Abstract
Mutations in apeR, a regulatory locus of the outer membrane esterase apeE from Salmonella enterica serovar Typhimurium, were shown to be alleles of the pstSCAB-phoU high-affinity phosphate transport operon. Expression of apeE was induced by phosphate limitation, and this induction required the phoBR phosphate regulatory system.
The apeE gene of Salmonella enterica serovar Typhimurium encodes an outer membrane esterase (3, 4). The gene has been sequenced (3) and is similar to LipI, a secreted lipase from the insect pathogen Photorhabdus luminescens (15), and to EstA an outer membrane esterase from Pseudomonas aeruginosa (17). However, Southern analysis and sequence comparisons have shown that apeE is not present in Escherichia coli (3). In the initial identification of apeE Collin-Osdoby and Miller (4) isolated mutations in an unlinked locus that resulted in a 60-fold increase in apeE transcription. They named this regulatory locus apeR and suggested that it was a gene for a transcriptional repressor of apeE. No conditions were identified which affected apeE expression (4).
In this study we cloned the apeR gene and showed that apeR mutations are alleles of the pstSCAB-phoU operon that encodes a high-affinity phosphate transport system. We also showed that apeE expression is induced by phosphate limitation and that this induction is dependent on the phoBR regulatory system.
Cloning and identification of apeR.
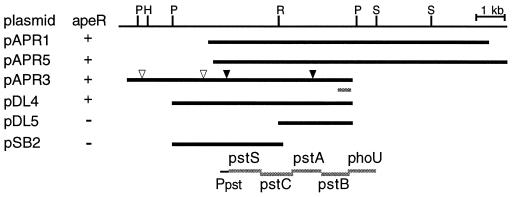
In an apeR wild-type background an apeE::lacZYA fusion makes about two Miller units of β-galactosidase and consequently forms white colonies on MacConkey agar. The apeR1 mutation increases this to 225 U, and the colonies are red. To clone the apeR gene, the original MudI fusion in apeE was first replaced with a more stable MudI-1734 insertion (strain CAC13). Plasmid libraries containing random 8- to 12-kb fragments of Salmonella chromosomal DNA in the vector pBR328 (7) were screened for plasmids that complemented the MacConkey phenotype of an apeR1 mutant. Three different plasmids were isolated that complemented both the apeR1 and apeR47::Tn5 mutations.
To demonstrate that these plasmids contained apeR, they were integrated into the chromosome of a polA strain by recombination between the cloned DNA on the plasmid and its homologous DNA on the Salmonella chromosome (6). Antibiotic resistance on the integrated plasmid was 82 to 84% phage P22 cotransducible with insertion zic868::Tn10, which had previously been shown to be 78% cotransducible with apeR1. This demonstrated that the cloned DNA was from the apeR region of the Salmonella chromosome.
Restriction enzyme analysis indicated that the three plasmids shared a 4.5-kb fragment (Fig. 1). Subcloning showed that DNA on both sides of an EcoRI site in the middle of this region was required for complementation. Four Tn1000 insertions were isolated in the insert region of plasmid pAPR3. Two of these, located 3 kb apart, eliminated complementation. DNA sequence was obtained from the right end of the insert in pAPR3. This sequence was 93% identical to the last 261 bases of the pstB gene and the first 73 bases of the phoU gene in the E. coli pstSCAB-phoU operon. Alignment of the E. coli sequence with the restriction map and the Tn1000 insertions indicated that the apeR1 mutation must be an allele of pstC, pstA, or pstB. This is consistent with the equivalent map positions of apeR on the Salmonella chromosome (11) and pstSCAB-phoU operon on the E. coli chromosome (1).
FIG. 1.
apeR complementing plasmids. Bars indicate chromosomal DNA contained on the plasmids. Restriction enzyme sites are indicated as follows: H, HindIII; P, PvuII; R, EcoRI; and S, SalI. Triangles indicate Tn1000 insertions: closed-triangle insertions eliminated complementation, open-triangle insertions did not. The E. coli pstSCAB-phoU operon is aligned with the restriction map based on DNA sequence determined from the right end of plasmid pAPR3.
Regulation of apeE by phosphate limitation and phoBR.
The pstSCAB-phoU operon encodes a high-affinity phosphate transport system. Mutations in the pst operon often result in constitutive expression of genes that are induced by phosphate limitation. This is particularly true of members of the PhoBR regulon, such as the E. coli phoA gene (16). This led us to examine the effect of phosphate limitation on apeE induction. Strain CAC13, containing an apeE::lacZYA fusion, was grown in minimal morpholinepropanesulfonic acid medium with limiting phosphate (0.1 mM) or excess phosphate (2 mM) as previously described (8), and β-galactosidase activity was measured (10). Limiting phosphate induced apeE expression 140-fold, from 1.5 to 210 U of β-galactosidase (Table 1), explaining the effect of the apeR (pst) mutation.
TABLE 1.
β-Galactosidase activity of apeE::lacZ fusions in limiting and excess phosphate
| Strain | Genotype | β-Galactosidase activitya (Miller units) in:
|
|
|---|---|---|---|
| 2 mM phosphate | 0.1 mM phosphate | ||
| CAC13 | ΔleuBCD485 apeE14::MudI-1734 | 1.5 | 210 |
| CAC47 | ΔleuBCD485 apeE14::MudI-1734 apeR1 | 225 | 241 |
| CAC49 | ΔleuBCD485 apeE14::MudI-1734 ΔphoB1::cat | 1.8 | 1.6 |
| CAC50 | ΔleuBCD485 apeE14::MudI-1734 ΔphoB1::cat apeR1 | 1.9 | 2.0 |
| CAC39 | ΔleuBCD485 apeE14::MudI-1734 katF::bla | 1.0 | 272 |
| CAC97 | ΔleuBCD485 apeE14::MudI-1734 phoP53::Tn10dTet | 0.8 | 134 |
| CAC99 | ΔleuBCD485 apeE14::MudI-1734 ntrA209::Tn10 | 0.8 | 149 |
Strains were grown overnight in morpholine propanesulfonic acid minimal medium with either 2 mM K2HPO4 and 0.06% glucose or 0.1 mM K2HPO4 and 0.4% glucose (8). The β-galactosidase activity was assayed as described by Miller (10). Each assay was performed in triplicate, and all strains were assayed at least twice.
To determine whether apeE is regulated by one of the known phosphate limitation regulatory genes, we used phage P22 transduction to construct isogenic strains that contained both the apeE::lacZYA fusion and mutations in either ntrA (rpoN), phoP, katF (rpoS), or phoBR. β-Galactosidase activity was measured for these strains in both limiting and excess phosphate. As seen in Table 1, only the phoBR deletion significantly affected phosphate induction, completely eliminating induction by phosphate limitation, as well as the effect of the apeR mutation. This indicated that apeE is a previously uncharacterized member of the phoBR regulon.
Analysis of the apeE promoter region.
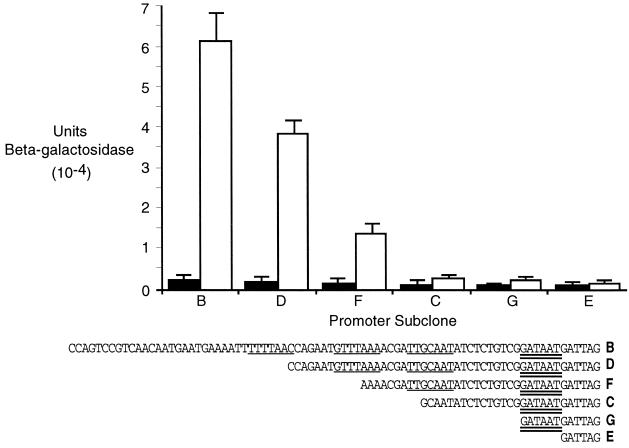
The promoters of genes that are regulated by PhoB contain a PhoB binding site (PHO box) in the −35 region of the promoter. A consensus PHO box consists of two 7-bp direct repeats separated by a 4-bp AT spacer (16). Although there is no obvious consensus PHO box upstream of the apeE coding region, by using a less-stringent definition of the PHO box based on mutational analysis of the PhoB binding site by Makino et al. (9) and examination of other phoBR regulated genes (14), we have identified three potential half PHO boxes located 19 bp upstream of a likely −10 sequence (GATAAT) (Fig. 2).
FIG. 2.
Phosphate regulation of apeE promoter subclones. Plasmid strains were grown on 2 mM (solid bars) or 0.1 mM (open bars) phosphate, and β-galactosidase activity was assayed (10). Assays were done in triplicate, and each strain was assayed three times. The 5′ ends of the subclones are shown below the graph. Single underlines indicate PHO box half-sites, and the double underline indicates a potential −10 region.
To begin identifying regions of the apeE promoter that are required for phosphate control, we performed deletion analysis of the promoter region. We used the PCR to generate fragments with a constant 3′ end 1413 nucleotides downstream of the start of translation and various 5′ ends as indicated in Fig. 2. These fragments were inserted into the promoter detection vector pRS415 (13), where they control the expression of a promoter-less lac operon. Plasmid-containing strains of S. enterica strain TN1379 were grown in either 2 or 0.1 mM phosphate, and the β-galactosidase activity was determined. As shown in Fig. 2, successive removal of potential PHO box half-sites reduced phosphate induction. Subclone B, which included all three proposed PHO box half sites, was induced 27-fold by phosphate limitation. Subclone D, which contained two half-sites, was induced 19-fold, and subclone F, with one complete half-site, was induced only 8.6-fold. Subclones C, G, and E containing no intact half-sites were essentially uninducible. These results support the identification of the putative PHO boxes in the apeE promoter region. Further analysis, such as site-directed mutagenesis and DNA footprinting with PhoB protein, will be needed to confirm this. The differences in induction ratios between the plasmids and the chromosomal MudI-1734 insertion were probably caused by the increased copy number of the plasmids and cryptic promoter activity in the approximately 600 bp between the MudI-1734 insertion site and the 3′ end of the subclones.
Possible function of ApeE.
As mentioned above, apeE is not present in E. coli, but a search of preliminary genomic sequence data showed that it is clearly present in the S. enterica serovars Typhi and Paratyphi A, as well as S. enterica serovar Typhimurium. Although this suggests that apeE could be involved in Salmonella virulence, it has not been identified in screens for Salmonella virulence factors. This is consistent with the report by Jiang et al. that a phoBR deletion itself failed to attenuate virulence (8), and therefore it is unlikely that PhoBR-regulated genes would either.
We have previously reported that apeE is required for the utilization of the model lipid substrate Tween 80 (polyoxyethylene sorbitan monooleate) and for the hydrolysis of methylumbelliferyl caprylate (3). Combined with the phosphate regulation data, this suggests that apeE could play an important role in the use of phospholipids as phosphate sources. The products of ApeE deacylation of phospholipids would be either sn-glycerol-3-phosphate or glycerophosphoryl diesters. In both E. coli and serovar Typhimurium these organic phosphate sources are transported across the inner membrane by the PhoB-dependent Ugp transport system and so can be used as sole phosphate sources (2, 5, 12). Whereas E. coli, which lacks ApeE, uses PhoA to remove the phosphate from the phospholipid, Salmonella spp., which lack PhoA but have ApeE, would not need PhoA to use phospholipids as phosphate sources since the deacylated products would be transported by the Ugp system. Additional studies are needed to confirm this hypothesis.
Acknowledgments
This work was supported by Public Health Service grant GM52697 from the National Institutes of Health and a Faculty Research Grant from Minnesota State University, Mankato, to C.A.C.
We thank C. G. Miller and B. L. Wanner for providing strains and the Genome Sequencing Center, Washington University, St. Louis, Mo., and the Sanger Center for communication of DNA sequence data prior to publication.
REFERENCES
- 1.Berlyn M K B, Low K B, Rudd K E, Singer M. Linkage map of Escherichia coli K-12, ed. 9. In: Neidhardt F C, Curtiss III R I, Gross C A, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W, Schaechter M, Umbarger H E, Riley M, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1715–1902. [Google Scholar]
- 2.Brzoska P, Boos W. Characterization of a ugp-encoded and phoB-dependent glycerophosphoryl diester phosphodiesterase which is physically dependent on the ugp transport system of Escherichia coli. J Bacteriol. 1988;170:4125–4235. doi: 10.1128/jb.170.9.4125-4135.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carinato M E, Collin-Osdoby P, Yang X, Knox T M, Conlin C A, Miller C G. The apeE gene of Salmonella typhimurium encodes an outer membrane esterase not present in Escherichia coli. J Bacteriol. 1998;180:3517–3521. doi: 10.1128/jb.180.14.3517-3521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collin-Osdoby P, Miller C G. Mutations affecting a regulated, membrane-associated esterase in Salmonella typhimurium LT2. Mol Gen Genet. 1994;243:674–680. doi: 10.1007/BF00279577. [DOI] [PubMed] [Google Scholar]
- 5.Hengge R, Larson T J, Boos W. sn-Glycerol-3-phosphate transport in Salmonella typhimurium. J Bacteriol. 1983;155:186–195. doi: 10.1128/jb.155.1.186-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hmiel S P, Snavely M D, Florer J B, Maguire M E, Miller C G. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J Bacteriol. 1989;171:4742–4751. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hmiel S P, Snavely M D, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986;168:1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang W, Metcalf W W, Lee K-S, Wanner B L. Molecular cloning, mapping, and regulation of pho regulon genes for phosphonate breakdown by the phosphonatase pathway of Salmonella typhimurium LT2. J Bacteriol. 1995;177:6411–6421. doi: 10.1128/jb.177.22.6411-6421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makino K, Amemura M, Kawamoto T, Kimura S, Shinagawa H, Nakata A, Suzuki M. DNA binding of PhoB and its interaction with RNA polymerase. J Mol Biol. 1996;259:15–26. doi: 10.1006/jmbi.1996.0298. [DOI] [PubMed] [Google Scholar]
- 10.Miller J H, editor. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 11.Sanderson K E, Hessel A, Liu S-L, Rudd K. The genetic map of Salmonella typhimurium, ed. VIII. In: Neidhardt F C, Curtiss III R I, Gross C A, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W, Schaechter M, Umbarger H E, Riley M, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1903–1999. [Google Scholar]
- 12.Schweizer H, Boo W. Regulation of ugp, the sn-glycerol-3-phosphate transport system of Escherichia coli K-12 that is part of the pho regulon. J Bacteriol. 1985;163:392–394. doi: 10.1128/jb.163.1.392-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 14.VanBogelen R A, Olson E R, Wanner B L, Neidhardt F C. Global analysis of proteins synthesized during phosphorous restriction in Escherichia coli. J Bacteriol. 1996;178:4344–4366. doi: 10.1128/jb.178.15.4344-4366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Dowds B C A. Phase variation in Xenorhabdus luminescens: cloning and sequencing of the lipase gene and analysis of its expression in primary and secondary phases of the bacterium. J Bacteriol. 1993;175:1665–1673. doi: 10.1128/jb.175.6.1665-1673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wanner B L. Phosphorous assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtiss III R I, Gross C A, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W, Schaechter M, Umbarger H E, Riley M, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1357–1381. [Google Scholar]
- 17.Wilhelm S, Tommassen J, Jaeger K-E. A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J Bacteriol. 1999;181:6977–6986. doi: 10.1128/jb.181.22.6977-6986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]