Abstract
Pancreatic primary cilia are active and dynamic, not static antenna-like sensors as previously thought. This movement may be an important mechanism to glucose regulation.
Textbooks classify eukaryotic cilia as two types: motile and nonmotile (primary) cilia. Primary cilia have long been regarded as nonmotile passive sensors that monitor environmental signals to maintain tissue homeostasis in the pancreas. These microtubule-based sensory organelles protrude out of the cell surface. Recently, primary cilia in pancreatic β cells were found to be involved in glucose homeostasis (1–3), but their functional roles are still vague, and the mechanisms of action are unclear.
In this issue of Science Advances, Cho et al. demonstrate that primary cilia are active and dynamic, rather than simple static antenna-like sensors. The researchers also link the motility of the β cells cilia to glucose sensing, which is critical in the physiology of blood sugar level regulation. This work leads to a plausible hypothesis that primary cilium motility may be a core function for glucose-stimulated calcium influx and insulin release (4). This insight could lead to new understanding of diabetes.
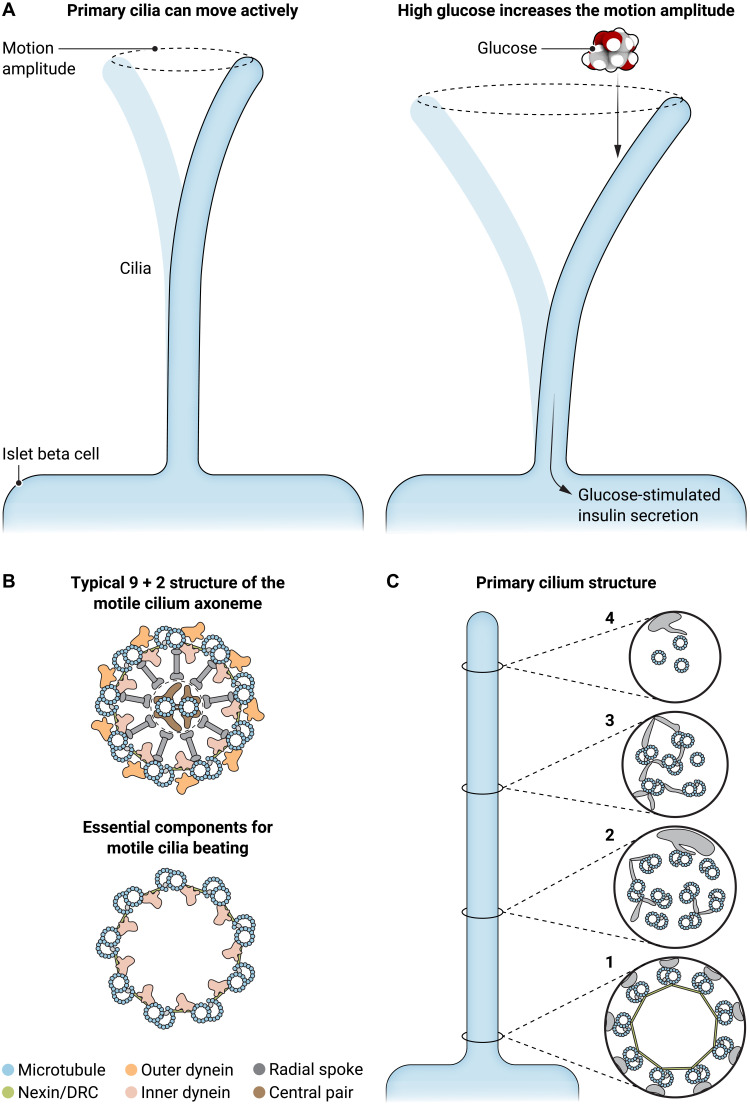
Cho et al. (4) used live-cell imaging to characterize the motility of pancreatic β cell cilia and its dependence on glucose. Since dynein inhibition or ATP depletion largely diminished the motility of the islet cilia, the motility is likely a proactive behavior instead of a passive motion. In addition, the motility of islet primary cilia is sensory function related, as an increase in glucose concentration leads to a motion pattern change. Such a motility change is associated with the degree of cellular calcium response and thereby insulin secretion (Fig. 1A).
Fig. 1. Primary cilia play a key role in glucose regulation through active motion.
(A) Proactive motion of primary cilia in islet β cells displays a low frequency of about 0.04 Hz. The movement amplitude increases significantly under a high glucose concentration, which is associated with the calcium response and thereby insulin secretion. (B) The typical structure (top) and architecture of the essential components (bottom) for the proactive beating of motile cilia. Typical primary cilia lack such characteristics. (C) If the β cell cilia display a similar structure to that of most primary cilia, they may not share the same proactive motion mechanism as that of motile cilia. Illustration credit: Ashley Mastin, Science Advances.
The mechanism underlying the proactive motility of the islet primary cilia is a fundamental question raised by this study. Data from immunofluorescence microscopy suggest that the β cell cilia possess some of the key proteins that are required for motile cilium motility, i.e., component proteins of the dynein arm (intermediate chain DNAI1, light intermediate chain DNALI1, and heavy chain DNAH5), the nexin-dynein regulatory complex (GAS8), and the sperm flagellar assembly protein SPEF2, in addition to the kinesin motor (KIF9) (4). Based on the data from decades of studies on motile cilia, we know that these component proteins must assemble into a distinctive 3D structure in motile cilia to enable the proactive beating function.
In typical motile cilia, the microtubule-based framework (axoneme) is constructed in a cylindrical configuration of nine microtubule doublets (5). Together with the dynein arms (6) and nexin links (dynein-regulatory complex) bridging the neighboring microtubule doublets, they represent the essential structural components for motile cilia beating (7) (Fig. 1B). Although the central pair complex is not an essential component for the beating function of the motile cilium axoneme, it is present in most motile cilia and regulates the beating motion pattern through radial spokes (Fig. 1B).
Proactive motion of motile cilia requires the essential component proteins to be properly assembled into the correct regular architecture, and defects of this regular architecture may abolish the beating function of motile cilia. In contrast to motile cilia, the structural features of the typical primary cilium axoneme change gradually from the base to the tip with a decreasing diameter (Fig. 1C, 1 to 4) (8). In primary cilia, no structural features exist like the array of dynein arms to make neighboring microtubule complexes slide against each other for proactive beating. Therefore, it is important to determine whether/how islet primary cilia utilize the dynein arms, nexin link, and microtubules identified by the immunofluorescence studies for proactive beating. Do these components present in a structural configuration similar to either of the two shown in Fig. 1B? If so, these islet cilia of β cells should be termed as islet motile cilia based on the structural classification definition. But if not, what is the structural basis for the proactive beating of these islet cilia?
The appearance of the cilia imaged by Cho et al. seems to agree with the structure of primary cilia in kidney cells (Fig. 1C) (8), echoing previously published electron microscopy (EM) results (9). Therefore, the mechanism of islet cilia motion may not share the same structural basis of the mechanism underlying typical motile cilia beating. The current EM data are not clear enough to draw a conclusion. A high-quality 3D structure of islet β cell cilia and its comparation with motile cilia could offer substantial insight into the mechanism of islet cilia motility.
Current data suggest that the motility of the β cell primary cilia is affected by glucose concentration and is associated with the Ca2+ response, which is critical to insulin secretion. This is compatible with the proposed idea that the islet cilium motility may be a required function by glucose sensing (4). However, while the mechanism of islet cilium motility is indecipherable, it is also possible that the motion of these primary cilia is simply a collateral consequence of glucose sensing.
Alternatively, intraflagellar transport (IFT) (10), which is a constantly active motility source in all cilia, may be involved in primary cilium motility. Movement of IFT trains along the microtubules relies on kinesin-II (kif3 in human) and dynein-2 motors that can be inhibited by antimycin A, ciliobrevin D, or erytho-9-(2-hydroxynonyl) adenine, all used in the study of Cho et al. (4). If IFT is involved in the primary cilium motility, the motility reduction following the use of these drugs may be explained as a result of IFT termination. The motion pattern change of the islet primary cilia under a high-glucose condition may result from a possible change(s) in IFT activity or cargo types in response to the glucose concentration change. Therefore, clarifying the motility mechanism of primary cilia is important for understanding the functional role(s) of islet β cell cilia motility in glucose sensing.
Although conclusive interpretation of some of the experimental data in the report requires more experimental work, the discovery that primary cilia proactively move and that their motion behavior is associated with glucose sensing is important. This work offers novel insights into the functional roles and mechanisms of islet β cell cilia in glucose sensing. It will also stimulate further investigation on primary cilium motility and its potential roles in sensory functions of other cell types.
REFERENCES
- 1.Volta F., Scerbo M. J., Seelig A., Wagner R., O’Brien N., Gerst F., Fritsche A., Häring H. U., Zeigerer A., Ullrich S., Gerdes J. M., Glucose homeostasis is regulated by pancreatic β-cell cilia via endosomal EphA-processing. Nat. Commun. 10, 5686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes J. W., Cho J. H., Conway H. E., DiGruccio M. R., Ng X. W., Roseman H. F., Abreu D., Urano F., Piston D. W., Primary cilia control glucose homeostasis via islet paracrine interactions. Proc. Natl. Acad. Sci. U.S.A. 117, 8912–8923 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C. T., Hilgendorf K. I., Bevacqua R. J., Hang Y., Demeter J., Kim S. K., Jackson P. K., Discovery of ciliary G protein-coupled receptors regulating pancreatic islet insulin and glucagon secretion. Genes Dev. 35, 1243–1255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.J. H. Cho, Z. A. Li, L. Zhu, B. D. Muegge, H. F. Roseman, T. Utterback, L. G. Woodhams, P. V. Bayly, J. W. Hughes, Islet primary cilia motility controls insulin secretion. Science Advances, eabq8486 (2022). [DOI] [PMC free article] [PubMed]
- 5.Ma M., Stoyanova M., Rademacher G., Dutcher S. K., Brown A., Zhang R., Structure of the decorated ciliary doublet microtubule. Cell 179, 909–922.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao Q., Han L., Wang Y., Chai P., Kuo Y. W., Yang R., Hu F., Yang Y., Howard J., Zhang K., Structures of outer-arm dynein array on microtubule doublet reveal a motor coordination mechanism. Nat. Struct. Mol. Biol. 28, 799–810 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolley D. M., Studies on the eel sperm flagellum. I. The structure of the inner dynein arm complex. J. Cell Sci. 110 ( Pt. 1), 85–94 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Sun S., Fisher R. L., Bowser S. S., Pentecost B. T., Sui H., Three-dimensional architecture of epithelial primary cilia. Proc. Natl. Acad. Sci. U.S.A. 116, 9370–9379 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aughsteen A. A., The ultrastructure of primary cilia in the endocrine and excretory duct cells of the pancreas of mice and rats. Eur. J. Morphol. 39, 277–283 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Pigino G., Intraflagellar transport. Curr. Biol. 31, R530–R536 (2021). [DOI] [PubMed] [Google Scholar]