SUMMARY
A survey of national animal influenza surveillance programmes was conducted to assess the current capacity to detect influenza viruses with zoonotic potential in animals (i.e. those influenza viruses that can be naturally transmitted between animals and humans) at regional and global levels. Information on 587 animal influenza surveillance system components was collected for 99 countries from Chief Veterinary Officers (CVOs) (n = 94) and published literature. Less than 1% (n = 4) of these components were specifically aimed at detecting influenza viruses with pandemic potential in animals (i.e. those influenza viruses that are capable of causing epidemic spread in human populations over large geographical regions or worldwide), which would have zoonotic potential as a prerequisite. Those countries that sought to detect influenza viruses with pandemic potential searched for such viruses exclusively in domestic pigs. This work shows the global need for increasing surveillance that targets potentially zoonotic influenza viruses in relevant animal species.
Key words: Emerging infections, influenza A, pandemic, surveillance, surveillance system
INTRODUCTION
Influenza A viruses originating from animals can adapt to infect humans following mutation or gene exchange [1]. Such viruses might cause the next influenza pandemic given that the human population has not previously acquired immunity. Identification and characterization of influenza A viruses circulating in animals are prerequisites for pandemic preparedness and should be combined with a decision-making process to allow for appropriate preparation and response. Surveillance in animals is, however, mostly implemented with the objective of safeguarding animal health and international trade. Activities therefore mainly target viruses notifiable to the World Organisation for Animal Health (OIE), such as H5 and H7 highly pathogenic influenza viruses in poultry [2] and equine influenza (EI), or investigate H1N1pdm09, H1N2 or H3N2 viruses in swine [3].
In order for an influenza surveillance system to detect the emergence of a potentially zoonotic virus in a timely way, several integrated components of active-representative or risk-based surveillance should be in place, coupled with timely communication of results, including genetic sequence data, to the international community. Since influenza virus strains can circulate in animals without causing clinical signs, healthy as well as diseased animal populations should be targeted. Through a review of surveillance strategies currently implemented in animals (excluding production or market environments), this survey contributed to assessing the capacity of countries to detect viruses with zoonotic potential and mechanisms in place to inform pandemic preparedness at the international level. The aim was to identify how existing efforts may be improved and where existing components could be adapted to inform human pandemic risk. Results identified geographical areas and/or target species that merit strengthened influenza surveillance activities to enhance pandemic preparedness.
METHODS
Data collection
A global cross-sectional survey directed at Chief Veterinary Officers (CVOs) of 183 countries was conducted to collect more comprehensive and detailed information on influenza surveillance in different animal species in the period 2010–2012, as well as information sharing mechanisms instigated upon detection of positives. Only 183 countries were contacted for the survey since FAO communication channels were used to send out the questionnaire and these excluded small island states and overseas territories. The 242 countries used as baseline data in the analysis were derived from the United Nations (UN) classification of regions and subregions, based on the fact that surveillance systems implemented in overseas territories are expected to differ compared to their larger governing states, owing to different geographical conditions and varying infection status.
A database and related questionnaire were designed in Excel® (Microsoft, USA) and the information entered by surveillance system component, characterized by surveillance type (active risk-based, active-representative, passive, outbreak investigation, sentinel, slaughterhouse, other), purpose (national, regional or international surveillance system, research project, other), objective (early detection, to reveal new cases, monitor presence and assess trends, estimate prevalence, demonstrate freedom, other), influenza targeted (avian, equine, swine, pandemic or other influenza) and target population (domestic, wild, companion animals, other). Any one country potentially could have several entries if several components are incorporated (e.g. active risk-based surveillance in domestic poultry, passive surveillance in wild birds, slaughterhouse surveillance in pigs, etc.).
Terms and definitions were adapted from the International Conference on Animal Health Surveillance (ICAHS) [4].
A total of 183 personalized e-mails were sent out to CVOs containing a supporting document with the objectives of the study, data-entry guidelines, a glossary with definitions of the terms used, a country-specific database with the information collected for review and clearance and a link to an OIE/Food and Agriculture Organization of the United Nations (FAO) support document highlighting the importance of this study and encouraging CVOs to respond to the survey (http://www.offlu.net/fileadmin/home/en/human-animal-interface/pdf/flurisk_en.pdf). All documents were supplied in English, French or Spanish, depending on countries' working language; the database was maintained in English only.
An initial deadline of 4 weeks was given. To increase the response rate, individual reminders were sent out by e-mail to non-responders giving a final deadline of a further 4 weeks. FAO regional and national officers and FAO country representatives were asked to follow up with the CVO office at country level.
A literature review revealed that the only published study aimed at characterizing global surveillance efforts was implemented by the OIE/FAO Network of Expertise on Animal Influenza (OFFLU) in 2009, but was restricted to avian influenza (AI) [5]. Furthermore, the European Surveillance Network for Influenza in Pigs 3 (ESNIP 3) [6] collected information on swine influenza (SI) surveillance system components in European countries and shared this data ahead of publication. Results from these studies have been integrated here.
The review was subsequently extended to include other sources, such as national ministry of agriculture (MoA) websites, national surveillance reports or bulletins, websites of non-governmental organizations (NGOs) or other institutions involved in surveillance activities, surveillance and control activities as reported by countries to the OIE and reports on surveillance projects funded by the European Commission (EC) or FAO. The search revealed a general lack of data and the information found was often insufficiently detailed.
Data collection was completed on 3 May 2013. For those countries included in the analysis where information was collected from the OFFLU survey or ESNIP-3 project, information on avian and equine surveillance was added from OIE's World Animal Health Information Database (WAHID) [7].
For notifiable AI viruses (AIVs) (H5 or H7) and EI, a country's infection status (infection occurred: yes/no, between 2010 and 2012) was added according to reports made to WAHID and outbreak information stored in FAO's EMPRES animal health database, EMPRES-i [8].
Data analysis
Descriptive analysis was performed using the surveillance system component as a unit of analysis. The data were analysed at global and regional levels using the following parameters: (1) response rate to the survey and overall data availability; (2) number of surveillance system components implemented and frequency of sampling; (3) surveillance type, objectives, purpose and funding source; (4) influenza virus, animal population and production sector targeted; and, (5) communication of and access to results.
Maps were created using ArcMap10 [9]. Regions and subregions were assigned according to UN classification [10], with the only modification of dividing Palestine in the analysis into West Bank and Gaza Strip.
RESULTS
Data availability
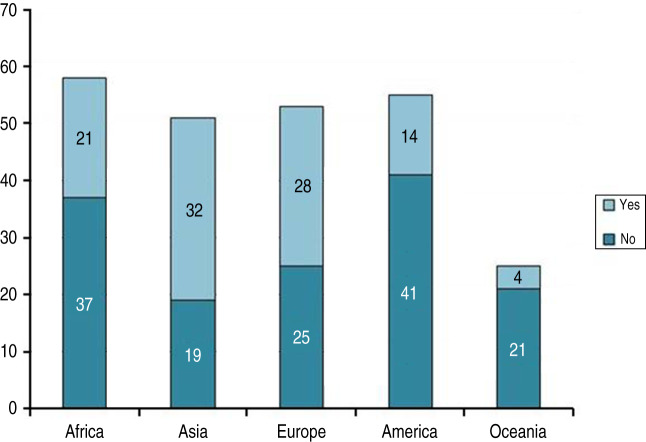
The response rate of the CVO survey was 51·4% (94/183) (Fig. 1). One country notified that no influenza surveillance was taking place.
Fig. 1.
Number of responding (94) and non-responding (89) countries by geographical region (51·4% response rate, 94/183).
On a regional scale, geographical distribution of responding countries was fair with regional response rates as follows: Africa (37·0%, 20/54); Asia (60·4%, 29/48); Europe (68·3%, 28/41); Americas (37·1%, 13/35); Oceania (80·0%, 4/5) (Fig. 1).
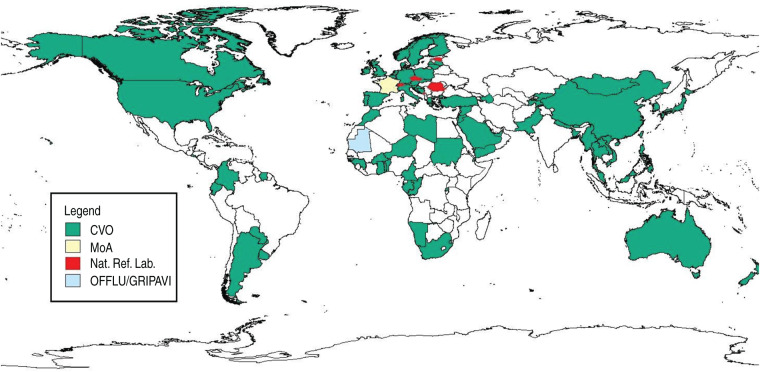
Using additional data sources (OFFLU, ESNIP-3, OIE WAHID) resulted in overall data availability for 99 of the 242 countries, thus 41% (Table 1). The regions with the largest number of countries contributing data to the analysis were Asia (32 countries, 63% data availability) and Europe (28 countries, 53%). The geographical distribution of these countries is shown at the subregional level (Table 1). Few individual subregions had relatively low data coverage, namely Eastern Africa (15%), the Caribbean (14%) and Central America (13%), but this largely improved at the regional level. An exception was Oceania, where no data was available for seven countries in Micronesia and 10 countries in Polynesia, thereby decreasing the overall data coverage to 16%. The geographical distribution of countries contributing data to the analysis is illustrated in Figure 2 by main information source.
Table 1.
Countries contributing data, by geographical subregion (40·9% coverage)
| Region | Subregion | No. of countries | Countries with data | Data coverage (%) |
|---|---|---|---|---|
| Africa | Eastern Africa | 20 | 3 | 15·00 |
| Middle Africa | 9 | 3 | 33·33 | |
| Northern Africa | 7 | 3 | 42·86 | |
| Southern Africa | 5 | 3 | 60·00 | |
| Western Africa | 17 | 9 | 52·94 | |
| Asia | Total | 58 | 21 | 36·21 |
| Central Asia | 5 | 2 | 40·00 | |
| Eastern Asia | 7 | 4 | 57·14 | |
| Southern Asia | 9 | 5 | 55·56 | |
| South-Eastern Asia | 11 | 8 | 72·73 | |
| Western Asia | 19 | 13 | 68·42 | |
| Europe | Total | 51 | 32 | 62·75 |
| Eastern Europe | 10 | 4 | 40·00 | |
| Northern Europe | 18 | 9 | 50·00 | |
| Southern Europe | 16 | 9 | 56·25 | |
| Western Europe | 9 | 6 | 66·67 | |
| America | Total | 53 | 28 | 52·83 |
| Caribbean | 28 | 4 | 14·29 | |
| Central America | 8 | 1 | 12·50 | |
| South America | 14 | 7 | 50·00 | |
| North America | 5 | 2 | 40·00 | |
| Oceania | Total | 55 | 14 | 25·45 |
| Australia/New Zealand | 3 | 2 | 66·67 | |
| Melanesia | 5 | 2 | 40·00 | |
| Micronesia | 7 | 0 | 0·00 | |
| Polynesia | 10 | 0 | 0·00 | |
| Total | 25 | 4 | 16·00 | |
| Grand total | 242 | 99 | 40·91 |
Fig. 2.
Geographical distribution of countries contributing data to the analysis, by main information source: CVO, Chief Veterinary Officer (names of contributing CVOs are given in the online Supplementary material); MoA, Ministry of Agriculture; Nat. Ref. Lab., National Reference Laboratory; OFFLU, OIE/FAO Network of Expertise on Animal Influenza; GRIPAVI, Ecology and Epidemiology of Avian Influenza in Developing Countries.
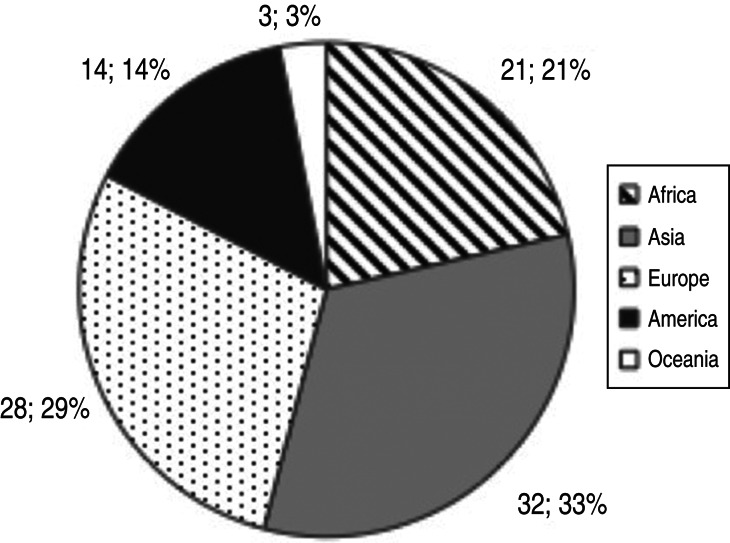
Figure 3 illustrates the number and percentage of countries contributing data by region. The country with no surveillance activities has been excluded from the analysis. Data availability was highest for Asia (33% of countries in the region contributing data to the analysis) and Europe (29%), and lowest for Oceania (3%). However, Oceania includes a number of small island states and the main land mass is concentrated in Australia/New Zealand and Melanesia, for which data availability was higher.
Fig. 3.
Number and percentage of countries contributing data on surveillance systems implemented, by region (total = 98 countries).
Number of components implemented and frequency of sampling
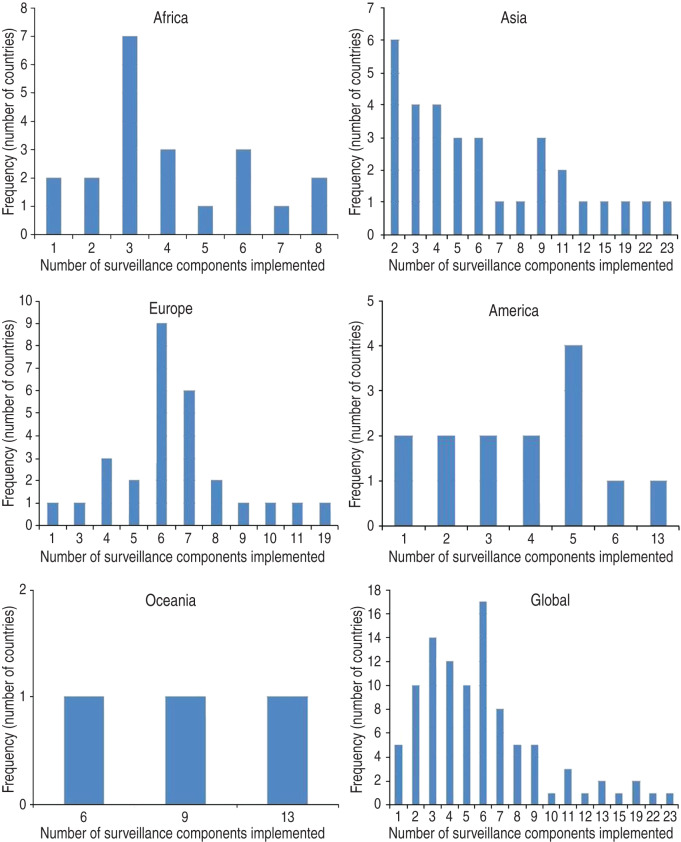
In total, information on 587 animal influenza surveillance system components was obtained. Regional comparison is facilitated by looking at the average number (and range) of components reported by countries. On average, countries in Oceania reported implementing nine components (range 6–13), Asian and European countries seven components (ranges 2–23 and 1–19, respectively), and American and African countries, four components (ranges 1–13 and 1–8, respectively). Frequency distributions are illustrated in Figure 4. All but five countries (from Africa, Europe and the Americas) indicated implementation of more than one component.
Fig. 4.
Frequency distribution of the number of surveillance system components implemented by geographical region and globally.
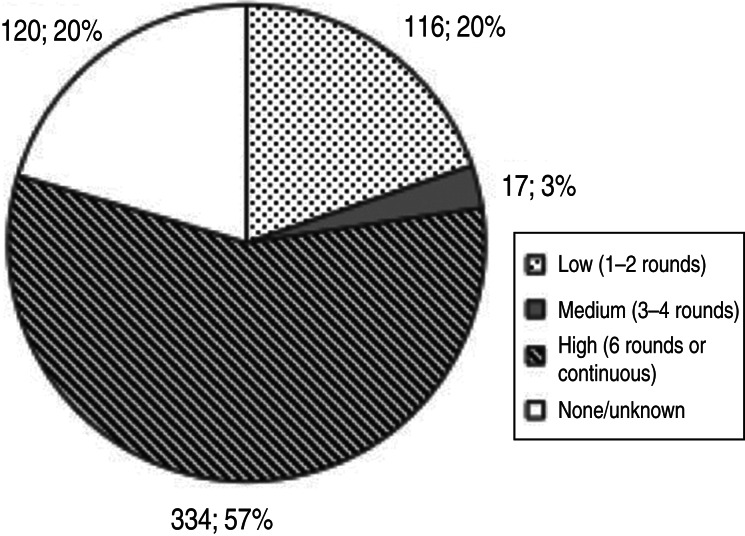
When stratifying by frequency of sampling rounds per year, almost 57% of the surveillance system components analysed were implemented at high frequency, either in six sampling rounds per year or continuous (see Table 2). Most regions reported to have implemented around half of their components at such high frequency, with the exception of Oceania that reached 90%. Ten (Africa) to around 22% (Asia, Europe, Americas) of components were implemented with low frequency at 1–2 rounds per year. Again, Oceania was the exception with only 3·6% of components at low frequency. Both in the Americas and in Oceania, none of the components analysed was implemented at medium frequency. In the other regions, medium sampling frequency (3–4 rounds per year) was indicated for around 2·5% (Africa and Asia) or 4·8% (Europe) of components. Remaining components were either not organized in rounds or continuously, or the information on sampling frequency was not provided or unknown (Fig. 5).
Table 2.
Number of surveillance system components by region and sampling frequency
| Region | Number of components by frequency of sampling rounds (and relative percentage by region, in %) | Grand total by region | |||
|---|---|---|---|---|---|
| Low (1–2 rounds) | Medium (3–4 rounds) | High (6 rounds or continuous) | None/unknown | ||
| Africa | 9 (10·59%) | 2 (2·35%) | 42 (49·41%) | 32 (37·65%) | 85 (100%) |
| Asia | 52 (22·81%) | 6 (2·63%) | 135 (59·21%) | 35 (15·35%) | 228 (100%) |
| Europe | 40 (21·39%) | 9 (4·81%) | 102 (54·55%) | 36 (19·25%) | 187 (100%) |
| America | 14 (23·73%) | 0 | 30 (50·85%) | 15 (25·42%) | 59 (100%) |
| Oceania | 1 (3·57%) | 0 | 25 (89·29%) | 2 (7·14%) | 28 (100%) |
| Grand total by sampling frequency | 116 (19·8%) | 17 (2·9%) | 334 (56·9%) | 120 (20·4%) | 587 |
Fig. 5.
Number and percentage of surveillance system components by frequency of sampling as expressed in number of surveillance rounds implemented per year (total = 587 components).
Surveillance type, objectives, purpose and funding
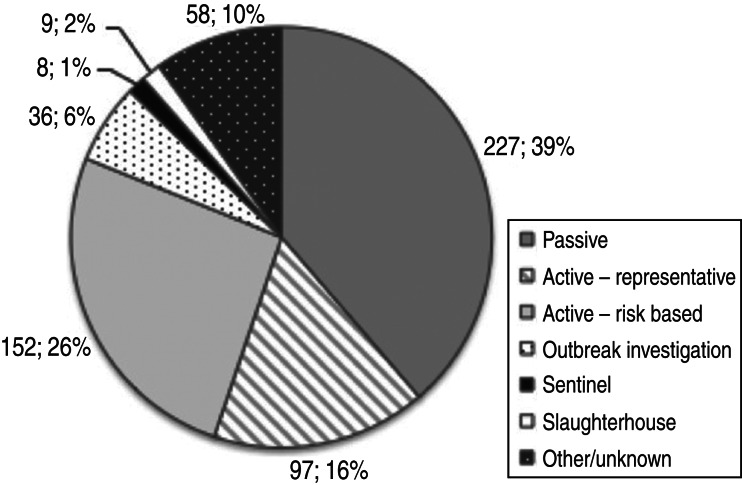
Almost half of the components analysed (42%) were active, including active risk-based and active-representative (Fig. 6). Passive surveillance was also widely practised (39%) and in Africa, this approach was dominant among surveillance system components (Table 3).
Fig. 6.
Number and percentage of surveillance system components by surveillance type (total = 587 components).
Table 3.
Number of surveillance system components by region and type of surveillance
| Region | Africa | Asia | Europe | America | Oceania | Globally |
|---|---|---|---|---|---|---|
| Surveillance type | ||||||
| Active: representative | 3 | 55 | 28 | 9 | 2 | 97 (16·5%) |
| Active: risk based | 13 | 73 | 45 | 14 | 7 | 152 (25·9%) |
| Passive | 40 | 72 | 80 | 22 | 13 | 227 (38·7%) |
| Outbreak investigation | 9 | 12 | 6 | 4 | 5 | 36 (6·1%) |
| Sentinel | 3 | 2 | 3 | 8 (1·4%) | ||
| Slaughterhouse | 4 | 4 | 1 | 9 (1·5%) | ||
| Other | 13 | 8 | 6 | 27 (4·6%) | ||
| Unknown | 4 | 2 | 25 | 31 (5·3%) | ||
| Grand total by region | 85 (14·5%) | 228 (38·8%) | 187 (31·9%) | 59 (10·1%) | 28 (4·8%) | 587 (100%) |
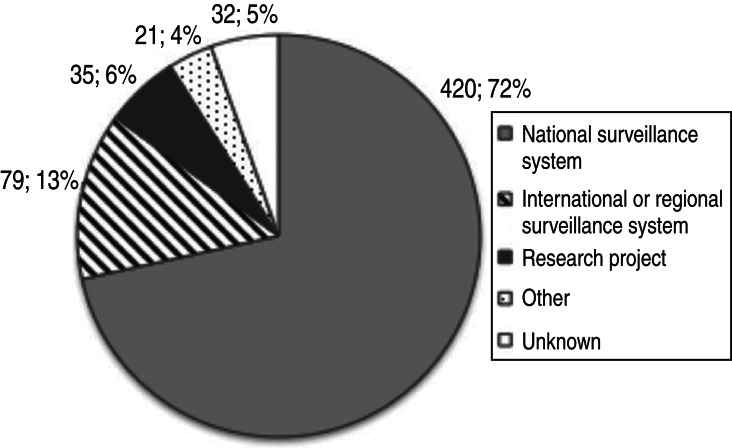
The majority (52%) of components analysed were being implemented with the main objectives of early detection of infection, monitoring the presence and assessing trends, or demonstrating freedom from infection (Table 4, Fig. 7). Seventy-two percent of components were implemented in the framework of a national surveillance system, while regional or international surveillance systems were the purpose for 14% (Table 5). Six percent of components were reportedly implemented in the framework of research projects and for the remaining 53 components (9%) the purpose was not specified.
Table 4.
Number of surveillance system components by region, purpose and objective
| Number of surveillance system components by surveillance purpose/objective | Africa | Asia | Europe | America | Oceania | Grand total |
|---|---|---|---|---|---|---|
| National surveillance system | 77 | 192 | 73 | 51 | 27 | 420 |
| Early detection | 45 | 107 | 52 | 20 | 24 | 248 |
| To reveal new cases | 2 | 2 | ||||
| Monitor presence and assess trends | 10 | 41 | 6 | 6 | 1 | 64 |
| Estimate prevalence | 1 | 9 | 8 | 1 | 1 | 20 |
| Demonstrate freedom | 5 | 19 | 6 | 10 | 1 | 41 |
| Other | 6 | 6 | ||||
| Unknown | 14 | 16 | 1 | 8 | 39 | |
| Regional or international surveillance system | 2 | 2 | 73 | 2 | 79 | |
| Early detection | 2 | 37 | 39 | |||
| Monitor presence and assess trends | 2 | 4 | 1 | 7 | ||
| Estimate prevalence | 23 | 23 | ||||
| Other | 1 | 1 | ||||
| Unknown | 1 | 1 | ||||
| Research project | 3 | 24 | 6 | 2 | 35 | |
| Early detection | 1 | 7 | 1 | 9 | ||
| Monitor presence and assess trends | 2 | 9 | 2 | 13 | ||
| Estimate prevalence | 7 | 3 | 1 | 11 | ||
| Other | 1 | 1 | ||||
| Unknown | 1 | 1 | ||||
| Other | 2 | 8 | 6 | 4 | 1 | 21 |
| Early detection | 7 | 4 | 11 | |||
| Monitor presence and assess trends | 1 | 1 | 2 | 4 | ||
| Other | 2 | 1 | 2 | 1 | 6 | |
| Unknown | 1 | 2 | 29 | 32 | ||
| Monitor presence and assess trends | 1 | 1 | ||||
| Unknown | 1 | 2 | 28 | 31 | ||
| Grand total | 85 | 228 | 187 | 59 | 28 | 587 |
Fig. 7.
Number and percentage of surveillance system components by surveillance purpose (total = 587 components).
Table 5.
Number of surveillance system components by region, purpose and funding source
| Number of surveillance system components by surveillance purpose/funding source | Africa | Asia | Europe | America | Oceania | Grand total |
|---|---|---|---|---|---|---|
| National surveillance system | 77 | 192 | 73 | 51 | 27 | 420 |
| International organization/foundation | 12 | 49 | 4 | 1 | 66 | |
| Mixed national/international | 19 | 9 | 23 | 51 | ||
| National government | 28 | 100 | 45 | 32 | 20 | 225 |
| Non-governmental organization | 2 | 2 | ||||
| Other | 3 | 3 | 8 | 7 | 21 | |
| Unknown | 15 | 31 | 1 | 8 | 55 | |
| Regional or international surveillance system | 2 | 2 | 73 | 2 | 79 | |
| International organization/foundation | 2 | 2 | 30 | 2 | 36 | |
| Mixed national/international | 25 | 25 | ||||
| National government | 8 | 8 | ||||
| Unknown | 10 | 10 | ||||
| Research project | 3 | 24 | 6 | 2 | ||
| International organization/foundation | 1 | 19 | 1 | 21 | ||
| National government | 1 | 3 | 2 | 6 | ||
| Other | 1 | 4 | 2 | 7 | ||
| Unknown | 1 | 1 | ||||
| Other | 2 | 8 | 6 | 4 | 1 | 21 |
| International organization/foundation | 2 | 2 | ||||
| National government | 2 | 1 | 3 | 1 | 7 | |
| Other | 2 | 4 | 4 | 1 | 11 | |
| Unknown | 1 | 1 | ||||
| Unknown | 1 | 2 | 29 | 32 | ||
| National government | 1 | 1 | ||||
| Unknown | 1 | 2 | 28 | 31 | ||
| Grand total | 85 | 228 | 187 | 59 | 28 | 587 |
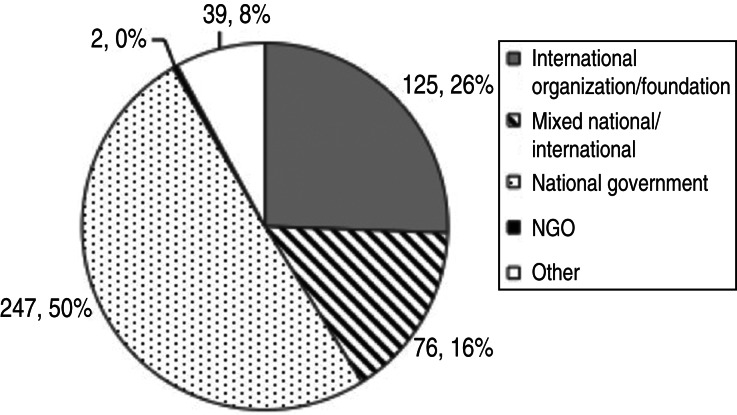
Considering only those surveillance system components where information on funding source was available (n = 489), 50% were funded by national governments and 16% had mixed funding, national and international (Fig. 8). Joint funding was particularly practised in Europe. Six percent of the components analysed were implemented in the framework of research with the main objective to monitor presence and prevalence of infection.
Fig. 8.
Number and percentage of surveillance system components by funding source (total = 489 components).
Influenza virus, animal population and production sector targeted, case definition used
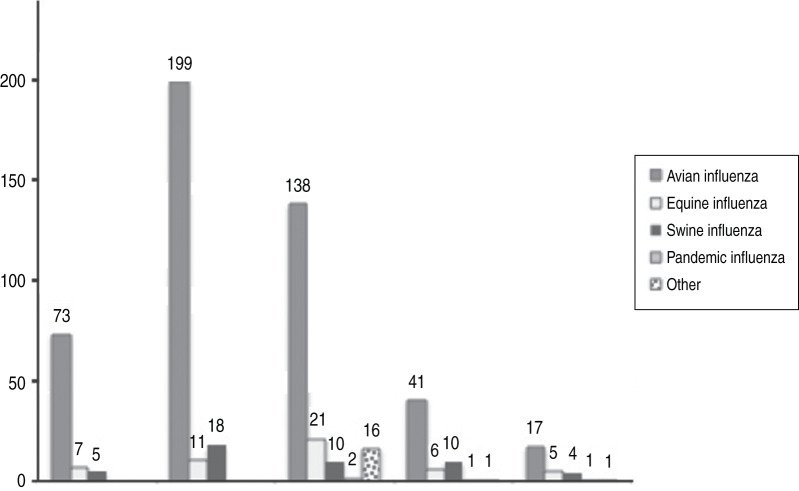
The majority of surveillance efforts worldwide (80% of components analysed) targeted AI (Fig. 9, Table 6). In Africa and Asia, AI was targeted by as much as 86% and 87% of components, respectively. Most regions prioritized domestic poultry over wild birds, with the exception of Europe and Oceania targeting domestic and wild birds equally (Table 6). Few individual components targeted captive birds in Asia (n = 2), imported zoo birds in the Americas (n = 1) or companion animals during outbreak investigation in Asia (n = 1). Generally, different poultry production systems were surveyed with similar attention (Table 7). The exception were those components (n = 7) implemented in African countries that reported AI occurrence; here intensive production systems were targeted with priority (i.e. by six of the components).
Fig. 9.
Number of surveillance system components (total = 587) by influenza targeted and geographical region.
Table 6.
Number of surveillance system components by region, influenza virus and population targeted
| Number of surveillance system components by virus/target population | Africa | Asia | Europe | America | Oceania | Grand total |
|---|---|---|---|---|---|---|
| Avian influenza | 73 | 199 | 138 | 41 | 17 | 468 |
| (%) | 85·88 | 87·28 | 73·80 | 69·49 | 60·71 | 79·73 |
| Poultry | 58 | 152 | 74 | 28 | 11 | 323 |
| Wild birds, including game birds | 15 | 41 | 64 | 12 | 6 | 138 |
| Companion animals | 1 | 1 | ||||
| Other | 5 | 1 | 6 | |||
| Equine influenza | 7 | 11 | 21 | 6 | 5 | 50 |
| (%) | 8·24 | 4·82 | 11·23 | 10·17 | 17·86 | 8·52 |
| Equines | 7 | 11 | 21 | 6 | 4 | 49 |
| Companion animals | 1 | 1 | ||||
| Swine influenza | 5 | 18 | 10 | 10 | 4 | 47 |
| (%) | 5·88 | 7·89 | 5·35 | 16·95 | 14·29 | 8·01 |
| Domestic pigs | 3 | 17 | 9 | 9 | 4 | 42 |
| Wild boar and other suids | 2 | 1 | 1 | 1 | 5 | |
| Pandemic influenza | 2 | 1 | 1 | 4 | ||
| (%) | 1·07 | 1·69 | 3·57 | 0·68 | ||
| Domestic pigs | 2 | 1 | 1 | 4 | ||
| Other | 16 | 1 | 1 | 18 | ||
| (%) | 8·56 | 1·69 | 3·57 | 3·07 | ||
| Companion animals | 16 | 1 | 17 | |||
| Other | 1 | 1 | ||||
| Grand total | 85 | 228 | 187 | 59 | 28 | 587 |
Table 7.
Number of surveillance system components by region, infection status, influenza virus targeted and target population or production system
| Targeted virus/infection status | Target population or production system | Africa | Asia | Europe | America | Oceania | Grand total | Percentage (%) |
|---|---|---|---|---|---|---|---|---|
| Avian influenza | All | 73 | 199 | 138 | 41 | 17 | 468 | 79·73 |
| Infection present | Poultry | 3 | 72 | 14 | 7 | 4 | 100 | 17·04 |
| Wild birds, including game birds | 1 | 23 | 11 | 4 | 2 | 41 | 6·98 | |
| Companion animals | 1 | 1 | 0·17 | |||||
| Other | 1 | 1 | 2 | 0·34 | ||||
| Infection not present | Poultry | 55 | 80 | 60 | 21 | 7 | 223 | 37·99 |
| Wild birds, including game birds | 14 | 18 | 53 | 8 | 4 | 97 | 16·52 | |
| Other | 4 | 4 | 0·68 | |||||
| Infection present | Backyard or free range | 1 | 53 | 14 | 8 | 6 | 82 | |
| Intensive with low biosecurity | 3 | 54 | 13 | 9 | 6 | 85 | ||
| Intensive with high biosecurity | 3 | 35 | 12 | 8 | 6 | 64 | ||
| Infection not present | Backyard or free range | 54 | 67 | 52 | 14 | 3 | 190 | |
| Intensive with low biosecurity | 56 | 63 | 47 | 13 | 7 | 186 | ||
| Intensive with high biosecurity | 22 | 61 | 48 | 16 | 1 | 148 | ||
| Equine influenza | All | 7 | 11 | 21 | 6 | 5 | 50 | 8·52 |
| Infection present | Equines | 2 | 7 | 5 | 14 | 2·39 | ||
| Infection not present | Equines | 7 | 9 | 14 | 1 | 4 | 35 | 5·96 |
| Companion animals | 1 | 1 | 0·17 | |||||
| Infection present | Backyard or free range | 1 | 1 | 2 | ||||
| Intensive with low biosecurity | 1 | 1 | 2 | |||||
| Intensive with high biosecurity | 1 | 1 | ||||||
| Infection not present | Backyard or free range | 4 | 2 | 6 | ||||
| Intensive with low biosecurity | 5 | 2 | 1 | 8 | ||||
| Intensive with high biosecurity | 5 | 1 | 6 |
Even if EI ranked second in the level of frequency of surveillance reported in Africa, Europe and Oceania and third in Asia and the Americas (Fig. 8), it was targeted by only 8·5% of surveillance system components analysed (Table 6). Almost all of these components (98%) focused on equines. Only one component in a country in Oceania investigated companion animals. A fairly balanced pattern for targeting different production systems could also be observed for EI surveillance in Africa and Asia. However, Europe and Oceania only targeted low biosecurity systems, including backyards.
SI was targeted by only 8% of components worldwide, with percentages amounting to 14% and 17% in Oceania and the Americas, respectively (Table 6). The majority of these components (89%) targeted domestic pigs in intensive production systems. Less than 1% (n = 4) of all reported components (in Europe, the Americas and Oceania) specifically searched for pandemic influenza viruses in domestic pigs.
Seventeen components (2·9%) were specified to target influenza in companion animals, most of them in Europe (n = 16) and one in Oceania.
The most common case definition used to confirm a positive influenza case in animals was based on virus isolation or reverse transcription–polymerase chain reaction (RT–PCR) (49·4%, 290/587) (Table 8). This accounted for almost all geographical regions, the only exception being Africa where most components were using clinical signs to confirm surveillance positives. Europe, America and Oceania used clinical signs in only very few of their surveillance system components implemented. Virus isolation or RT–PCR was predominantly used in the majority of surveillance types, with the exception of active representative surveillance where serology was being equally applied at the global level (Table 8). In Europe, serology was the preferred case definition for active representative surveillance. For 87 components (14·8%) the case definition used was not provided.
Table 8.
Number of surveillance system components by region, surveillance type and case definition used
| Case definition based on | Africa | Asia | Europe | America | Oceania | Grand total | Percentage (%) | |
|---|---|---|---|---|---|---|---|---|
| Global total | Virus isolation or RT–PCR | 18 | 104 | 112 | 36 | 20 | 290 | 49·40 |
| Serology | 11 | 43 | 41 | 6 | 6 | 107 | 18·23 | |
| Clinical signs | 40 | 51 | 6 | 6 | 103 | 17·55 | ||
| Other | 1 | 7 | 1 | 2 | 11 | 1·87 | ||
| Unknown | 15 | 23 | 28 | 10 | 76 | 12·95 | ||
| By surveillance type | ||||||||
| Active: representative | Virus isolation or RT–PCR | 1 | 31 | 8 | 7 | 47 | ||
| Serology | 1 | 17 | 20 | 1 | 2 | 41 | ||
| Clinical signs | 1 | 4 | 1 | 6 | ||||
| Unknown | 3 | 3 | ||||||
| Active: risk based | Virus isolation or RT–PCR | 4 | 46 | 36 | 11 | 4 | 101 | |
| Serology | 1 | 13 | 8 | 1 | 3 | 26 | ||
| Clinical signs | 6 | 6 | 12 | |||||
| Other | 2 | 2 | ||||||
| Unknown | 2 | 6 | 1 | 2 | 11 | |||
| Passive | Virus isolation or RT–PCR | 4 | 16 | 61 | 12 | 13 | 106 | |
| Serology | 5 | 4 | 11 | 20 | ||||
| Clinical signs | 29 | 41 | 5 | 3 | 78 | |||
| Other | 3 | 1 | 4 | |||||
| Unknown | 2 | 8 | 3 | 6 | 19 | |||
| Outbreak investigation | Virus isolation or RT–PCR | 4 | 9 | 3 | 2 | 3 | 21 | |
| Serology | 1 | 3 | 2 | 1 | 7 | |||
| Clinical signs | 3 | 1 | 1 | 5 | ||||
| Other | 2 | 2 | ||||||
| Unknown | 1 | 1 | ||||||
| Sentinel | Virus isolation or RT–PCR | 2 | 3 | 5 | ||||
| Serology | 2 | 2 | ||||||
| Clinical signs | 1 | 1 | ||||||
| Slaughterhouse | Virus isolation or RT–PCR | 1 | 2 | 3 | ||||
| Serology | 1 | 2 | 1 | 4 | ||||
| Other | 2 | 2 | ||||||
| Other/unknown | Virus isolation or RT–PCR | 3 | 1 | 1 | 2 | 7 | ||
| Serology | 3 | 3 | 1 | 7 | ||||
| Clinical signs | 1 | 1 | ||||||
| Other | 1 | 1 | ||||||
| Unknown | 10 | 6 | 24 | 2 | 42 | |||
RT–PCR, Reverse transcription–polymerase chain reaction.
Communication of and access to results
Surveillance results were being reported at regular intervals in the majority of components (64%), which ranged from yearly to four times a year (Table 9). In instances where positive animal cases were identified, the public health sector was alerted for about half of the components targeting avian, swine, and pandemic influenza (61%, 51%, and 50%, respectively) (Table 10).
Table 9.
Frequency of communication of results by number of surveillance system components and region
| Frequency of reporting | Africa | Asia | Europe | America | Oceania | Grand total | Percentage (%) |
|---|---|---|---|---|---|---|---|
| Irregularly/case by case | 25 | 30 | 15 | 13 | 12 | 95 | 16·18 |
| Daily or monthly | 3 | 70 | 11 | 6 | 90 | 15·33 | |
| Quarterly or twice a year | 46 | 69 | 63 | 17 | 14 | 209 | 35·60 |
| Yearly | 1 | 4 | 61 | 8 | 2 | 76 | 12·95 |
| Other/unknown | 10 | 55 | 37 | 15 | 117 | 19·93 | |
| Grand total | 85 | 228 | 187 | 59 | 28 | 587 | 100·00 |
Table 10.
Number of surveillance system components in which the public health (PH) sector is alerted in case positives are found, by influenza targeted and region
| Influenza targeted | Africa | Asia | Europe | America | Oceania | Global total | |
|---|---|---|---|---|---|---|---|
| Avian influenza | PH sector alerted | 43 | 120 | 80 | 27 | 17 | 287 |
| (%) | 58·90 | 60·30 | 57·97 | 65·85 | 100·00 | 61·32 | |
| All components for AI | 73 | 199 | 138 | 41 | 17 | 468 | |
| Equine influenza | PH sector alerted | 0 | 1 | 2 | 1 | 1 | 5 |
| (%) | 0·00 | 9·09 | 9·52 | 16·67 | 20·00 | 10·00 | |
| All components for EI | 7 | 11 | 21 | 6 | 5 | 50 | |
| Swine influenza | PH sector alerted | 4 | 10 | 6 | 4 | 24 | |
| (%) | 80·00 | 55·56 | 0·00 | 60·00 | 100·00 | 51·06 | |
| All components for SI | 5 | 18 | 10 | 10 | 4 | 47 | |
| Pandemic influenza | PH sector alerted | 0 | 0 | 0 | 1 | 1 | 2 |
| (%) | 0 | 0 | 2 | 1 | 1 | 4 | |
| All components pandemic influenza | 0 | 0 | 0 | 100 | 100 | 50 | |
| Other influenza | PH sector alerted | 0 | 0 | 1 | 1 | 2 | |
| (%) | 0 | 0 | 6·25 | 100·00 | 0·00 | 11·11 | |
| All components other influenza | 0 | 0 | 16 | 1 | 1 | 18 |
AI, Avian influenza; EI, equine influenza; SI, swine influenza.
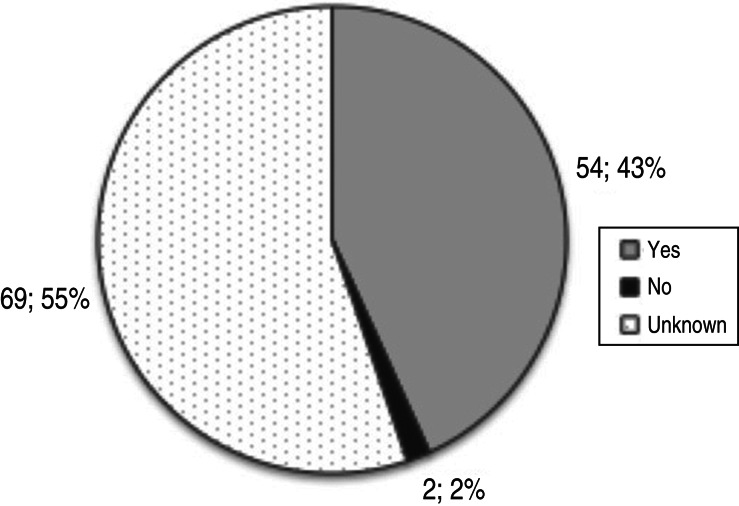
For 24·2% (142/587) of the components analysed it was indicated that isolates were sequenced (Table 11). Countries also reported their sequencing activities for components where no surveillance positives were found between 2010 and 2012. Regarding the extent of sequencing performed for those surveillance system components that reportedly rendered positive results (21·3%), for 43% of these components it was indicated that sequence information had been published in online genetic databases (Fig. 10). Overall, for 21·8% (128/587) of components it was reported that sequences generated by the surveillance activities would be submitted to one of the public genetic databases; for 37·5% (48/128) of components this would be GenBank, for 8·6% (11/128) of components Global Initiative on Sharing All Influenza Data (GISAID; http://platform.gisaid.org) would be used, for 3·1% (4/128) of components sequences would be submitted to the Influenza Research Database (IRD) and in 50·8% (65/128) of components other public databases would be used (Table 11). In one component (1%), implemented in the framework of a master thesis, the sequence(s) were not published, even if full genome sequencing had been performed.
Table 11.
Extent of sequencing and sequence availability by number of surveillance system components and region
| Africa | Asia | Europe | America | Oceania | Grand total | Percentage (%) | |
|---|---|---|---|---|---|---|---|
| Were isolates sequenced? | |||||||
| Full genome sequenced | 20 | 9 | 5 | 2 | 36 | 6·13 | |
| HA and NA | 23 | 15 | 1 | 39 | 6·64 | ||
| HA only | 2 | 10 | 8 | 20 | 3·41 | ||
| NA only | 2 | 2 | 0·34 | ||||
| Not sure to what extent | 4 | 31 | 7 | 3 | 45 | 7·67 | |
| Total of isolates sequenced | 6 | 76 | 41 | 16 | 3 | 142 | 24·19 |
| Not sequenced | 18 | 24 | 45 | 11 | 11 | 109 | 18·57 |
| Unknown | 61 | 128 | 101 | 32 | 14 | 336 | 57·24 |
| Are sequences publicly available? | |||||||
| GenBank | 33 | 8 | 4 | 3 | 48 | 8·18 | |
| GISAID | 11 | 11 | 1·87 | ||||
| Influenza Research database (IRD) | 4 | 4 | 0·68 | ||||
| Other | 5 | 51 | 4 | 5 | 65 | 11·07 | |
| Total of sequences in public domain | 9 | 84 | 23 | 9 | 3 | 128 | 21·77 |
| No | 2 | 3 | 5 | 0·85 | |||
| Unknown | 74 | 141 | 164 | 50 | 25 | 454 | 77·34 |
| Grand total | 85 | 228 | 187 | 59 | 28 | 587 | 100·00 |
GISAID, Global Initiative on Sharing All Influenza Data.
Fig. 10.
Percentage of surveillance system components rendering positive results (n = 125) for which sequence information was published in online databases (e.g. GenBank, GISAID™, OpenFluDB, IRD or other).
DISCUSSION
Data on national animal influenza surveillance strategies from 99 countries worldwide show that such systems are reportedly widely implemented. The outcome of our survey was the description of intensity and design of influenza surveillance systems, particularly their use of different components and the resulting capacity for early detection of emerging virus strains with zoonotic potential. Ideally, to improve pandemic preparedness, subtypes which in the past demonstrated higher zoonotic potential should be targeted, regardless of their importance for animal production, health or trade. Apart from H5 and H7 highly pathogenic avian influenza (HPAI) or low pathogenic avian influenza, priority should be given to H9 isolates from domestic birds, H1 and H3 isolates from domestic pigs and to any new subtype isolated from a mammalian host (i.e. a subtype which has not been previously isolated from a mammalian species) (O. Munoz et al., unpublished data). Moreover, influenza subtypes causing epidemic or non-epidemic episodes characterized by unusual epidemiological patterns (e.g. unexpected incidence, morbidity, mortality) should be prioritized.
All but five countries (from Africa, Europe and the Americas) indicated having several surveillance system components in place, each with different attributes. Those countries with only few components could be encouraged to increase active surveillance efforts in order to identify a wider diversity of influenza viruses from a range of animal populations. Both active and passive surveillance approaches are almost equally implemented and both have their place in influenza surveillance strategies. Active surveillance provides representative information for the population of interest and is the surveillance design of choice when aiming to determine prevalence. Passive surveillance, on the other hand, is less costly since it utilizes existing, readily available data, but cannot provide representative information. Passive surveillance can be used for detection of clinical cases but is ineffective for the investigation of zoonotic influenza viruses that do not produce overt disease signs in animals. A recent example is influenza A(H7N9) being non-pathogenic for poultry despite causing clinical disease in humans [11].
Around half of components analysed are being implemented with the objective of early detection. However, the fact that some influenza viruses are notifiable to national and international bodies, while others are not, is likely to strongly influence laboratory screening protocols. Routine influenza testing of poultry or pigs, however, can and should be used to screen for potentially zoonotic viruses concurrently. Given that influenza pandemics so far have been caused by H1, H2 or H3 viruses [12, 13], laboratory protocols should be adapted accordingly and advanced characterization performed, e.g. screening for markers for human pathogenicity or other molecular markers known or suspected to enhance zoonotic potential (O. Munoz et al., unpublished data). A limitation in our survey was not being able to determine if further subtyping was performed on positive samples, which would have facilitated the assessment of how existing animal health-focused components could potentially detect zoonotic viruses. Pandemic influenza viruses in recent decades have likely emerged from either poultry or pig viruses that gained pandemic potential through re-assortment with circulating human viruses [14–16]. Pigs potentially act as a mixing vessel between avian and mammalian influenza viruses [1, 17]. Among poultry species, quail (and possibly turkeys) could play a role as virus amplifiers and bridging species for avian viruses originating from wild waterfowl, the viruses natural reservoir [18–20]. Therefore, with regards to investigating zoonotic risk, influenza surveillance in these species should be given priority, especially when husbandry production systems and human living spaces are intermingled.
The analysis revealed that AI is targeted by most surveillance components implemented worldwide with most regions prioritizing domestic poultry over wild birds. In this context it is important to keep in mind that the survey has been implemented following a period of increased financial investment related to the recent H5N1 HPAI events.
SI is targeted by only few components, predominantly surveying intensive pig production systems. One of the reasons for lesser efforts could be that SI is not notifiable, with the exception of H5 or H7 subtypes, and generally has no adverse trade implications. Although morbidity rates may reach 100% in affected herds, mortality rates are generally low [21]. Therefore, countries have less incentive to survey pig herds. This has changed, however, with the recent emergence of H1N1pdm09. Consumer fears that eating pork meat might result in infection led to a downturn in domestic and international pork markets and several pork-importing countries officially imposed bans on swine and pork products [22]. As a consequence, some pork-exporting countries started implementing H1N1pdm09 surveillance in pig herds. However, surveillance conducted by the private sector, such as the pig industry, may not have been reported to us if national authorities are not informed via official communications. It is therefore important that the private sector shares its surveillance results with the national authorities, including non-notifiable influenzas or negative results, to aid the understanding of animal influenza virus circulation and viral diversity in a country. For example, following H1N1pdm09, the USA developed standard operating procedures for data sharing between the pig industry and the US Department of Agriculture, safeguarding the identity of individual farmers [23].
Very few (<1%), sporadically implemented surveillance system components were indicated to specifically search for influenza viruses with pandemic potential, all of them targeting domestic pigs. We note a general integration into national surveillance systems, which is an indication for government leadership and funding in these few countries and shows the interest and commitment of these governments to invest in pandemic preparedness through targeted surveillance. Such structures would also indicate a minimum level of sustainability of efforts. As much as we defined pandemic influenza in the glossary provided to CVOs prior to the survey as ‘influenza strains causing epidemic spread in human populations over large geographical regions or even worldwide’, this term may have been confused with H1N1pdm09, which could explain that pandemic viruses were indicated to be monitored only in swine populations. Nevertheless, the results reveal the global need for increasing surveillance targeting influenza viruses with pandemic potential in different animal species.
For AI different production systems were targeted by surveillance activities in a fairly balanced manner, while for SI intensive production systems were prioritized. Backyard and intensive systems usually represent very separate environments in which influenza viruses may evolve differently. Hence we suggest targeting extensive as well as intensive production systems, prioritised according to their characteristics, e.g. biosecurity aspects (allowing for increased intra- and inter-species contact) and trade connectivity. The backyard and free range systems or those with low biosecurity are vulnerable to influenza virus introductions and co-infections in animals may occur, providing influenza viruses opportunity to exchange genes and re-assort. Intensive production systems, as opposed to backyard systems, may be characterised by continuous introduction of naïve animals if the good practices of all-in/all-out are not followed. Endemic circulation of influenza viruses may be sustained in such environments and viruses may modify and further adapt over time. In addition, poorly implemented vaccination campaigns may encourage the evolution of escape mutants. This has been shown for H5N1 HPAI viruses in Egypt, where different virus lineages circulate in backyard and intensive production systems [24, 25].
Scientific value would be added by implementing targeted, risk-based influenza surveillance also in species other than pigs and poultry, especially when these are in close contact with domestic pigs and humans in low biosecurity settings. Survey results show that those countries targeting also companion animals (along with poultry) during outbreak investigations as well as wild or captive mammals provide opportunity for detecting potential zoonotic influenza viruses.
The survey further revealed that surveillance is mostly implemented at a high frequency and results are being reported at regular intervals in the majority of components. The overall level of international access to surveillance results was, however, difficult to assess, since several communication mechanisms can be initiated in a single component. Timely reporting of results from transboundary animal disease surveillance, such as influenza, and facilitation of national as well as international access to findings has clear benefits to the international community. This has recently been demonstrated during the emergence of influenza A(H7N9), where China rapidly shared information on human cases, surveillance plans and findings in humans as well as animals, control measures implemented and genetic sequences. The latter in turn allowed for other countries to augment their preparedness and surveillance plans according to findings in China [26–28].
The most common case definition used to confirm a positive influenza case in animals was reported to be based on virus isolation or RT–PCR, which constitute the laboratory methods of choice to confirm a positive influenza case. Any current infection with influenza viruses can only be confirmed through antigen or virus detection and any serological positives from suspect outbreaks should be followed up accordingly. Additionally, in order to trace the evolution of animal influenza viruses over time or to discover new genetic traits of known circulating viruses, e.g. acquired human pathogenicity, it is necessary to regularly select a subset of viruses for advanced genetic characterization based on criteria such as invasion of new hosts or new geographical areas or representative of endemic strains. Making such information available to the scientific community by submitting sequence information to public databases and sharing live viruses of particular concern is equally important. Even if countries indicated that isolates generated from only 24% of components were sequenced, virus sequences were submitted to public genetic databases in the majority of cases.
The public health sector is only alerted for about half of the components targeting avian, swine and pandemic influenza in the instance that positive animal cases are identified. Failure to do so may be related to a lack of established communication channels. Communication between veterinary and public health services may also be impaired or non-existent, since they are usually governed by different ministries. However, when investigating animal influenza viruses that may have severe consequences in the human population, it is of utmost importance that these services inform each other of any findings and promote joint activities. A One Health approach would encourage standardised communication and collaboration between veterinary services and public health officials. Routine operating procedures should be put in place and officials adequately trained. Procedures for emergency response should also be established and regularly tested in simulation exercises.
Adapting existing surveillance activities to include capture of influenza viruses with zoonotic potential may be a practical way forward to make efficient use of resources. In general and if resources are available, targeted surveillance system components should be implemented to survey animal populations that are not included in existing surveillance programmes. Additionally, research addressing specific questions should be encouraged and funded to help filling surveillance gaps.
Main recommendations from the study can be summarized as follows:
-
•
Animal influenza surveillance systems have high potential to inform human pandemic risk if several integrated components of representative or risk-based surveillance are in place. Those countries only implementing few components are encouraged to diversify surveillance efforts in order to target more influenza viruses and/or animal populations.
-
•
To improve pandemic preparedness, subtypes with higher zoonotic risk should be targeted regardless of their importance for animal production, health or trade.
-
•
Routine testing of poultry or pigs for purposes other than pandemic preparedness can and should be used to screen also for potentially pandemic viruses. As part of the routine laboratory investigation of viruses suspected to have zoonotic potential, screening for human-adaptive traits should be performed.
-
•
Laboratory methods such as PCR or virus isolation constitute the case definition of choice. Countries are further encouraged to regularly select a subset of viruses for genetic characterization through sequencing.
-
•
Timely reporting of surveillance results, including surveillance activities by the private sector or in the framework of research, and international access to important or particular findings are as important as submitting animal influenza virus sequences to public genetic databases and sharing live virus with the international research community.
ACKNOWLEDGEMENTS
This study was undertaken as part of the FLURISK project funded by the European Food Safety Authority (EFSA). The project has been coordinated by the Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe), Italy, bringing together recognized human and veterinary medicine European research institutes, reference laboratories and universities, fostering cross-disciplinary expertise and collaboration.
The authors are grateful to the OIE, who co-signed the supporting documents and encouraged this project. Likewise, FAO country representatives and field animal health officers provided their invaluable assistance in following up with non-responding countries.
The authors also express their gratitude to all CVOs and their staff who took the time to respond to the survey. Likewise, the sharing of SI surveillance information by the ESNIP-3 Consortium before publication was highly appreciated and added value to the analysis.
APPENDIX. FLURISK Consortium members (in alphabetical order, by institute)
Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe): Roberta Bassan, Ilaria Capua (Project Coordinator), Giovanni Cattoli, Marco De Nardi (Project Manager), Isabella Monne, Olga Munoz.
Royal Veterinary College (RVC): Dirk Pfeiffer, Katharina Stärk, Kim Stevens, Sophie von Dobschuetza, Barbara Wieland.
Animal Health and Veterinary Laboratories Agency (AHVLA): Jill Banks, Andrew Breed, Sharon Brookes, Ian Brown, Tamsin Dewe, Kate Harris, Andy Hillb, Louise Kelly, Rowena Kosmider.
National Institute of Public Health and Environment (RIVM): Gudrun Freidl, Arie Havelaar, Marion Koopmans, Adam Meijer.
University of Ghent (UniGhent): Karen van der Meule, Kristien van Reeth.
Institut Pasteur (IP): Vincent Enouf, Jean Claude Manuguerra, Sylvie van der Werf.
Food and Agricultural Organization of the United Nations (FAO): Gwenaelle Dauphin.
World Organisation for Animal Health (OIE): Gounalan Pavade.
Centers for Disease Control and Prevention (CDC): Sue Trock.
a Also at FAO.
b Also at RVC.
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814002106.
click here to view supplementary material
REFERENCES
- 1.Alexander DJ, Brown IH. Recent zoonoses caused by influenza A viruses. Revue Scientifique et Technique OIE 2000; 19: 197–225. [DOI] [PubMed] [Google Scholar]
- 2.OIE. Infection with avian influenza viruses. Terrestrial Animal Health Code 2013; vol. II, chapter 10.4. [Google Scholar]
- 3.Peiris J, Poon L, Guan Y. Surveillance of animal influenza for pandemic preparedness. Science 2012; 335: 1173–1174. [DOI] [PubMed] [Google Scholar]
- 4.Hoinville L, et al. Animal Health Surveillance Terminology – Final report from pre-ICAHS workshop. International Conference on Animal Health Surveillance (ICAHS), 2013. [Google Scholar]
- 5.Pavade G, et al. OFFLU Review of avian influenza surveillance and epidemiological projects in some European, African, and Asian countries. OIE/FAO Network of Expertise on Animal Influenza (OFFLU), 2010. [Google Scholar]
- 6.Brown IH. ESNIP 3 Project – European Surveillance Network for Influenza in Pigs. European Union, 2012.
- 7.OIE. World animal health information database – WAHID (http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home). Accessed 15 March 2013.
- 8.FAO. EMPRES-i, Global animal disease information system (http://empres-i.fao.org). Accessed 15 March 2013.
- 9.ESRI. ArcGIS Desktop: release 10. Environmental Systems Research Institute, Redlands, CA, USA. 2011.
- 10.UN. Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings (http://millenniumindicators.un.org/unsd/methods/m49/m49regin.htm). 2013.
- 11.Swayne DE, et al. Understanding the 2013 H7N9 avian influenza outbreak in poultry: field epidemiology and experimental pathogenesis studies. One Influenza, One World and One Health. Influenza 2013 Conference, Oxford, UK, 2013.
- 12.Horimoto T, Kawaoka Y. Pandemic threat posed by avian influenza A viruses. Clinical Microbiology Reviews 2001; 14: 129–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Evolution of a pandemic: A(H1N1) 2009, April 2009–August 2010. 2013.
- 14.Reid AH, et al. Origin and evolution of the 1918 ‘Spanish’ influenza virus hemagglutinin gene. Proceedings of the National Academy of Sciences USA 1999; 96: 1651–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nature Reviews Microbiology 2004; 2: 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith GJ, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009; 459: 1122–1125. [DOI] [PubMed] [Google Scholar]
- 17.Ma W, Kahn RE, Richt JA. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. Journal of Molecular and Genetic Medicine 2009; 3: 158–166. [PMC free article] [PubMed] [Google Scholar]
- 18.Makarova N, et al. Replication and transmission of influenza viruses in Japanese quail. Virology 2003; 310: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain M, Hickman D, Perez D. Evidence of expanded host range and mammalian-associated genetic changes in a duck H9N2 influenza virus following adaptation in quail and chickens. PLoS One 2008; 3: e3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannecchini S, et al. Molecular adaptation of an H7N3 wild duck influenza virus following experimental multiple passages in quail and turkey. Virology 2010; 408: 167–173. [DOI] [PubMed] [Google Scholar]
- 21.OIE. Swine influenza. Technical Disease Cards, 2009.
- 22.Attavanich W, McCarl BA, Bessler D. The effect of H1N1 (swine flu) media coverage on agricultural commodity markets. Applied Economic Perspectives and Policy 2011; 33: 241–259. [Google Scholar]
- 23.USDA. USDA swine influenza surveillance update, 2012.
- 24.Arafa A, et al. Phylogenetic analysis of hemagglutinin and neuraminidase genes of highly pathogenic avian influenza H5N1 Egyptian strains isolated from 2006 to 2008 indicates heterogeneity with multiple distinct sublineages. Avian Diseases 2010; 54: 345–349. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Moneim A, Afifi M, El-Kady M. Genetic drift evolution under vaccination pressure among H5N1 Egyptian isolates. Journal of Virology 2011; 283: doi: 10.1186/1743-422X-8-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kageyama T, et al. Genetic analysis of novel avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013. Eurosurveillance 2013; 18: pii = 20453. [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis K, et al. Detection of mild to moderate influenza A/H7N9 infection by China's national sentinel surveillance system for influenza-like illness: case series. British Medical Journal 2013; 346: f3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinese Government. Ministry of Agriculture, People's Republic of China, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814002106.
click here to view supplementary material
Data Availability Statement
The response rate of the CVO survey was 51·4% (94/183) (Fig. 1). One country notified that no influenza surveillance was taking place.
Fig. 1.
Number of responding (94) and non-responding (89) countries by geographical region (51·4% response rate, 94/183).
On a regional scale, geographical distribution of responding countries was fair with regional response rates as follows: Africa (37·0%, 20/54); Asia (60·4%, 29/48); Europe (68·3%, 28/41); Americas (37·1%, 13/35); Oceania (80·0%, 4/5) (Fig. 1).
Using additional data sources (OFFLU, ESNIP-3, OIE WAHID) resulted in overall data availability for 99 of the 242 countries, thus 41% (Table 1). The regions with the largest number of countries contributing data to the analysis were Asia (32 countries, 63% data availability) and Europe (28 countries, 53%). The geographical distribution of these countries is shown at the subregional level (Table 1). Few individual subregions had relatively low data coverage, namely Eastern Africa (15%), the Caribbean (14%) and Central America (13%), but this largely improved at the regional level. An exception was Oceania, where no data was available for seven countries in Micronesia and 10 countries in Polynesia, thereby decreasing the overall data coverage to 16%. The geographical distribution of countries contributing data to the analysis is illustrated in Figure 2 by main information source.
Table 1.
Countries contributing data, by geographical subregion (40·9% coverage)
| Region | Subregion | No. of countries | Countries with data | Data coverage (%) |
|---|---|---|---|---|
| Africa | Eastern Africa | 20 | 3 | 15·00 |
| Middle Africa | 9 | 3 | 33·33 | |
| Northern Africa | 7 | 3 | 42·86 | |
| Southern Africa | 5 | 3 | 60·00 | |
| Western Africa | 17 | 9 | 52·94 | |
| Asia | Total | 58 | 21 | 36·21 |
| Central Asia | 5 | 2 | 40·00 | |
| Eastern Asia | 7 | 4 | 57·14 | |
| Southern Asia | 9 | 5 | 55·56 | |
| South-Eastern Asia | 11 | 8 | 72·73 | |
| Western Asia | 19 | 13 | 68·42 | |
| Europe | Total | 51 | 32 | 62·75 |
| Eastern Europe | 10 | 4 | 40·00 | |
| Northern Europe | 18 | 9 | 50·00 | |
| Southern Europe | 16 | 9 | 56·25 | |
| Western Europe | 9 | 6 | 66·67 | |
| America | Total | 53 | 28 | 52·83 |
| Caribbean | 28 | 4 | 14·29 | |
| Central America | 8 | 1 | 12·50 | |
| South America | 14 | 7 | 50·00 | |
| North America | 5 | 2 | 40·00 | |
| Oceania | Total | 55 | 14 | 25·45 |
| Australia/New Zealand | 3 | 2 | 66·67 | |
| Melanesia | 5 | 2 | 40·00 | |
| Micronesia | 7 | 0 | 0·00 | |
| Polynesia | 10 | 0 | 0·00 | |
| Total | 25 | 4 | 16·00 | |
| Grand total | 242 | 99 | 40·91 |
Fig. 2.
Geographical distribution of countries contributing data to the analysis, by main information source: CVO, Chief Veterinary Officer (names of contributing CVOs are given in the online Supplementary material); MoA, Ministry of Agriculture; Nat. Ref. Lab., National Reference Laboratory; OFFLU, OIE/FAO Network of Expertise on Animal Influenza; GRIPAVI, Ecology and Epidemiology of Avian Influenza in Developing Countries.
Figure 3 illustrates the number and percentage of countries contributing data by region. The country with no surveillance activities has been excluded from the analysis. Data availability was highest for Asia (33% of countries in the region contributing data to the analysis) and Europe (29%), and lowest for Oceania (3%). However, Oceania includes a number of small island states and the main land mass is concentrated in Australia/New Zealand and Melanesia, for which data availability was higher.
Fig. 3.
Number and percentage of countries contributing data on surveillance systems implemented, by region (total = 98 countries).