Abstract
Fungi of the genus Trichoderma have been marketed for the management of diseases of crops. However, some Trichoderma species may produce toxic secondary metabolites and it should receive due attention to ensure human safety. In this study, we investigated the in vitro antagonistic potential of T. asperellum AU131 and T. longibrachiatum AU158 as microbial biocontrol agents (MBCAs) against Fusarium xylarioides and the associated antagonistic mechanism with bioactive substances. Swiss albino mice were used to evaluate the in vivo toxicity and pathogenicity of T. asperellum AU131 and T. longibrachiatum AU158 methanolic extracts and spore suspensions, respectively, in a preliminary safety assessment for use as biofungicides. Gas Chromatography-Mass Spectrometry (GC-MS) was used to profile volatile organic metabolites (VOCs) present in the methanolic extracts. The agar diffusion assay of the methanolic extracts from both T. asperellum AU131 and T. longibrachiatum AU158 were effective at a concentration of 200 μg/mL (1×107 spores/mL), causing 62.5%, and 74.3% inhibition, respectively. A GC-MS analysis of methanolic extracts from both bioagents identified 23 VOCs which classified as alcohols, acids, sesquiterpenes, ketones and aromatic compounds. The oral administration of methanolic extracts and spore suspensions of each Trichoderma species to female Swiss albino mice over 14 days did not show any significant signs of toxicity, mortality or changes to body weight. It can be concluded that the tested spore suspensions and methanolic extracts were not pathogenic or toxic, respectively, when administered to Swiss albino mice at various doses.
Introduction
Given the large socio-economic impact of crop monocultures and the environmental hazards of chemical pesticides [1], microbial biocontrol agents (MBCAs) have recently become a strategic option for controlling plant diseases [2], insects [3] and weeds [4]. A number of biopesticides that contain bioagents such as T. asperellum, T. harzianum and T. viride as the active ingredients are currently marketed in Europe, USA, Asia and Africa [5, 6]. In contrast to chemical pesticides, which have been reported to induce resistance among pests and cause residual toxic effects, biopesticides are highly selective against the target pest [6]. Microbial biocontrol agents (MBCAs) have been designed to grow and reproduce, survive in the environment for prolonged periods in symbiotic consortia with in the host. Hence, a relatively small amount of MBCAs are needed to be applied to a certain location compared to chemical pesticides [5, 6]. Although the vast majority of MBCAs are generally regarded as safe for humans and the environment [7, 8], some studies have demonstrated that increased exposure to fungal substances among agricultural workers may affect the immune system [13, 14].
There are evidences that members of the genus Trichoderma are effective MBCAs against various plant pathogens across different agro-ecological systems [6, 9–12]. However, Trichoderma species are also known to cause opportunistic infections in humans, varying from localized to fatal disseminated diseases; these are particularly dangerous for risk populations, including patients undergoing peritoneal dialysis, transplant recipients and patients with hematological malignancies [13, 14]. In one of our previous studies, it has been reported that a comparatively high Trichoderma species diversity in coffee ecosystem in Ethiopia among which T. asperellum AU131 and T. longibrachiatum AU158 were found effective against F. xylarioides causing coffee wilt disease (CWD) [15]. Based on subsequent bioassay analysis under different conditions, we formulated a biofungicide from T. asperellum AU131 and T. longibrachiatum AU158 for the control of CWD caused by F. xylarioides [16]. However, the toxicological assessment of these bioagents were not conducted. Thus, systematic study is very important to evaluate and ensure the safety of biofungicides for agricultural use [17]. On the other hand, understanding and evaluating the mechanism of action of secondary metabolites of these potential MBCAs against F. xylarioides is very important. Several Trichoderma strains were widely studied due to their capacity to compete for nutrients and space [18], parasitize other fungi [19, 20], enact antibiosis by producing secondary metabolites or antimicrobial compounds [8, 21, 22], induce defense responses in plants [23], and to promote plant growth [24, 25]. Some strains of T. longibrachiatum and T. orientale are found to be toxic to humans, especially in immunocompromised patients [13, 14].
Since the 1960s, toxicity studies have been used as a vital step in the approval of new products onto the market. The present study was conducted in animal models following the protocols laid out by the Organization for Economic Cooperation and Development (OECD) [26]. More specifically, the study applied the experimental design described in Tier 1 of the Toxicological Evaluation of Microbial Pest Control Agents (MPCA), which has the purpose of assessing the pathogenicity and toxicity of biopesticide products [27]. In Europe, Council Directive 91/414/EEC and its successive amendments identify which requirements an applicant must achieve for the authorization to produce and market pesticides, including those that have a bioactive substances [28, 29]. In particular, the directive requires the provision of information concerning the short-term toxicity of any relevant metabolites produced by the candidate MBCA [29–31].
The present study provides toxicological data that are of importance in assessing the toxicological relevance of two potential MBCAs, T. asperellum AU131 and T. longibrachiatum AU158. To this end, it is conceivable that it would be more effective to determine the toxicological risks associated with a particular MBCAs by assaying a mixture of metabolites, like those in methanolic extracts, on model animals that are sensitive to a large spectrum of molecules, instead of assessing the toxicity of single and pure metabolites [28, 29]. Moreover, secondary metabolites produced by Trichoderma species should be identified and profiled, which is very helpful to categorize them according to their toxicological effects. Thus, the volatile metabolites were analysed by gas chromatography with a single quadrupole mass spectrometer detector (GC-MS) analysis [32, 33].
In the present study, Swiss albino mice were used to assess the acute toxicity of spore suspensions and methanolic extracts of T. asperellum AU131 and T. longibrachiatum AU158 in short term assays. Thus, the study aimed to: (i) evaluate the in vitro antagonistic activity of methanolic extracts against F. xylarioides (ii) evaluate the sensitivity of albino mice to spore suspensions and methanolic extracts; (iii) generate toxicological data that can be used when assessing the risk associated with the toxicity Trichoderma species; and (iv) profile volatile secondary metabolites produced by these fungi through GC-MS analysis.
Materials and methods
Trichoderma species and preparation of spore suspensions
When studying acute oral toxicity, spore suspensions (live cells) were prepared by adding 5 mL (0.9%) of NaCl to mature Trichoderma species on PDA plates to dislodge the spores from the mycelium [34]. The spore density was counted using a haemocytometer (Neuberger GmbH, Germany) to determine the spore concentrations.
Effect of temperature and pH on mycelial growth of Trichoderma species
To evaluate the virulence of the MBCAs, mycelial discs of test fungus at different temperatures, from 25 to 37°C, were inoculated onto Petri dishes containing minimal (0.5% glucose, 0.1% KH2PO4, 0.1% (NH4)2SO4, 0.1% MgSO4.7H2O, 2% agar in distilled water) or yeast extract (0.5% glucose, 0.2% yeast extract, 1% KH2PO4, 2% agar in distilled water) agar medium. The effect of pH on mycelial growth was determined by using buffer solutions to adjust the pH of both minimal and yeast extract agar medium to values ranging from 2 to 9 [13]. Mycelial discs (5 mm in diameter) cut from the active growing culture were used as inocula. After incubation of inoculated plates in completely randomised desgin (CRD) at different temperatures, mycelial growth was determined by colony diameter measurements.
Extraction of secondary metabolites
For secondary metabolite extraction, Trichoderma species were grown in 500 mL flasks containing 100 g (dry weight) solid substrate (wheat bran to white rice (2:1 w/w)) supplemented with 1% (v/v) glycerol (98.9%) and 1% (w/v) (NH4)2SO4) (99.5%) to moisten the substrate [16]. The flasks were sterilized at 121°C for 15 min and, after cooling, inoculated with 20 mL spore suspension (1 × 107 spores/mL) followed by incubation at 28°C for 21 days [16]. The cultures were homogenized by adding methanol, followed by centrifugation at 5000 rpm for 15 min. The supernatant was collected and dried under a vacuum; the obtained methanolic extracts was purified using Sephadex LH-20 column (Sigma-Aldrich, St. Louis, MO) and stored at -20°C until use for oral administration in mice and GC-MS analysis.
Toxicological bioassay of secondary metabolites against F. xylarioides
Seeded agar assay
The methanolic extracts of T. asperellum AU131 and T. longibrachiatum AU158 were filtered through 0.22 μm nylon membranes to obtain purified extracts. The methanolic extracts were mixed with warm SFM agar (200, 100, 50 and 10 μg/mL of methanolic extracts) and plated in sterile Petri plate to analyze the effect of non-volatile metabolites on the test pathogen [35]. A 5 mm mycelial disc of the test pathogen was placed at the center of the Petri plate-containing SFM agar. Plates without methanolic extracts was served as control. These plates were maintained at 28°C until the control plate was fully-grown. The diameter (mm) of the fungal colony growth was measured, and the percent of growth inhibition (PGI) was calculated using the formula,
where, C = radial growth of the pathogen in control plate; T = radial growth of the pathogen in treatment.
Agar diffusion assays
To evaluate antifungal activity of the methanolic extracts, 1 mL of spore suspension (5 × 105 spore/mL) of F. xylarioides, was spread on the surface of the plate containing King B (KB) medium [36, 37]. Holes (5 mm diameter) were made in the center of the agar plates into which the test solutions or solvent control (MeOH) were applied [36, 37]. Fifty μL of the series dilution of the methanolic extracts were poured into the well and methanol was used as negative control. The plates were incubated in CRD at 25°C for 3 days. All bioassays were performed in triplicate and compared with identically prepared solvent controls. The inhibition zones were measured as the diameter of inhibition zones (clear zones) and expressed as the PGI. The size of the F. xylarioides growth inhibition zone was calculated in mm. The plates were photographed with a Canon digital camera and analyzed by using the graphic software “Fiji ImageJ” (GNU General Public License). Finally, the PIG was calculated using the following formula [38].
Where T = diameter of inhibition zones in in the treatment; I = initial hole diameter (5 mm) and C = diameter of inhibition of the solvent control.
GC-MS metabolite profiling of Trichoderma species methanolic extracts
The volatile metabolites were analysed by gas chromatography with a single quadrupole mass spectrometer detector (GC-MS) analysis [32, 33]. Prior to the analysis, dried methanolic extracts obtained from Trichoderma species were dissolved in ethyl acetate and placed in a 1 mL glass vial. The GC system was equipped with an HP-5MS (30 m × 0.25 mm and 0.25 μm 5% diphenyl/95% dimethylpolysiloxane) capillary column (J and W Scientific, Folsom, CA, USA). The instrument was programmed to start at 40°C (held for 2 min) and to increase to 160°C by 6°C /min, after which it increased to 260°C by 10°C/min (held for 4 min). Helium (99.9%) was used as the carrier gas, and the flow rate was 1 mL/min. The ion source temperature was set at 230°C, the ionizing electron energy was 70 eV, and the mass range was 40–450 Da in full-scan acquisition mode [37, 38]. The spectra of the identified volatile metabolites were compared with the spectra of known compounds in the GC-MS of National Institute of Standards and Technology (NIST) database. The threshold for identification was ≥ 90% similarity. The analysis was carried out in triplicate to monitor the repeatability of the analysis. The instrumental responses obtained were interpreted using mass hunter ChemStation software.
Experimental animal and ethical approval
Adult female Swiss albino mice (6–8 weeks old), nulliparous, non-pregnant and weighing 25–30 g [31, 39], were obtained from the Ethiopian Public Health Institute (Addis Ababa, Ethiopia). Female mice were allocated in treatment groups (five animals/per group). All of the animals were kept in polypropylene cages and acclimatized for a period of seven days prior to the start of the experiment. They were placed under standard conditions (23 ± 2°C with a 12 h light-dark cycle), and were fed commercial rodent chow ad libitum and given clean tap water during the experimental period [17]. All of the animals were cared for in compliance with the internationally accepted guide for the care and use of laboratory animals [40], as well as the Addis Ababa University institutional guidelines for animal ethics, which were previously approved by the College of Natural and Computational Sciences Institutional Review Board (CNCS-IRB) under the protocol number IRB/44/2020.
Experimental design
For the effective administration of methanolic extracts and spore suspensions to mice through oral gavage, water was not provided in the morning to induce thirst. After fasting for 3 h, methanolic extracts and live cells (spore suspensions) were administered to each treatment group [31]. Prior to the administration of methanolic extracts and spore suspensions, the body weights of the mice were measured using analytical balance to prepare appropriate doses [31, 41, 42]. The animals in the experimental groups (n = 5) were administered with four distinct concentrations of spores (1 × 106, 1 × 107, 1 × 108 and 1 × 109 spores/mL) and four distinct concentrations of methanolic extracts (600, 1200, 2000 and 5000 mg/kg bw). A total of five female mice received each of the tested doses. Mice in the control group received the same volume of 0.9% NaCl [43].
Acute oral toxicity
Single-dose acute oral toxicity was evaluated based on OECD Guidelines [39, 44]. The general behavior of mice and signs of toxicity (hypo-activity, breathing difficulty, tremors, and convulsion) were continuously observed for 1 h after oral treatment, after which these signs were intermittently observed for 4 h as well as over a period of 24 h [17, 39]. Attention was given to signs of tremors, convulsions, salivation, diarrhea, lethargy, sleep and coma. During the subsequent post-dosing period (14 days), the animals were observed at least once per day. All of the observations were systematically recorded, with individual records maintained for each animal. Body weights were measured at the initiation of treatment, as well as 7 and 14 days after administration [40]. The LD50 value was determined according to the Dragstedt and Lang method described by El Allaoui, Filali [45].
Statistical analysis
Prism software (version 8.0; GraphPad, Inc., San Diego, CA) was used to perform the statistical analysis. Comparisons between the control and treatment groups were conducted using one-way analysis of variance (ANOVA), followed by a Tukey post-hoc test. All of the results from experiments performed in triplicates were presented as the mean value ± standard error of the mean; the threshold for statistical significance was set at p ≤ 0.05.
Results
Effect of temperature and pH on mycelial growth of Trichoderma species
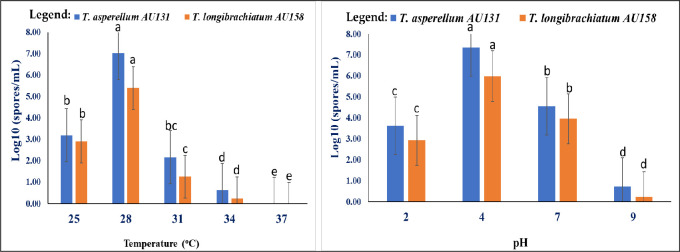
The investigated Trichoderma species showed optimum growth at 28°C on both minimal and yeast extract agar media, with neither species growing at 37°C (Fig 1). They were able to grow at all of the tested pH values (ranging from 2 to 9) at 28°C, with optimum growth observed at pH 4 (Fig 1). It should be stated that both MBCAs were able to grow at pH 7, which is a precondition of growth within the human body.
Fig 1. Effect of temperature and pH on Trichoderma species growth.
Different alphabets depicted in superscript indicate mean treatments that are significantly different according to Tukey’s HSD posthoc test at p ≤ 0.05 each value is an average of 3 replicate samples ± standard error.
Toxic effects of secondary metabolites against F. xylarioides
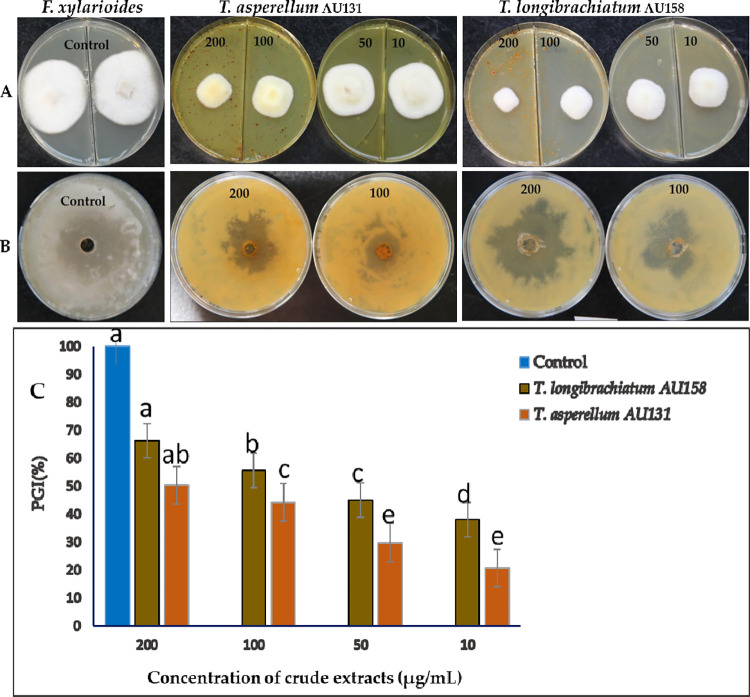
Methanolic extracts from both Trichoderma species amended with PDA medium were capable of inhibiting the mycelial growth of F. xylarioides (p ≤ 0.05). Trichoderma longibrachiatum AU158 showed the highest defined level of in vitro antagonistic activity in both seeded agar assay and agar diffusion assay methods (Fig 2A–2C). ANOVA analysis from seeded agar assay method revealed statistically significant (p ≤ 0.05) differences in the mycelial growth inhibition of F. xylarioides at different methanolic extracts concentrations, with inhibition percentages ranging from 38 to 66.2% (T. longibrachiatum AU158) and 20 to 50.2% (T. asperellum AU131) (Fig 2C). Moreover, the methanolic extracts from T. longibrachiatum AU158 at maximum concentrations incited a change in the colony morphology of the pathogen with significant reduction in growth. The mean inhibitory effect against F. xylarioides restricted almost completely in plates as compared to the control, F. xylarioides, grown alone (Fig 2A). The agar diffusion test of the methanolic extracts from both T. asperellum AU131 and T. longibrachiatum AU158 were the most effective at a concentration of 200 μg/mL, causing 62.5%, and 74.3% inhibition, respectively (Fig 2B). The inhibition was not visible on the test pathogen at a concentration ≤ 50 mg/mL. The radial growth inhibition of F. xylarioides in both bioassay methods are attributed to inhibitory secondary metabolites released by bioagents through competition, mycoparasitism and production of cell wall degrading enzymes.
Fig 2. Bioassay activity of methanolic crude extracts of T. asperellum AU131 and T. longibrachiatum AU158 against F. xylarioides.
A and C: Seeded agar assays: The picture shows the antifungal activity at different concentrations (200, 100, 50 and 10 μg/mL of crude extracts amended with PDA medium) and B: Agar diffusion assays (200 and 100 μg/mL of crude extracts). Different alphabets depicted in superscript indicate mean treatments that are significantly different according to Tukey’s HSD posthoc test at p ≤ 0.05, each value is an average of 3 replicate samples ± standard error. PGI = percentage of growth inhibition.
GC-MS identification of volatile metabolites
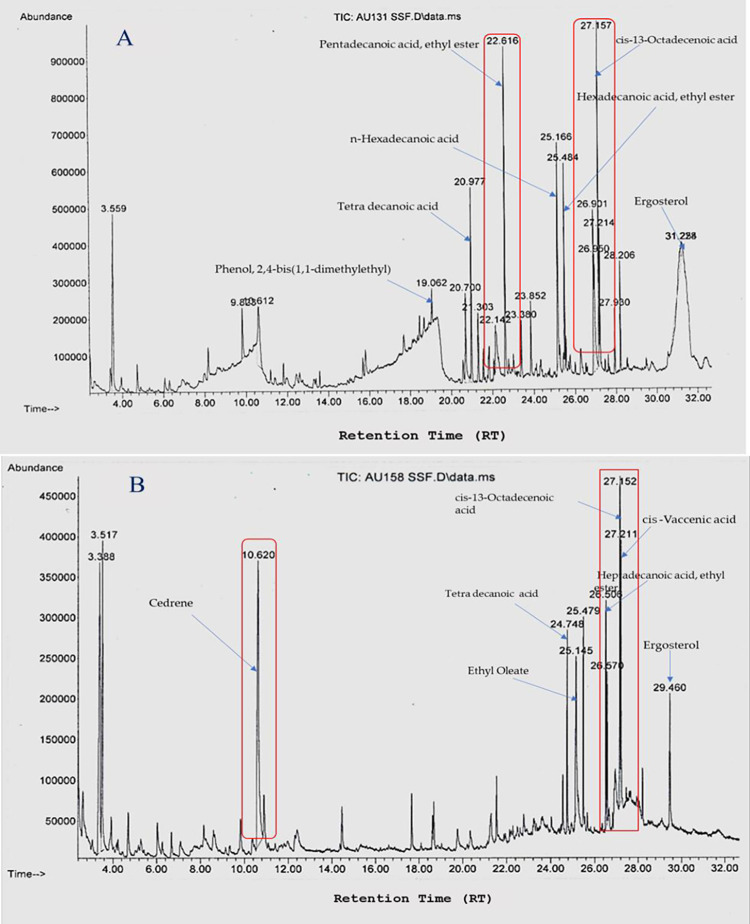
A GC-MS analysis of the methanolic extracts of both BCAs revealed the presence of 23 volatile secondary metabolites. The chromatogram illustrated that various volatile metabolites were present in the analyte, with these compounds then identified based on molecular weight, retention time, and molecular formula. Typical chromatograms and mass spectra of the compounds identified from the methanolic extracts of T. asperellum AU131 and T. longibrachiatum AU158 were shown in Fig 3A and 3B, respectively. The identified compounds were classified as alcohols, acids, sesquiterpenes, aromatics, ketones and esters according to the chemical class (Table 1). The most commonly identified VOCs from the T. asperellum AU131 methanolic extracts were; cis-13-Octadecenoic acid (21.48%), 2,4-bis(1,1-dimethylethyl)-Phenol (17.34%), n-hexadecanoic acid (15.6%), hexadecanoic acid ethyl ester (14.14%), and linoleic acid ethyl ester (11.46%), whereas cis-13-Octadecenoic acid (23.48%), 2,4-bis(1,1-dimethylethyl)-Phenol (18.5%), ethyl oleate (17.83%) and n-hexadecanoic acid (16.72) were detected from the T. longibrachiatum AU158 methanolic extracts (Table 1).
Fig 3.
Chromatograms of the volatile organic compounds identified from T. asperellum AU131 (A) and T. longibrachiatum AU158 (B) crude extracts.
Table 1. Volatile organic compounds of T. asperellum AU131 and T. longibrachiatum AU158 identified by GC-MS.
| Secondary Metabolites | T. asperellum AU131 | T. longibrachiatum AU158 | Chemical class | Molecular formula | Molecular weight (g/mol) | ||
|---|---|---|---|---|---|---|---|
| RT (min) | Area (%) | RT (min) | Area (%) | ||||
| Benzene, 4-ethenyl-1,2-dimethoxy | 15.68 ± 0.2 | 0.23 ± 0.1 | ND | Aromatic | C10H12O2 | 164.20 | |
| 2-(4-Methoxyphenyl) ethanol | 15.81 ± 0.4 | 0.44 ± 0.3 | ND | Alcohol | C9H12O2 | 152.19 | |
| Cedrene | ND | 10.62 ± 0.5 | 0.16 ± 0.1 | Sesquiterpenes | C15H24 | 204.19 | |
| 2,4-bis(1,1-dimethylethyl)-Phenol | 19.06 ± 1.3 | 17.34 ± 0.5 | 19.35 ± 0.4 | 18.5 ± 0.5 | Sesquiterpenes | C14H28O | 206.17 |
| Tetradecanoic acid | 20.98 ± 1.1 | 0.60 ± 0.5 | 24.75 ± 0.9 | 0.75 ± 0.9 | Acid | C14H22O2 | 228.38 |
| Pentadecanoic acid, ethyl ester | 22.62 ± 0.9 | 0.16 ± 0.3 | 24.76 ± 1.1 | 0.38 ± 0.1 | Esters | C17H34O2 | 270.46 |
| Ethyl 13-methyl-tetradecanoate | 23.38 ± 2.1 | 1.05 ± 0.4 | 24.8 ± 0.7 | 0.4 ± 0.2 | Esters | C17H34O2 | 270.46 |
| Palmitoleic acid | ND | 24.97 ± 2.2 | 3.4 ± 0.2 | Acid | C16H30O2 | 254.41 | |
| n-Hexadecanoic acid | 25.17 ± 0.5 | 15.60 ± 0.7 | 25.48 ± 0.4 | 16.72 ± 0.7 | Acid | C16H32O2 | 256.43 |
| Hexadecanoic acid, ethyl ester | 25.48 ± 1.6 | 14.14 ± 0.4 | 25.58 ± 1.6 | 11.84 ± 1.3 | Ester | C18H36O2 | 284.48 |
| Heptadecanoic acid | ND | 26.22 ± 0.5 | 0.1 ± 0.2 | Acid | C17H34O2 | 270.46 | |
| Heptadecanoic acid, ethyl ester | ND | 26.51 ± 1.2 | 0.28 ± 0.1 | Ester | C19H38O2 | 298.51 | |
| Ethyl 15-methyl-hexadecanoate | ND | 26.57 ± 0.1 | 8.1 ± 0.4 | Ester | C19H38O2 | 298.51 | |
| Z,Z-9,12-Octadecadienoic acid | 26.90 ± 2.1 | 13.48 ± 0.4 | 27.05 ± 0.7 | 9.85 ± 0.2 | Acid | C18H32O2 | 280.24 |
| Oleic acid | 26.95 ± 0.6 | 6.38 ± 0.8 | ND | Acid | C18H34O2 | 282.47 | |
| 9,17-Octadecadienal, (Z) | 26.95 ± 1.5 | 6.78 ± 0.6 | ND | Ketones | C18H32O | 264.45 | |
| cis-13-Octadecenoic acid | 27.15 ± 0.2 | 21.48 ± 1.1 | 27.15 ± 2.3 | 23.48 ± 0.4 | Acids | C18H34O2 | 282.47 |
| cis -Vaccenic acid | ND | 27.21 ± 1.5 | 5.49 ± 0.4 | Acids | C18H34O2 | 282.47 | |
| Linoleic acid ethyl ester | 27.21 ± 1.2 | 11.46 ± 0.2 | 27.32 ± 1.7 | 12.87 ± 0.1 | Esters | C20H36O2 | 308.51 |
| Ethyl Oleate | 27.21 ± 1.4 | 5.65 ± 0.3 | 27.25 ± 1.3 | 17.83 ± 0.3 | Esters | C20H38O2 | 310.52 |
| Beta-Sitosterol | 27.98 ± 2.6 | 0.09 ± 0.2 | ND | Alcohol | C29H50O | 414.72 | |
| Ergosta-4,7,22-trien-3.beta.-ol | 28.21 ± 0.7 | 2.15 ± 0.9 | ND | Alcohol | C28H44O | 396.34 | |
| Ergosterol | 31.28 ± 1.7 | 5.33 ± 0.1 | 29.46 ± 0.5 | 4.3 ± 0.4 | Alcohol | C28H44O | 396.66 |
* Not detected, RT = Retention time
Acute toxicological evaluation
The survival rate in the assay was 100%. In the acute toxicity study, methanolic extracts doses under 5000 mg/kg bw and spore doses under 1 × 109 spores/mL did not cause any mortality or signs of toxicity in female mice over the observed period. No obvious clinical signs, including hair loss, scabbing, soft or mucoid feces, and lowered defecation, were noted in the study animals. All of the experimental animals showed normal behavior 14 days after administration, which demonstrates that the metabolites produced by the studied species as well as direct administration of live cells are safe for mice. Furthermore, the administration of metabolites or live cells did not cause any inflammatory or enterotoxigenic effects that could have induced gastroenteritis, e.g., diarrhea. On the other hand, it was not possible to calculate the mean lethal dose (LD50) because the administered doses did not cause death in any of the mice. In other words, the LD50 test results concerning methanolic extracts and spore suspensions mean that the metabolites and Trichoderma species do not cause any lethal effects in mice at doses below 5000 mg/kg bw and 1 × 109 spores/mL, respectively.
Effect of methanolic extracts and spore suspensions on body weight of mice
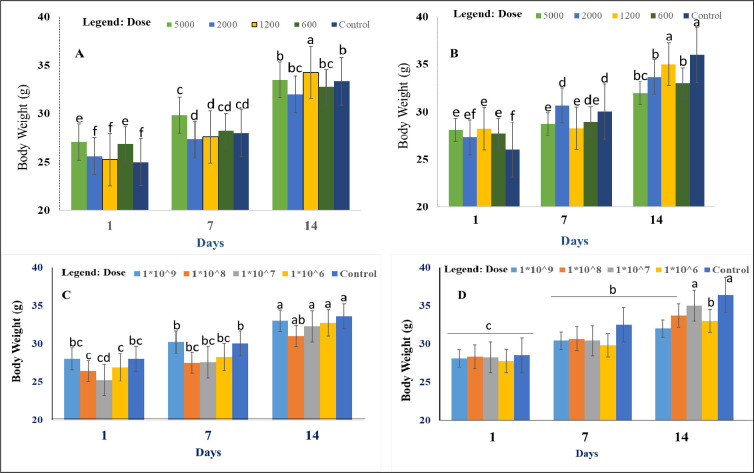
Animals in the treatment group, i.e., which were administered with methanolic extracts or live cells, did not show any apparent changes in body weight, with the values similar to what was observed in the control group, which were administered with a 0.9% NaCl solution. The results illustrated in Fig 4A–4D demonstrate that mice in both the treatment and control groups showed a gradual increase in body weight over the study period. The results presented in this study showed that the oral administration of either methanolic extracts (Fig 4A and 4B) or cellular suspensions Fig 4C and 4D did not cause any significant (p > 0.05) changes in the weight of mice. Furthermore, the body weights of animals in the treatment group did not noticeably differ from the body weights of animals in the control group. This suggests that the methanolic extracts and spores of the two tested Trichoderma species neither support weight loss nor stimulate weight gain.
Fig 4. Changes in the body weights of mice administered with crude extracts and spore suspensions from Trichoderma species.
(A and C) T. asperellum AU131 and (B and D) T. longibrachiatum AU158. (A and B) Mice that were administered with methanolic crude extracts and (C and D) mice that were administered with spore suspensions. Different alphabets depicted in superscript indicate mean treatments that are significantly different according to Tukey’s HSD posthoc test at p ≤ 0.05, each value is an average of 5 replicate samples ± standard error. Each point represents the mean value ± SD (n = 5).
Discussion
The present study investigated the in vitro bioassay and acute toxicity of these MBCAs through short-term assays in animal models to ensure their safety in coffee farm applications. Most of the species involved in Trichoderma infections are considered as opportunistic pathogens [46], with the virulence factors including mycelial growth at 37°C and neutral pH, hemolytic activity and toxicity to mammalian cells [13, 14], along with resistance to antifungal compounds [47]. Acute toxicity refers to the toxic effects of substances that result either from a single or multiple doses in a short period of time (usually less than 24 h) and can be described within 14 days [42]. The results of the present study showed that neither T. asperellum AU131 nor T. longibrachiatum AU158 grew on minimal media at 37°C. However, both species were able to grow at physiological pH, which agrees with the reports of Antal et al. [13] and Kredics et al. [14]. More specifically, Antal et al. [13] reported that T. longibrachiatum strains showed optimum growth at 30°C on both minimal and yeast extract agar media, while all of the strains were also able to grow at 40°C. Mikkola et al. [48] reported that toxic T. longibrachiatum strains grew on malt extract agar (MEA) at 22°C and 37°C. Hence, the concerns and risks associated with biopesticides can differ from one species to another, most likely requiring a case-by-case approach in associated risk assessment.
Trichoderma species are known to produce a wide range of bioactive secondary metabolites that are known to have antifungal, antibacterial, and toxic properties to control a wide range of phytopathogens, such as Fusarium species, Botrytis cinerea, Pythium species, Rhizoctonia solani, Sclerotinia sclerotiorum, and Ustilago maydis [8, 21, 37]. In the present study, the results of the antifungal activity of methanolic extracts obtained from both T. asperellum AU131 and T. longibrachiatum AU158 showed that the extracted secondary metabolites inhibited the growth of F. xylarioides in the seeded agar and agar diffusion assays, as shown in Fig 2A and 2B. Metabolic extracts from both bioagents have antifungal activity against F. xylarioides, however, the extract from T. longibrachiatum AU158 was more effective, as it was able to inhibit at the minimum concentration (10 mg/mL) in seeded agar assay. Many species of Trichoderma, such as T. harzianum, T. viride, T. hamatum, T. atroviride, and T. virens, have been reported to be effective in control of a wide range of soil borne plant pathogens [49, 50]. However, the biocontrol efficacy varies with Trichoderma species and target plant diseases [51]. A significant advancement from the present study is the finding that the SSF and methanolic extracts of the AU131 and AU158 strains provided a significant inhibitory effect on F. xylarioides, a causative agent of coffee wilt disease. Our findings suggest that both strains can be considered as a biocontrol agent in the effort of using alternative approaches to control coffee wilt.
Analysis of the GC-MS chromatograms showed that both MBCAs produced large quantities of VOCs that were identified as alcohols, acids, esters, sesquiterpenes and ketones. This wide range of VOCs emitted by both Trichoderma species is in line with the diverse VOCs produced by Trichoderma species reported in previous studies [25, 37]. The volatile secondary metabolites identified from methanolic extracts of the two investigated Trichoderma species (T. asperellum AU131 and T. longibrachiatum AU158) also agreed with what has previously been reported in the literature [32, 33, 37, 52]. The fungal production of VOCs is a dynamic process in that it is directly affected by both genetic and environmental factors, which include community composition, substrate, temperature, moisture level, and pH [53–56]. The identified metabolites play important roles in mycoparasitic interactions as well as induced systemic resistance (ISR) in plants via the upregulation of jasmonic acid and salicylic acid synthesis [57]. For instance, 2,4-bis(1,1-dimethylethyl)-phenol (2,4-DTBP) was found to be effective against the agriculturally important root-rot fungus; Fusarium oxysporum based on the inhibition of spore germination and hyphal growth [58]. During fungal spore germination, 2,4-DTBP completely inhibited germination by preventing the emergence of a normal germ tube, eventually leading to abnormal branching and swelling of the hyphae [58].
As GC-MS is the first step towards profiling the metabolites present in methanolic extracts, the present study showed presence of five major VOCs from both BCAs, viz., cis-13-Octadecenoic acid, 2,4-bis(1,1 dimethylethyl)-Phenol, n-hexadecanoic acid, ethyl oleate and hexadecanoic acid ethyl ester (Fig 3A and 3B). Most of the metabolites identified in this study are widely used as antimicrobials, food additives, cancer drugs, herbicides and pesticides. Specifically, cis-13-Octadecenoic acid and n-hexadecanoic acid are reported to have anti-inflammatory, cancer preventive and hepatoprotective properties [59]. The other identified linoleic acid ester and Z,Z-9-12-Octadecenoic acid also have anti-inflammatory, antiandrogenic, and anemiagenic properties [60]. Moreover, two of the identified volatile compounds–tetradecanoic acid and n-hexadecanoic acid methyl ester–are antioxidants [61]. Since there are no complete toxicity reports for all the secondary metabolites from Trichoderma species, we tested their toxicological levels using animal models (mice). We used methanolic extracts to assess the toxicological risks associated with a particular MBCA rather than using purified commercial metabolites as per the guidelines of the OECD.
Methanolic extracts and spore suspensions were first administered by oral gavage, since this is the most common route of human exposure. Thus, the acute toxicity assays were performed to monitor the harmful effects of an MBCAs to the organism following single or short-term exposure [62]. The performed experiments mainly evaluated mortality, changes in behavior, body weight, and other characteristics relative to the overall well-being of mice. In the present study, the in vivo toxicity evaluation of methanolic extracts and spore suspensions of Trichoderma species did not reveal any mortality among the Swiss albino mice; this suggests that the extracts and spore suspensions are not deadly to mammals. A report by the European Food Safety Authority EFSA [63] indicated that T. harzianum Rifai strain T-22 and T. asperellum T25 did not result in adverse effects following the oral, intratracheal, subcutaneous and intravenous administration of doses ranging from 6.4 × 106 to 1.5 × 107 colony forming unit (cfu)/animal. Moreover, the report indicated complete clearance of the spore suspension within two days of oral administration, a dramatic decrease in levels in the lungs by day 21 post-intratracheal administration, and a marked decrease in the number of cfu from the colonized organs following intravenous administration. No signs of pathogenicity were observed for any of the routes of exposure, which is in agreement with the results of the present study.
However, methanolic extracts of various T. longibrachiatum isolates have been reported to contain heat-resistant substances that inhibit the motility of boar spermatozoa and quench the mitochondrial transmembrane potential (ΔΨm) of sperm cells at low concentrations [64]. Several clinical isolates originally identified based on their morphological characters were recently re-identified by sequence-based molecular techniques as T. longibrachiatum, which provided the most frequently occurring clinical etiological agent within the genus Trichoderma [65, 66]. Thus, the biotechnological and agricultural application of T. longibrachiatum strains should be carefully monitored to minimize possible health risks.
Methanolic extracts from T. asperellum AU131 showed no toxicity up to concentrations of 5000 mg/kg bw. Moreover, acute oral toxicity studies conducted with both T. asperellum AU131 and T. longibrachiatum AU158 cellular suspensions did not result in treatment-related adverse effects across doses ranging from 1 × 106 to 1 × 109 spores/mL. This was supported by previous observations that the oral administration of methanolic extracts and cellular suspensions representing various fungal BCAs demonstrated very low toxicity in rodents and ruminants [28, 67]. Moreover, a report from the USA Environmental Protection Agency [27] demonstrated that T. asperellum strain ICC 012 is non-toxic to rats at a dose of 2000 mg/kg bw and 4.2 × 109 cfu/g. The product formulated from this species contains 99.9% w/w T. asperellum strain ICC 012 as the active ingredient, with a minimum and nominal content of 1 × 109 and 2.5 × 109 cfu/g dry weight), respectively. The report clearly stated that exposure to this species does not cause any acute, sub-chronic, chronic, immune, endocrine, or non-dietary issues.
On the other hand, the oral median lethal dose (LD50) could not be calculated since the administered doses did not cause any death to the mice. These results suggest that the lethal doses for both T. asperellum AU131 and T. longibrachiatum AU158 methanolic extracts exceed 5000 mg/kg bw, which represents the highest reference dose [17]. In this regard, methanolic extracts may be considered safe at the tested levels, i.e., 5000 mg/kg bw and below. Changes in body weight is an important index for evaluating the toxicity of bioformulated products [68]. In the present study, both the treatment and control groups demonstrated a gradual increase in mean body weight. However, the difference in weight gain between the control and treatment groups was statistically insignificant (p ≥ 0.05).
Moreover, the study evaluate the acute toxicity of T. asperellum AU131 and T. longibrachiatum AU158 using Swiss albino mice and GC-MS to identify volatile metabolites of methanolic extracts. However, non-volatile metabolites produced by Trichoderma species and showing antimicrobial activity such as gliotoxin, peptaibols, gliovirin, polyketides, and pyrones [69–71] were not addressed in this study since they are not detected by GC-MS. Thus, further study is in progress to quantify and assess their toxicity related risks by using untargeted liquid chromatography–high resolution mass spectrometry (LC–HRMS).
Conclusion
In conclusion, methanolic extracts and spore suspensions from T. asperellum AU131 and T. longibrachiatum AU158 were neither toxic nor pathogenic to Swiss albino mice across the tested range of concentrations. A GC-MS analysis of the extracts from both bioagents revealed the presence of 23 VOCs that were classified as alcohols, acids, sesquiterpenes, ketones and aromatic compounds. Both Trichoderma species showed optimum growth at 28°C on minimal and yeast extract agar media, with neither species growing at 37°C. In addition, methanolic extracts from T. longibrachiatum showed the highest inhibition activity and was active even at a low concentration (10μg/mL). In general, the study provides good insight to use these mBCAs as antagonists in biocontrol of CWD, F. xylarioides.
Acknowledgments
We would like to thank the Department of Microbial, Cellular and Molecular Biology and Department of Chemistry of Addis Ababa University (AAU), Ethiopia, for the laboratory facilities. Special thanks goes to the Department of Plant Breeding of the Swedish University of Agricultural Sciences (SLU), Sweden, for valuable support to the project.
Data Availability
All relevant data are within the article.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Roossinck MJ, García-Arenal F. Ecosystem simplification, biodiversity loss and plant virus emergence. Curr opin Virol. 2015;10:56–62. doi: 10.1016/j.coviro.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ab Rahman SFS, Singh E, Pieterse CM, Schenk PM. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018;267:102–11. doi: 10.1016/j.plantsci.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 3.Coppola M., Cascone P., Lelio I.D., Woo S.L., Lorito M., Rao R., Pennacchio F., Guerrieri E. and Digilio M.C. Trichoderma atroviride P1 Colonization of Tomato Plants Enhances Both Direct and Indirect Defense Barriers Against Insects. Front Physiol. 2019; 10: 813–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javaid A. and Ali S. Herbicidal activity of culture filtrates of Trichoderma spp. against two problematic weeds of wheat. Nat Prod Res. 2011; 25: 730–740. [DOI] [PubMed] [Google Scholar]
- 5.Zamanizadeh HR, Hatami N, Aminaee MM, Rakhshandehroo F. Application of biofungicides in control of damping disease off in greenhouse crops as a possible substitute to synthetic fungicides. Inter J Environ Sci Technol. 2011;8(1):129–36. doi: 10.1007/bf03326202 [DOI] [Google Scholar]
- 6.Wu Q, Sun R, Ni M, Yu J, Li Y, Yu C, et al. Identification of a novel fungus, Trichoderma asperellum and comprehensive evaluation of its biocontrol efficacy. PLoS One. 2017;12(6):e0179957. Epub 2017/06/24. doi: 10.1371/journal.pone.0179957 ; PubMed Central PMCID: PMC5482467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstantinovas C, de Oliveira Mendes TA, Vannier-Santos MA, Lima-Santos J. Modulation of human immune response by fungal biocontrol agents. Front Microbiol. 2017;8:39. doi: 10.3389/fmicb.2017.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan RAA, Najeeb S, Mao Z, Ling J, Yang Y, Li Y, et al. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and Root-knot nematode. Microorganisms. 2020;8(3):401–412. doi: 10.3390/microorganisms8030401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Xu B, Zhang J, Gan Y. Identification of the antifungal activity of Trichoderma longibrachiatum T6 and assessment of bioactive substances in controlling phytopathgens. Pestic Biochem Phys. 2018;147:59–66. [DOI] [PubMed] [Google Scholar]
- 10.Shahid M, Srivastava M, Singh A, Kumar V, Rastogi S, Pathak N, et al. Comparative study of biological agents, Trichoderma harzianum (Th-Azad) and Trichoderma viride (01PP) for controlling wilt disease in pigeon pea. J Microb Biochem Technol. 2014;6(2): 110–5. [Google Scholar]
- 11.Eltem R, Sayit S, Sozer S, Sukan FV, inventors; Google Patents, assignee. Production of Trichoderma citrinoviride micropropagules as a biocontrol agent by means of an economical process 2017. [Google Scholar]
- 12.de Rezende LC, de Andrade Carvalho AL, Costa LB, de Almeida Halfeld-Vieira B, Silva LG, Pinto ZV, et al. Optimizing mass production of Trichoderma asperelloides by submerged liquid fermentation and its antagonism against Sclerotinia sclerotiorum. World J Microbiol Biotechnol. 2020;36(8):1–14. [DOI] [PubMed] [Google Scholar]
- 13.Antal Z, Kredics L, Pakarinen J, Dóczi I, Andersson M, Salkinoja-Salonen M, et al. Comparative study of potential virulence factors in human pathogenic and saprophytic Trichoderma longibrachiatum strains. Acta Microbiol Immunol Hung. 2005;52(3–4):341–50. [DOI] [PubMed] [Google Scholar]
- 14.Kredics L, Antal Z, Szekeres A, Manczinger L, Dóczi I, Kevei F, et al. Production of extracellular proteases by human pathogenic Trichoderma longibrachiatum strains. Acta Microbiol Immunol Hung. 2004;51(3):283–95. [DOI] [PubMed] [Google Scholar]
- 15.Mulatu A, Megersa N, Abena T, Kanagarajan S, Liu Q, Alemu T, et al. Biodiversity of the Genus Trichoderma in the Rhizosphere of Coffee (Coffea arabica) Plants in Ethiopia and their Potential Use in Biocontrol of Coffee Wilt Disease. Crops. 2022; 2(2): 120–141. doi: 10.3390/crops2020010 [DOI] [Google Scholar]
- 16.Mulatu A, Alemu T, Megersa N, Vetukuri RR. Optimization of Culture Conditions and production of Bio-Fungicides from Trichoderma species under Solid-State Fermentation using Mathematical Modeling. Microorganisms. 2021;9(8):1675–700. Epub 2021/08/28. doi: 10.3390/microorganisms9081675 ; PubMed Central PMCID: PMC8400879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhandary BSK, Sharmila K, Kumari NS, Bhat SV. Acute and subacute toxicity study of the ethanol extracts of Punica granatum (Linn). Whole fruit and seeds and synthetic ellagic acid in Swiss albino mice. Asian J Pharm Clin Res. 2013;6(4):192–8. [Google Scholar]
- 18.Alfiky A, Weisskopf L. Deciphering Trichoderma–Plant Pathogen Interactions for Better Development of Biocontrol Applications. J Fungi 2021;7:61–79. doi: 10.3390/jof7010061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amira MB, Lopez D, Mohamed AT, Khouaja A, Chaar H, Fumanal B, et al. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol Control. 2017;110:70–8. [Google Scholar]
- 20.Filizola PRB, Luna MAC, de Souza AF, Coelho IL, Laranjeira D, Campos-Takaki GM. Biodiversity and phylogeny of novel Trichoderma isolates from mangrove sediments and potential of biocontrol against Fusarium strains. Microb Cell Fact. 2019;18(1):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermosa R, Cardoza RE, Rubio MB, Gutiérrez S, Monte E. Secondary metabolism and antimicrobial metabolites of Trichoderma. Biotechnology and Biology of Trichoderma: Elsevier; 2014. p. 125–37. [Google Scholar]
- 22.Keswani C, Mishra S, Sarma BK, Singh SP, Singh HB, biotechnology. Unraveling the efficient applications of secondary metabolites of various Trichoderma species. J Appl Microbiol. 2014;98(2):533–44. [DOI] [PubMed] [Google Scholar]
- 23.Ramírez-Valdespino CA, Casas-Flores S, Olmedo-Monfil V. Trichoderma as a Model to Study Effector-Like Molecules. Front Microbiol. 2019;10. doi: 10.3389/fmicb.2019.01030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan RAA, Najeeb S, Hussain S, Xie B, Li Y. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms. 2020;8(6):817. doi: 10.3390/microorganisms8060817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva LR, Valadares-Inglis MC, Peixoto GHS, de Luccas BEG, Muniz PHPC, Magalhães DM, et al. Volatile organic compounds emitted by Trichoderma azevedoi promote the growth of lettuce plants and delay the symptoms of white mold. Biol Contr. 2021;152:104447. [Google Scholar]
- 26.Macinko J, Starfield B, Shi L. The contribution of primary care systems to health outcomes within Organization for Economic Cooperation and Development (OECD) countries, 1970–1998. Health Serv Res. 2003;38(3):831–65. doi: 10.1111/1475-6773.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Firestone M, Kavlock R, Zenick H, Kramer M. The US Environmental Protection Agency strategic plan for evaluating the toxicity of chemicals. J Toxicol Environ Health, Part B. 2010;13(2–4):139–62. [DOI] [PubMed] [Google Scholar]
- 28.Favilla M, Macchia L, Gallo A, Altomare C. Toxicity assessment of metabolites of fungal biocontrol agents using two different (Artemia salina and Daphnia magna) invertebrate bioassays. Food Chem Toxicol. 2006;44(11):1922–31. [DOI] [PubMed] [Google Scholar]
- 29.Altomare C, Pernfuss B, Strasser H, editors. Assessing potential cytotoxicity of biocontrol microorganisms using invertebrate assays 2012. [Google Scholar]
- 30.Wijffels R. Biosafety and the Environmental Uses of Micro-Organisms. Harmonisation of Regulatory Oversight in Biotechnology Paris: OECD Publishing. Paris, France: OECD Publishing; 2015. [Google Scholar]
- 31.OECD. Guideline for the Testing of Chemicals. Acute Oral Toxicity e Acute Toxic Class Method: Test No-423. 2001. [Google Scholar]
- 32.Stoppacher N, Kluger B, Zeilinger S, Krska R, Schuhmacher RJJoMM. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J Microbiol Methods. 2010;81(2):187–93. [DOI] [PubMed] [Google Scholar]
- 33.Siddiquee S, Cheong BE, Taslima K, Kausar H, Hasan MM. Separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC-MS using three different capillary columns. J Chromatogr Sci. 2012;50(4):358–67. doi: 10.1093/chromsci/bms012 [DOI] [PubMed] [Google Scholar]
- 34.Skrobek A, Boss D, Défago G, Butt TM, Maurhofer M. Evaluation of different biological test systems to assess the toxicity of metabolites from fungal biocontrol agents. Toxicol Lett. 2006;161(1):43–52. doi: 10.1016/j.toxlet.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 35.Dubey SC, Suresh M, Singh B. Evaluation of Trichoderma species against Fusarium oxysporum f. sp. ciceris for integrated management of chickpea wilt. Biol Contr. 2007;40(1):118–27. doi: 10.1016/j.biocontrol.2006.06.006 [DOI] [Google Scholar]
- 36.Bano N, Musarrat J. Characterization of a novel carbofuran degrading Pseudomonas sp. with collateral biocontrol and plant growth promoting potential. FEMS Microbiol Lett. 2004;231(1):13–7. doi: 10.1016/s0378-1097(03)00894-2 . [DOI] [PubMed] [Google Scholar]
- 37.Hernández-Rodríguez A, Heydrich-Pérez M, Acebo-Guerrero Y, Velazquez-Del Valle MG, Hernandez-Lauzardo AN. Antagonistic activity of Cuban native rhizobacteria against Fusarium verticillioides (Sacc.) Nirenb. in maize (Zea mays L.). Appl Soil Ecol. 2008;39(2):180–6. [Google Scholar]
- 38.Khan MR, Chonhenchob V, Huang C, Suwanamornlert P. Antifungal Activity of Propyl Disulfide from Neem (Azadirachta indica) in Vapor and Agar Diffusion Assays against Anthracnose Pathogens (Colletotrichum gloeosporioides and Colletotrichum acutatum) in Mango Fruit. Microorganisms. 2021;9(4). Epub 20210414. doi: 10.3390/microorganisms9040839 ; PubMed Central PMCID: PMC8070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.OECD. Repeated dose 28-day oral toxicity study in rodents. Paris, France: 2008. [Google Scholar]
- 40.Jia R-l, Xu S, Guo Y-f, Yin Z-q, Fei L, Xiong J, et al. Acute and subchronic toxicity as well as evaluation of safety pharmacology of modified pulsatilla granules. J Integrat Agri. 2017;16(3):671–8. [Google Scholar]
- 41.Mancebo A, Molier T, González B, Lugo S, Riera L, Arteaga M, et al. Acute oral, pulmonary and intravenous toxicity/pathogenicity testing of a new formulation of Bacillus thuringiensis var israelensis SH-14 in rats. Regul Toxicol Pharmacol. 2011;59(1):184–90. [DOI] [PubMed] [Google Scholar]
- 42.Goudah A, Abdo-El-Sooud K, Yousef MA. Acute and subchronic toxicity assessment model of Ferula assa-foetida gum in rodents. Vet World. 2015;8(5):584. doi: 10.14202/vetworld.2015.584-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flood J. Coffee wilt disease: CABI, London; 2010. [Google Scholar]
- 44.OECD. Organization for Economic Cooperation and Development (OECD) Guidelines For Testing of Chemicals, no. 423. Paris, France: 2001. [Google Scholar]
- 45.El Allaoui A, Filali FR, Oumokhtar B, Ibijbijen J. Evaluation de la toxicité aigue du colorant (Rhodamine B) utilisé dans la fabrication des saucisses traditionnelles dans la ville de Meknès au Maroc. La Science en Liberté. 2011;3:1–15. [Google Scholar]
- 46.Trabelsi S, Hariga D, Khaled S. First case of Trichoderma longibrachiatum infection in a renal transplant recipient in Tunisia and review of the literature. La Tunisie Medicale. 2010;88(1):52–7. [PubMed] [Google Scholar]
- 47.Alınç T, Cusumano A, Peri E, Torta L, Colazza S. Trichoderma harzianum strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity. J Chem Ecol. 2021;47(4):455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikkola R, Andersson MA, Kredics L, Grigoriev PA, Sundell N, Salkinoja‐Salonen MS. 20‐Residue and 11‐residue peptaibols from the fungus Trichoderma longibrachiatum are synergistic in forming N a+/K+‐permeable channels and adverse action towards mammalian cells. FEBS J. 2012;279(22):4172–90. [DOI] [PubMed] [Google Scholar]
- 49.Mukherjee PK, Mehetre ST, Sherkhane PD, Muthukathan G, Ghosh A, Kotasthane AS, et al. A novel seed-dressing formulation based on an improved mutant strain of Trichoderma virens, and its field evaluation. Front Microbiol. 2019;10:1910. doi: 10.3389/fmicb.2019.01910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaverri P, Branco-Rocha F, Jaklitsch W, Gazis R, Degenkolb T, Samuels GJ. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia. 2015;107(3):558–90. Epub 2015/02/11. doi: 10.3852/14-147 ; PubMed Central PMCID: PMC4885665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2(1):43–56. [DOI] [PubMed] [Google Scholar]
- 52.Riba M, Sans A, Bau P, Grolleau G, Renou M, Guerrero A. Pheromone response inhibitors of the corn stalk borer Sesamia nonagrioides. Biological evaluation and toxicology. J Chem Ecol. 2001;27(9):1879–97. [DOI] [PubMed] [Google Scholar]
- 53.Hung R, Lee S, Bennett JW. Fungal volatile organic compounds and their role in ecosystems. Appl Microbiol Biotechnol. 2015;99(8):3395–405. doi: 10.1007/s00253-015-6494-4 [DOI] [PubMed] [Google Scholar]
- 54.Mutawila C, Vinale F, Halleen F, Lorito M, Mostert L. Isolation, production andin vitro effects of the major secondary metabolite produced by Trichoderma species used for the control of grapevine trunk diseases. Plant Pathol. 2016;65(1):104–13. doi: 10.1111/ppa.12385 [DOI] [Google Scholar]
- 55.Lee S, Behringer G, Hung R, Bennett J. Effects of fungal volatile organic compounds on Arabidopsis thaliana growth and gene expression. Fungal Ecol. 2019;37:1–9. [Google Scholar]
- 56.Singh A, Shahid M, Srivastava M, Pandey S, Sharma A, Kumar V. Optimal physical parameters for growth of Trichoderma species at varying pH, temperature and agitation. Virol Mycol. 2014;3(1):127–34. [Google Scholar]
- 57.Singh S, Nair V, Jain S, Gupta Y. Evaluation of anti-inflammatory activity of plant lipids containing ⍺–linolenic acid. Ind J Exper Biol. 2008;46:453–6. [PubMed] [Google Scholar]
- 58.Pullaiah T, Murthy K, Goud P, Kumar T, Vijayakumar R. Medicinal plants used by the tribals of Nallamalais, Eastern Ghats of India. J Trop Med Plants. 2003;4(2):237–44. [Google Scholar]
- 59.Guasch-Ferré M, Bhupathiraju SN, Hu FB. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin Chem. 2018;64(1):82–98. Epub 2017/10/19. doi: 10.1373/clinchem.2017.272344 ; PubMed Central PMCID: PMC5975233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajeswari G, Murugan M, Mohan V. GC-MS analysis of bioactive components of Hugonia mystax L.(Linaceae). Res J Pharm Biol Chem Sci. 2012;3(4):301–8. [Google Scholar]
- 61.Shivprasad M, Varsha J. GC-MS Screening of Some Bioactive Compounds from Methanolic Extracts of Medicinally Relevant Wild Edible Plant Parts. Int J Sci Res. 2018;4: 49–56. [Google Scholar]
- 62.Melo KM, Oliveira R, Grisolia CK, Domingues I, Pieczarka JC, de Souza Filho J, et al. Short-term exposure to low doses of rotenone induces developmental, biochemical, behavioral, and histological changes in fish. Environ Sci Poll Res. 2015;22(18):13926–38. [DOI] [PubMed] [Google Scholar]
- 63.EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance Trichoderma harzianum Rifai strains T‐22 and ITEM‐908. Eur Food Safety Auth J. 2013;11(10):3055. [Google Scholar]
- 64.Szekeres A, Leitgeb B, Kredics L, Antal Z, Hatvani L, Manczinger L, et al. Peptaibols and related peptaibiotics of Trichoderma. Acta Microbiol Immunol Hung. 2005;52(2):137–68. [DOI] [PubMed] [Google Scholar]
- 65.Druzhinina IS, Komoń-Zelazowska M, Kredics L, Hatvani L, Antal Z, Belayneh T, et al. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiol. 2008;154(11):3447–59. [DOI] [PubMed] [Google Scholar]
- 66.Kuhls K, Lieckfeldt E, Samuels GJ, Meyer W, Kubicek CP, Börner T. Revision of Trichoderma sect. Longibrachiatum including related teleomorphs based on analysis of ribosomal DNA internal transcribed spacer sequences. Mycologia. 1997;89(3):442–60. [Google Scholar]
- 67.Ryu D. The occurrence, production and stability of zearalenone and other Fusarium toxins: The University of Nebraska-Lincoln; 1997. [Google Scholar]
- 68.Rivas CAB, Castillo AA, Martínez HS, Zapata EP, Hernández JB, Tassé YM. Acute oral toxicity of Azadirachta indica (Neem Tree). Rev Cuba de Plantas Medicinales. 2013;18(3):502–7. [Google Scholar]
- 69.Zhang SH, Yang J, Ma H, Yang Y, Zhou GF, Zhao X, et al. Longibramides A-E, Peptaibols Isolated from a Mushroom Derived Fungus Trichoderma longibrachiatum Rifai DMG-3-1-1. Chem Biodivers. 2021;18(5):e2100128. Epub 2021/03/13. doi: 10.1002/cbdv.202100128 . [DOI] [PubMed] [Google Scholar]
- 70.Stoppacher N, Reithner B, Omann M, Zeilinger S, Krska R, Schuhmacher R. Profiling of trichorzianines in culture samples of Trichoderma atroviride by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(24):3963–70. Epub 2007/11/17. doi: 10.1002/rcm.3301 . [DOI] [PubMed] [Google Scholar]
- 71.Rebuffa S, Prigent Y, Auvin-Guette C, Bodo B. Tricholongins BI and BII, 19‐residue peptaibols from Trichoderma longibrachiatum: Solution structure from two‐dimensional NMR spectroscopy. Eur J Biochem. 1991;201(3):661–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the article.