Abstract
Studies of Escherichia coli membranes that were highly enriched in the Salmonella enterica serovar Typhimurium PhoQ protein showed that the presence of ATP and divalent cations such as Mg2+, Mn2+, Ca2+, or Ba2+ resulted in PhoQ autophosphorylation. However, when Mg2+ or Mn2+ was present at concentrations higher than 0.1 mM, the kinetics of PhoQ autophosphorylation were strongly biphasic, with a rapid autophosphorylation phase followed by a slower dephosphorylation phase. A fusion protein lacking the sensory and transmembrane domains retained the autokinase activity but could not be dephosphosphorylated when Mg2+ or Mn2+ was present at high concentrations. The instability of purified [32P]phospho-PhoP in the presence of PhoQ-containing membranes indicated that PhoQ also possesses a phosphatase activity. The PhoQ phosphatase activity was stimulated by increasing the Mg2+ concentration. These data are consistent with a model in which Mg2+ binding to the sensory domain of PhoQ coordinately regulates autokinase and phosphatase activities.
Two-component signal transduction systems allow bacteria to adapt to changing environmental conditions by modulating the transcription of specific genes. Two-component systems usually are characterized by a transmembrane protein histidine kinase and a cytoplasmic response regulator (reviewed in references 19 and 22). Upon detection of environmental stimuli through its sensory domain, the histidine protein kinase autophosphorylates by transfer of the γ-phosphoryl group from ATP to a highly conserved histidine residue in the transmitter domain of the protein (23). Through its N-terminal receiver domain, the response regulator catalyzes the transfer of the phosphoryl group from the conserved histidine residue to an invariant aspartate residue (23). This results in the activation of the output domain of the response regulator, which in most cases possesses DNA binding properties and functions as a transcriptional regulator. In addition to autokinase activity and phosphorylation of the response regulator, many histidine protein kinases possess a phosphatase activity (1, 11, 17). The balance between autokinase activity and phosphatase activity controls the level of phosphorylated response regulator (14, 20, 24). However, it is not clear whether external signals affect the autokinase activity and/or the phosphatase activity of histidine protein kinases (3, 12, 16).
In the case of the facultative intracellular pathogen Salmonella enterica serovar Typhimurium, a two-component system that consists of PhoP (the response regulator) and PhoQ (the histidine protein kinase) has been shown to be a major regulator of virulence. By repressing or activating the expression of more than 40 different genes, the PhoP/PhoQ system controls entry into epithelial cells, survival within macrophages, resistance to antimicrobial peptides, and lipopolysaccharide modifications (reviewed in references 5 and 9). In contrast to many histidine protein kinases for which the physiological ligand is still unknown, the PhoQ protein has been shown to sense changes in the external concentration of Mg2+ (6, 7).
The PhoQ protein (487 amino acids) is organized into an N-terminal periplasmic sensory domain flanked at both ends by transmembrane segments and a C-terminal cytoplasmic signaling domain. By analogy with other histidine protein kinases, PhoQ is considered to be present at the cytoplasmic membrane as a homodimer in which the kinase domain from one subunit phosphorylates the conserved histidine residue in the second subunit (2, 18). Upon depletion of external Mg2+, a signal is propagated across the membrane by an unknown mechanism, resulting in activation of the phosphorylation cascade (6, 10). In this study, we expressed the S. enterica serovar Typhimurium PhoQ protein in Escherichia coli and showed that the recombinant protein possesses both autokinase and phosphatase activities.
Expression of PhoQ in membranes.
For expression of the PhoQ protein of S. enterica serovar Typhimurium, the phoQ gene was amplified by PCR and cloned into expression vector pET-3a (Novagen) to generate plasmid pET-Q. E. coli BL21(λDE3)/pLysE cells, transformed with pET-Q, were grown at 37°C in Luria-Bertani medium supplemented with the appropriate antibiotics. Transcription of the phoQ gene was induced at an optical density of ≈0.8 by adding 0.5 mM isopropyl-B-d-thiogalactopyranoside (IPTG). Membranes were prepared as described previously (15) and analyzed for PhoQ production by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). A protein that migrated on SDS-PAGE with approximately the mobility expected for the phoQ gene product (55 kDa) was detected in the membrane fraction of cells that were induced by IPTG (approximately 10% of total membrane proteins) (data not shown). This protein was not detected in membranes prepared from uninduced cells. To establish that the overproduced protein corresponds to PhoQ, we constructed a vector encoding the PhoQ protein fused to a C-terminal tag of six histidine residues (PhoQ-His) and performed Western blot analysis by using a chromogenic conjugate directed against the His tag (Qiagen). As expected, the PhoQ-His protein (56 kDa) was detected by the chromogenic conjugate, whereas wild-type PhoQ, which does not harbor a His tag, was not recognized (data not shown).
Autokinase activity of PhoQ.
Autokinase assays were performed by incubating PhoQ-containing membranes (12 μg of total membrane protein) in a 15-μl total volume with 0.1 mM [γ-32P]ATP (10 Ci/mmol) in phosphorylation buffer (50 mM Tris-HCl [pH 7.5], 200 mM KCl, 0.1 mM EDTA, 10% glycerol) supplemented with increasing concentrations of various divalent cations. Reactions were carried out at 22°C and stopped by the addition of SDS sample buffer. Samples were heated at 37°C for 5 min and applied to 10% polyacrylamide gels. Dried gels were analyzed with a PhosphorImager. Under these experimental conditions, the relationship between incorporated radioactivity and the amount of membrane protein (8 to 32 μg) was linear, indicating that PhoQ was not in excess. The radiolabeled product was identified as PhoQ since control membranes prepared from E. coli BL21(λDE3)/pLysE cells transformed with the parent vector pET-3a were devoid of the phosphorylated species (data not shown).
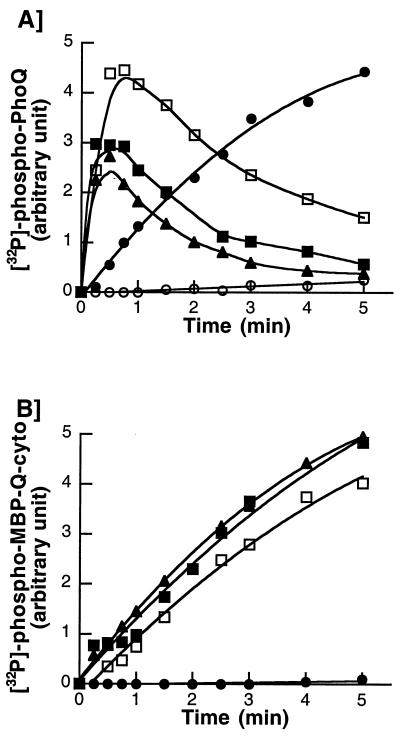
For autophosphorylation, histidine protein kinases require certain divalent cations, mostly Mg2+, present in their cytoplasmic catalytic domain (23). In the case of the PhoQ sensor kinase, it has been shown that Mg2+ also binds to the periplasmic sensory domain and transmits inhibitory signals to the cytoplasmic catalytic domain (6, 7). Thus, we postulated that high concentrations of Mg2+ might interfere with PhoQ autophosphorylation in vitro. To explore the possibility, we determined the kinetics of PhoQ autophosphorylation in the presence of increasing concentrations of MgCl2. In the absence of added MgCl2, PhoQ autophosphorylation was barely detectable (Fig. 1A). In the presence of 0.1 mM MgCl2, PhoQ autophosphorylation reached a plateau within 5 min (Fig. 1A), and [32P]phospho-PhoQ was stable for at least 20 min. When higher concentrations of MgCl2 (0.2 to 10 mM) were added to the reaction mixtures, kinetics of PhoQ autophosphorylation were strongly biphasic, with a rapid phosphorylation phase followed by a slower dephosphorylation phase (Fig. 1A). At these concentrations of MgCl2, the initial rates of PhoQ autophosphorylation were higher than that obtained in the presence of 0.1 mM MgCl2. The levels of [32P]phospho-PhoQ peaked within 30 to 45 s, and then decreased with increasing concentrations of MgCl2 (Fig. 1A). These data are consistent with the idea that the early phosphorylation phase is dependent on occupation of the C-terminal catalytic Mg2+-binding site, whereas the slower dephosphorylation phase results from the occupation of the N-terminal sensory Mg2+-binding site.
FIG. 1.
Time course of PhoQ autophosphorylation in the presence of increasing concentrations of MgCl2. Autokinase assays were performed at 22°C in a 15-μl volume of phosphorylation buffer supplemented with MgCl2, as indicated. Reactions were initiated by the addition of 0.1 mM [γ-32P]ATP (10 Ci/mmol). At various time points, reactions were stopped by the addition of 5 μl of 4× SDS sample buffer. Samples (10 μl) were subjected to SDS-PAGE, and the amount of 32P associated with PhoQ was quantitated with a PhosphorImager. (A) PhoQ-containing membranes (12 μg of total protein) were autophosphorylated in the absence (○) or presence of 0.1 mM (●), 0.2 mM (□), 1 mM (■), or 10 mM (▴) MgCl2. (B) The C-terminal catalytic domain of PhoQ, MBP-PhoQ-cyto (8 μg), was autophosphorylated in the presence of 0.1 mM (●), 0.2 mM (□), 1 mM (■), or 10 mM (▴) MgCl2.
To determine whether the N-terminal sensory domain of PhoQ was responsible for the observed Mg2+-induced dephosphorylation, we constructed a vector encoding MBP-Q-cyto, which consists of the C-terminal catalytic domain of PhoQ (residues 247 to 487) fused to the maltose binding protein (MBP). MBP-Q-cyto was expressed in E. coli and purified using an amylose-agarose affinity column. In contrast to the membrane-bound PhoQ, MBP-Q-cyto was unable to autophosphorylate in the presence of 0.1 mM MgCl2 but able to autophosphorylate at concentrations of MgCl2 above 0.2 mM (Fig. 1B). This suggests that the isolated C-terminal catalytic domain of PhoQ may have a slightly lower affinity for Mg2+ than the intact protein. In contrast to what was observed for the membrane-bound PhoQ, high concentrations of MgCl2 (1 to 10 mM) did not promote the dephosphorylation of MBP-Q-cyto (Fig. 1B). Thus, the N-terminal sensory domain of PhoQ appears to be required for the Mg2+-induced dephosphorylation observed for membrane-bound PhoQ (Fig. 1A).
To examine the possibility that a contaminating Mg2+-dependent phosphatase was present in the E. coli washed membranes, we examined the stability of phosphorylated MBP-Q-cyto in the presence of E. coli control membranes lacking PhoQ. MBP-Q-cyto (8 μg) was first subjected to autophosphorylation for 10 min in the presence of 1 mM MgCl2. Then E. coli membranes (12 μg of total protein) were added to the reaction mixtures. Reactions were stopped at various time points and analyzed by SDS-PAGE. We found that the phosphorylated PhoQ cytoplasmic domain was 87% stable over a 15-min period (data not shown). From these data, it appears that the presence of a Mg2+-dependent phosphatase present in E. coli membranes cannot explain the rapid dephosphorylation observed for the membrane-bound PhoQ at concentrations of MgCl2 higher than 0.2 mM.
Autokinase activity of PhoQ in the presence of other divalent cations.
Similarly, we determined the kinetics of PhoQ autophosphorylation in the presence of increasing concentrations of MnCl2, CaCl2, or BaCl2. Kinetic data obtained in the presence of MnCl2 (data not shown) were very similar to those obtained in the presence of MgCl2 (Fig. 1A), indicating that Mn2+ is also able to promote PhoQ dephosphorylation. In contrast, when increasing concentrations of CaCl2 or BaCl2 were used, PhoQ autophosphorylation reached a plateau within minutes and remained stable for at least 20 min (data not shown). Thus, neither Ca2+ nor Ba2+ can induce the dephosphorylation of PhoQ.
Chemical stability of the phospholinkage and autophosphorylation of PhoQ-H277N.
To assess further the nature of the phosphorylated residue, we examined the stability of the incorporated phosphate following in vitro autophosphorylation under alkali and acidic conditions. We found that the PhoQ phospholinkage was resistant to treatment with 3 N NaOH but was highly sensitive to 1 N HCl treatment (data not shown). Thus, the phosphorylated residue had the stability expected for an N-phosphorylated amino acid such as histidyl-phosphate (4). The His-277 residue of PhoQ corresponds to the highly conserved histidine residue found in all histidine protein kinases (19). To determine whether His-277 is the site of PhoQ autophosphorylation, an asparagine residue was substituted for the histidine by site-directed mutagenesis. Incubation of membranes overproducing the resultant PhoQ-H277N mutant with [γ-32P]ATP and MgCl2 (0.1 mM), MnCl2 (0.1 mM), CaCl2 (2 mM), or BaCl2 (2 mM) did not result in phosphate incorporation (data not shown). This showed that residue His-277 of PhoQ is critical for autophosphorylation. These results taken together indicated that His-277 is the site of PhoQ autophosphorylation.
Phosphotransfer from PhoQ to PhoP.
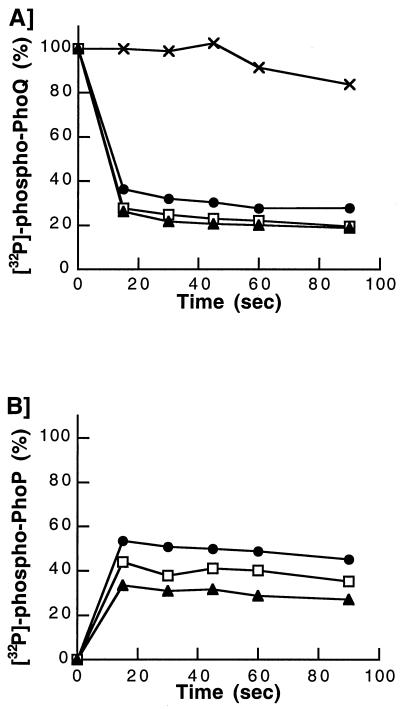
To study the transfer of phosphate from PhoQ to PhoP, we used membranes containing [32P]phospho-PhoQ and PhoP-His (a PhoP variant that possesses a C-terminal His tag). PhoP-His was expressed in E. coli and purified by immobilized metal affinity chromatography. PhoQ-containing membranes were first autophosphorylated for 5 min at room temperature in the presence of [γ-32P]ATP and 0.1 mM MgCl2. To remove unincorporated ATP, membranes were pelleted by high-speed centrifugation at 625,000 × g for 10 min at 4°C, washed twice with phosphorylation buffer, and resuspended in the same buffer. Phosphotransfer reactions were performed by incubating purified membranes containing [32P]phospho-PhoQ (12 μg of total protein) in a 20-μl total volume with excess PhoP-His (4.2μg) in phosphorylation buffer supplemented with MgCl2. At various time points, reactions were stopped and analyzed by SDS-PAGE. A control reaction in which PhoP-His was omitted was performed to verify that [32P]phospho-PhoQ was stable during the reaction period (Fig. 2A). Kinetics of phosphotransfer were performed in the presence of increasing concentrations of MgCl2. In all cases, the initiation of phosphotransfer resulted in an extremely rapid dephosphorylation of PhoQ (Fig. 2A) and a concomitant phosphorylation of PhoP-His (Fig. 2B). The dephosphorylation of PhoQ was 75% complete within 15 s of the phosphotransfer reaction (Fig. 2A). Increasing the concentration of MgCl2 in the reaction mixtures resulted in slight increases of the initial rates of PhoQ dephosphorylation (Fig. 2A) but decreased the initial rates of PhoP-His phosphorylation (Fig. 2B). Thus, the total amount of protein-bound radiolabeled phosphate decreased by increasing the concentration of MgCl2. These data suggest that high concentrations of Mg2+ stimulate the dephosphorylation of PhoP-His, possibly through a phosphatase activity associated to PhoQ.
FIG. 2.
Phosphotransfer from PhoQ to purified PhoP-His. PhoQ-containing membranes were first autophosphorylated and purified of unincorporated ATP. Phosphotransfer assays were performed at 22°C in a 20-μl volume of phosphorylation buffer supplemented with MgCl2, as indicated. Reactions were initiated by mixing purified membranes containing [32P]phospho-PhoQ with purified PhoP-His. Reactions were stopped by the addition of SDS sample buffer and analyzed by SDS-PAGE. Dried gels were exposed to a PhosphorImager. Total levels of radioactivity present on PhoQ and PhoP at the initial time of the phosphotransfer assay were considered 100 and 0%, respectively. (A) Time course of [32P]phospho-PhoQ dephosphorylation. The phosphotransfer reaction was performed in the absence of PhoP-His (×). Phosphotransfer reactions were carried out in the presence of 1 mM (●), 5 mM (□), and 10 mM (▴) MgCl2. (B) Time course of PhoP-His phosphorylation. Phosphotransfer reactions were carried out in the presence of 1 mM (●), 5 mM (□), and 10 mM (▴) MgCl2.
PhoQ functions as a phosphatase for phospho-PhoP.
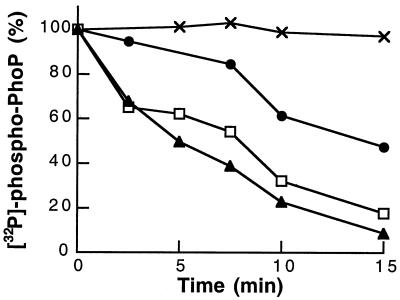
To explore the possibility that PhoQ possesses phosphatase activity, we examined the stability of purified [32P]phospho-PhoP-His in the presence of PhoQ-containing membranes. Phosphorylation of PhoP-His was achieved by incubating membranes containing [32P]phospho-PhoQ and PhoP-His, as described above. The reaction mixture was centrifuged at high-speed (625,000 × g for 10 min at 4°C) to remove the membrane fraction, and the supernatant containing [32P]phospho-PhoP-His was recovered. Unincorporated ATP, ADP generated during the autokinase reaction, and MgCl2 were removed by buffer exchange into phosphorylation buffer using centrifugal filter devices (Millipore). For the phosphatase assay, excess of [32P]phospho-PhoP-His (4.2μg) was incubated with either control membranes or PhoQ-containing membranes (12μg of total protein) in phosphorylation buffer, in the presence of increasing concentrations of MgCl2. At various time points, reactions were stopped and analyzed by SDS-PAGE. A control reaction in which [32P]phospho-PhoP-His was incubated with control membranes lacking PhoQ indicated that [32P]phospho-PhoP-His was stable during the reaction period (Fig. 3). When [32P]phospho-PhoP-His was incubated with PhoQ-containing membranes, the amount of [32P]phospho-PhoP-His decreased over time (Fig. 3). The rates of PhoP dephosphorylation were stimulated by increasing the concentration of MgCl2 (Fig. 3). These data indicate that PhoQ possesses phosphatase activity that is regulated by Mg2+.
FIG. 3.
Dephosphorylation of phospho-PhoP by PhoQ. [32P]phospho-PhoP-His was purified from ATP, ADP, and Mg2+ by high-speed centrifugation and ultrafiltration. Phosphatase assays were performed at 22°C in a 20-μl volume of phosphorylation buffer supplemented with MgCl2, as indicated. Reactions were initiated by the addition of membranes. At indicated time points, reactions were stopped by the addition of SDS sample buffer. Samples (13 μl) were subjected to SDS-PAGE, and the amount of 32P associated with PhoP-His was quantitated with a PhosphorImager. The total radioactivity present on PhoP at the initial time of the phosphatase assay was considered as 100%. Purified [32P]phospho-PhoP-His was incubated with control membranes in the presence of 1 mM MgCl2 (×). Purified [32P]phospho-PhoP-His was incubated with PhoQ-containing membranes in the presence of 1 mM (●), 5 mM (□), and 10 mM (▴) MgCl2.
Concluding remarks.
In the present study, we examined the catalytic activities of the S. enterica serovar Typhimurium PhoQ histidine kinase and showed that like most histidine kinases, PhoQ exhibits both autokinase and phosphatase activities. The main finding of this study is that MgCl2 concentrations above 0.1 mM affected the stability of [32P]phospho-PhoQ and stimulated PhoQ dephosphorylation (Fig. 1A). Since Mg2+-induced dephosphorylation of PhoQ was not observed with the isolated transmitter domain (MBP-Q-cyto) (Fig. 1B), it appears that Mg2+ promotes PhoQ dephosphorylation by binding to the periplasmic sensory domain and signaling through the membrane. This finding is in contrast to that from a study by Castelli et al. (3), who found that MgCl2 concentrations higher than 0.2 mM had no inhibitory effect on the autokinase activity of PhoQ. The main variation in the experimental procedure that may account for differences between the two studies is that Castelli et al. used an S. enterica serovar Typhimurium strain for the isolation of PhoQ-containing membranes, whereas we used an E. coil strain. One possibility is that a membrane-bound Mg2+-dependent phosphatase is present in the E. coli washed membranes but absent from the S. enterica membranes. This is unlikely, because we showed that the phosphorylated transmitter domain of PhoQ (MBP-Q-cyto) is 87% stable over a 15-min period after the addition of control E. coli membranes. Moreover, it is unlikely that such a phosphatase is absent from S. enterica, which is closely related to E. coli. However, we cannot rule out this possibility entirely.
Two possibilities could account for the Mg2+-induced dephosphorylation of PhoQ. One possibility is that conformational alterations upon Mg2+ binding to the periplasmic domain destabilize the PhoQ phospholinkage by altering its molecular environment. Another possibility is that Mg2+ binding triggers a reverse autokinase reaction in which the phosphoryl group is transferred to ADP to form ATP. The fact that ADP, which is generated upon PhoQ autophosphorylation, is necessary to the reverse autokinase reaction would explain the biphasic kinetic curves shown in Fig. 1A. Such a reverse autokinase reaction has been described previously for other two-component systems (8, 13, 21). Studies are under way to further characterize the mechanism by which Mg2+ induces PhoQ dephosphorylation.
In good agreement with Castelli et al. (3), we found that the phosphatase activity of PhoQ is stimulated by the concentration of MgCl2. These results taken together are consistent with a model in which PhoQ responds to external ligands by modulating both autokinase and phosphatase activities. Both the alteration of the autokinase activity and the increase of the phosphatase activity would result in decreased levels of phospho-PhoP and, in turn, in the repression of PhoP-activated genes. Similar conclusions have been reached for other histidine kinase sensors including, FixL and NtrB (12, 16).
Acknowledgments
This work was supported by grant MT-15551 from the Medical Research Council of Canada and by a fellowship to H. Le Moual. from Fonds de la Recherche en Santé du Québec, M. Montagne and A. Martel were the recipients of fellowships from the Faculty of Medicine, Université de Sherbrooke.
REFERENCES
- 1.Aiba H, Mizuno T, Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1989;264:8563–8567. [PubMed] [Google Scholar]
- 2.Bilwes A M, Alex L A, Crane B R, Simon M I. Structure of CheA, a signal-transducing histidine kinase. Cell. 1999;96:131–141. doi: 10.1016/s0092-8674(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 3.Castelli M E, Garcia Vescovi E, Soncini F C. The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J Biol Chem. 2000;275:22948–22954. doi: 10.1074/jbc.M909335199. [DOI] [PubMed] [Google Scholar]
- 4.Duclos B, Marcandier S, Cozzone A J. Chemical properties and separation of phosphoamino acids by thin-layer chromatography and/or electrophoresis. Methods Enzymol. 1991;201:10–21. doi: 10.1016/0076-6879(91)01004-l. [DOI] [PubMed] [Google Scholar]
- 5.Ernst R K, Guina T, Miller S I. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J Infect Dis. 1999;179(Suppl. 2):S326–330. doi: 10.1086/513850. [DOI] [PubMed] [Google Scholar]
- 6.Garcia Vescovi E, Soncini F C, Groisman E A. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia Vescovi E, Ayala Y M, Di Cera E, Groisman E A. Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+ J Biol Chem. 1997;272:1440–1443. doi: 10.1074/jbc.272.3.1440. [DOI] [PubMed] [Google Scholar]
- 8.Gilles-Gonzalez M A, Gonzalez G. Regulation of the kinase activity of heme protein FixL from the two-component system FixL/FixJ of Rhizobium meliloti. J Biol Chem. 1993;268:16293–16297. [PubMed] [Google Scholar]
- 9.Groisman E A. The ins and outs of virulence gene expression: Mg2+ as a regulatory signal. Bioessays. 1998;20:96–101. doi: 10.1002/(SICI)1521-1878(199801)20:1<96::AID-BIES13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Gunn J S, Hohmann E L, Miller S I. Transcriptional regulation of Salmonella virulence: a PhoQ periplasmic domain mutation results in increased net phosphotransfer to PhoP. J Bacteriol. 1996;178:6369–6373. doi: 10.1128/jb.178.21.6369-6373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igo M M, Ninfa A J, Stock J B, Silhavy T J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 12.Jiang P, Ninfa A J. Regulation of autophosphorylation of Escherichia coli nitrogen regulator II by the PII signal transduction protein. J Bacteriol. 1999;181:1906–1911. doi: 10.1128/jb.181.6.1906-1911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang P, Peliska J A, Ninfa A J. Asymmetry in the autophosphorylation of the two-component regulatory system transmitter protein nitrogen regulator II of Escherichia coli. Biochemistry. 2000;39:5057–5065. doi: 10.1021/bi992921w. [DOI] [PubMed] [Google Scholar]
- 14.Jin T, Inouye M. Ligand binding to the receptor domain regulates the ratio of kinase to phosphatase activities of the signaling domain of the hybrid Escherichia coli transmembrane receptor, Tazl. J Mol Biol. 1993;232:484–492. doi: 10.1006/jmbi.1993.1404. [DOI] [PubMed] [Google Scholar]
- 15.Le Moual H, Quang T, Koshland D E., Jr Conformational changes in the cytoplasmic domain of the Escherichia coli aspartate receptor upon adaptive methylation. Biochemistry. 1998;37:14852–14859. doi: 10.1021/bi980343y. [DOI] [PubMed] [Google Scholar]
- 16.Lois A F, Weinstein M, Ditta G S, Helinski D R. Autophosphorylation and phosphatase activities of the oxygen-sensing protein FixL of Rhizobium meliloti are coordinately regulated by oxygen. J Biol Chem. 1993;268:4370–4375. [PubMed] [Google Scholar]
- 17.Ninfa A J, Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci USA. 1986;83:5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ninfa E G, Atkinson M R, Kamberov E S, Ninfa A J. Mechanism of autophosphorylation of Escherichia coli nitrogen regulator II (NRII or NtrB): trans-phosphorylation between subunits. J Bacteriol. 1993;175:7024–7032. doi: 10.1128/jb.175.21.7024-7032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 20.Russo F D, Silhavy T J. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J Mol Biol. 1991;222:567–580. doi: 10.1016/0022-2836(91)90497-t. [DOI] [PubMed] [Google Scholar]
- 21.Shi L, Liu W, Hulett F M. Decay of activated Bacillus subtilis Pho response regulator, PhoP P, involves the PhoR P intermediate. Biochemistry. 1999;38:10119–10125. doi: 10.1021/bi990658t. [DOI] [PubMed] [Google Scholar]
- 22.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stock J B, Surrette M G, Levitt M, Park P. Two-component signal transduction systems: structure-function relationships and mechanisms of catalysis. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 25–51. [Google Scholar]
- 24.Yang Y, Inouye M. Intermolecular complementation between two defective mutant signal-transducing receptors of Escherichia coli. Proc Natl Acad Sci USA. 1991;88:11057–11061. doi: 10.1073/pnas.88.24.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]