASA5, PmGASA8, and PmGASA10 were preferentially high-expressed in flower bud tissues (Figure 6a). PmGASA1, PmGASA3, PmGASA11, and PmGASA14 were relatively high-expressed in fruits rather than other organs (Figure 6a). PmGASA2, PmGASA6, PmGASA7, PmGASA9, PmGASA12, PmGASA13, and PmGASA15 were more high-expressed in stems (Figure 6a). PmGASA7, PmGASA13, and PmGASA15 were also expressed in flower bud tissues (Figure 6a). All PmGASA genes were relatively low-expressed in root tissues (Figure 6a).
Figure 6.
Expression analysis of PmGASA genes across different organs and during floral bud development in P. mume. (a) Expression profile of PmGASAs in flower bud, leaf, root, and fruit tissues. (b) Expression profile of PmGASAs during four developmental stages of floral bud break. Hierarchical clustering was used to compare the gene expression levels of PmGASAs across different samples, with highly expressed genes shown in red and weakly expressed in blue.
2.8. Expression Patterns of PmGASA Genes during Floral Bud Development
To understand the functional role of PmGASA genes in floral bud development, we analyzed the expression levels of PmGASAs during the flower bud break process (Figure 6b). The PmGASA genes can be clustered into three major groups based on their expression profiles. PmGASA1, PmGASA5, PmGASA7, PmGASA9, PmGASA10, PmGASA11, PmGASA13, PmGASA14, and PmGASA15 were highly induced during the flower bud flushing stage (Figure 6b). The transcription levels of PmGASA2, PmGASA6, and PmGASA12 were relatively high in the endodormant floral bud, and then significantly dropped after flower buds exited dormancy (Figure 6b). PmGASA16 was strongly induced after endodormancy release, but was then found to be repressed in flushed flower buds (Figure 6b).
2.9. Expression Patterns of PmGASA Genes in Response to Gibberellin Treatments
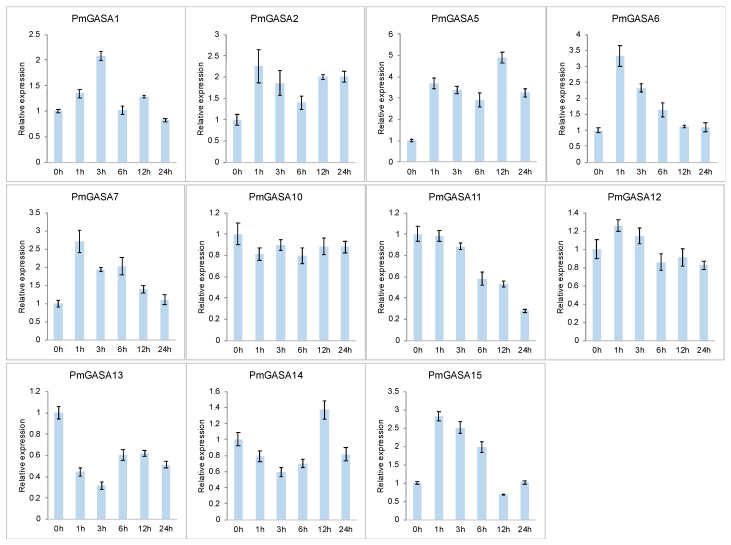
To further explore the function of GASA genes during gibberellin responses, we first investigated the expression patterns of PmGASA genes in leaves after hormone treatment (Figure 7). It was shown that PmGASA1, PmGASA2, PmGASA5, PmGASA6, PmGASA7, and PmGASA15 were strongly induced within one hour after the GA treatment (Figure 7). Among them, PmGASA6 and PmGASA7 showed the highest up-regulation within one hour after GA treatment, approximately three-fold (Figure 7). On the contrary, we observed that PmGASA11, PmGASA13, and PmGASA14 were firstly down-regulated within a short period of time in response to gibberellin treatment (Figure 7). The expression of PmGASA11 maintained a constant level within the first three hours, but then dropped after six hours, while the transcript levels of PmGASA13 and PmGASA14 increased slightly 12 hours after gibberellin treatment (Figure 7).
Figure 7.
Relative expression levels of PmGASAs in leaves over two days, after gibberellin treatment. Error bars represent the standard deviations assessed from three technical replicates.
To assess the effects of GA treatment on PmGASA genes in floral bud, we also analyzed the expression patterns of PmGASA in flower buds treated with GA3. The transcription of PmGASA1, PmGASA2, PmGASA5, PmGASA6, PmGASA7, and PmGASA11 was highly induced in flower buds within three hours after GA treatment and was found slowly repressed after six hours (Figure S6). Meanwhile, the expression levels of PmGASA10 and PmGASA15 first dropped, but were slightly increased three hours after GA treatment (Figure S6). PmGASA12 and PmGASA13, on the other hand, were repressed at all time after GA treatment in the flower buds of P. mume (Figure S6). However, the rest of the PmGASA genes, such as PmGASA4, PmGASA9, and PmGASA16 were barely detected in the GA-treated leaves or floral buds.
2.10. Expression Patterns of PmGASA Genes in Response to ABA and IAA Treatments
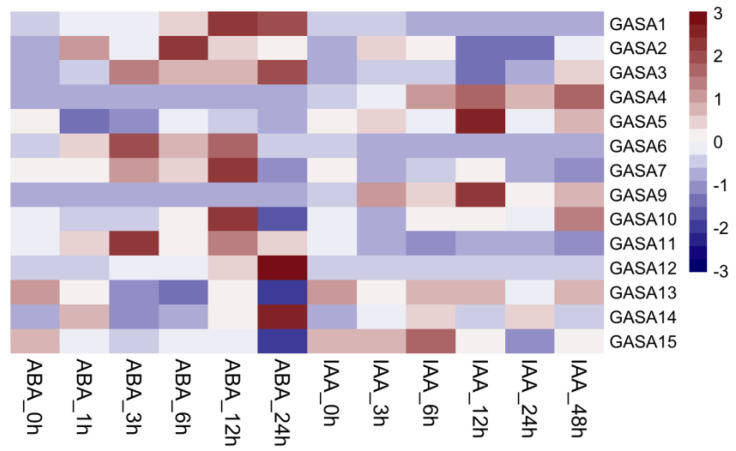
To test the involvement of PmGASA genes in the signaling transduction of other phytohormones, we analyzed the expression profile of GASA genes in leaves treated with exogenous ABA and IAA (Figure 8). Upon the application of ABA, the expression levels of PmGASA1, PmGASA2, PmGASA6, PmGASA11, PmGASA12, and PmGASA14 were instantly up-regulated, while PmGASA5, PmGASA13, and PmGASA15 were down-regulated within a few hours and were then slightly increased (Figure 8). After the treatment of IAA, PmGASA1 and PmGASA2 were strongly induced within one hour, but PmGASA4, PmGASA14, and PmGASA15 displayed slowly increasing expression patterns during the first 12 h (Figure 8). On the other hand, PmGASA6, PmGASA11, PmGASA7, and PmGASA12 were down-regulated after IAA treatment (Figure 8). However, the expression levels of PmGASA7 and PmGASA12 were strongly increased approximately at 12 h after the IAA treatment (Figure 8).
Figure 8.
Expression analysis of PmGASAs in response to ABA and IAA treatments. The heatmap displayed the average relative expression levels of PmGASAs from the qRT-PCR assays.
3. Discussion
Gibberellins are a class of phytohormones involved in many important plant developmental processes. GASA/GAST is a multi-gene family regulated by gibberellin acid and encode small cysteine-rich peptides that are implicated in regulating plant growth and development [1]. The GASA family gene, GAST1, was firstly discovered in tomato (Solanum lycopersicum) as a GA-stimulated protein [6]. So far, GASA gene members have been identified in a wide range of plant species, such as Arabidopsis thaliana [21], soybean (Glycine max) [30], cotton (Gossypium hirsutum) [31,32], potato (Solanum tuberosum) [33], strawberry (Fragaria × ananassa) [15], rice (Oryza sativa) [2,8], and grapevine (Vitis vinifera) [34] etc. GASA family genes were reported to regulate seed germination [23], the development of lateral root [35], flower organ formation [16], fruit development [15], stem growth and flowering time [13], and environmental stress responses [24,36].
Despite previous studies of GASA family genes in different plant species, their functional roles in perennial tree species have not been fully uncovered. In poplar tree, PeuGASAs were found to be induced or inhibited by different hormones, including ABA, SA, and MeJA, and were responsive to drought, mechanical damage, and other abiotic stresses [10]. MdGASA genes were also found responsive to GA and ABA treatment and were potentially related to flower induction in apple (Malus domestica) [18]. In white pear (Pyrus pyrifolia), PpyGAST1 potentially regulates bud dormancy by integrating GA and ABA signaling [19]. To obtain a deeper understanding of the structure and function of GASA members in woody perennials, we comprehensively investigated the phylogenetic relationship, exon-intron structure, protein motif, and cis-element analysis of GASA family genes. We also analyzed the molecular evolution of GASA genes and explored their functional roles in P. mume, combining transcriptional regulatory network prediction and expression analysis.
In our study, we identified 16 GASA protein encoding genes in P. mume and 90 GASA genes from the other five species (A. thaliana, M. domestica, P. mume, P. persica, Populus tricocarpa, and O. sativa). The gene number of GASA gene family is approximately even among species, except M. domestica. We detected 28 MdGASA genes in M. domestica, approximately two-fold of that in Prunus species. This is likely due to a whole genome duplication event occurring in Maleae species after splitting from Prunus [37]. By examining the molecular structure of PmGASAs, we observed relatively consistent exon-intron placement and protein motifs among GASA genes, which is in compliment with that of GASA genes in other tree species [10,18]. The slight structural variation among GASA genes is likely due to the gain or loss of exons/introns during chromosomal evolution [30]. PmGASA proteins consist of 88 to 219 amino acids, which is similar to the AtGASA proteins of 80–275 amino acid residues [21]. The GASA domain within most PmGASA proteins possessed the featured cys-motif of twelve cysteine residues, which is essential to maintain the spatial structure and function of GASA proteins [20]. The cysteine residues are required for the formation of disulfide bonds, which are critical for GASA protein folding and interaction with other proteins [38,39]. The PmGASA proteins were predicted to be hydrophilic and mostly unstable, except for PmGASA1, PmGASA12, and PmGASA15. The subcellular localization of PmGASA proteins is in the nucleus or golgiosome. In previous studies, the GASA proteins were detected in the plasma membrane, cell wall, cytoplasm, nuclei, or extracellular space in different plant species [8,13,24]. Despite the presence of an N-terminal signal peptide, post-translational modifications, protein-protein interaction, and covalent bonds to lipids may be responsible for the proper localization of PmGASA proteins [8,13,24].
The phylogenetic analysis revealed that the GASA gene members of six plant species are clustered into three distinct clades, which is commonly observed in angiosperms [23]. The PmGASAs are grouped with GASA family members from P. persica, then with other dicotyledonous species, including M. domestica, Populus tricocarpa and A. thaliana, and finally with the monocotyledonous species O. sativa. The GASA gene phylogeny coincides with the species phylogenic tree [40]. To understand the evolutionary trajectory of GASA family genes, we inferred the gene duplication events of GASA family genes among five plant species. In dicotyledonous species, dispersed duplications, tandem duplications, and WGD or segmental duplications have played a dominant role during the GASA gene family evolution. Dispersed and singleton duplications, however, are two predominant duplication modes observed in monocotyledonous species. As for P. mume, the WGD/segmental duplications generated four gene pairs, while tandem duplications generated one gene pair. The Ka/Ks ratio between the paralogous genes of five PmGASA gene pairs were all less than 1, suggesting that the duplicated gene pairs are under strong purifying selection restraining their coding sequence variation. With protein selection analysis, we observed strong purifying selection acting on PmGASA proteins within Clade 1 and Clade 3 [30]. However, adaptive selection pressure was detected at a few specific residues for PmGASA proteins in Clade 2, indicating the selected amino acids may be important for the functional divergence of proteins within Clade 2 [41]. The extensive colinear relationship among GASA genes of dicotyledonous species has also been observed in previous studies [3]. However, the lack of colinear GASA gene pairs between A. thaliana and O. sativa may suggest rounds of chromosomal rearrangements during the independent evolution of O. sativa after monocotyledonous plant speciation.
The promoter analysis revealed many hormonal responsive cis-acting elements within PmGASA promoters. We detected abundant ABA responsive ABRE-element, auxin responsive elements (AuxRR-core/TGA), GA responsive elements (GARE/TATC-box/P-box), SA responsive elements (TCA), and MeJA responsive elements (TGACG/CGTCA) present within the promoter region of different PmGASA members. Among them, all three types of elements (GA, ABA, and IAA responsive elements) were present in only PmGASA1 and PmGASA11 at the same time. The expression analysis confirmed the results that PmGASA1 and PmGASA11 were responsive to exogenous treatments of IAA, GA, and ABA. The RT-PCR assays revealed GA inducibility in most PmGASA genes. Interestingly, PmGASA6, PmGASA7, and PmGASA14, lacking GA responsive element within their promoters, also displayed induced or repressed expression patterns within three hours after GA treatment. It is possible that these PmGASA genes are regulated indirectly by GA responsive regulators. In addition to PmGASA1 and PmGASA11, the expression patterns of PmGASA2, PmGASA5, PmGASA6, PmGASA7, PmGASA13, PmGASA14, and PmGASA15 all displayed responsiveness to exogenous GA, ABA, and IAA treatments, indicating their possible role in integrating the gibberellin acid-, abscisic acid- and auxin-signaling pathways in P. mume.
The transcription regulatory network analysis of PmGASA family genes also predicted a number of TFs related to hormonal signaling. For example, RGA1 encodes a GARS family transcription factor that may act upstream of PmGASA1, PmGASA13, PmGASA14, PmGASA15, and PmGASA16 in repressing gibberellin responses. ABF2 [42], ABI5 [43], and AREB3 [43] are three transcription factors known to regulate the ABA-mediated seed germination, development, and maturation in Arabidopsis [42,43]. These three TFs may regulate PmGASA7 and PmGASA12 transcription through binding to ABRE-motifs during ABA signaling pathways in P. mume. ARFs is a family of transcription factors that specifically binds to the 5′-TGTCTC-3′ element of auxin-responsive genes to regulate early auxin responses [44]. ARF10 and ARF16 were predicted to regulate the transcription of PmGASA7 through binding to its AuxRR-core motifs in P. mume. These results suggested that PmGASA genes are possibly involved in a wide range of plant hormone signal transduction networks. Additionally, the abiotic stress responsive cis-elements were detected within PmGASA promoters, indicating the possible involvement of PmGASA genes in relevant biological processes.
In addition to hormonal responses, GASA genes are also known to play important roles in plant organ growth and development. To further elucidate the functional role of PmGASA genes, we examined the expression patterns of GASA genes across different plant organs. The PmGASA genes displayed distinct spatial expression patterns across stem, leaf, root, fruit, and flower bud tissues. PmGASA5, PmGASA8, and PmGASA10 were specifically expressed in flowers; PmGASA1, PmGASA3, PmGASA11, and PmGASA14 were relatively high-expressed in fruits; and PmGASA2, PmGASA6, PmGASA7, PmGASA9, PmGASA12, PmGASA13, and PmGASA15 were mostly transcribed in stems. We failed to detect the transcript of PmGASA4 and PmGASA16 in any of the five plant organs. The divergent tissue-specific expression patterns of PmGASAs may suggest their functional divergence in regulating the development and formation of different organs in P. mume.
Bud dormancy cycling is an adaptive process crucial to the survival of temperate tree species during harsh winters [45]. Perennial tree species usually enter endodormancy upon exposure to short day or low temperatures after growth cessation and bud set [46]. When buds accumulate sufficient chilling, endodormancy is released and trees enter ecodormancy [47]. Gibberellic acid is a class of plant hormones involved in regulating bud dormancy establishment and dormancy release in perennial trees [48]. During endodormancy release, the long-term chilling induces GA biosynthesis, which mediates the transcription of FT and promotes bud flush [49]. Induced by gibberellins, GASA family genes may play important roles in regulating hormonal signal transduction during bud dormancy cycling. In white pear (Pyrus pyrifolia), PpyGAST1 is highly induced during endodormancy release [19]. The over-expression of PpyGAST1 in Arabidopsis leads to early seed germination through up-regulating GA biosynthetic genes AtGA20OX2 and AtGA3OX1, and the downstream AtEXPA1, indicating the functional role of PpyGAST1 in promoting dormancy release [19]. However, whether GASA gene members are involved in regulating floral bud flush and dormancy release in other perennial fruit trees is still unknown. Therefore, we analyzed the PmGASA expression patterns during floral bud development. We observed strong induction of PmGASA16 after endodormancy exit in floral buds, indicating its possible role in promoting the endodormancy break in P. mume. We also observed significant up-regulation of PmGASA1, PmGASA5, PmGASA7, PmGASA9, PmGASA10, PmGASA11, PmGASA13, PmGASA14, and PmGASA15 during flower bud flush. These PmGASA genes are likely regulated by hormonal level change or may be responsible for modulating related hormonal responses during floral organ development and bud flushing in P. mume [50]. The repression of PmGASA2, PmGASA6, and PmGASA12 during the whole process of floral bud break may be required for the complex developmental process occurring in the flushing flower bud.
4. Materials and Methods
4.1. Identification of GASA Genes in Six Plant Species
We first obtained the genomes of Arabidopsis thaliana, Malus domestica, Prunus mume, Prunus persica, Populus tricocarpa, and Oryza sativa from public datasets including TAIR (http://www.arabidopsis.org), GDR (https://www.rosaceae.org), and Phytozome (https://phytozome-next.jgi.doe.go) (accessed all databases on 24 June 2022). To identify GASA family genes in each species, we used the Hidden Markov Model of the GASA domain (PF02704) as a query to search for all putative GASA proteins using the HMMER v3.3.2 software (https://www.ebi.ac.uk/Tools/hmmer/; accessed on 4 September 2022) [51]. On the other hand, the protein sequences of 15 AtGASA genes were used to blast against the genomes of five other species (M. domestica, P. mume, P. persica, Populus tricocarpa, and O. sativa), and the top hits with e-value ≤ 1.0 × 10−10 were considered as candidate genes. By combining all GASA candidate genes identified using HMMER and BLASTP approaches, we verified the presence and completeness of the GASA domain in each GASA protein using NCBI CDD search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi; accessed on 24 June 2022) and the Simple Modular Architecture Research Tool (SMART: http://smart.embl.de/; accessed on 24 June 2022) [40]. Only the non-redundant genes with complete GASA domains were included in the following analysis.
4.2. Physicochemical Property Analysis of PmGASA Proteins
The physicochemical characteristics of PmGASA proteins were analyzed using the ExPASy online tool (http://web.expasy.org/protparam/; accessed on 3 July 2022), including the isoelectric point (pI), number of amino acids, and molecular weight (MW) [52]. The protein tertiary structures and transmembrane helices were predicted using the PHYRE2 program (https://www.genscript.com/wolf-psort.html; accessed on 3 July 2022) and TMHMM server v2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/; accessed on 3 July 2022). Finally, the protein subcellular locations were predicted using the Plant-mPLoc program (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/; accessed on 3 July 2022) [53].
4.3. Gene Structure and Protein Motif Analysis of PmGASA Genes
The genome annotation information was retrieved for PmGASA family genes. The exon/intron arrangement and chromosome locations of PmGASA genes were visualized using TBtools software [54]. The conserved protein motifs of PmGASAs were analyzed using MEME (https://meme-suite.org/meme/tools/meme; accessed on 4 July 2022) [55] with their sequences generated and visualized using WebLogo platform (http://weblogo.berkeley.edu/logo.cgi; accessed on 4 July 2022) [56]. All genes were renamed according to their positions across chromosomes.
4.4. Phylogenetic Analysis of GASA Family Genes
Multiple sequence alignment was performed by aligning the whole protein sequences of the GASA family genes with ClustalW within the MEGA 11.0 software (www.megasoftware.net) [57]. Subsequently, a phylogenetic tree of GASA family genes among A. thaliana, M. domestica, P. mume, P. persica, Populus tricocarpa, and O. sativa was constructed with MEGA 11.0 using the neighbor-joining (NJ) method with 1000 bootstrap replicates. Similarly, the phylogenetic tree of PmGASAs was built following the same protocol. The phylogenetic tree was finally visualized using the iTOL online tool (http://itol2.embl.de; accessed on 5 July 2022). We also extracted the amino acids within the GASA domains of PmGASA proteins and aligned them with ClustalW. The amino acid sequence alignment was finally visualized using GeneDoc software [58].
4.5. Microsynteny and Selection Analysis of GASA Family Genes
To identify the syntenic relationship among GASA family genes, we first performed all-against-all BLASTP among genomes of six investigated plant species. Based on the top BLASTP hits (e-value < 1.0 × 10−10), we detected the GASA gene pairs located within the colinear genome blocks across species using MCScanX [59]. The duplicated GASA family genes were detected based on the self-blast approach, followed by duplication event detection using ‘duplicate_gene_classifier’ implemented in MCscanX [59]. The intra-species and inter-species synteny among GASA family genes were visualized using the software TBtools [54]. Non-synonymous (Ka) and synonymous (Ks) substitution ratios were calculated for duplicated PmGASA gene pairs by aligning their protein sequences using ClustalW followed by the PAL2NAL program (http://www.bork.embl.de/pal2nal/; accessed on 6 August 2022). The mode of selection (positive, purifying, or neutral) acting on the duplicated gene pairs was determined with Ka/Ks ratio >1, <1, or =1, respectively. We also evaluated the selection pressure among codons of PmGASA genes with the software Selecton (http://selecton.tau.ac.il; accessed on 6 August 2022) [60].
4.6. Cis-Acting Element Analysis of PmGASA Genes
The promoter sequences of PmGASA genes were extracted as the 2kb sequence upstream of the start codon for each gene based on the genome sequences of P. mume. The cis-elements were predicted and analyzed using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; accessed on 24 July 2022). The cis-regulatory elements related to hormonal responses and abiotic stress were further classified.
4.7. Tissue Specific Expression of PmGASA Genes
To understand the expression pattern of PmGASA genes across different plant organs, we obtained the tissue transcriptome data of P. mume (GSE4760162) from the NCBI SRA database [26]. Raw sequencing reads were preprocessed with Trimmomatic software, followed by aligning clean-paired reads to their corresponding reference genomes using HISAT2 [40]. The FPKM values were obtained for GASA genes and were compared across different tissues using the R package ‘edgeR’ [61]. The expression patterns were visualized using the ‘pheatmap’ package in R. Hierarchical clustering was performed on expression profiles of PmGASAs based on the Euclidean distance matrix using ‘hclust’ in R (with agglomeration method set as ‘ward.D’).
4.8. Expression Pattern of PmGASA Genes during Floral Bud Development
To investigate the functional roles of PmGASAs during floral bud break, we retrieved the RNA-seq data of flower buds at four developmental stages, including endodormancy, ecodormancy, bud break, and bud flush [62]. The raw data were cleaned, analyzed, and normalized to FPKM values following the pipeline described above. The expression profiles of PmGASAs during the floral bud development process were obtained and visualized with heatmap using R.
4.9. Plant Materials and qRT-PCR Analysis of PmGASA Genes in Gibberellin Treatment
A five-year-old P. mume tree cultivated at Beijing Forestry University (China) was used in this study. We performed hormonal treatments by applying 100 mM GA3, 100 mM IAA, or 300 mM ABA to newly sprouted leaves and dormant floral buds. The flower buds of ecodormant stage and young leaves from current-year branches were mixed and collected at 0, 1, 3, 6, 12, 24, and 48 h after hormonal treatments. Samples were immediately frozen with liquid nitrogen and stored at −80 °C.
Total RNA was extracted from plant tissue samples with a Plant RNA Kit (Omega Bio-tek, Norcross, GA, USA) following the manufacturer’s protocols, and the integrity was verified using 1% agarose gel electrophoresis. The cDNA was synthesized using the PrimeScript RT reagent kit (Takara, Kusatsu, Japan). The relative expression of GASA genes was assessed with qRT-PCR assays, with three technical replicates with standard deviations assessed. The qRT-PCR experiments were performed on the PikoReal real-time PCR platform (Thermo Fisher Scientific, Waltham, MA, USA) with temperature settings: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 30 s. The protein phosphatase 2A (PP2A) gene was used as an internal reference to calculate the relative gene expression levels using the 2−ΔΔCt method [29]. The primers used for qRT-PCR experiments are provided in Table S1.
4.10. Transcriptional Regulatory Network Prediction of PmGASAs
Putative transcription factors (TFs) regulating PmGASA genes were predicted using the Plant Transcriptional Regulatory Map (PTRM) website (http://plantregmap.gao-lab.org/regulation_prediction.php; accessed on 18 August 2020) at the threshold p-value ≤ 1 × 10−6 with PmGASA gene promoter sequences as input [63]. The TF-gene regulatory network was visualized using Cytoscape software [64].
5. Conclusions
In our study, we identified a total of 16 PmGASA genes in the P. mume genome and analyzed their chromosomal location, gene structure, protein physicochemical characteristics, and conserved motifs. The phylogenetic analysis revealed three major gene clades among PmGASAs. With promoter analysis, we identified various cis-acting regulatory elements and constructed the putative transcriptional regulatory networks involving PmGASAs. By combing synteny analysis and selection analysis, we inferred the expansion and evolutionary trajectory of the GASA family genes in P. mume. Furthermore, we analyzed the spatial and temporal expression patterns of PmGASA genes across different plant organs, during floral bud break and hormonal responses. Overall, these findings suggested the possible involvement of PmGASAs in mediating floral bud break and hormonal signaling in P. mume. Our study provided insights into the evolution and functional implication of GASA family genes in P. mume and has laid theoretical basis for future studies exploring the molecular mechanism of GASA genes in other woody perennial trees.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231810923/s1.
Author Contributions
Conceptualization, M.Z. and Q.Z.; methodology, M.Z.; validation, W.C. and M.Z.; formal analysis, M.Z.; investigation, M.Z.; resources, T.C.; data curation, W.C.; writing—original draft preparation, M.Z.; writing—review and editing, Q.Z.; visualization, W.C.; supervision, Q.Z.; project administration, J.W.; funding acquisition, M.Z. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used in this study are publicly available. All analyzed data can be found in the article or in the supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Fundamental Research Funds for the Central Universities (2021ZY44), National Natural Science Foundation of China (31902045), and the Special Fund for Beijing Common Construction Project.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar A., Singh A., Kumar P., Sarkar A.K. Giberellic Acid-Stimulated Transcript Proteins Evolved through Successive Conjugation of Novel Motifs and Their Subfunctionalization. Plant Physiol. 2019;180:998–1012. doi: 10.1104/pp.19.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L., Wang Z., Xu Y., Joo S.H., Kim S.K., Xue Z., Xu Z., Wang Z., Chong K. OsGSR1 is involved in crosstalk between gibberellins and brassinosteroids in rice. Plant J. 2009;57:498–510. doi: 10.1111/j.1365-313X.2008.03707.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu T., Zhong Y., Chen M., Wu B., Wang T., Jiang B., Zhong G. Analysis of CcGASA family members in Citrus clementina (Hort. ex Tan.) by a genome-wide approach. BMC Plant Biol. 2021;21:565. doi: 10.1186/s12870-021-03326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tam J.P., Wang S., Wong K.H., Tan W.L. Antimicrobial Peptides from Plants. Pharmaceuticals. 2015;8:711–757. doi: 10.3390/ph8040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverstein K.A.T., Moskal W.A., Jr., Wu H.C., Underwood B.A., Graham M.A., Town C.D., VandenBosch K.A. Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant J. 2007;51:262–280. doi: 10.1111/j.1365-313X.2007.03136.x. [DOI] [PubMed] [Google Scholar]
- 6.Shi L., Gast R.T., Gopalraj M., Olszewski N.E. Characterization of a shoot-specific, GA3- and ABA-regulated gene from tomato. Plant J. 1992;2:153–159. [PubMed] [Google Scholar]
- 7.Aubert D., Chevillard M., Dorne A.M., Arlaud G., Herzog M. Expression patterns of GASA genes in Arabidopsis thaliana: The GASA4 gene is up-regulated by gibberellins in meristematic regions. Plant Mol.Biol. 1998;36:871–883. doi: 10.1023/A:1005938624418. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa T., Sakaguchi N., Shimada H. Two OsGASR genes, rice GAST homologue genes that are abundant in proliferating tissues, show different expression patterns in developing panicles. Genes Genet Syst. 2006;81:171–180. doi: 10.1266/ggs.81.171. [DOI] [PubMed] [Google Scholar]
- 9.Nahirñak V., Rivarola M., Gonzalez de Urreta M., Paniego N., Hopp H.E., Almasia N.I., Vazquez-Rovere C. Genome-wide Analysis of the Snakin/GASA Gene Family in Solanum tuberosum cv. Kennebec. Am. J. Potato Res. 2016;93:172–188. doi: 10.1007/s12230-016-9494-8. [DOI] [Google Scholar]
- 10.Han S., Jiao Z., Niu M.X., Yu X., Huang M., Liu C., Wang H.L., Zhou Y., Mao W., Wang X., et al. Genome-Wide Comprehensive Analysis of the GASA Gene Family in Populus. Int J Mol Sci. 2021;22:12336. doi: 10.3390/ijms222212336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García A.N., Ayub N.D., Fox A.R., Gómez M.C., Diéguez M.J., Pagano E.M., Berini C.A., Muschietti J.P., Soto G. Alfalfa snakin-1 prevents fungal colonization and probably coevolved with rhizobia. BMC Plant Biol. 2014;14:1–13. doi: 10.1186/s12870-014-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roxrud I., Lid S.E., Fletcher J.C., Schmidt E.D., Opsahl-Sorteberg H.G. GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol. 2007;48:471–483. doi: 10.1093/pcp/pcm016. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S., Yang C., Peng J., Sun S., Wang X. GASA5, a regulator of flowering time and stem growth in Arabidopsis thaliana. Plant Mol. Biol. 2009;69:745–759. doi: 10.1007/s11103-009-9452-7. [DOI] [PubMed] [Google Scholar]
- 14.Trapalis M., Li S.F., Parish R.W. The Arabidopsis GASA10 gene encodes a cell wall protein strongly expressed in developing anthers and seeds. Plant Sci. 2017;260:71–79. doi: 10.1016/j.plantsci.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Moyano-Cañete E., Bellido M.L., García-Caparrós N., Medina-Puche L., Amil-Ruiz F., González-Reyes J.A., Caballero J.L., Muñoz-Blanco J., Blanco-Portales R. FaGAST2, a Strawberry Ripening-Related Gene, Acts Together with FaGAST1 to Determine Cell Size of the Fruit Receptacle. Plant Cell Physiol. 2012;54:218–236. doi: 10.1093/pcp/pcs167. [DOI] [PubMed] [Google Scholar]
- 16.Kotilainen M., Helariutta Y., Mehto M., Pöllänen E., Albert V.A., Elomaa P., Teeri T.H. GEG Participates in the Regulation of Cell and Organ Shape during Corolla and Carpel Development in Gerbera hybrida. Plant Cell. 1999;11:1093–1104. doi: 10.1105/tpc.11.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han M., Jin X., Yao W., Kong L., Huang G., Tao Y., Li L., Wang X., Wang Y. A Mini Zinc-Finger Protein (MIF) from Gerbera hybrida Activates the GASA Protein Family Gene, GEG, to Inhibit Ray Petal Elongation. Front. Plant Sci. 2017;8:1649. doi: 10.3389/fpls.2017.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan S., Zhang D., Zhang L., Gao C., Xin M., Tahir M.M., Li Y., Ma J., Han M. Comprehensive analysis of GASA family members in the Malus domestica genome: Identification, characterization, and their expressions in response to apple flower induction. BMC Genom. 2017;18:827. doi: 10.1186/s12864-017-4213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q., Niu Q., Tang Y., Ma Y., Yan X., Li J., Tian J., Bai S., Teng Y. PpyGAST1 is potentially involved in bud dormancy release by integrating the GA biosynthesis and ABA signaling in ‘Suli’ pear (Pyrus pyrifolia White Pear Group) Environ. Exp. Bot. 2019;162:302–312. doi: 10.1016/j.envexpbot.2019.03.008. [DOI] [Google Scholar]
- 20.Zhang S., Wang X. One new kind of phytohormonal signaling integrator: Up-and-coming GASA family genes. Plant Signal. Behav. 2017;12:e1226453. doi: 10.1080/15592324.2016.1226453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S., Wang X. Expression pattern of GASA, downstream genes of DELLA, in Arabidopsis. Sci. Bull. 2008;53:3839–3846. doi: 10.1007/s11434-008-0525-9. [DOI] [Google Scholar]
- 22.Chen K., Liu W., Li X., Li H. Overexpression of GmGASA32 promoted soybean height by interacting with GmCDC25. Plant Signal Behav. 2021;16:1855017. doi: 10.1080/15592324.2020.1855017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong C., Xu H., Ye S., Wang S., Li L., Zhang S., Wang X. Gibberellic Acid-Stimulated Arabidopsis6 Serves as an Integrator of Gibberellin, Abscisic Acid, and Glucose Signaling during Seed Germination in Arabidopsis. Plant Physiol. 2015;169:2288–2303. doi: 10.1104/pp.15.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun S., Wang H., Yu H., Zhong C., Zhang X., Peng J., Wang X. GASA14 regulates leaf expansion and abiotic stress resistance by modulating reactive oxygen species accumulation. J. Exp. Bot. 2013;64:1637–1647. doi: 10.1093/jxb/ert021. [DOI] [PubMed] [Google Scholar]
- 25.An B., Wang Q., Zhang X., Zhang B., Luo H., He C. Comprehensive transcriptional and functional analyses of HbGASA genes reveal their roles in fungal pathogen resistance in Hevea brasiliensis. Tree Genet Genomes. 2018;14:41. doi: 10.1007/s11295-018-1256-y. [DOI] [Google Scholar]
- 26.Zhang Q., Chen W., Sun L., Zhao F., Huang B., Yang W., Tao Y., Wang J., Yuan Z., Fan G., et al. The genome of Prunus mume. Nat. Commun. 2012;3:1–8. doi: 10.1038/ncomms2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailly C. Anticancer properties of Prunus mume extracts (Chinese plum, Japanese apricot) J. Ethnopharmacol. 2020;246:112215. doi: 10.1016/j.jep.2019.112215. [DOI] [PubMed] [Google Scholar]
- 28.Gao Z., Luo W. The Prunus mume Genome. Compendium of Plant Genomes. Springer; Cham, Switzerland: 2019. Origin and Evolution of Prunus mume; pp. 5–7. [Google Scholar]
- 29.Zhang M., Yang Q., Yuan X., Yan X., Wang J., Cheng T., Zhang Q. Integrating Genome-Wide Association Analysis With Transcriptome Sequencing to Identify Candidate Genes Related to Blooming Time in Prunus mume. Front. Plant Sci. 2021;12:1226. doi: 10.3389/fpls.2021.690841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad M.Z., Sana A., Jamil A., Nasir J.A., Ahmed S., Hameed M.U., Abdullah A genome-wide approach to the comprehensive analysis of GASA gene family in Glycine max. Plant Mol. Biol. 2019;100:607–620. doi: 10.1007/s11103-019-00883-1. [DOI] [PubMed] [Google Scholar]
- 31.Qiao K., Ma C., Lv J., Zhang C., Ma Q., Fan S. Identification, characterization, and expression profiles of the GASA genes in cotton. J. Cotton Res. 2021;4:7. doi: 10.1186/s42397-021-00081-9. [DOI] [Google Scholar]
- 32.Liu Z.H., Zhu L., Shi H.Y., Chen Y., Zhang J.M., Zheng Y., Li X.B. Cotton GASL genes encoding putative gibberellin-regulated proteins are involved in response to GA signaling in fiber development. Mol. Biol. Rep. 2013;40:4561–4570. doi: 10.1007/s11033-013-2543-1. [DOI] [PubMed] [Google Scholar]
- 33.Berrocal-Lobo M., Segura A., Moreno M., López G., García-Olmedo F., Molina A. Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol. 2002;128:951–961. doi: 10.1104/pp.010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad B., Yao J., Zhang S., Li X., Zhang X., Yadav V., Wang X. Genome-Wide Characterization and Expression Profiling of GASA Genes during Different Stages of Seed Development in Grapevine (Vitis vinifera L.) Predict Their Involvement in Seed Development. Int. J. Mol. Sci. 2020;21:1088. doi: 10.3390/ijms21031088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann R., Sakai H., Hochholdinger F. The Gibberellic Acid Stimulated-Like gene family in maize and its role in lateral root development. Plant Physiol. 2010;152:356–365. doi: 10.1104/pp.109.149054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko C.-B., Woo Y.-M., Lee D.J., Lee M.-C., Kim C.S. Enhanced tolerance to heat stress in transgenic plants expressing the GASA4 gene. Plant Physiol. Biochem. 2007;45:722–728. doi: 10.1016/j.plaphy.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Wu J., Wang Z., Shi Z., Zhang S., Ming R., Zhu S., Khan M.A., Tao S., Korban S.S., Wang H., et al. The genome of the pear (Pyrus bretschneideri Rehd.) Genome Res. 2012;23:396–408. doi: 10.1101/gr.144311.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darby N., Creighton T.E. Disulfide bonds in protein folding and stability. Methods Mol. Biol. 1995;40:219–252. doi: 10.1385/0-89603-301-5:219. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Nissan G., Lee J.Y., Borohov A., Weiss D. GIP, a Petunia hybrida GA-induced cysteine-rich protein: A possible role in shoot elongation and transition to flowering. Plant J. 2004;37:229–238. doi: 10.1046/j.1365-313X.2003.01950.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M., Li P., Yan X., Wang J., Cheng T., Zhang Q. Genome-wide characterization of PEBP family genes in nine Rosaceae tree species and their expression analysis in P. mume. BMC Ecol. Evol. 2021;21:1–23. doi: 10.1186/s12862-021-01762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meslin C., Mugnier S., Callebaut I., Laurin M., Pascal G., Poupon A., Goudet G., Monget P. Evolution of genes involved in gamete interaction: Evidence for positive selection, duplications and losses in vertebrates. PLoS ONE. 2012;7:e44548. doi: 10.1371/journal.pone.0044548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S., Kang J.-y., Cho D.-I., Park J.H., Kim S.Y. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J. 2004;40:75–87. doi: 10.1111/j.1365-313X.2004.02192.x. [DOI] [PubMed] [Google Scholar]
- 43.Bensmihen S., Giraudat J., Parcy F. Characterization of three homologous basic leucine zipper transcription factors (bZIP) of the ABI5 family during Arabidopsis thaliana embryo maturation. J. Exp. Bot. 2005;56:597–603. doi: 10.1093/jxb/eri050. [DOI] [PubMed] [Google Scholar]
- 44.Guilfoyle T.J., Ulmasov T., Hagen G. The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cell Mol. Life Sci. 1998;54:619–627. doi: 10.1007/s000180050190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beauvieux R., Wenden B., Dirlewanger E. Bud Dormancy in Perennial Fruit Tree Species: A Pivotal Role for Oxidative Cues. Front. Plant Sci. 2018;9:657. doi: 10.3389/fpls.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M., Suren H., Holliday J.A., Gaut B. Phenotypic and Genomic Local Adaptation across Latitude and Altitude in Populus trichocarpa. Genome Biol. Evol. 2019;11:2256–2272. doi: 10.1093/gbe/evz151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Q., Gao Y., Wu X., Moriguchi T., Bai S., Teng Y. Bud endodormancy in deciduous fruit trees: Advances and prospects. Hortic. Res. 2021;8:139. doi: 10.1038/s41438-021-00575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eriksson M.E., Hoffman D., Kaduk M., Mauriat M., Moritz T. Transgenic hybrid aspen trees with increased gibberellin (GA) concentrations suggest that GA acts in parallel with FLOWERING LOCUS T2 to control shoot elongation. New Phytol. 2015;205:1288–1295. doi: 10.1111/nph.13144. [DOI] [PubMed] [Google Scholar]
- 49.Rinne P.L.H., Welling A., Vahala J., Ripel L., Ruonala R., Kangasjärvi J., van der Schoot C. Chilling of Dormant Buds Hyperinduces FLOWERING LOCUS T and Recruits GA-Inducible 1,3-β-Glucanases to Reopen Signal Conduits and Release Dormancy in Populus. Plant Cell. 2011;23:130–146. doi: 10.1105/tpc.110.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang M., Cheng W., Yuan X., Wang J., Cheng T., Zhang Q. Integrated transcriptome and small RNA sequencing in revealing miRNA-mediated regulatory network of floral bud break in Prunus mume. Front. Plant Sci. 2022;13:931454. doi: 10.3389/fpls.2022.931454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Potter S.C., Luciani A., Eddy S.R., Park Y., Lopez R., Finn R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018;46:W200–W204. doi: 10.1093/nar/gky448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R.D., Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids. Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newbigin E., Chou K.-C., Shen H.-B. Plant-mPLoc: A Top-Down Strategy to Augment the Power for Predicting Plant Protein Subcellular Localization. PLoS ONE. 2010;5:e11335. doi: 10.1371/journal.pone.0011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Bailey T.L., Williams N., Misleh C., Li W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids. Res. 2006;34:W369–W373. doi: 10.1093/nar/gkl198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crooks G.E., Hon G., Chandonia J.M., Brenner S.E. WebLogo: A sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicholas K.B. GeneDoc: Analysis and visualization of genetic variation, EMBNEW. Embnew News. 1997;4:14. [Google Scholar]
- 59.Wang Y., Tang H., DeBarry J.D., Tan X., Li J., Wang X., Lee T.-h., Jin H., Marler B., Guo H., et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stern A., Doron-Faigenboim A., Erez E., Martz E., Bacharach E., Pupko T. Selecton 2007: Advanced models for detecting positive and purifying selection using a Bayesian inference approach. Nucleic Acids Res. 2007;35:W506–W511. doi: 10.1093/nar/gkm382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z., Zhuo X., Zhao K., Zheng T., Han Y., Yuan C., Zhang Q. Transcriptome Profiles Reveal the Crucial Roles of Hormone and Sugar in the Bud Dormancy of Prunus mume. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-23108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian F., Yang D.-C., Meng Y.-Q., Jin J., Gao G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2019;48:D1104–D1113. doi: 10.1093/nar/gkz1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this study are publicly available. All analyzed data can be found in the article or in the supplementary material.