SUMMARY
As feral swine continue to expand their geographical range and distribution across the United States, their involvement in crop damage, livestock predation, and pathogen transmission is likely to increase. Despite the relatively recent discovery of feral swine involvement in the aetiology of a variety of pathogens, their propensity to transmit and carry a wide variety of pathogens is disconcerting. We examined sera from 2055 feral swine for antibody presence to six serovars of Leptospira that can also infect humans, livestock or domestic animals. About 13% of all samples tested positive for at least one serovar, suggesting that Leptospira infection is common in feral swine. Further studies to identify the proportion of actively infected animals are needed to more fully understand the risk they pose.
Key words: Disease, feral swine, Leptospira, leptospirosis, Sus scrofa
INTRODUCTION
Leptospirosis is a disease of global importance and is one of the most widespread zoonoses worldwide [1], infecting most mammalian species. Leptospirosis is caused by bacterial spirochaetes and includes both saprophytic and pathogenic species belonging to the genus Leptospira. The bacteria are classified genetically into 19 species, [2], with at least 12 of these being pathogenic and including more than 250 pathogenic serovars. Most serovars are host adapted, but the leptospires are harboured in the kidney and can serve as a source of infection for other animals or humans [1].
Human infection typically occurs after direct exposure to contaminated animal urine, or indirectly through contaminated water, when it comes in contact with the skin, eyes, or mucous membranes [1]. Severity of the disease depends on the age and health of the person infected. Symptoms typically include fever, chills, and intense headache, although some infections including jaundice, acute renal and hepatic failure, pulmonary distress and haemorrhage can result in death [2, 3]. In the continental United States, cases are usually linked to people who are infected through occupational exposure (i.e. slaughter workers, farmers, etc.) [4], and to residents of Hawaii, where Leptospira thrive due to the tropical and subtropical climate [5]. However, over the last 20 years, outdoor enthusiasts have more commonly been the victims of infection with the pathogen [4, 6, 7] due to recreational exposure to contaminated water. Leptospirosis is now classified as a re-emerging zoonotic infection because of this increase in the number of human outbreaks over the last decade [8] resulting in increased attention worldwide.
Although originally introduced into the United States in the 16th century, feral swine (Sus scrofa) have markedly increased their range and population size over the last 20 years [9]. This is due to a number of factors including natural range expansion, accidental escape or intentional release, translocation into new areas specifically for hunting purposes, and their adaptability to a wide range of habitats [10]. As this expansion continues across the country, and recreational hunting and wild pig meat consumption become more popular, the possibility of direct contact between feral swine and humans will increase, and could result in increased opportunities for disease transmission [11]. The rate of expansion is disconcerting since feral swine can serve as reservoirs for a number of diseases of agricultural and zoonotic concern [12, 13]. Leptospirosis, in particular, is a problem because it can infect both humans and livestock, and can survive in moist soil and freshwater environments for long periods [2]. In domestic swine Leptospira is an important cause of reproductive failure, most frequently with serovar Bratislava [14]. Whether or not this pathogen causes similar reproductive effects in swine is unknown, but feral swine populations have continued to increase in both population size and geographical range which suggests that if there is reproductive failure associated with Leptospira in feral swine, it is not having a population-level effect. Although Leptospira antibodies have been detected in feral swine in the United States previously, these studies were focused on specific geographical areas [15–17]. We are unaware of a nationwide comprehensive effort to determine the geographical distribution and apparent prevalence of this pathogen in the United States. In order to provide insight into this disease system, we tested feral swine sera from across the United States to characterize the antibody prevalence of feral swine to six serovars of Leptospira of agricultural or zoonotic concern (Bratislava, Canicola, Grippotyphosa, Hardjo, Icterohaemorrhagiae, Pomona).
METHODS
Sample collection
Samples are routinely collected for disease surveillance from feral swine removed for wildlife damage management purposes by the U.S. Department of Agriculture's Wildlife Services (WS), across the United States. Although samples are tested for various diseases at the time of collection, additional serum samples are stored at −80 °C as part of a tissue archive operated by the WS' National Wildlife Disease Program.
For this study, 2055 feral swine serum samples collected between February 2007 and June 2011 were selected from the archive. Counties with 10 or more samples available in the archive were prioritized, resulting in at least one county (107 counties total) per state for 28 states (Supplementary Table S1). The samples were comprised of 1091 females, 953 males, and 11 of unspecified sex (Table 1).
Table 1.
Number of feral swine tested by sex and test result for exposure to Leptospira* by microscopic agglutination testing (MAT)
| No. tested* | No. positive | % positive (95% CI) | |
|---|---|---|---|
| Sex† | |||
| Males | 953 | 138 | 14·5% (12·3–16·9) |
| Females | 1091 | 131 | 12·0% (10·1–14·1) |
CI, Confidence interval.
MAT included serovars Bratislava, Canicola, Grippotyphosa, Hardjo, Icterohaemorrhagiae, and Pomona.
Eleven additional pigs of unspecified gender were negative.
Sample testing
All samples were tested at Colorado State University with the microscopic agglutination test (MAT) [18] to detect antibodies against six Leptospira serovars: Hardjo, Icterohaemorrhagiae, Canicola, Grippotyphosa, Pomona, and Bratislava. A titre of ⩾1:200 was considered positive. MAT results were reported as the endpoint dilution of serum where 50% agglutination of cells was observed (1:50, 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:>6400). Titres >1:6400 were not measured to their endpoint since titres ⩾1:800 were considered evidence of recent or current infection [19]. The serovar in which agglutination was detected at the highest dilution was considered the infective serogroup, which for some samples included multiple serovars.
Statistical analysis
Mean Leptospirosis exposure and associated 95% confidence intervals for the different sexes were calculated using a binomial distribution. Potential disease associated risk factors were analysed using a mixed model (Proc Glimmix) and run using SAS version 9.2 [20]. The analysis was conducted using a logistic link function and binary error. Leptospira exposure (positive or negative) was the dependent variable. The primary regression factor of interest (α = 0·10) was the sex of sample animals.
RESULTS
Of 2055 samples tested, 269 (13·1%) were positive for at least one serovar and 97 (36%) of these were infected with multiple serovars. Co-infections with Pomona and Bratislava were more common than any other serovar combination. Samples tested positive for at least one serovar in 20 of 28 states and 71% of 107 counties represented in this study (Supplementary Table S1).
Sex was significantly associated with exposure (F = 2·78, P = 0·095), with slightly more males having been exposed than females (OR 1·28, Table 1).
Pomona was the most common serovar identified followed by Bratislava, Grippotyphosa, Icterohaemorrhagiae, Hardjo and Canicola, respectively (Table 2). Current or recent infections (titres of ⩾800) were identified most commonly as Pomona, followed by Bratislava and Grippotyphosa (Table 2).
Table 2.
Antibody titres to six serovars of Leptospira detected in feral swine in the United States
| Serovar* | Positive (n) | 1:200 | 1:400 | 1:800 | 1:1600 | 1:3200 | 1:>6400 |
|---|---|---|---|---|---|---|---|
| Bratislava | 106 | 50 | 25 | 16 | 6 | 8 | 1 |
| Canicola | 12 | 10 | 2 | ||||
| Grippotyphosa | 75 | 29 | 15 | 18 | 8 | 3 | 2 |
| Hardjo | 32 | 16 | 13 | 1 | 1 | 1 | |
| Icterohaemorrhagiae | 59 | 32 | 18 | 8 | 1 | ||
| Pomona | 124 | 39 | 26 | 22 | 12 | 10 | 15 |
Some feral swine were positive for multiple serovars.
DISCUSSION
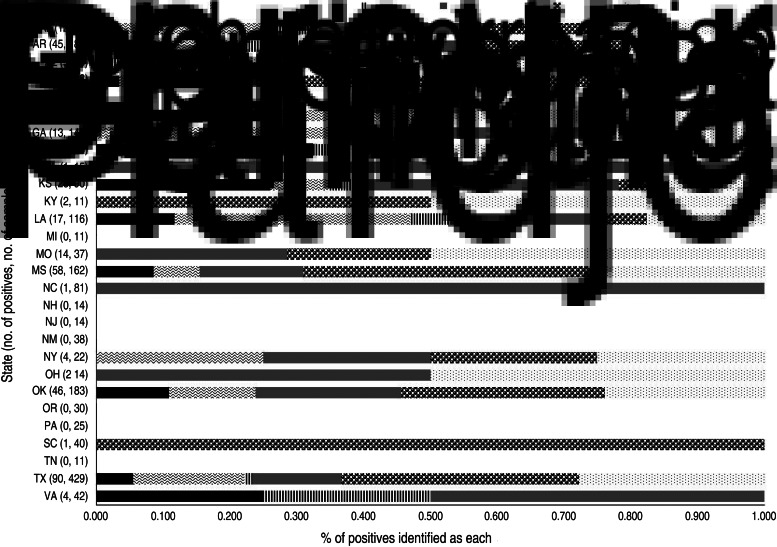
We determined that exposure to Leptospira is common in feral swine, and is not limited to certain regions of the country since antibody-positive feral swine were identified in 70% of the states included in this study (Fig. 1). Although no definitive patterns of distribution were identified based on the samples available, it is clear that feral swine, regardless of location, are exposed to pathogenic Leptospira and thus may be involved in the epidemiology of the pathogen. Domestic pigs can harbour the spirochaete in the kidneys and intermittently shed the organism [11] suggesting that feral swine could do the same.
Fig. 1.
Proportion of feral swine samples identified by microagglutination as specific serovars of Leptospira (serovars: Hardjo, Icterhaemorrhagiae, Canicola, Grippotyphosa, Pomona, Bratislava) by state in the United States from February 2007 to June 2011.
Feral swine typically use wet areas for wallowing and rooting which may result in contamination of the habitat [21]. Feral swine root in agricultural fields and utilize artificial water sources such as stock tanks or those created by irrigation [22] which may perpetuate transmission. The high rate of Leptospira exposure observed in feral swine relative to other pathogens [22], may be a result of their propensity to utilize habitats which may have been contaminated by other wildlife species such as raccoons (Procyon lotor), squirrels (family Sciuridae) or rats (genus Rattus) [19]. Although transmission most likely occurs through these indirect routes, direct transmission may also occur through direct contact with infected urine, placental fluids, or milk and through venereal or placental transmission. Swine may also serve as a reservoir and subsequent source of environmental contamination in formerly pathogen-free areas. As feral swine continue to expand their geographical range throughout the United States, it will be important to understand their aetiological role, if any, in leptospirosis outbreaks. This may be especially important in areas where freshwater-based activities, such as swimming, kayaking, or adventure races are popular, consequently increasing the risk for zoonotic transmission of this organism.
We detected a significant difference in antibody prevalence between males and females. Feral swine social groups, called sounders, are comprised of sows and their offspring. Adult males are solitary [23], after they disperse from the natal social group. As a result, males occupy larger home ranges than females [24]. This transient social behaviour may explain why males were more frequently exposed to Leptospira. This distinction between sexes (Table 1) was not replicated in other studies [25, 26], but may have been merely a function of sample size since we sampled at least five times the number of animals as these other studies.
Our data suggest that males may be important contributors in the transmission cycle of Leptospira; their propensity to occupy larger home ranges compared to females may lead to increased opportunities for both contraction and dispersal of the pathogen via indirect contact with contaminated environments, or directly through contact with infected animals. Surveillance efforts may be most cost effective by targeting males rather than females that may be less likely to be involved in transmission.
Pomona is the primary serovar that infects domestic swine [27]; therefore it is not surprising that it was also the most common serovar that we detected in feral swine. This finding is consistent with previous studies [25, 28]; however, these high rates of exposure to Pomona are alarming since this serovar is associated with infections in humans [27] and domestic swine [29]. Similarly, the high prevalence of Bratislava was consistent with other studies [19] that have identified the serovar frequently in domestic swine [14, 29].
Cross-reaction of serovars is a known limitation of serological testing [30] and cross-reactivity with Pomona in particular has been well documented [31]. For the purposes of this study, the serovar with the highest antibody titre was determined to be the infecting serovar per individual animal. This design was used to account for the potential for cross-reactivity. However, this does not take into account the potential for individual feral swine to have antibodies to more than one serovar, indicating the potential for multiple exposures to multiple Leptospira during the life of the feral swine. In addition, although identification of a certain serovar may suggest a specific animal source, because of the strong associations of some serovars with particular species, cross-reactivity between serovars may prevent pinpointing a specific source and also may prevent a robust diagnosis [4]. Although cross-reactivity likely occurred in these data since 36% of the positives had multiple serovars with titres ⩾200, it is clear that Leptospira exposure is widespread in feral swine and that they serve as reservoir hosts for Pomona and Bratislava similar to domestic swine. Infections with serovar Bratislava in domestic swine tend to occur at low titres (i.e. titres <200) [29], and if this same pattern holds true in feral swine it is possible that many of the feral swine we identified as Bratislava-negative were actually infected with this serovar, but below the cut-off value of 200 that we used in this study.
As feral swine populations continue to expand into urban areas and their ranges overlap with domestic swine and human activities, transmission of pathogens such as Leptospira will become of increasing concern. Since widespread exposure was documented during this study, we recommend that further studies be conducted to more fully understand the role of feral swine in shedding this pathogen.
ACKNOWLEDGEMENTS
We thank all of the wildlife biologists and technicians who participated by collecting the samples included in this paper and spending many hours trapping feral swine and preparing samples for testing. We also thank Cindy Hirota and Kathryn Brown who provided technical support for this project. Mention of trade names or commercial products in this work is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003148.
click here to view supplementary material
REFERENCES
- 1.Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Veterinary Microbiology 2010; 140: 287–296. [DOI] [PubMed] [Google Scholar]
- 2.Evangelista KV, Coburn J. Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future Microbiology 2010; 5: 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharti AR, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infectious Diseases 2003; 3: 757–771. [DOI] [PubMed] [Google Scholar]
- 4.Meites E, et al. Reemerging leptospirosis, California. Emerging Infectious Diseases 2004; 10: 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz AR, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974–1998. Clinical Infectious Diseases 2001; 33: 1834–1841. [DOI] [PubMed] [Google Scholar]
- 6.Morgan J, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clinical Infectious Diseases 2002; 34: 1593–1599. [DOI] [PubMed] [Google Scholar]
- 7.Stern EJ, et al. Outbreak of leptospirosis among Adventure Race participants in Florida, 2005. Clinical Infectious Diseases 2010; 50: 843–849. [DOI] [PubMed] [Google Scholar]
- 8.Vke L. Leptospirosis: a re-emerging infection. Malaysian Journal of Pathology 2011; 33: 1–5. [PubMed] [Google Scholar]
- 9.Cooper SM, et al. Distribution and interspecies contact of feral swine and cattle on rangeland in South Texas: implications for disease transmission. Journal of Wildlife Diseases 2010; 46: 152–164. [DOI] [PubMed] [Google Scholar]
- 10.Seward NW, et al. Feral swine impacts on agriculture and the environment. Sheep & Goat Research Journal 2004; 19: 34–40. [Google Scholar]
- 11.Meng X, Lindsay D, Sriranganathan N. Wild boars as sources for infectious diseases in livestock and humans. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 2009; 364: 2697–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witmer GW, Sanders RB, Taft AC. Feral swine – are they a disease threat to livestock in the United States? Proceedings of the Wildlife Damage Management Conference 2003; 10: 316–325.
- 13.Corn JL, et al. Pathogen exposure in feral swine populations geographically associated with high densities of transitional swine premises and commercial swine production. Journal of Wildlife Diseases 2009; 45: 713–721. [DOI] [PubMed] [Google Scholar]
- 14.Bolin CA, Cassells JA. Isolation of Leptospira interrogans serovars bratislava and hardjo from swine at slaughter. Journal of Veterinary Diagnostic Investigation 1992; 4: 87–89. [DOI] [PubMed] [Google Scholar]
- 15.New JC, et al. A serologic survey of selected viral and bacterial diseases of European wild hogs, Great Smoky Mountains National Park, USA. Journal of Wildlife Diseases 1994; 30: 103–106. [DOI] [PubMed] [Google Scholar]
- 16.Corn J, et al. Survey of selected diseases in wild swine in Texas. Journal of American Veterinary Medical Association 1986; 189: 1029–1032. [PubMed] [Google Scholar]
- 17.Clark R, et al. Serologic survey of California wild hogs for antibodies against selected zoonotic disease agents. Journal of American Veterinary Medical Association 1983; 183: 1248–1251. [PubMed] [Google Scholar]
- 18.OIE. Leptospirosis. In: Manual of Diagnostic Tsts and Vaccines for Terrestrial Animals. Paris, France: Office International des Epizooties, 2008, pp. 251–264. [Google Scholar]
- 19.Chatfield J, et al. Serosurvey of leptospirosis in feral hogs (Sus scrofa) in Florida. Journal of Zoo and Wildlife Medicine 2013; 44: 404–407. [DOI] [PubMed] [Google Scholar]
- 20.SAS. Base SAS® 9·2 Procedures Guide. Cary, NC: SAS Institute Inc., 2011. [Google Scholar]
- 21.Campbell TA, Long DB. Feral swine damage and damage management in forested ecosystems. Forest Ecology and Management 2009; 257: 2319–2326. [Google Scholar]
- 22.Bevins SN, et al. Consequences associated with the recent range expansion of nonnative feral swine. Bioscience 2014; 64: 291–299. [Google Scholar]
- 23.Martys MF. Social organization and behavior in the suidae and tayassuidae. In: Spitz F, Barrett RH, eds. Biology of Suidae. Briancon, France: Imprimerie des Escartons, 1991, pp. 65–77. [Google Scholar]
- 24.Gabor TM, et al. Demography, sociospatial behaviour and genetics of feral pigs (Sus scrofa) in a semi-arid environment. Journal of Zoology 1999; 247: 311–322. [Google Scholar]
- 25.Mason R, et al. Leptospira interrogans antibodies in feral pigs from New South Wales. Journal of Wildlife Diseases 1998; 34: 738–743. [DOI] [PubMed] [Google Scholar]
- 26.Vengust G, et al. Leptospira antibodies in wild boars (Sus scrofa) in Slovenia. European Journal of Wildlife Research 2008; 54: 749–752. [Google Scholar]
- 27.Burnstein T, Baker JA. Leptospirosis in swine caused by Leptospira pomona. Journal of Infectious Diseases 1954; 94: 53–64. [DOI] [PubMed] [Google Scholar]
- 28.Jansen A, et al. Leptospirosis in urban wild boars, Berlin, Germany. Emerging Infectious Diseases 2007; 13: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolin CA. Diagnosis of leptospirosis in swine. Swine Health and Production 1994; 2: 23. [Google Scholar]
- 30.Levett P. Leptospirosis. Clinical Microbiology Reviews 2001; 14: 296–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang RW, Morse E. Serologic cross reactions among leptospirae observed with sera from animals infected with Leptospira Pomona. Journal of Immunology 1959; 82: 471–476. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003148.
click here to view supplementary material