SUMMARY
Tularemia is a contagious infectious disease due to Francisiella tularensis that can cause serious clinical manifestations and significant mortality if untreated. Although the frequency and significance of the disease has diminished over the last decades in Central Europe, over the past few years, there is new evidence suggesting that tularemia has re-emerged worldwide. To know the real epidemiology of the disease is at the root of correct control measures. In order to evaluate whether tularemia is re-emerging in Italy, data on mortality and morbidity (obtained by the National Institute of Statistics; ISTAT), Italian cases described in the scientific literature and data concerning hospitalizations for tularemia (obtained by the National Hospital Discharge Database) were analysed. From 1979 to 2010, ISTAT reported 474 cases and no deaths. The overall number of cases obtained from the literature review was at least 31% higher than that reported by ISTAT. Moreover, the number of cases reported by ISTAT was 3·5 times smaller than hospitalized cases. In Italy tularemia is sporadic, rarely endemic and self-limiting; but, although the trend of reported tularemia does not support the hypothesis of a re-emerging disease, the study demonstrates a wide underreporting of the disease. The real frequency of the disease should be carefully investigated and taken into account in order to implement specific prevention measures.
Key words: Compulsory reporting, discharge forms, epidemiology, Italy, tularemia
INTRODUCTION
Tularemia is a contagious infectious disease due to Francisiella tularensis, a small (0·1–1 × 0·1–3 μm), pleomorphic non-motile, facultative intracellular Gram-negative bacillus, obligately aerobic and non-spore forming [1]. F. tularensis can remain for weeks in soil, mud, water and putrefied carcasses. Infection with F. tularensis can occur via several routes: via skin contact, inhalation, and consumption of contaminated water or meat (especially hare meat) [2].
Several subspecies (biovars) are recognized: F. tularensis subsp. tularensis, also known as type A, F. tularensis subsp. holarctica also known as type B, F. tularensis subsp. mediasiatica and F. tularensis subsp. novicida [3]. Human diseases are primarily associated with two F. tularensis biovars: F. tularensis subsp. tularensis (type A) and F. tularensis subsp. holarctica (type B).
F. tularensis type A is one of the most infectious pathogens known in human medicine [4]. The infective dose in humans is extremely low: 10 bacteria when injected subcutaneously and 25 when given as an aerosol [4]. The bacterium can cause serious clinical manifestations and significant mortality if untreated. Ticks, mosquitoes and other insects are important vectors. F. tularensis subsp. tularensis, before antibiotic treatment was available, showed a fatality rate as high as 30%, and currently is less than 2% [3, 4]. Tularemia mainly occurs in the in North America [3, 4], but also in European countries like the former Soviet Union [4] and in Scandinavian countries [3]. In Italy F. tularensis type A has been reported only once in an outbreak involving three subjects of the same family; insects were not involved [5] and there were no fatalities.
F. tularensis type B rarely causes fatal disease. It is mainly associated with streams, ponds, lakes, rivers and semi-aquatic animals such as muskrats and beavers. Therefore, some authors consider type B tularemia to be a waterborne disease [4]. In support of this, subsp. holarctica has been shown to survive and replicate in protozoa [1]. Nevertheless, subsp. holarctica has been found also in hares and other animals. The bacterium is transmitted to humans by direct contact with infectious animals, arthropod bites, aerosols, or ingestion of contaminated water and food [4]. F. tularensis type B is endemic in Europe, Russia, Northern Asian countries and Japan [6] and it is the biovar most frequently reported in Italy [7–9].
F. tularensis subsp. mediasiatica has been reported from Central Asia (Kazakhstan and Turkmenistan) and East European countries [4, 10]. Experimental studies in rabbits have shown a moderate virulence comparable to that of subsp. holarctica [4]. F. tularensis subsp. novicida was reported in Australia, but it is endemic primarily in North America and rarely isolated [10].
Although tularemia is a long-known disease, its significance has diminished over the last decades in Central Europe. However, over the past few years, there is new evidence suggesting that tularemia has re-emerged worldwide [2], with recent descriptions of outbreaks in the USA, Asia and Europe [2, 6, 11, 12]. In Germany, several lines of evidence indicate that tularemia has re-emerged since 2004 [6]. Recently, other episodes have also been described in Austria [13]. In 2000 and 2003, two outbreaks of tularemia occurred in Kosovo, each involving more than 300 cases and presenting predominantly as the oropharyngeal form [14]. In Kosovo prior to 2000 tularemia was considered endemic. Farmers left their farms in Kosovo during the war, leaving unharvested fields vulnerable to rodents. Rats and mice entered farm houses, leaving excrement and carcasses in water wells [14]. Cases have been described in Scandinavia from 2000 to 2005, with an average of 3·4 cases/100 000 people [3]. Several outbreaks of oropharyngeal tularemia recently occurred in Turkey which were related to consumption of untreated natural spring water [15] or unchlorinated environmental water [16].
In Italy, human tularemia was recognized for the first time in 1962, in the province of Pavia (Lombardy), in hares imported from Eastern Europe for the purpose of restocking [17]. Since then, the disease has been occasionally diagnosed in hares [9], in farmed rabbits and mice [8, 18–21]. Sera of numerous wild or domestic animals tested positive for F. tularensis, not only in areas where human infections had occurred, but also in neighbouring areas, documenting the circulation of the organism in the territory [8, 21, 22]. The disease is self-limiting in these areas for several reasons: one, is that rodents and lagomorphs develop a septicaemic disease leading to rapid death [23]. The droppings or the carcasses of infected animals, however, can contaminate aqueduct or spring water and sometimes outbreaks of oropharyngeal tularemia have been reported in the Appenines area of Liguria and Tuscany [4, 5]. Sporadic human cases are reported each year [24–28]. Recently, another water-related outbreak of 44 cases was described in Tuscany [7]. This large epidemic occurred about 20 years after the last outbreak of tularemia and confirms the circulation of F. tularensis in Tuscany. Considering that in 2010 some batches of hares imported into Italy for restocking from Eastern Europe (Hungary and Romania) were found serologically positive for F. tularensis subsp. holarctica (type B), the import of hares from these countries carries a real risk for the introduction of F. tularensis strains having different virulence characteristics from those found in the native wildlife populations [23].
The aim of this study is to investigate the time trend of tularemia in Italy, in order to evaluate whether the disease is re-emerging and to suggest appropriate preventive measures.
METHODS
Three sources of information were compared: data from the registers of mortality and morbidity by the National Institute of Statistics (ISTAT), data from National Hospital Discharge Database (NHDD) held by the Italian Ministry of Health (MoH) and data from the scientific literature.
In Italy, information on mortality and morbidity for tularemia are available, on a national basis, from death certificates and from the surveillance system of infectious diseases to which notification is mandatory. To assess the frequency of the disease we reviewed mortality and morbidity data from 1979 (year of the first registered cases in this database) to 2010 (the last year for which information on morbidity and causes of death are available). Since no deaths from tularemia were notified from 1979 to 2010, this outcome was not considered in this paper.
Morbidity data were described by year of occurrence, while cumulative morbidity data were stratified by province, season of occurrence, gender and age of subjects.
Moreover, the NHDD contains anonymized administrative and health data regarding discharged patients, which all public and private hospitals are legally required to notify. For each patient, the main discharge diagnosis represents the clinical condition which took up the greatest amount of resources and involved the greatest cost for the hospital. Up to five additional secondary diagnoses may be listed. Diagnoses are coded using the nomenclature of the International Classification of Diseases, 9th revision – Clinical Modification (ICD-9-CM). We collected and examined data regarding tularemia hospitalizations for the period 2001–2010, considering the following codes: 0210 (ulceroglandular tularemia); 0211 (typhoidal tularemia); 0212 (pneumonic tularemia); 0213 (oculoglandular tularemia); 0218 (other forms of tularemia); 0219 (not specified tularemia). Multiple hospital admissions for the same case were identified by a unique key and excluded from the analysis. Hospitalization rates and interquartile range (IQR) were calculated by year, age group and gender.
The age-specific annual resident populations data obtained from ISTAT were used to calculate the ratio of tularemia hospitalizations. The ratio of tularemia hospitalizations to reported tularemia cases, by year and geographical area, was also calculated.
With regard to case definitions of tularemia, no specific and standardized case definitions are provided in the national documents regulating the reporting to the national morbidity register (ISTAT) or to the NHDD. Nevertheless, all physician in their reporting activities are asked to meet the European case definition provided by the European Commission after the publication of the Decision of the European Parliament and of the Council setting up the network for the epidemiological surveillance and control of communicable diseases in the Community [29, 30].
No information concerning the laboratory test conducted on patients are reported from ISTAT and NHDD; therefore, it was not possible to obtain information with regard to diagnostic methods used.
Moreover, since these official databases do not provide information concerning any epidemiological link among cases, it is not possible to understand whether the reported cases are independent or belong to one or more outbreaks. Therefore, a review of all the scientific papers reporting data on tularemia cases occurring in Italy during the period 1960–2012 was performed. The literature search consulted the main scientific search engines (Web of Science, PubMed, Google, Google Scholar); peer-reviewed proceedings of meetings were also investigated. The search included combinations of the following key words: tularemia, Italy, F. tularensis.
The number of cases described in the literature was compared with those reported in the ISTAT dataset and matched in terms of period of onset and geographical location.
The research does not report any experiment on human or biological human samples, nor information on identifiable human material and data because it was conducted by consulting national public databases, which collect aggregated anonymous epidemiological data; thus, no identifiable human data are reported.
RESULTS
Morbidity data from the surveillance system of infectious diseases
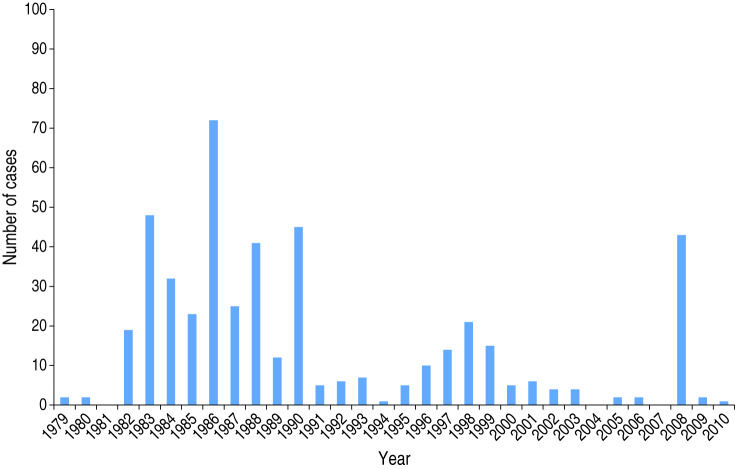
From 1979, when the first case of tularemia was included in the ISTAT database, to 2010, National Statistics (ISTAT) reported 474 cases and no deaths. Figure 1 shows distribution of cases by year. Usually, years with a high number of cases alternated with years in which the disease was virtually absent. The highest number of cases reported occurred in the 1980s, although 42 cases were reported in Tuscany in 2008.
Fig. 1.
F. tularensis cases occurring in Italy from 1979 to 2010.
Cases included both genders (48·3% males, 51·7% females) and all age groups (0–14 years: 25·0%; 15–24 years: 10·4%; 25–64 years: 52·4%; >64 years: 12·2%). The largest percentage of cases (60·6%) occurred in winter, although cases occurred all year round.
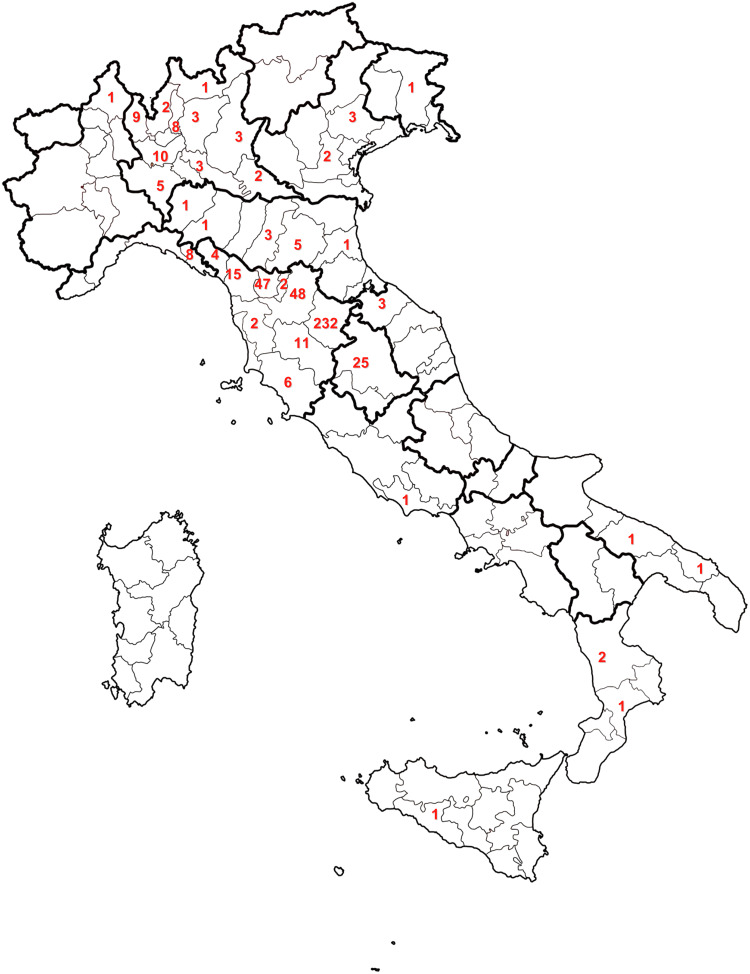
The Apennine Mountains located in Liguria, Emilia Romagna, Tuscany, and Umbria and the province of Pavia in Lombardy are the geographical areas at highest risk of infection, producing 96·4% of reported cases (Fig. 2). Cases are sporadically described in other regions from the Northern, Central and Southern parts of the country.
Fig. 2.
Distribution by province of F. tularensis cases occurring in Italy from 1978 to 2010.
NHDD
After excluding for multiple admissions for the same case, there were 222 admissions for tularemia during the period 2001–2010, corresponding to an average annual hospitalization rate of 0·38/1 000 000 population (range 0·27–0·52).
Tularemia was listed as the primary discharge diagnosis in 116/222 admissions (52·2%). Ulceroglandular tularemia was the most frequent hospitalized form (46·4%), followed by other forms of tularemia (15·3%), typhoidal tularemia (12·6%), oculoglandular tularemia (10·8%), not specified tularemia (8·1%) and pneumonic tularemia (6·8%).
The average annual rates of hospitalization were 0·28/1 000 000 population in Northern Italy, 0·60/1 000 000 in Central Italy and 0·38/1 000 000 in Southern Italy.
The median age of hospitalized cases was 57 years (IQR 33); 39·6% (88/222) were aged >64 years, 31·5% (70/222) were aged between 45 and 64 years, 23·4% (52/222 cases) were aged between 25 and 44 years, 1·8% (4/222 cases) between 15 and 24 years, and 3·6% (8/222 cases) were children aged <15 years.
Overall, 66·2% of hospital admissions for tularemia involved female patients. The median length of hospital stay was 6 days (IQR 9); but this varied with age, increasing from patients aged <15 years to >65 years [median 4 days (IQR 7); median 7 days (IQR 9), respectively].
Not all hospitalized cases of tularemia are notified, as illustrated in the data shown in Table 1, and the annual number of hospitalizations was 4·2–12 times greater than the number of notifications in the same year; but in 2008 the number of reported cases was greater than the number hospitalized. With regard to 2008, it should be noted that, according to the compulsory notification system, out of the 43 cases reported, 42 came from Tuscany (41 from Pistoia province); by contrast, according to discharge forms data, only 10/31 hospitalized cases were from Tuscany (of which only six were from Pistoia province).
Table 1.
Ratio of tularemia hospitalizations and reported tularemia cases, by year and geographical area, Italy 2001–2010
| No. of tularemia hospitalizations | No. of reported tularemia cases | Ratio hospitalizations/ reported cases | |
|---|---|---|---|
| Year | |||
| 2001 | 25 | 6 | 4·2 |
| 2002 | 17 | 4 | 4·3 |
| 2003 | 30 | 4 | 7·5 |
| 2004 | 14 | 0 | — |
| 2005 | 16 | 2 | 8 |
| 2006 | 24 | 2 | 12·0 |
| 2007 | 24 | 0 | — |
| 2008 | 31 | 43 | 0·7 |
| 2009 | 16 | 2 | 8·8 |
| 2010 | 25 | 1 | 25 |
| Geographical area | |||
| Northern Italy | 75 | 2 | 37·5 |
| Central Italy | 68 | 61 | 1·1 |
| Southern Italy | 79 | 1 | 79·0 |
| Total | 222 | 64 | 3·5 |
Differences between overall numbers of notified tularemia cases and tularemia hospitalizations were observed in two of the three geographical areas: Central Italy did not significantly differ (68 vs. 61) (Table 1).
Additional data from the literature review
On the basis of published data, in the last 50 years (1960–2010), several outbreaks and sporadic cases occurred in Italy [17, 20]. F. tularensis biovar holarctica (type B) was mainly involved [7–9]. Only in one outbreak was biovar tularensis (type A) implicated [5].
Table 2 shows tularemia cases reported in the literature and those of ISTAT database, by year and geographical area.
Table 2.
Ratio of tularemia cases described in the literature and reported by ISTAT, by year and geographical area in Italy
| Reference | Years | Region | No. of cases (literature review) | No. of cases (ISTAT) | Ratio |
|---|---|---|---|---|---|
| Bianchi [17] | 1962–1964 | Lombardy | 6 | — | — |
| Senaldi et al. [20] | 1979–1981 | Lombardy | 11 | 2 | 5·5 |
| Tasselli et al. [21] | 1982–1987 | Tuscany | 328 | 198 | 1·7 |
| Mignani et al. [31] | 1987–1988 | Liguria | 61 | 6 | 10·2 |
| Castro et al. [28] | 1987–1997 | Tuscany | 134 | 118 | 1·1 |
| Fabbi et al. [7] | 2007–2008 | Tuscany | 44 | 41 | 1·1 |
| Total | 584 | 365 | 1·6 |
ISTAT, National Institute of Statistics
Although the literature describes suspected cases of tularemia as early as 1931 [17], the first confirmed outbreak, dating back to 1962 [17], occurred in Lombardy, involving six cases and was not reported by ISTAT. In the same area a second outbreak was described in the years 1979–1981 [20] resulting in 11 cases of tularemia, of which only five were reported in the national register.
In the years 1982–1983 several outbreaks occurred in Tuscany [5, 24, 25, 27, 31]. The cases notified to the Regional Health Authorities of Tuscany from 1982 to 1987 [21] were 328 vs. 198 reported by ISTAT for the same geographical area and years.
A new outbreak occurred in 1987–1988 in Liguria, due to a contamination of the water supply [26, 31]. It caused four hospital admissions and at least 61 cases, both symptomatic and asymptomatic. For the same period ISTAT reported only six cases in Liguria.
For the decade 1987–1997, Castro et al. [28] reported 134 cases vs. 118 cases in Tuscany from the ISTAT database.
Finally, another water-related outbreak due to F. tularensis biovar holarctica (type B) occurred in Pistoia (Tuscany) [7], resulting in 44 cases from December 2007 to March 2008, of which 19 cases occurred in 2007; however, ISTAT does not report any cases of tularemia in that year.
DISCUSSION
In Italy tularemia is sporadic, rarely endemic or epidemic and self-limiting. The cases collected by ISTAT underestimate the real number of cases compared to those reported in the literature or in the NHDD.
The level of underreporting is evident when results of the hospitalization data analysis are considered: during the period 2001–2010, 63 cases were reported to the notification system, but 222 patients were hospitalized for tularemia. In particular, in each year the number of hospitalized cases was greater than the numbers notified; with the exception of 2008, when 43 cases were reported, 42 of which were from Tuscany. This peak is due to an epidemic of 41 cases in Pistoia (one of Tuscany's provinces), and the highest notification rate may be related, as usual, to the greater attention paid to the reporting of cases during an outbreak.
In 2008, 31 cases were hospitalized, six in Pistoia province. Therefore, it is possible to assume that, during the epidemic in Pistoia, 14·6% (6/41) of reported cases were hospitalized. If we extend this proportion to the total of 222 hospitalized cases, it is possible to postulate an expected number of cases for the period 2001–2010 that exceeds1500 patients. This, of course, is an estimate affected by several potential errors (e.g. a potential mismatching between cases and hospitalizations, although in the same province), but it could be useful to have a hypothetical idea of the level of underestimation.
Moreover, tularemia notification rates were significantly lower in Southern and Northern Italy compared to Central Italy, but the same evidence was not found from the hospitalization rate which was consistent in different areas (0·28/1 000 000 population in Northern Italy, 0·60/1 000 000 in Central Italy, 0·38/1 000 000 in Southern Italy). This suggests that tularemia does not occur more frequently in a specific area. These data confirm the results of a seroepidemiological study performed at the end of the 1980s in three Italian cities (Naples, Rome, Siena) that showed a percentage of positivity comparable in the three locations, although two of the selected cities were not considered at-risk areas [32].
As shown by notification and in the literature, the disease occurs mainly between ages 25–44 years and <15 years, by contrast hospitalization rate was higher for those aged 44 years (71·2%), with a high proportion of cases occurring in women.
Most cases of F. tularensis type B occurred in winter and spring [7, 19, 24–26, 31, 33]. This monthly distribution is not surprising: tularemia outbreaks that are associated with the consumption of hunted animals usually occur in the summer and early autumn, whereas waterborne tularemia outbreaks usually occur in the autumn and winter [15]. In fact, in water-borne cases, the low temperatures of the water in the winter months and the lack of chlorination of spring water create better conditions for the survival of F. tularensis [8]. Furthermore, the ability of F. tularensis subsp. holarctica strains to survive in association with protozoa indicates that ubiquitous aquatic protozoa might be an important environmental reservoir for the bacterium [34] that could be concentrated by molluscs, crayfish, frogs and water-borne rodents that ingest infected protozoa [1]. Therefore it is important to stress the need of periodic control of surface water and aqueducts, to avoid dangerous infestations (rodents).
Regarding the cases of tularemia not related to water, the seasonality was less evident [5, 8, 17, 19, 20, 21]. Some peaks of cases were concurrent with the hunting season for contact with infected hares [17, 33].
The analysis of hospitalizations per month during the period 2001–2010 does not confirm a marked seasonality, although the highest percentage (34·2%) of hospitalizations was observed in winter.
Most tularemia cases were concentrated in the Apennines between Tuscany and Liguria and all the water-related outbreaks described occurred in these areas. This can be dependent on the fact that tularemia is maintained in nature by various species of small rodents inhabiting forest environments, like those present in the Apennines.
The outbreaks in Italy do not seem to have been tick-borne, although Ixodes ricinus is commonly observed throughout the country [35]. Currently, it is difficult to establish a firm conclusion about the role of ticks because the studies performed often involved a small number of specimens [8, 21, 35]. Considering the recent restocking in which hares were found serologically positive for F. tularensis [23] and that the hares are hosts for different stages of several tick species [36], it can be expected that higher numbers of infected rodents and lagomorphs are the hosts of a growing proportion of infected ticks and, thus, support an increased transmission of F. tularensis subsp. holarctica. Therefore increased veterinary controls on animals imported for restocking should be enforced to prevent the spread of disease through the trade of animals.
Although the time trend of reported tularemia in Italy does not support the hypothesis of an emerging disease, the study of discharge forms has demonstrated a wide underreporting of the disease, particularly in Northern and Southern Italy; and moreover, a systematic comparison among data provided by different epidemiological databases could be useful to better understand the real burden of an infectious disease. The problem of under-diagnosis and underreporting is common to other infectious diseases; however, it is more serious for diseases, such as tularemia, for which effective preventive measures (e.g. water control or veterinary surveillance) can avoid further cases. Therefore some interventions need to be implemented considering the following points.
First, the cyclical nature of the disease in animals [37]. Recently a survey performed on sera of imported hares showed positivity for F. tularensis [23]. Outbreaks of the disease in humans often follow outbreaks of tularemia in rodents, as observed in Russian Federation and Sweden [4]. Tularemia transmission patterns may also change over time [4]. Therefore additional and systematic veterinary controls, not only on animals imported for restocking, is crucial, which should be supported by environmental studies focused on the ecology of F. tularensis in epidemic areas.
Second, global warming has been suspected to be involved in the recent increase of cases of the disease in other countries [13, 38]. Often, the number of cases shows wide variations from one year to another and this is probably related to climatic factors such as temperature and precipitation. In fact the rainy climate might play a facilitative role in the contamination of water sources with F. tularensis [39]. During the rainy season, the stream lines of the natural spring water can overflow due to the high level of rainfall and, in this conditions, it is easier to find rodents (mice) dead along those lines [15]. Regarding Italy, it has to be considered that in some areas of the country (including Liguria and Tuscany) several floods have occurred in recent years, causing considerable damage to aqueducts. At present there is virtually no data linking specific climatic conditions and outbreaks of tularemia. However, it is a crucial area of research that may yield important conclusions for predicting and possibly preventing new outbreaks [4].
Third, the role of insects in the transmission of the disease (ticks, mosquitos), since mosquito bites have recently been suspected as being responsible for changes in the epidemiology of tularemia in Germany [6]. In an investigation performed by Ciceroni et al. [35] around Florence, positivity for F. tularensis was not found, but, according to Heyman et al. [40], tick-borne diseases are likely to become real infectious threats, one of the main concerns for public health in Europe in the next few years; therefore, well planned, efficient, and cost-effective surveillance systems need to be implemented. As for other infectious diseases [41], integrated human, veterinary and entomological surveillance can be implemented in order to control the spread of the disease, in a ‘one health’ perspective [42].
The preventive measures described above are especially important considering the increasing number of people who spend holidays in at-risk areas, and also the increasing habit of keeping small rodents as pets, or contact during leisure activities with the wildlife habitat. Therefore, over the next years, the implementation of adequate preventive measures, addressed to the public of all ages, has to be carefully taken into account.
ACKNOWLEDGEMENTS
The research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Foley JE, Nieto NC. Tularemia. Veterinary Microbiology 2010; 140: 332–338. [DOI] [PubMed] [Google Scholar]
- 2.Maurin M, et al. Human tularemia in France, 2006–2010. Clinical Infectious Diseases 2011; 53: 133–141. [DOI] [PubMed] [Google Scholar]
- 3.Keim P, Johansson A, Wagner DM. Molecular epidemiology, evolution and ecology of Francisella. Annals of the New York Academy of Sciences 2007; 1105: 30–66. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Guidelines on Tularemia. Geneva: WHO Press, 2007. (http://www.cdc.gov/tularemia/resources/whotularemiamanual.pdf). [Google Scholar]
- 5.Greco D, Ninu E. A family outbreak of tularemia. European Journal of Epidemiology 1983; 3: 232–233. [DOI] [PubMed] [Google Scholar]
- 6.Hanke C, et al. Ulceroglandular tularemia in toddler in Germany after a mosquito bite. European Journal of Pediatrics 2009; 168: 937–940. [DOI] [PubMed] [Google Scholar]
- 7.Fabbi M, et al. An outbreak of tularemia in Tuscany, central Italy, linked to a natural spring water. In: Abstract Book of the 6th International Conference on Tularemia, 13–15 September 2009; Berlin, Germany. Abstract 108.
- 8.Magnino S, et al. Epidemiological investigation on tularemia in Oltrepò-Pavese area (Italy) [in Italian]. Archivio Veterinario Italiano 1990; 1: 1–22. [Google Scholar]
- 9.Rinaldi A. Research on Francisella tularensis isolates, Italy [in Italian]. La Clinica Veterinaria 1967, 90: 72–74. [Google Scholar]
- 10.Farlow J, et al. Francisella tularensis in the United States. Emerging Infectious Diseases 2005; 11: 1835–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitadze N, et al. Water-borne outbreak of oropharingeal and glandular tularemia in Georgia: investigation and follow-up. Infection 2009; 37: 514–521. [DOI] [PubMed] [Google Scholar]
- 12.Petersen JM, et al. Multiple Francisella tularensis subspecies and clades, tularemia outbreak, Utah. Emerging Infectious Diseases 2008; 14: 1928–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang S, Kleines M. Two at one blow: re-emergence of tularemia in Upper Austria. The New Microbiologica 2012; 35: 349–352. [PubMed] [Google Scholar]
- 14.Tärnvik Al, Priebe HS, Grunow R. Tularemia in Europe: an epidemiological overview. Scandinavian Journal of Infectious Diseases 2004; 36: 350–355. [DOI] [PubMed] [Google Scholar]
- 15.Meric M, et al. Tularemia outbreaks in Sakarya, Turkey: case-control and environmental studies. Singapore Medical Journal 2010; 51: 655. [PubMed] [Google Scholar]
- 16.Simşek Hl, et al. Identification of Francisella tularensis by both culture and real-time TaqMan PCR methods from environmental water specimens in outbreak area where tularemia cases not previously reported. European Journal of Clinical Microbiology and Infectious Disease 2012; 31: 2353–2357. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi L. On some cases of tularemia found in Lombardy. Sero-immunological and histopathological observations [in Italian]. Giornale di Malattie Infettive e Parassitarie 1966; 7: 443–448. [PubMed] [Google Scholar]
- 18.Palarchi M, Micozzi G. Francisella tularensis isolation in mice (Apodemus) caught during a human epidemic of tularemia [in Italian]. Il Progresso Veterinario 1989; 4: 142–144. [Google Scholar]
- 19.Pippi L, et al. A case of erythema nodosum in tularemia [in Italian]. Quaderni Sclavo di Diagnostica Clinica e di Laboratorio 1988; 24: 165–170. [PubMed] [Google Scholar]
- 20.Senaldi G, et al. A recurrent anthropozoonosis in Oltrepò Pavese: tularemia [in Italian]. Giornale delle Malattie Infettive e Parassitarie 1987; 5: 491–493. [Google Scholar]
- 21.Tasselli E, et al. Tularemia in Tuscany during the period 1982–1987 [in Italian]. Obiettivi e Documenti Veterinari 1988; 9: 23–28. [Google Scholar]
- 22.Ercolini C, et al. Sero-epidemiological investigation on tularemia in La Spezia province, Italy [in Italian]. Il Progresso Veterinario 1991; 10: 358–361. [Google Scholar]
- 23.Corrò M, et al. Tularemia in hares imported in Italy from Eastern Europe. In: Abstracts of the VII International Symposium on Wild Fauna: 20–21 October 2011, Edinburgh, UK. Abstract 307.
- 24.Paci P, Leoncini F, Paoli M. Eighty-five cases of tularemia in Tuscany. Epidemiological considerations [in Italian]. Recenti Progressi in Medicina 1983; 4: 431–437. [PubMed] [Google Scholar]
- 25.Greco D, et al. A waterborne tularemia outbreak. European Journal of Epidemiology 1987; 1: 35–38. [DOI] [PubMed] [Google Scholar]
- 26.Mignani E, et al. Italian epidemic of waterborne tularemia. Lancet 1988; 17: 1423. [DOI] [PubMed] [Google Scholar]
- 27.Pintus GC, et al. Two cases of tularemia observed at Monte Amiata [in Italian]. Minerva Medica 1984; 75: 1961–1964. [PubMed] [Google Scholar]
- 28.Castro R, et al. Tularemia: considerations on a new case in the Monte Amiata [in Italian]. Minerva Medica 1999; 90: 81–3. [PubMed] [Google Scholar]
- 29.Official Journal of the European Union. Decision No. 2119/98/EC of the European Parliament and of the Council of 24 September 1998 setting up a network for the epidemiological surveillance and control of communicable diseases in the Community (http://eur-lex.europa.eu/resource.html?uri=cellar:b97ab1a4-21f5-49de-9964-bc25617d3485.0008.02/DOC_1&format=PDF). Accessed 1 September 2014.
- 30.Official Journal of the European Union. Commission Decision 2003/534/EC of 17 July 2003 amending Decision No 2119/98/EC of the European Parliament and of the Council and Decision 2000/96/EC as regards communicable diseases listed in those decisions and amending Decision 2002/253/EC as regards the case definitions for communicable diseases (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:184:0035:0039:EN:PDF). Accessed 1 September 2014.
- 31.Mignani E, et al. Epidemic episodes of tularemia in Vara Valley (La Spezia) [in Italian]. Giornale di Malattie Infettive e Parassitarie 1991; 11: 999–1001. [Google Scholar]
- 32.Zardi O, et al. From a randomized assay, Francisella turned out to be epidemiologically present in the studied areas [in Italian]. Giornale di batteriologia, virologia ed immunologia 1986; 74: 42–46. [PubMed] [Google Scholar]
- 33.Pippi L, Toti M, Alegente G. An epidemic focus of tularemia in the Province of Siena [in Italian]. Minerva Medica 1985; 76: 1353–1356. [PubMed] [Google Scholar]
- 34.Lundström JO, et al. Transstadial transmission of Francisella tularenis holarctica in mosquitoes, Sweden. Emerging Infectious Diseases 2011; 5: 794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciceroni L, et al. Risk of tick-borne bacterial diseases in human in the Florence Area, Tuscany. Annals of the New York Academy of Sciences 2003; 990: 346–349. [DOI] [PubMed] [Google Scholar]
- 36.Gyuranecz M, et al. Factors influencing emergence of tularemia, Hungary 1984–2010. Emerging Infectious Diseases 2012; 8: 1379–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tärnvik A, Sandström G, Sjöstedt A. Epidemiological analysis of tularemia in Sweden 1931–1993. FEMS Immunology & Medical Microbiology 1996; 13: 201–220. [DOI] [PubMed] [Google Scholar]
- 38.Akalin H, Helvaci S, Gedikoglu S. Re-emergence of tularemia in Turkey. International Journal of Infectious Diseases 2009; 13: 547–555. [DOI] [PubMed] [Google Scholar]
- 39.Celebi G, et al. Tularemia, a reemerging disease in Northwest Turkey: Epidemiological investigation and evaluation of treatment responses. Japanese Journal of Infectious Diseases 2006; 59: 229–234. [PubMed] [Google Scholar]
- 40.Heyman P, et al. A clear and present danger: tick-borne diseases in Europe. Expert Review of Anti-Infective Therapy 2010; 8: 33–50. [DOI] [PubMed] [Google Scholar]
- 41.Napoli C, et al. Integrated human surveillance systems of West Nile virus infections in Italy: the 2012 experience. International Journal of Environmental Research and Public Health 2013; 10: 7180–7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinsstag J, et al. From ‘one medicine’ to ‘one health’ and systemic approaches to health and well-being. Preventive Veterinary Medicine 2011; 101: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]