Abstract
A Thermus thermophilus HB27 strain was constructed in which the malate dehydrogenase (mdh) gene was deleted. The Δmdh colonies are recognized by a small-colony phenotype. Wild-type phenotype is restored by transformation with Thermus plasmids or integration vector containing an intact mdh gene. The wild-type phenotype provides a positive selection tool for the introduction of plasmid DNA into Thermus spp., and because mdh levels can be readily quantified, this host-vector system is a convenient tool for monitoring gene expression.
Thermus spp. are gram-negative thermophilic microorganisms that grow at temperatures between 50 and 82°C (1, 11). Plasmid vectors that have been used in Thermus spp. have been constructed that encode tryptophan, leucine, or pyrimidine synthesis genes to complement auxotrophic and deleted hosts, providing for positive selection of transformants (4, 12). The host-vector system described here is an improvement on previously reported plasmid vectors for Thermus spp. because not only is the Escherichia coli-Thermus shuttle vector easily selected and maintained in Thermus spp., but malate dehydrogenase activity, encoded by the mdh gene present in this vector can also be readily and accurately quantified (2).
Construction of Thermus thermophilus HB27 and Thermus flavus hosts with deletions of the mdh gene.
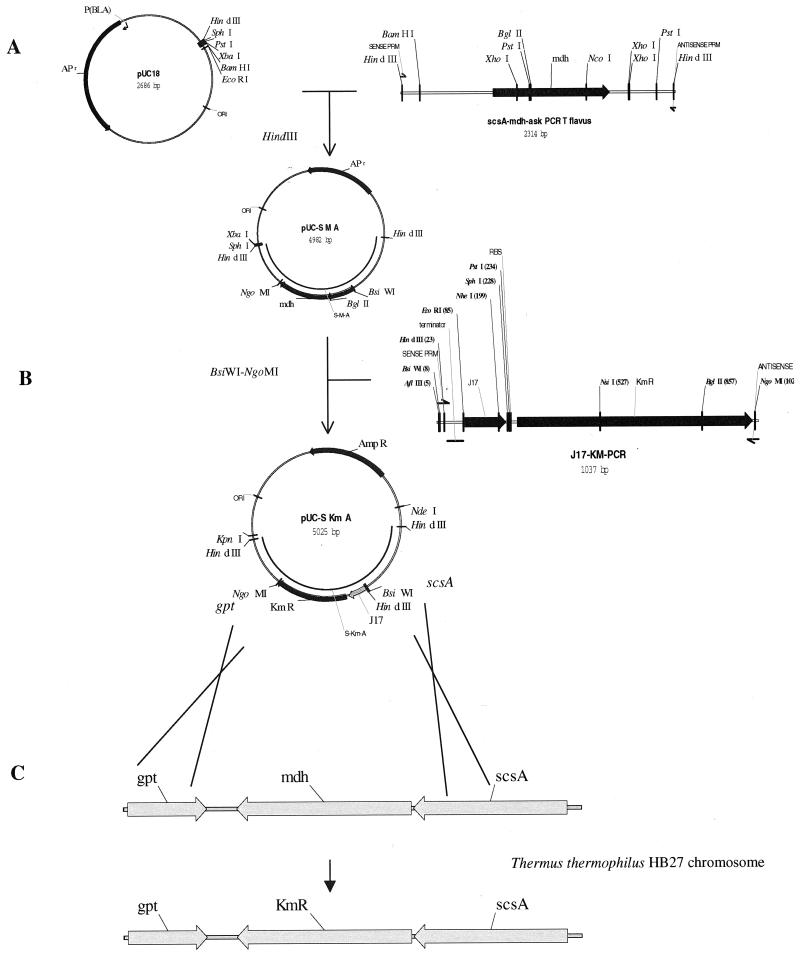
To create the Δmdh strain of T. thermophilus HB27, we constructed an integration vector designated pUC-S KmA. The construction is detailed in Fig. 1. A 2.3-kb PCR fragment containing a region spanning three separate genes, succinate coenzyme A ligase (scsA), malate dehydrogenase (mdh), and purine phosphoribosyltransferase (gpt), was amplified from the T. flavus chromosome and cloned into pUC18. A 10-ul PCR amplification reaction was conducted in an Idaho Technologies Rapid Air Thermo-Cycler in the presence of 8% glycerol and 1% dimethyl sulfoxide. The PCR amplification cycle was run for 40 cycles at 94°C, 55°C, and 1 min of holding at 72°C. The 984-bp mdh gene is located near the center of this 2.3-kb fragment, so that the chromosomal regions that flank mdh are 780 bp (5′) and 560 bp (3′). The entire coding sequence of the mdh gene was removed by restriction enzymes and replaced with a thermotolerant kanamycin resistance cassette (Kmr) (7). This vector, designated pUC-SKmA, can replicate in E. coli but not in Thermus. However, homologous recombination between the scsA and gpt gene sequences allows pUC-SKmA to integrate into the chromosome when it was used to transform T. thermophilus HB27 (5). Transformants were screened at 55°C on TT rich medium (6) supplemented with kanamycin (40 μg/ml). Approximately 104 kanamycin-resistant transformants per μg of DNA were observed. Two distinct colony types arose after 5 days of incubation. The majority of the colonies were very small (0.1 to 0.5 mm) even after 5 days. A few colonies (60 to 100 CFU) were much larger (1.5 to 2.8 mm), the same size as wild-type T. thermophilus HB27 colonies. The two colony types were subcultured, and total DNA (plasmid and chromosomal) was dot blotted onto a nylon membrane.
FIG. 1.
Construction of T. thermophilus Δmdh Kmr MM8-5. (A) The region surrounding the mdh gene from T. flavus was amplified from chromosomal DNA using PCR primers forward. (5′-ACAACAAAGCTTCGGGCAAAGGGGGAACGGAGGTCCT-3′) and reverse (5′-ACAACAAAGCTTGAGCCTTTTGACCTCGTCCTGGGG-3′). These primers were designed to amplify the sequence of the malate dehydrogenase gene and the regions immediately flanking the malate dehydrogenase gene from T. flavus according to published DNA sequences (8–10). Restriction sites were added to the PCR primers to give 5′ and 3′ HindIII sites. The 2.3-kb PCR product containing the mdh gene and flanking regions was cloned into the HindIII site of pUC18. The resulting plasmid is 4,982 bp and was designated pUCS-M-A. (B) Plasmid pUCS-M-A was digested with BsiWI and NgoMI, removing the all but the first 20 bp of the mdh gene sequence. A kanamycin resistance cassette was amplified using plasmid pTEXJ17 as the template and using PCR primers forward (5′-ACAACACGTACGGATTACGCCAAGCTTCATGGCCTAA-3′) and reverse (5′-ACAACAGCCGGCTCGTTCAAAATGGTATGCGTTTTG-3′). Restriction sites were added to the PCR primers to give a 5′ BsiWI and 3′ NgoMI site. The cassette contains a strong constitutive Thermus promoter (J17) upstream of the themostable kanamycin nucleotidyltransferase cassette (Kmr). To prevent transcription readthrough from the native mdh promoter, a transcription termination sequence was cloned upstream of the J17 promoter. The kanamycin resistance cassette was digested with BsiWI and NgoMI and ligated into BsiWI-NgoMI-digested pUCS-M-A. The mdh gene was replaced with a kanamycin resistance cassette, and the resulting plasmid was designated pUC-SKmA. (C) After transformation of plasmid pUC-SKmA into T. thermophilus HB27, a double-crossover homologous recombination event replaces mdh with the Kmr determinant. The Δmdh Kmr T. thermophilus HB27 strain subsequently isolated was designated MM8-5. RBS, ribosome-binding site; PRM, primer.
The dot blot was probed with a digoxigenin-11-dUTP (DIG)-labeled T. flavus mdh gene. DNA prepared from the smaller colonies did not hybridize to the T. flavus mdh gene, whereas DNA harvested from the larger colonies hybridized to the mdh probe. The membrane was also probed with a DIG-labeled Kmr cassette, and both small and large colony types hybridized to it. Because malate dehydrogenase is a key enzyme in the tricarboxylic acid cycle, the colonies resulting from double-crossover integration events (Δmdh) are recognized by this small-colony phenotype. The larger Kmr colonies were single-crossover integration events in which the chromosomal mdh is intact and the entire plasmid is in the chromosome. The T. thermophilus Δmdh Kmr mutant strain was designated MM8-5. T. thermophilus Δmdh Kmr MM8-5 was used as a recipient in further transformation experiments.
Construction of Thermus vectors containing mdh as a reporter gene.
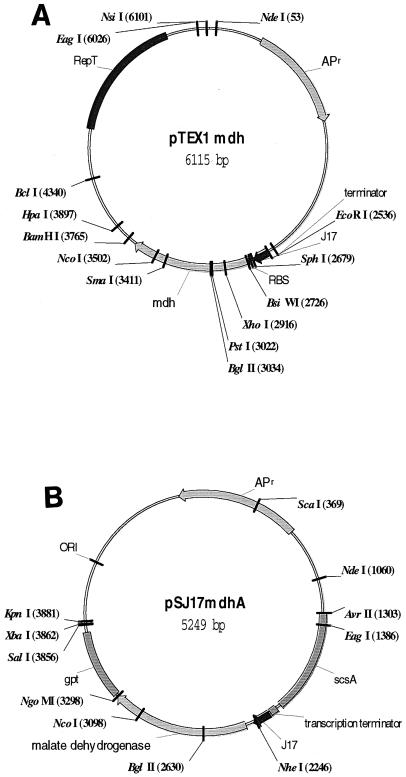
The malate dehydrogenase (mdh) gene from T. flavus was amplified by PCR and cloned into Thermus-E. coli expression vector pTEXI. The expression vector pTEXI is capable of replication in both Thermus spp. and E. coli, and the promoter (J17) employed in this expression vector functions in both bacterial hosts. J17 is a constitutive promoter isolated in our lab from T. thermophilus chromosomal DNA. The expression vector containing the mdh gene, designated pTEXI-mdh, is diagramed in Fig. 2A.
FIG. 2.
(A) Expression vector pTEX1-mdh contains the T. flavus malate dehydrogenase gene downstream of the strong constitutive Thermus promoter J17. The mdh gene from T. flavus was amplified from chromosomal DNA using PCR primers forward mdh (5′-ACACAGAATTCGCATGCTCAAGAAGGCCCTGGGCTAA-3′) and reverse mdh (5′-ACACACGGATCCTGCGCCAGCATGGGGTGGTATAAA-3′). Restriction sites were added to the PCR primers to give 5′ EcoRI and 3′ BamHI sites. Plasmid pTEX1 was digested with EcoRI and BamHI, and an EcoRI-BamHI-digested mdh PCR product was ligated into the vector to create pTEX1-mdh. (B) Integrative vector pSJ17mdhA has the J17 promoter-mdh gene cassette flanked by the scsA and gpt chromosomal regions.
The J17 promoter from pTEXI-J17mdh was replaced by two constitutive Thermus promoters isolated in our laboratory (D50-3 and P2-100). These promoters have low and medium levels of expression, respectively, in Thermus spp. relative to J17. The resulting plasmids were designated pTEX1-D50-3 and pTEX1-P2-100. An integrative vector was constructed to examine the expression of the mdh gene under control of the J17 promoter present as a single integrated copy. This construct was designated pSJ17mdhA and is shown in Fig. 2B. pSJ17mdhA contains pUC19 sequences and can replicate in E. coli. pSJ17mdhA does not replicate in Thermus spp. as a plasmid but integrates into the chromosome by a double-crossover event. pSJ17mdhA has the J17 promoter-mdh gene cassette flanked by the scsA and gpt chromosomal regions. A transcription termination sequence from the T. flavus phenylalanyl tRNA synthetase operon (3) was cloned upstream of the cassette to prevent transcription readthrough from the native succinateCoA ligase/malate dehydrogenase operon promoter.
Plasmids pTEXI-mdh, pTEXI-D50-3, and pTEXI-P2-100 and the integrative vector pSJ17mdhA were transformed into MM8-5. Transformants were easily detected by the restoration of cultures to the wild-type or larger and faster-growing colonies by the expression of the malate dehydrogenase gene located on these expression vectors. Typically, T. thermophilus strain MM8-5 takes 4 to 5 days to form small visible colonies at 55°C in TT supplemented with 40 μg of kanamycin per ml. T. thermophilus MM8-5 transformants that received an expression vector carrying the mdh gene yielded larger colonies in 2 to 3 days.
Expression vector pTEXI-mdh and the alternative promoter pTEX derivatives are very stable in T. thermophilus MM8-5. After more than 20 generations of growth under nonselective conditions, pTEX plasmids were detected in all of the colonies examined (100 for each species). This result is expected because T. thermophilus MM8-5 cells that possess expression vectors containing the mdh gene grow more rapidly than plasmid-free cells that lack a functional mdh gene.
Malate dehydrogenase activity of Thermus constructs.
The levels of malate dehydrogenase (2) being produced by plasmid and integrative expression vectors were evaluated in both T. thermophilus HB27 and MM8-5. Crude lysates prepared from each culture were assayed for enzyme activity at two temperatures (25 and 50°C), and the results are shown in Table 1. T. thermophilus MM8-5 had slight to no malate dehydrogenase activity, confirming complete deletion of the mdh gene from the chromosome of this strain. The activity observed in assays performed at 50°C reflect a slight amount of background due to the conversion of NADH to NAD by unidentified components of cell lysates rather than the malate dehydrogenase-dependent conversion of oxaloacetate and NADH to malate and NAD.
TABLE 1.
Malate dehydrogenase activity of Thermus vector constructsa
| Strain | Promoter expressing mdh | MDH activity (U/mg)
|
|
|---|---|---|---|
| 25°C | 50°C | ||
| HB27 | Wild type | 2.5 ± 0.12 | 19.9 ± 0.25 |
| MM8-5/pSJ17mdhA* | J17 | 0.6 ± 0.01 | 5.5 ± 0.17 |
| HB27/pTEX1-mdh | J17 | 3.7 ± 0.06 | 29.6 ± 1.39 |
| MM8-5 | None | 0.0 | 0.5 ± 0.01 |
| MM8-5/pTEXI-mdh | J17 | 4.2 ± 0.13 | 32.6 ± 0.72 |
| MM8-5/pTEX1-D50-3 | D50-3 | 0.3 | 2.7 ± 0.07 |
| MM8-5/pTEX1-P2-300 | P2-300 | 3.0 ± 0.07 | 23.2 ± 0.99 |
Activity values recorded are averages of three replicate samples from three separate experiments, for a total of nine data points. Standard deviation is less than 5%. One unit of activity is defined as the amount of enzyme needed to convert 1 μmol of NADH to NAD in 1 min. *, integrative vector.
The malate dehydrogenase activity of crude extracts assayed at 50°C is on average nine times higher than the activity levels measured at 25°C. The data in Table 1 clearly indicate that promoters D50-3, P2-300, and J17 have different strengths, resulting in enzyme levels in MM8-5 strains that are 0.28, 1.16, and 1.65 times the level in wild-type T. thermophilus HB27, respectively. Since each promoter is evaluated here in identical genetic constructs that differ only by the promoter driving expression of the mdh gene, these levels should serve to accurately quantify the strength of these promoters. Other strains whose enzyme activity is listed in Table 1 all use the same promoter, J17, to express the mdh gene in various backgrounds. MM8-5/pS-J17mdh-A contains a single copy of the mdh gene integrated into the chromosome under the control of the J17 promoter. MM8-5/pTEXI-mdh contains the mdh gene under the control of the J17 promoter on a plasmid vector, and HB27/pTEXI-mdh contains two separate sources of the mdh gene, a wild-type mdh gene on the chromosome and the mdh gene under the control of the J17 promoter on a plasmid vector. Since MM8-5/pTEXI-mdh yields 32.6 U of malate dehydrogenase per mg of protein, it is unexpected that HB27/pTEXI-mdh, which contains two separate copies mdh gene, a wild-type mdh gene on the chromosome as well as the mdh gene on a plasmid vector, shows nearly the same activity (29.6 U/mg). Plasmid instability may have contributed to homologous recombination between the two mdh gene copies.
We describe the construction of a T. thermophilus Δmdh strain with a deletion of the entire DNA sequence encoding the mdh gene and its use as a host for Thermus plasmids expressing an intact mdh gene. The tricarboxylic acid cycle in Thermus spp., like most microorganisms, plays a central role in metabolism. Malate dehydrogenase catalyzes the dehydrogenation of malate to oxaloacetate using NAD+ as a cofactor and is a key enzyme in the tricarboxylic acid cycle. This host-expression vector system offers a strong positive selection tool for the introduction of plasmid DNA into T. thermophilus, and mdh can be used as a reporter gene to quantify promoter strength in T. thermophilus. The growth rate advantage of mdh+ versus Δmdh cells enriches for cells that retain mdh-containing plasmids, which has the effect of stabilizing these plasmids in Δmdh hosts.
Acknowledgments
This work was prepared with the support of U.S. Department of Energy grant DE-FG02-97ER62464.
The technical assistance of Jung-Ho Kwak, Hoshin Park, and Arati Kolhatkar is gratefully acknowledged.
REFERENCES
- 1.Brock T D, Freeze H. Thermus aquaticus gen. nov. and sp. nov. a nonsporulating extreme thermophile. J Bacteriol. 1969;98:289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horinouchi S, Nishiyama M, Nakamura A, Beppu T. Construction and characterization of multicopy expression-vectors in Streptomyces spp. Mol Gen Genet. 1987;210:468–475. doi: 10.1007/BF00327199. [DOI] [PubMed] [Google Scholar]
- 3.Keller B, Kast P, Hennecke H. Cloning and sequence analysis of the phenylalanyl-tRNA synthetase genes (pheST) from Thermus thermophilus. FEBS Lett. 1992;301:83–88. doi: 10.1016/0014-5793(92)80215-3. . (Erratum, 310:204.) [DOI] [PubMed] [Google Scholar]
- 4.Koyama Y, Arikawa Y, Furukawa K. A plasmid vector for an extreme thermophile, Thermus thermophilus. FEMS Microbiol Lett. 1990;60:97–101. doi: 10.1016/0378-1097(90)90352-q. [DOI] [PubMed] [Google Scholar]
- 5.Koyama Y, Hoshino T, Tomizuka N, Furukawa K. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J Bacteriol. 1986;166:338–340. doi: 10.1128/jb.166.1.338-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasa I, de Grado M, de Pedro M A, Berenguer J. Development of Thermus-Escherichia shuttle vectors and their use for expression of the Clostridium thermocellum celA gene in Thermus thermophilus. J Bacteriol. 1992;174:6424–6431. doi: 10.1128/jb.174.20.6424-6431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumura M, Aiba S. Screening for thermostable mutant of kanamycin nucleotidyl transferase by the use of a transfromation system for a thermophile, Bacillus stearothermophilus. J Biol Chem. 1985;260:15298–15303. [PubMed] [Google Scholar]
- 8.Nishiyama M, Horinouchi S, Beppu T. Characterization of an operon encoding succinyl-CoA synthetase and malate dehydrogenase from Thermus flavus AT-62 and its expression in Escherichia coli. Mol Gen Genet. 1991;226:1–9. doi: 10.1007/BF00273580. [DOI] [PubMed] [Google Scholar]
- 9.Nishiyama M, Kukimoto M, Beppu T, Horinouchi S. An operon encoding aspartokinase and purine phosphoribosyltransferase in Thermus flavus. Microbiology. 1995;141:1211–1219. doi: 10.1099/13500872-141-5-1211. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama M, Matsubara N, Yamamoto K, Iijima S, Uozumi T, Beppu T. Nucleotide sequence of the malate dehydrogenase gene of Thermus flavus and its mutation directing an increase in enzyme activity. J Biol Chem. 1986;261:14178–14183. [PubMed] [Google Scholar]
- 11.Oshima T. Comparative studies on biochemical properties of an extreme thermophile, Thermus thermophilus HB 8. Seikagaku. 1974;46:887–907. . (In Japanese.) [PubMed] [Google Scholar]
- 12.Raven N. Genetics of Thermus. In: Williams R, editor. Thermus species. New York, N.Y: Plenum Press; 1995. pp. 157–184. [Google Scholar]