Abstract
Background
Co-infection of the four major species of human malaria parasite Plasmodium falciparum (Pf), P. vivax (Pv), P. malariae (Pm), and P. ovale sp. (Po) is regularly observed, but there is limited understanding of between-species interactions. In particular, little is known about the effects of multiple Plasmodium species co-infections on gametocyte production.
Methods
We developed molecular assays for detecting asexual and gametocyte stages of Pf, Pv, Pm, and Po. This is the first description of molecular diagnostics for Pm and Po gametocytes. These assays were implemented in a unique epidemiological setting in Papua New Guinea with sympatric transmission of all four Plasmodium species permitting a comprehensive investigation of species interactions.
Findings
The observed frequency of Pf-Pv co-infection for asexual parasites (14.7%) was higher than expected from individual prevalence rates (23.8%Pf x 47.4%Pv = 11.3%). The observed frequency of co-infection with Pf and Pv gametocytes (4.6%) was higher than expected from individual prevalence rates (13.1%Pf x 28.2%Pv = 3.7%). The excess risk of co-infection was 1.38 (95% confidence interval (CI): 1.09, 1.67) for all parasites and 1.37 (95% CI: 0.95, 1.79) for gametocytes. This excess co-infection risk was partially attributable to malaria infections clustering in some villages. Pf-Pv-Pm triple infections were four times more frequent than expected by chance alone, which could not be fully explained by infections clustering in highly exposed individuals. The effect of co-infection on parasite density was analyzed by systematic comparison of all pairwise interactions. This revealed a significant 6.57-fold increase of Pm density when co-infected with Pf. Pm gametocytemia also increased with Pf co-infection.
Conclusions
Heterogeneity in exposure to mosquitoes is a key epidemiological driver of Plasmodium co-infection. Among the four co-circulating parasites, Pm benefitted most from co-infection with other species. Beyond this, no general prevailing pattern of suppression or facilitation was identified in pairwise analysis of gametocytemia and parasitemia of the four species.
Trial registration
This trial is registered with ClinicalTrials.gov, Trial ID: NCT02143934.
Author summary
The majority of malaria research focuses on the Plasmodium falciparum and P. vivax parasite species, due to their large public health burden. The epidemiology of P. malariae and P. ovale parasites has been comparatively neglected, due to a lack of research tools, most notably diagnostics. We present new molecular diagnostic assays for detecting P. malariae and P. ovale gametocytes, the sexual stage of the malaria parasite transmitted to mosquitoes. These assays were applied to samples collected in Papua New Guinea, a rare region with high transmission of the four major malaria parasite species. Patterns of co-infections were characterized accounting for interactions between pairs and triples of parasites. Heterogeneity in exposure to mosquito bites was identified as a key driver of patterns of co-infection. The effect of co-infection on parasite density was analyzed by systematic comparison of all pairwise interactions. The most significant within-host interaction of parasites was the large increase in P. malariae parasite density due to co-infection with P. falciparum. This finding was replicated for P. malariae gametocytes (but did not attain statistical significance due to low sample numbers) suggesting that co-infection provides a key transmission advantage to P. malariae.
Introduction
Interactions of multiple Plasmodium species co-infecting a single human host have been investigated since the early days of the malariological literature. Data from malariatherapy in syphilis patients suggested interaction between different Plasmodium species [1,2], for instance, one species dominated the other after simultaneous inoculations with P. falciparum (abbreviated to Pf) and P. vivax (abbreviated to Pv) or with Pv and P. malariae (abbreviated to Pm). Since then, there have been many reports on Plasmodium species interactions, mainly targeting Pf-Pv and Pf-Pm co-infections [3–6].
In many previous reports (summarized in Table A in S1 Text), one species dominated the other. However, the outcome of within-host co-infection may not only consist of within-host competition and suppression of one of the infecting species. One of the species may also be favored by facilitation, or both species may be positively or negatively affected [6,7]. Although many earlier reports indicated interactions between different Plasmodium species, it was not obvious if these were positive or negative. Interactions may be experimentally measured as changes in parasite density caused by the presence of a co-infecting genotype. A positive association between two parasite species indicates that co-infection is more likely than expected under the hypothesis of independence, and vice versa for negative associations. Heterogeneity in exposure to infectious mosquitoes poses a further challenge to understanding between-species interactions. For instance, two species may be frequently co-observed because they are transmitted by the same mosquito, rather than because of facilitating interactions.
As Pm and Pf are sympatrically distributed in many endemic areas, mixed infections of both species are frequent [8–11]. Although P. ovale sp. (abbreviated to Po) is often detected in mixed infections with the other three major Plasmodium species in the Pacific, Asia and Africa [8,10,11], the low prevalence of this species limits meaningful statistical analyses of interactions. Several studies describe positive associations of Pf with Pm and/or Po [3,4]; while in contrast others describe seasonal fluctuations of Pf prevalence and Pm density which can be interpreted as negative interaction, i.e. suppression of Pm by Pf [3]. Many of the published prevalence rates for Pm were determined by light microscopy (LM) and therefore other low density co-infecting species may be overlooked. This compromises any comparison with more recent PCR-based data, particularly when assessing the effects of co-infection on prevalence and density of the other species.
Suppression occurring in Pf-Pv co-infection was suggested by a systematic review of LM data [6]. Considerable previous research, particularly on Pf-Pv co-infection, has focused on the fact that species co-infection may also involve immunological interaction [12]. The host’s allocation of immune response against co-infecting parasites might involve complex trade-offs, for example, between investment in defense against one parasite species on the cost of controlling the other species. Complex interactions likely depend on life-history traits of both co-infecting species, such as different host cell requirements or length of life cycle or duration of a natural infection. Several studies reported that Pv and Pm infections reduce the severity of falciparum malaria in co-infections [13–15]. These findings gave rise to speculation that Pv infection, or in general infection with a less virulent parasite [13], may protect against subsequent severe Pf malaria by inducing cross-species immunity and acting as a hypothetical natural vaccine in areas where both parasites co-exist [13]. However, other studies found that Pf-Pv co-infections were present in severe malaria cases, arguing against Pv protecting against Pf infection [16,17].
Gametocytes, the sexual stages of Plasmodium species, are the only life stages transmissible to mosquito vectors and therefore essential for onward transmission. There is limited knowledge on the effects of multiple species co-infection on gametocyte production and transmission [18] (summarized in Table B in S1 Text).
Many previous studies are limited by the low sensitivity of LM. When no molecular detection is used, low-density Plasmodium species co-infection may fall below the limit of detection of LM and remain undetected. Here we present molecular diagnostic data on interactions among the four major human Plasmodium species. In addition to quantifying the parasitemia of all species by qPCR, the carriage of sexual stages (gametocytes) of each species was measured by RT-qPCR to assess effects of co-infection on onward transmission. To detect gametocytes of all four species, we developed molecular assays for Pm and Po gametocyte detection, for which no assays are published to date. These assays were based on the Pm and Po orthologues of pfs25 and pvs25 genes and used in combination with earlier developed Pf- and Pv-specific RT-qPCRs [19].
The present study aims to identify the effect of co-infection on parasite density and gametocyte carriage for all possible combinations of the four major human Plasmodium species using state of the art molecular diagnostics [19].
Methods
Ethics statement
Clinical trial registration: ClinicalTrials.gov NCT02143934. Human subjects: The study received ethical clearance from the Papua New Guinea Institute of Medical Research Institutional Review Board (0908), the PNG Medical Advisory Committee (09.11), the Ethics Committee of Basel 237/11 and was conducted in full concordance with the Declaration of Helsinki. Written informed consent was obtained from the parents/guardians of all children enrolled in the study.
Study site and population
A comprehensive investigation of co-infection was possible through a unique setting of four sympatric Plasmodium species in Papua New Guinea (PNG). At the study site in East Sepik Province, Pv prevalence is among the highest reported globally [20,21]. Pf in PNG can locally reach holo-endemic transmission levels otherwise only seen in sub-Saharan Africa [22]. Pm and Po are often found as mixed-species infections in PNG [18]. During the study period, East Sepik Province had amongst the highest rates of malaria prevalence in PNG [23,24].
Nucleic acid samples were collected from the Albinama cohort study conducted between 2009 and 2010 in villages of Maprik District, East Sepik Province, PNG. Full details of the study are provided in Robinson et al [21] and a CONSORT checklist is provided in Fig A in S1 Text. In brief, 504 children aged 5–10 years were recruited and randomized to receive anti-malaria treatment targeting blood-stage parasites, or treatment targeting both blood- and liver-stages. Children were actively monitored for infection by qPCR and illness for a total of 32 weeks. Finger-prick blood samples were taken every two weeks for the first 12 weeks and every 4 weeks from week 14–32.
Detection of blood-stage parasites and gametocytes
Previously published data was incorporated in the present analysis, namely qPCR-based and RT-qPCR-based detection of Pf, Pv, Pm and Po parasitemia, as well as RT-qPCR-based Pf and Pv gametocytemia [25]. Data on Pm and Po gametocytemia was newly generated by RT-qPCR using previously extracted RNA samples stored at -80C. Plasmodium species blood-stage infections were detected using a generic qPCR assay to detect Plasmodium infection by any species [19,26], followed by species-specific (Pf, Pv, Pm and Po) qPCRs targeting the 18S rRNA genes [27]. The Po qPCR detects both Po curtisi and Po wallikeri. Pf and Pv gametocytes were detected and quantified using RT-qPCRs targeting the transcripts of pfs25 or orthologues [19]. qPCR assays detect DNA of all parasite stages present in the blood, including genomic DNA of gametocytes. Because asexual parasite stages contribute the overwhelming fraction of blood stages (except for rare cases of persisting gametocytes but cleared asexuals), we used the term "asexual" to denote the total blood stage parasites based on qPCR. This asexual fraction we contrast with the pure gametocyte fraction obtained by detecting stage-specific gametocyte RNA.
For detection and quantification of Pm and Po gametocytes, two RT-qPCR assays targeting pms25 and pos25 (PlasmoDB: PmUH01_10042200 and PocGH01_06024100, respectively) transcripts were developed and validated. Pfs25 and pvs25 are among the highest transcribed genes in mature gametocytes [26]. Primers as well as HEX-BHQ1-labelled and FAM-MGB-EQ-labelled probes of pms25 and pos25, respectively, were selected within regions of maximal diversity to its orthologues. Owing to the lack of pos25 and pms25 sequences in GenBank, the target region was amplified and sequenced from field isolates to identify conserved regions. Sequences of oligonucleotides as well as reaction mixes and thermal profiles are shown in Tables C-E in S1 Text. Although there is evidence that P. ovale wallikeri and P. ovale curtisi are distinct species that are sympatric and do not recombine [28], in this study we analyze these parasites as a single species complex P. ovale sp.
Analytical specificity of the pms25 and pos25 RT-qPCRs was assessed both in silico using sequences of human Plasmodium species identified by PlasmoDB and BLAST searches. Specificity was evaluated experimentally using Pf, Pv, and Po or Pm gDNA and human gDNA from a malaria-free anonymous blood donor. The new gametocyte-specific pms25 and pos25 RT-qPCR assays did not show any cross-reactivity and were negative for human and non-malariae or non-ovale Plasmodium DNA samples. All parasite DNAs from field samples that were selected for this validation were negative for Pm or Po as confirmed by 18S qPCR.
Analytical sensitivity and RT-qPCR efficiency were validated on dilution rows of synthesized pCR 2.1-TOPO TA vectors (Invitrogen, Switzerland) containing inserts of the respective pms25 or pos25 amplicons. Details on plasmid dilution rows and performance of RT-qPCR assays are presented in Table F in S1 Text. Sensitivity was defined by the lowest concentration of the standard plasmid whereby at least 50% of the replicates were positive (see Tables G-H in S1 Text). Amplification efficiency was calculated for each assay as Efficiency = 10(-1/Slope) -1. Details on intra- and inter-assay coefficient of variation (CV) of the pms25 and pos25 RT-qPCR assays are shown in Tables J-K in S1 Text.
Statistical analysis
Let Xi be the asexual or gametocyte parasite prevalence of i ∈ {Pf, Pv, Pm, Po}. Denote Xij as the observed co-infection prevalence of parasites i and j. If parasites i and j are independently distributed, the expected co-infection prevalence is XiXj. The excess co-infection risk of parasites i and j is defined as . The excess co-infection risk is analysed across all pairs of parasites using the following linear regression:
The intercept is fixed at 0, and the estimated slope β is the average excess co-infection risk across all pairs of parasites.
Let Xijk be the observed triple infection prevalence of parasites i, j and k. The excess risk of triple infection can be defined as . The excess triple infection risk can be analysed using linear regression as follows:
The intercept is fixed at 0, and the estimated slope γ is the average excess triple infection risk across all triples of parasites. If excess triple infection risk was entirely attributable to the pairwise clustering of parasites, we would expect . If triple infections are more common than can be explained by pairwise co-infections, then we expect .
Denote Yi as the density of asexual parasites or gametocytes where i ∈ {Pf, Pv, Pm, Po}. The interaction between the densities of parasites i and j is analysed using the following linear regression:
where δij = 1 in the case of co-infection, and δij = 0 otherwise. θ is a covariate accounting for either pre-treatment status; post-treatment with blood-stage drugs; or post-treatment with blood-stage drugs plus PQ. Data were analysed using R v3.5.3 statistical software.
Results
Co-infections of 4 Plasmodium species in PNG
Co-infection patterns for Pf, Pv, Pm and Po asexual parasites and gametocytes are shown in Fig 1 for the 504 pre-treatment samples. The pattern of co-infections was also analyzed for all 5561 samples collected during the entire cohort study (Fig B in S1 Text). For the pre-treatment samples collected at enrolment there was substantial heterogeneity in asexual parasite and gametocyte prevalence between the five neighboring villages of the cohort study20. Compared to the reference village of Albinama, the village of Bolumita had significantly higher prevalence of asexual parasites and gametocytes for both Pf and Pm (Table L in S1 Text). With 504 samples collected at enrolment, no other associations were statistically significant (age, sex, bed net use). When the analysis was extended to 5561 observations from the entire cohort study, a number of associations observed at enrolment became statistically significant (Table O in S1 Text).
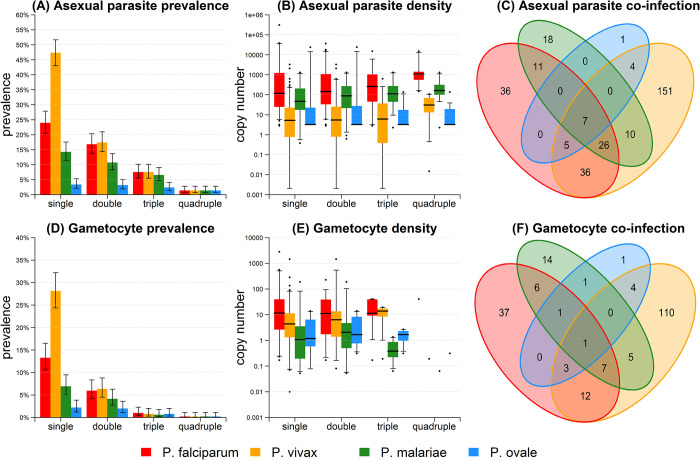
Fig 1. Malaria co-infection in pre-treatment samples (n = 504).
(A) Co-infection prevalence of asexual parasites. Double infection with Pf denotes the proportion of samples PCR positive for Pf and at least one other species. Triple infection with Pf denotes the proportion of samples PCR positive for Pf and at least two other species. Other bars are similarly defined. 95% confidence intervals were calculated using Wilson’s binomial method. (B) Asexual parasite density in co-infected samples. (C) Venn diagram of asexual parasite co-infection. (D) Co-infection prevalence of gametocytes. (E) Gametocyte density in co-infected samples. (F) Venn diagram of gametocyte co-infection.
Estimating excess co-infection risk
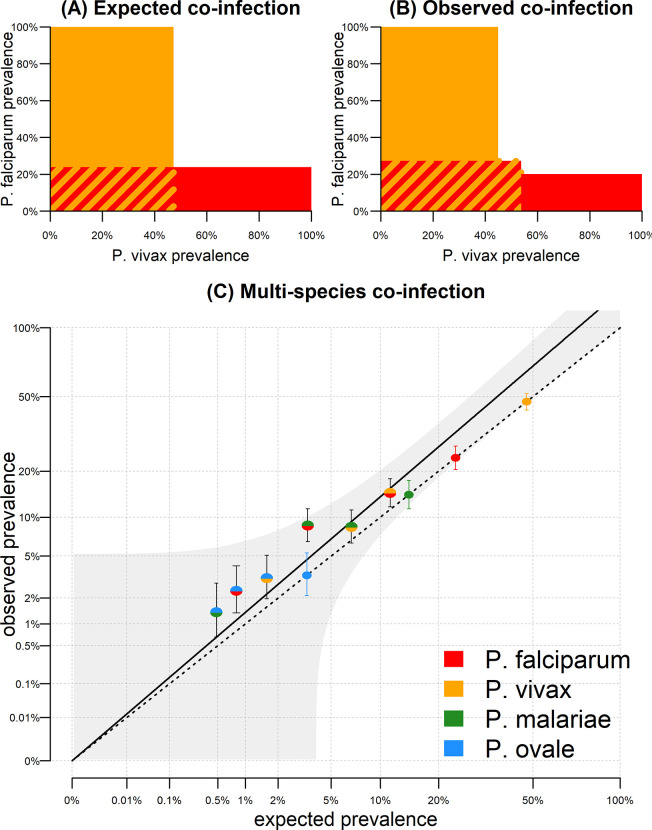
In the pre-treatment samples, Pf prevalence was 23.8% (120/504) and Pv prevalence was 47.4% (239/504). If these parasites were randomly distributed, we would expect co-infection prevalence of 0.238 * 0.474 = 11.3%. However, in the data we observe co-infection prevalence of 14.7% (74/504). The excess risk of Pf and Pv co-infection is therefore 14.7%/11.3% = 1.29. When we account for all six pairwise combinations of co-infection as shown in Fig 2, we estimate the average risk of excess co-infection as 1.38 (95% CI: 1.09, 1.68).
Fig 2. Excess asexual parasite co-infection in pre-treatment samples.
(A) In the pre-treatment samples, Pf asexual prevalence was 23.8% (120/504) and Pv asexual prevalence was 47.4% (239/504). If these parasites were randomly distributed, we would expect co-infection prevalence of 0.238 * 0.474 = 11.3% (red and yellow striped region). (B) Co-infection prevalence of 14.7% (74/504) was observed, in excess of what is expected by random mixing. (C) Expected versus observed co-infection prevalence for the six pairwise combinations of asexual parasites. The dashed line represents the scenario where observed co-infection prevalence equals expected prevalence. Mono-coloured points denote observed prevalence rates of the four species. The multi-coloured data points fall above this line. The solid line denotes a regression model fitted through these points, with 95% confidence intervals shown in grey.
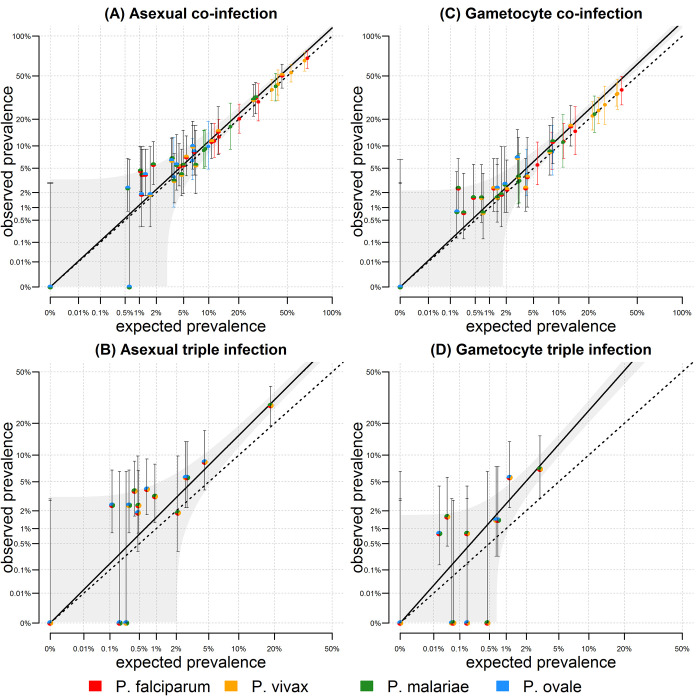
The analysis in Fig 2 was applied to the combined data from all five villages. Excess co-infection risk could be attributable to unaccounted heterogeneity, for example if malaria infections cluster in some villages. The analysis of asexual parasite co-infection was repeated with stratification by village in Fig 3A, with an estimate of excess co-infection risk of 1.11 (95% CI; 1.07, 1.16). Therefore, a proportion of the excess co-infection risk is due to the concentration of malaria of all species within high transmission villages. A similar pattern was observed for gametocyte co-infection (Fig 3C).
Fig 3. Co-infection in pre-treatment samples stratified by village.
(A) For the six pairwise combinations of two malaria species, the observed and expected co-infection asexual prevalence is plotted for each of the five villages. The dashed line represents the scenario where observed co-infection prevalence equals expected prevalence. The multi-coloured data points tend to fall above this line. The solid line denotes a regression model fitted through these points, with 95% confidence intervals shown in grey. (B) Observed and expected triple infection. If the prevalence of Pm is XPm, then the expected prevalence of Pf, Pv and Pm co-infection is XPf * XPv * XPm. The multi-coloured points fall above the dashed line indicating greater observed than expected prevalence. The solid line denotes a regression model fitted through these points. (C) Gametocyte co-infection. (D) Gametocyte triple infection.
This approach can be extended to account for triple infection. Considering Pf (120/504 = 23.8%), Pv (239/504 = 47.4%) and Pm (72/504 = 14.3%) in the pre-treatment samples, if parasite infection was independent, we would expect 0.238*0.474*0.143 = 1.6% of individuals with all three parasites. Instead, we observe 33/504 = 6.5% with Pf, Pv and Pm asexual parasites. This is 6.5/1.6 ~ 4 times higher than expected by chance. Using a statistical model to account for the four possible combinations we estimated that triple infections are 2.42 (95% CI; -1.62, 6.47) times more frequent than expected by chance alone (Table 1). This association is not significant, partially due to being based on four observations. We can further apply the analysis to 20 observations of triple infection by stratifying by the five villages (Fig 3B) in which case we estimate a significant excess risk of triple infections of 1.51 (95% CI; 1.38, 1.64).
Table 1. Estimated risk of co-infection in pre-treatment samples.
*The excess risk of triple infection accounting for the known pairwise clustering due to heterogeneity (e.g. 1.26 = 2.42/1.382).
| Asexual parasites | Gametocytes | |||
|---|---|---|---|---|
| risk | P value | risk | P value | |
| Pre-treatment samples | ||||
| excess risk of 2nd infection | 1.38 (1.09, 1.68) | 0.0489 | 1.38 (0.96, 1.80) | 0.13 |
| excess risk of 3rd infection | 2.42 (-1.62, 6.47) | 0.54 | 4.67 (-0.77, 10.12) | 0.28 |
| heterogeneity adjusted excess risk of 3rd infection * | 1.26 (-0.85, 3.37) | 0.82 | 2.44 (-0.40, 5.29) | 0.39 |
| Pre-treatment samples; stratified by village | ||||
| excess risk of 2nd infection | 1.11 (1.07, 1.16) | 1.4 x 10−5 | 1.22 (1.13, 1.32) | 5.5 x 10−5 |
| excess risk of 3rd infection | 1.51 (1.38, 1.64) | 7.3 x 10−8 | 2.42 (2.05, 2.79) | 1.2 x 10−7 |
| heterogeneity adjusted excess risk of 3rd infection * | 1.22 (1.11, 1.32) | 0.00046 | 1.61 (1.37, 1.86) | 6.3 x 10−5 |
A hypothesis for the excess prevalence of triple infection is that it is a consequence of the excess risk of co-infection with pairs of parasites. For the village-stratified data, the excess risk of co-infection with two parasites was 1.11. If the observed patterns were due to heterogeneity measured in this way, we would expect an excess risk of triple infection of 1.11*1.11 = 1.23. Thus, we can estimate an excess risk of triple infection adjusted for heterogeneity of 1.51/1.23 = 1.22 (95% CI; 1.11, 1.32) (Table 1). This implies that triple infections are even more common than can be explained by clustering of infections in highly exposed individuals.
Effect of co-infection on parasite density
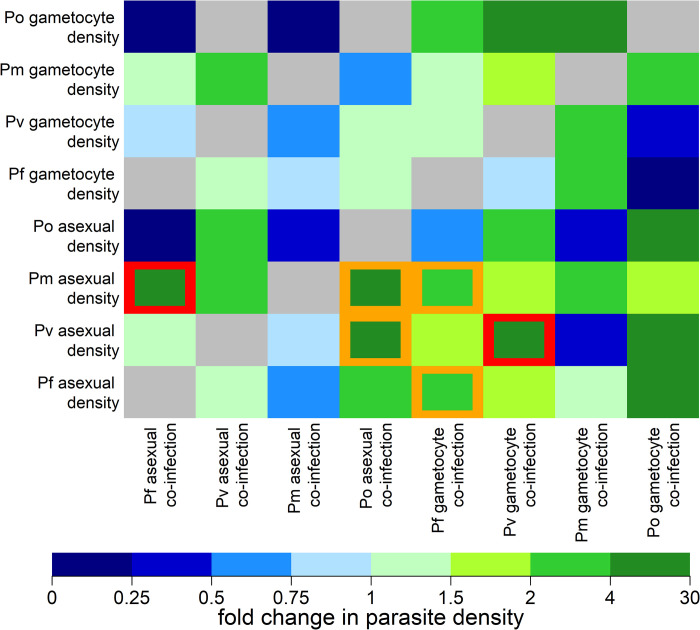
For the eight types of parasites measured (asexual parasites and gametocytes to Pf, Pv, Po, and Pm) there are 56 possible pairwise interactions. Fig 4 presents a systematic comparison of all pairwise interactions for the pre-treatment samples. For example, Pm asexual parasites had a geometric mean density of 14 copy numbers/μL in the absence of Pf parasites. However, when co-infected with Pf asexual parasites the geometric mean of Pm asexual parasite density increased to 94 copy numbers/μL. Therefore, Pf co-infection was associated with a 6.57 (95% CI; 2.93, 14.75; P = 2.1 x 10−5) increase in Pm density. This association was still significant after adjusting for multiple hypothesis testing (P = 0.0005). One other association remained significant after correction for multiple hypothesis testing: the density of Pv asexual parasite infection was greater when Pv gametocytes were also detected. This is likely a consequence of the high degree of correlation between Pv asexual and gametocyte densities, and the challenge of detecting low density infections [25]. Although not significant after multiple hypothesis testing, we observed that Pv and Pm asexual densities were higher in co-infection with Po compared to Pv densities in the absence of Po.
Fig 4. Effect of co-infection on parasite density in pre-treatment samples.
The y-axis denotes the measured parasite density and the x-axis denotes the confounding effect of co-infection. Each square denotes the fold change in parasite density due to co-infection. For example, for Pm asexual parasites, co-infection with Pf asexual parasites leads to a 6.57 (2.9, 14.8) fold increase in Pm asexual parasite density. Grey squares denote interactions where it was not possible to estimate an effect. Otherwise, all estimated effects are presented regardless of statistical significance. Orange squares denote significant associations with P values < 0.05. Red squares denote significant associations with P values < 0.05 after the Benjamini-Hochberg adjustment for multiple hypothesis testing.
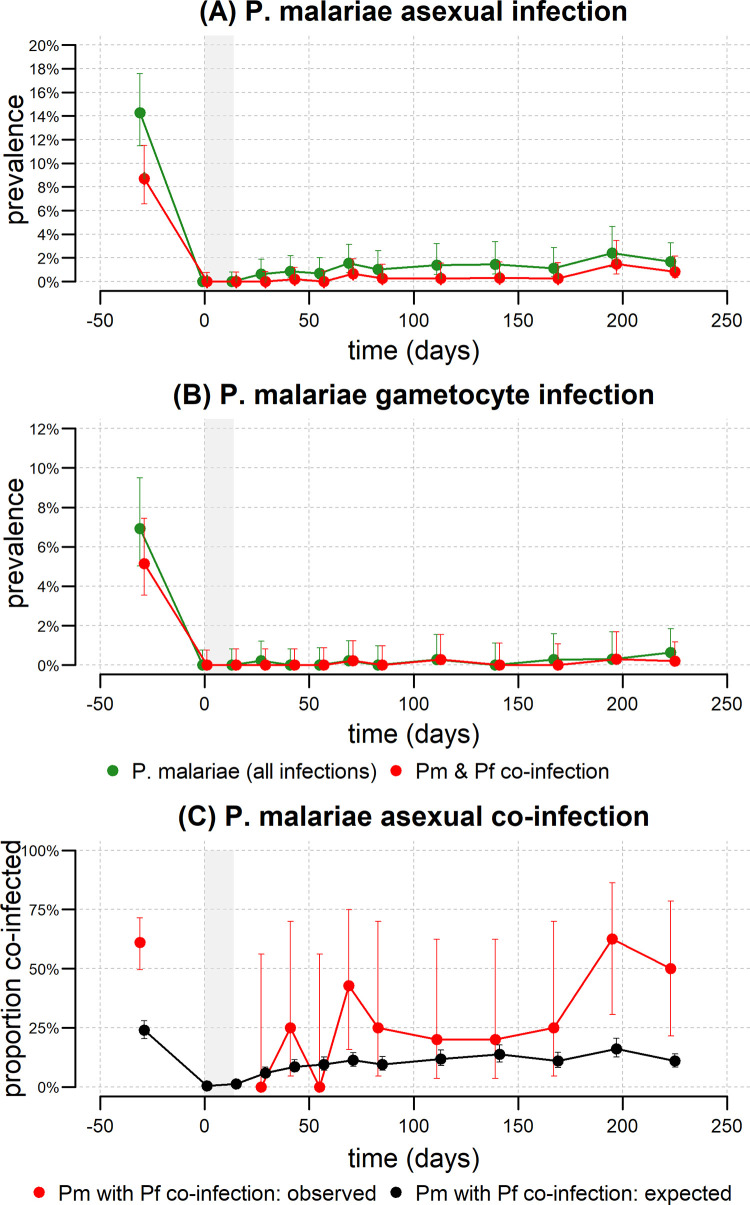
Following the finding on Pm densities being determined by co-infections, an analysis of the effects of Pm co-infection over time was performed. Enrolment prevalence of Pm asexual infection was 14.3% (Fig 5A) and 6.9% for gametocytes (Fig 5B). After treatment, prevalence dropped to zero and thereafter rose very slowly over time. A very high proportion of Pm infections also occurred as co-infection with another Plasmodium species. Before treatment, 61.1% (49.6%, 71.5%) of P. malariae infections were observed to be co-infected with P. falciparum (Fig 5C). Notably, this is greater than the expected proportion of P. malariae infections co-infected with P. falciparum under an assumption of random mixing. After treatment, the co-infection proportion increases over time, albeit with substantial variation.
Fig 5. Longitudinal analysis of P. malariae and the effect of co-infection.
The prevalence of (A) P. malariae asexual parasites, and (B) P. malariae gametocytes. The prevalence of all P. malariae infections is shown in green, and the prevalence of P. malariae and P. falciparum co-infection is shown in red. The grey shaded region denotes the period of treatment. 5561 samples were included over the entire time period. 123 samples were positive for P. malariae asexual parasites, and 61 of these were co-infected with P. falciparum asexual parasites. 43 samples were positive for P. malariae gametocytes, and 18 of these were co-infected with P. falciparum gametocytes. (C) The observed proportion of P. malariae asexual infections that are co-infected with P. falciparum is shown in red. The expected proportion of P. malariae infections co-infected with P. falciparum under an assumption of random mixing is shown in black.
Discussion
To investigate the prevalence of gametocytes of the four major Plasmodium species in humans and their within-host interactions, we developed new assays for Pm and Po gametocyte quantification to complement existing methods for Pf and Pv gametocytes. To our knowledge, this is the first study that analyzes quantitative molecular data of both asexual parasite stages and gametocytes for these four parasite species.
The present study showed an average risk of excess co-infection of 1.38 when accounting for all six pairwise combinations of co-infection in the pre-treatment samples from all five study villages. This excess in co-infections is partially due to transmission heterogeneity, i.e. when malaria infections cluster in some of the villages. After stratifying by village, an estimated excess risk of co-infection of 1.11 was found. Thus, a proportion of the excess co-infection risk was attributable to the concentration of all Plasmodium species within high transmission villages. A similar pattern was observed for gametocyte co-infection.
Several factors may contribute to a heterogeneous parasite distribution, such as host susceptibility, mosquito vector ecology and transmission seasonality [29]. Previous studies in PNG reported significant heterogeneity in malaria transmission that was attributed to the geographic diversity within the country and local population structure [22,23,30]. Earlier analyses of this study have found considerable micro-spatial heterogeneity in malaria transmission among both neighboring communities and individual children from the same village [20].
In mixed-species co-infections the number of transmission stages produced may be up- or downregulated by within-host interactions. For instance, any signal from a co-infecting or dominant species could induce gametocyte production. Such a signal was shown to facilitate induction of gametocytogenesis: lysophosphatidylcholine (LysoPC) is taken up by Pf and triggers gametocyte production depending on LysoPC plasma levels [31]. Since all four human Plasmodium species harbor the effector gene gametocyte development protein 1 (gdv1), LysoPC-driven facilitation of a co-infecting species’ investment in gametocytes may be expected. On the contrary, a so far unidentified signal could down-regulate the gametocyte production of a co-infecting species and thus influence gametocyte carriage.
A striking outcome of this study was that Pm density increased with any additional co-infection. Co-infections with Pf asexual parasites lead to a significant 6.57-fold increase in Pm asexual parasite density. It was also observed that in co-infection with Pm, the mean asexual and gametocyte densities of any other co-infecting species were reduced compared to those in single-species infections, although these effects were not statistically significant. These data could suggest that Pm thrives on the cost of co-infections and outcompetes these. In line with these observations in PNG, findings from retrospective analyses of malaria therapy data showed that primary Pm infections were protective against secondary Pf infections [32]. To investigate whether Pm-specific infection dynamics could be a driver of the observed effect, a longitudinal analysis of Pm infections (Fig 5) was performed. Pm prevalence increased very slowly over time—consistent with a low force of infection combined with a long duration of infection. This is consistent with Pm causing chronic infections that can last for years8, possibly facilitated by interactions with other parasites.
The Pm and Po gametocyte detection assays were designed based on the assumption of functional similarity because of sequence similarity to the highly expressed pfs25 orthologue i.e. pms25 and pos25 show similar high expression to pfs25. Because no transcriptome data for pms25 and pos25 are yet available to assess accurate transcription levels of these orthologues, it cannot be excluded that more suitable markers with even higher expression levels could exist. Our validation of the pfs25 orthologues showed good performance and sensitive detection of the pms25 and pos25 transcripts with our assays.
A major technical limitation of the study was that blood for RNA extraction was collected on filter paper and later stored in TRIzol for several years. RNA sample storage in TRIzol was shown to be suboptimal compared to direct transfer of blood into a RNA-stabilizing reagent [19]. Therefore, gametocyte densities could have been underestimated or remained undetected due to suboptimal sampling and storage, leading to RNA degradation. Another limitation consisted in the narrow age range of study participants (i.e. children aged 5–10 years). Thus the data may not be representative of the total population. We did not analyze morbidity because there were not enough clinical cases of malaria to assess inter-species interactions with sufficient statistical power. A further challenge of this study was the limited number of samples positive for P. malariae or P. ovale sp.. These limited sample numbers were also a barrier to the analysis of the sub-species of P. o. curtisi and P. o. wallikeri.
Our analysis of species interaction was essentially cross-sectional focusing on the pre-treatment samples of the cohort study. A full longitudinal analysis would be more powerful, but would need to account for the PQ treatment given randomly to 50% of participants after the baseline sample. We make the simplifying assumption that PQ should not affect co-infection analysis of the follow-up samples. However, in the PQ group newly appearing infections are predominantly derived from mosquito infections, while in the placebo group, newly appearing Pv or Po parasites could be either relapses or true new infections.
A further limitation is that the analyses presented did not account for individual-level heterogeneity in exposure, which would have been possible in Pf or Pv infected individuals by adjusting for molecular force of blood-stage infection (molFOB) [20]. This could potentially reduce some of the remaining heterogeneity, which was not removed by the village-level adjustment of our analysis.
To our knowledge, this is the first study that analyses the effect of co-infection on both parasite density and gametocyte carriage for all possible combinations of the four major human Plasmodium species using molecular data of asexual parasite stages and gametocytes. We found an excess risk of co-infection that was higher than expected by chance alone. Similarly, triple infections were more common than expected from clustering of infections in highly exposed individuals. We attributed these observations partly to heterogeneity in exposure. The effects of co-infection among Plasmodium species did not follow a generalized pattern. Instead, species-specific effects of within-host interaction seem to act in opposite directions. The clearest picture emerged from the total parasite density data, but less so from gametocyte data. Pm benefitted most from co-infections with any other Plasmodium species, as its asexual and gametocyte densities were increased in co-infections.
Parasite epidemiology and biology is complex, leading to both positive and negative effects on parasite prevalence and density for all pairs of co-infecting species. Despite their limitations for definitively inferring facilitation or suppression, the molecular assays and statistical analyses presented here allow for improved understanding of malaria parasite co-infection.
Supporting information
(DOCX)
Acknowledgments
We sincerely thank the children, their parents/guardians and communities for their willingness to participate in this study. We gratefully acknowledge the assistance of staff at Albinama Health Centre and of the network of village-based health workers. We acknowledge the efforts of the PNG Institute of Medical Research Maprik field, administration, and laboratory staff, as well as technical support through other PNGIMR branches. We specially thank Anna Rosanas-Urgell and Alice Ura from PNGIMR for preserving RNA of samples.
Data Availability
All data and code used for producing the results are freely available on GitHub: https://github.com/MWhite-InstitutPasteur/malaria_coinfection_PNG.
Funding Statement
This work was carried out with funding awarded to IF from the Swiss National Science Foundation (grants no. 310030-134889 and 310030-159580), NIH NIAID International Centres of Excellence in Malaria Research South West Pacific (U19 AI089686) and Asia Pacific (U19 AI129392-01) awarded to IM. Field work was supported by the TransEPI consortium funded by the Bill & Melinda Gates Foundation awarded to IM. IM (GNT11155075) and LJR (GNT1161627) are supported by NHMRC Fellowships. MTW is supported by the French Government's Laboratoire d'Excellence "Integrative Biology of Emerging Infectious Diseases" (Investissement d'Avenir grant n°ANR-10-LABX-62-IBEID), and the INCEPTION program (Investissement d’Avenir grant ANR-16-CONV-0005). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boyd MF, Kitchen SF. Simultaneous inoculation with Plasmodium vivax and Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1937; s1-17: 855–861. [Google Scholar]
- 2.Mayne B, Young MD. Antagonism between species of malaria parasites in induced mixed infections. Public Health Reports (1896–1970). 1938; 53: 1289–1291. [Google Scholar]
- 3.Molineaux L, Storey J, Cohen JE, Thomas A. A longitudinal study of human malaria in the West African Savanna in the absence of control measures: relationships between different Plasmodium species, in particular P. falciparum and P. malariae. Am. J. Trop. Med. Hyg. 1980; 29: 725–737. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie FE, Bossert WH. Multispecies Plasmodium infections of humans. J. Parasitol. 1999; 85: 12–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Howard SC, Donnelly CA, Chan MS. Methods for estimation of associations between multiple species parasite infections. Parasitology. 2001; 122: 233–251. doi: 10.1017/s0031182001007272 [DOI] [PubMed] [Google Scholar]
- 6.Haghdoost AA, Alexander N. Systematic review and meta-analysis of the interaction between Plasmodium falciparum and Plasmodium vivax in humans. J. Vector Borne Dis. 2007; 44: 33–43. [PubMed] [Google Scholar]
- 7.Choisy M, de Roode JC. Mixed infections and the evolution of virulence: effects of resource competition, parasite plasticity, and impaired host immunity. Am. Nat. 2010; 175: E105–118. doi: 10.1086/651587 [DOI] [PubMed] [Google Scholar]
- 8.Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale–the ‘bashful’ malaria parasites. Trends Parasitol. 2007; 23: 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinko B, Oguike MC, Larbi JA, Bousema T, Sutherland CJ. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int. J. Parasitol.: Drugs and Drug Resistance. 2013; 3: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doctor SM, Liu Y, Anderson OG, Whitesell AN, Mwandagalirwa MK, Muwonga J, et al. Low prevalence of Plasmodium malariae and Plasmodium ovale mono-infections among children in the Democratic Republic of the Congo: a population-based, cross-sectional study. Malar. J. 2016; 15: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woldearegai TG, Lalremruata A, Nguyen TT, Gmeiner M, Veletzky L, Tazemda-Kuitsouc GB, et al. Characterization of Plasmodium infections among inhabitants of rural areas in Gabon. Sci Rep. 2019; 9: 9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox FEG. Concomitant infections, parasites and immune responses. Parasitology. 2001; 122: S23–S38. doi: 10.1017/s003118200001698x [DOI] [PubMed] [Google Scholar]
- 13.Williams TN, Maitland K, Bennett S, Ganczakowski M, Peto TE, Newbold CI, et al. High incidence of malaria in α-thalassaemic children. Nature. 1996; 383: 522–525. [DOI] [PubMed] [Google Scholar]
- 14.Black J, Hommel M, Snounou G, Pinder M. Mixed infections with Plasmodium falciparum and P. malariae and fever in malaria. Lancet. 1994; 343: 1095. [DOI] [PubMed] [Google Scholar]
- 15.Akala HM, Watson OJ, Mitei KK, Juma DW, Verity R, Ingasia LA, et al. Plasmodium interspecies interactions during a period of increasing prevalence of Plasmodium ovale in symptomatic individuals seeking treatment: an observational study. Lancet Microbe. 2021; 2:4, 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie FE, Smith DL, O’Meara WP, Forney JR, Magill AJ, Permpanich B, et al. Fever in patients with mixed-species malaria. Clin. Infect. Dis. 2006; 42: 1713–1718. doi: 10.1086/504330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genton B, D’Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, et al. Plasmodium vivax and mixed infections are associated with severe malaria in children: A prospective cohort study from Papua New Guinea. PLOS Med. 2008; 5: e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bousema JT, Drakeley CJ, Mens PF, Arens T, Houben R, Omar SA, et al. Increased Plasmodium falciparum gametocyte production in mixed infections with P. malariae. Am, J. Trop. Med. Hyg. 2008; 78: 442–448. [PubMed] [Google Scholar]
- 19.Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, et al. Strategies for detection of Plasmodium species gametocytes. PLOS One. 2013; 8: e76316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann NE, Karl S, Wampfler R, Kiniboro B, Teliki A, Iga J, et al. The complex relationship of exposure to new Plasmodium infections and incidence of clinical malaria in Papua New Guinea. eLife. 2017; 6: e23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CSN, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: A randomised placebo-controlled trial and mathematical model. PLOS Med. 2015; 12: e1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller I, Bockarie M, Alpers M, Smith T. The epidemiology of malaria in Papua New Guinea. Trends Parasitol. 2003; 19: 253–259. doi: 10.1016/s1471-4922(03)00091-6 [DOI] [PubMed] [Google Scholar]
- 23.Hetzel MW, Morris H, Tarongka N, Barnadas C, Pulford J, Makita L, et al. Prevalence of malaria across Papua New Guinea after initial roll-out of insecticide-treated mosquito nets. Trop. Med. Int. Health. 2015; 20: 1745–1755. doi: 10.1111/tmi.12616 [DOI] [PubMed] [Google Scholar]
- 24.Hetzel MW, Reimer LJ, Gideon G, Koimbu G, Barnadas C, Makita L, et al. Changes in malaria burden and transmission in sentinel sites after the roll-out of long-lasting insecticidal nets in Papua New Guinea. Parasit. Vectors. 2016; 9: 340 doi: 10.1186/s13071-016-1635-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koepfli C, Robinson LJ, Rarau P, Salib M, Sambale N, Wampfler R, et al. Blood-stage parasitaemia and age determine Plasmodium falciparum and P. vivax gametocytaemia in Papua New Guinea. PLOS One. 2015; 10: e0126747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wampfler R, Hofmann NE, Karl S, Betuela I, Kiniboro B, Lorry L, et al. Effects of liver-stage clearance by primaquine on gametocyte carriage of Plasmodium vivax and P. falciparum. PLOS Negl. Trop. Dis. 2017; 11: e0005753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosanas-Urgell A, Mueller D, Betuela I, Barnadas C, Iga J, Zimmerman PA, et al. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar. J. 2010; 9: 361. doi: 10.1186/1475-2875-9-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oguike MC, Betson M, Burke M, Nolder D, Stothard JR, Kleinschmidt I, et al. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol. 2011; 41(6–10): 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman PA, Mehlotra RK, Kasehagen LJ, Kazura JW. Why do we need to know more about mixed Plasmodium species infections in humans? Trends Parasitol. 2004; 20: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller I, Widmer S, Michel D, Maraga S, McNamara DT, Kiniboro, et al. High sensitivity detection of Plasmodium species reveals positive correlations between infections of different species, shifts in age distribution and reduced local variation in Papua New Guinea. Malar. J. 2009; 8: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brancucci NMB, Gerdt JP, Wang CQ, De Niz M, Philip N, Adapa SR, et al. Lysophosphatidylcholine regulates sexual stage differentiation in the human malaria parasite Plasmodium falciparum. Cell. 2017; 171: 1532–1544.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins WE, Jeffery GM. A retrospective examination of sporozoite- and trophozoite-induced infections with Plasmodium falciparum in patients previously infected with heterologous species of Plasmodium: effect on development of parasitologic and clinical immunity. Am. J. Trop. Med. Hyg. 1999; 61: 36–43. [DOI] [PubMed] [Google Scholar]