SUMMARY
Two global (re-)emerging zoonoses, leptospirosis and hantavirus infections, are clinically indistinguishable. Thirty-one patients, hospitalized in Sri Lanka for acute severe leptospirosis, were after exclusion of other potentially involved pathogens, prospectively screened with IgM ELISA for both pathogens. Of these, nine (29·0%) were positive for leptospirosis only, one (3·2%) for hantavirus only, seven (22·5%) for both pathogens concomitantly, whereas 13 (41·9%) remained negative for both. Moreover, in a retrospective study of 23 former patients, serologically confirmed for past leptospirosis, six (26·0%) were also positive in two different IgG ELISA hantavirus formats. Surprisingly, European Puumala hantavirus (PUUV) results were constantly higher, although statistically not significantly different, than Asian Hantaan virus (HTNV), suggesting an unexplained cross-reaction, since PUUV is considered absent throughout Asia. Moreover, RT–PCR on all hantavirus IgM ELISA positives was negative. Concomitant leptospirosis-hantavirus infections are probably heavily underestimated worldwide, compromising epidemiological data, therapeutical decisions, and clinical outcome.
Key words: Acute kidney injury (AKI), co-infections, hantavirus, leptospirosis, rodents, scrub typhus, Sri Lanka
INTRODUCTION
Leptospirosis and hantavirus infections are the two most globally widespread zoonoses, occurring in the five continents. Both are (re-)emerging, partially due to global warming [1–3], and as one of the consequences of intense rainfall, are associated with an increasing severity of floods [4]. The estimated yearly number of leptospirosis cases now amounts to 350000–500000 [5], whereas for hantavirus infections, the annual incidence is estimated at 150000– 200000 cases [6]. Both figures are in fact significant underestimations, since only severe cases are hospitalized [5, 7]. Moreover, whereas hantavirus infections are a standard example of rodent-borne human infections, many leptospirosis cases are also rodent-borne, with the wild rat as the main reservoir and carrier, hence also with a surprisingly similar human epidemiology; both mainly afflict the young male adult population, have a local seasonality, and often occur after local floods [4, 5]. Further confusing for the attending physician is the fact that both zoonoses are indistinguishable in their clinical presentation. Indeed, the World Health Organization (WHO) recommended the following surveillance case definition of leptospirosis:
A suspected case involves a person presenting with acute febrile illness; headache, myalgia and prostration; associated with any of the following symptoms: conjunctival suffusion; meningeal irritation; anuria, oliguria, or proteinuria; jaundice; haemorrhage (from intestines; lung bleeding often notorious); cardiac arrhythmia or failure; skin rash; a history of exposure to infected animals or an environment contaminated with infected animal urine [8].
Except perhaps for the skin rash and the pulmonary haemorrhages, all these symptoms are characteristic also for hantavirus infections. In fact, the latter are the only viral haemorrhagic fevers present in the northern hemisphere. Since hantaviruses have protean presenting symptoms, but also have the kidney as the prime target organ [9, 10], the cardinal signs of presentation may perfectly mimic leptospirosis, i.e. fever, myalgia, conjunctival suffusion, haemorrhagic manifestations, hepatic and renal involvement, particularly acute kidney injury (AKI), and thrombocytopenia, which in both diseases is an index of severity. Most other laboratory anomalies are again markedly similar: signs of liver involvement, elevated CRP, hyponatraemia, hypokalaemia, etc. This extreme degree of clinical and biochemical similarities has recently provoked the question if relying on clinical suspicion only is still acceptable in diagnosing a so-called typical leptospirosis case, even in highly endemic regions [11]. Another great mimicker of leptospirosis, but present only in SW Asia, is scrub typhus, caused by the rickettsia Orienta tsutsugamushi [6, 12]. An explanation for this surprising parallelism between bacterial (leptospiral and rickettsial) and viral illnesses has rarely been discussed so far, but can presumably be found in common grounds of pathogenesis, stressing predominance of the host factor over the infecting agent itself: although still largely poorly understood, all these (re)emerging infections seem to induce an autoimmune reaction, resulting in the so-called ‘cytokine storm’, and in a temporary capillary hyperpermeability [9, 10, 12, 13]. This transient endothelial dysfunction, recently coined as ‘severe endothelial dysfunction syndrome’ (SEDS), induces generalized interstitial oedema and fluid accumulation, and has been suggested as the ‘common culprit’ in the rare examples of triple co-infection: dengue haemorrhagic fever (DHF), leptospirosis and hepatitis [14]. Even (rarely needed) biopsies of the target organ, i.e. the kidney, are indistinguishable in leptospirosis, hantavirus nephropathy, and in scrub typhus: tubulo-interstitial nephritis, with interstitial oedema, sometimes accompanied by a patchy monocellular infiltrate, and rather exceptionally by interstitial haemorrhages [9, 10, 12].
With this in mind, it can only be regretted that the WHO has not yet issued an officially agreed case definition and/or inclusion criteria for hantavirus infections, as it did for leptospirosis [15]. Of note, the first WHO-licensed denomination for hantavirus infections was ‘haemorrhagic fever with renal syndrome’ (HFRS), adopted after a 1982 meeting in Tokyo, Japan. [16]. However, an exact definition of what exactly should be understood under ‘a renal syndrome’ in HFRS was not specified, which still causes problems in interpretation for many borderline cases [17]. Indeed, AKI with clearly impaired renal function is far from present in all HFRS cases, as it is in leptospirosis, but seems present in up to 53% of scrub typhus cases according to a recent Indian series [12].
There are more historical and geographical reasons to explain a lower medical awareness or even lack of interest of the (Western) medical community for HFRS: in contrast to leptospirosis (1886), and scrub typhus (1930), the causative pathogen of HFRS was only discovered in 1978, with the isolation in Korea by H. W. Lee of Hantaan virus (HTNV), the Asian prototype of all ensuing hantaviruses, now totalling 24 ‘official’ species, and many others still awaiting classification [17–19]. The most global hantavirus is Seoul virus (SEOV), which is spread worldwide by the omnipresent wild rat. Despite this, HFRS was and still is perceived as a health problem mainly for the Far East [17]. Indeed, over 90% of all HFRS cases occur in China, where annual epidemics have been registered since 1950, first clinically and later serologically confirmed. Despite a yearly decline of cases, the total number of cases registered in China up to 2010 still amounts to over a 1·4 million, with over 46 000 registered deaths [20], astounding numbers still largely ignored by Western medicine, and even by nephrologists [17].
Leptospirosis and/or hantavirus infections worldwide
Assessing connections between leptospirosis and HFRS is not a new idea, and was applied in Belgium from the pioneering times (1983) of hantavirus serology, when in indirect immunofluorescent assay (IFA) IgG screening with the hantavirus prototype HTNV antigen, van der Groen et al. found hantavirus-positivity in 26/682 (3·8%) leptospirosis-suspected sera, compared to only 21/950 (2·2%) in healthy Belgian blood donors. Thus, more than three decades ago, a significantly higher hantavirus IgG prevalence in leptospirosis suspects compared to blood donors was demonstrated (relative risk 1·72, 95% confidence interval 1·08–2·76), suggesting that at least some of the Belgian patients suspected of leptospirosis did in fact have HFRS (or both) [21]. Expansion of this basic strategy during ten subsequent years resulted in 1993 in the most important leptospirosis vs. HFRS comparative study so far, confirming again a statistically significant difference of seroprevalence in both study cohorts, with IgG IFA hantavirus-positivity in 2·1% (44/2055) of suspected leptospirosis cases vs. only 1·3% (124/9413) in healthy blood donors (χ2 = 10·5, P < 0·005) in Belgium [22]. Thus, from this early era of hantavirus research onwards, looking for SEOV infection seemed promising for pinpointing unsuspected hantavirus impact in high-risk groups, particularly in suspected leptospirosis clinical series. Following this simple approach, systemic screening with a Chinese wild rat-derived SEOV strain R22VP30 in suspected leptospirosis patients led to the documentation of the first serologically proven HFRS cases in the New World (Brazil) [23], in Ireland [24], and in India [25].
Moreover, dual infections are possible, aggravating or at least complicating the clinical picture, but have rarely been diagnosed simultaneously in the same patient(s), although in fact both infections can be transmitted concomitantly to humans from one individual but doubly-infected rodent carrier, e.g. the wild rat, or another hantavirus rodent reservoir which may also transmit Leptospira. We found only three single case-reports in the literature, one in Scotland [26], one in Hungary [27], and on in Croatia [28]. In all these three cases, possible exposure of the patient to wild rodents was evident, but it was only in the Croatian case that Markotić et al. proved both infections with confirmation techniques, i.e. microscopic agglutination test (MAT) with two successive serum samples for leptospirosis, and IgM ELISA followed by reverse transcriptase–polymerase chain reaction (RT–PCR) for HFRS [28]. With only routine IgM ELISA screening for both pathogens, we recently found serological evidence of concomitant acute leptospirosis and hantavirus infection in five patients (0·3%) of a Belgian cohort of 1580 suspected leptosirosis patients. However, this prevalence rose to a noticeable 9% (5/55), only if the seroconfirmed leptospirosis group was considered [22].
Leptospirosis and hantavirus infections in Sri Lanka: prior findings
The first human hantavirus infections in Sri Lanka were demonstrated in 1986, presenting with what was then described as a ‘leptospirosis type’ illness [29, 30]. Serological examinations, including cumbersome plaque reduction neutralization tests (PRNT), on leptospirosis-suspected but MAT-negative sera were performed in the national hantavirus reference centre in Seoul, Korea, and suggested a hantaviral pathogen, related to, but not identical with the rat-borne SEOV. Moreover, a hantavirus was isolated from a wharf rat, captured in Colombo harbour, which appeared closely related to, but not identical with, the brown rat-borne SEOV [29]. So from the very beginning, the true nature of a hantaviral pathogen in Sri Lanka remained elusive.
By contrast, Sri Lanka in the following decades became established as a country hyperendemic for leptospirosis, ranking 6th, with an annual incidence of 5·4 cases/100 000 population, in a recent global survey of 28 countries [4]. Regrettably, China and neighbouring India were not included in this global leptospirosis list, due to unavailability of national data [4]. However, after repeated floods from 2008 to 2011, the incidence in Sri Lanka rose notably: in 2008, the total number of clinically suspected cases reported to the surveillance system was 7406 with 204 deaths, giving a reported incidence rate based on notification data of 35·7/100000. In 2009, 4980 cases and 145 deaths were reported, and the outbreak persisted in 2010 with 4553 cases and 121 deaths [31]. Thus, the probable case incidence remained more than 22·5/100000, making it the second highest reported incidence of leptospirosis worldwide. However, these cases are reported on the basis of clinical suspicion, and fewer than 10% were laboratory confirmed because of lack of diagnostic capacity [31]. Of note, the Seychelles are heading the list as the most endemic country in the world for leptospirosis (annual incidence of 43·21 cases/100 000), a region where we formerly could exclude HFRS as operative in pulmonary haemorrhage as a predominant cause of death in leptospirosis [32].
Several leptospirosis studies or reviews were dedicated to Sri Lanka [33–36], although never obtaining a percentage of >50% of confirmed cases, using either ELISA, with the gold standard MAT, or with PCR. In the 2005–2008 sentinel surveillance study, from the 4000 suspected leptospirosis cases, only 41 (1%) positive MAT results were obtained [35]. In a study after the flood-associated outbreak in 2011, only 33·3% (32/96) confirmations could be found with a quantitative PCR technique [36]. Given the delayed appearance of (detectable) antibodies, the suppressive effect of antibiotics, the many requirements of a reliable MAT test, etc., such a low seroconfirmation rate seems unavoidable, as is also the case for any other leptospirosis seroprevalence study worldwide. Nevertheless, one cannot help but wonder whatever infectious or other cause then prompted a substantial part of all the other suspected cases to seek costly hospitalization, even in developing countries, if they were not afflicted by leptospirosis, particularly after floods. The most rigorously conducted study on leptospirosis so far in Sri Lanka was performed by Agampodi et al. after the major 2008 outbreak. After enrolling study subjects according to the strictest WHO inclusion criteria, and using all available modern screening and confirmation techniques, they ended up with 61·3% (246/401) unconfirmed cases [31].
For all these reasons, we decided to conduct a prospective study in Sri Lankan cases, hospitalized for acute leptospirosis-like symptoms, but screening them immediately after admission for both hantaviral and leptospiral pathogens with a quick ELISA format, i.e. the same simple but quick approach as conducted recently in Belgium [22]. Moreover, a retrospective study using the same approach was also performed in a similar patient cohort, i.e. formerly hospitalized for suspected acute leptospirosis.
MATERIAL AND METHODS
Prospective study
Patients admitted at the university hospital of Kelaniya, Ragama, in Sri Lanka with severe leptospirosis-like symptoms (fever, myalgiae, nausea, and a majority with diminished renal function, and/or with icterus, see Results section) were selected after clinical or serological exclusion of other acute conditions mimicking leptospirosis, such as scrub typhus, malaria, enteric fever and dengue fever (data not given). Thirty-nine patients (aged 13–74 years, mean 35 years, 34/39 males), with a history of rodent exposure in 34/39, were selected, of which eight were not enrolled in the study because double successive serum sampling was not possible. Thus, 31 individuals were screened for leptospirosis by the Leptospira IgM ELISA assay (Panbio, Australia), and for hantaviruses by the Hantavirus Puumala IgM ELISA and Hantavirus Hantaan IgM ELISA test (Progen, Germany). The explanation of the very limited number of study subjects finally enrolled lies in the simple fact that for each separately screened pathogen, only one single 96-well ELISA test plate was available in this developing country, resulting from a generous gift from abroad. Moreover, it was decided from the start that for every study subject two consecutive serum samples would be examined, i.e. one acute and one convalescent sample, in order to assess eventual seroconversions and to better exclude eventual false-positive reactions. An extract of Leptospira biflexa serovar Patoc, an apathogenic but genus-specific saprophyte, is used as antigen in the Panbio IgM ELISA, whereas in the Progen IgM ELISA a recombinant nucleoprotein is used from the prototype European PUUV strain (Cg 18–20), and the prototype Asian HTNV strain (HTN 76–118). As required by Progen's manufacturer, rheumatoid factor absorption was performed prior to IgM measurements. Results of the Leptospira IgM ELISA were measured at 450 nm as optical densities (OD) and an OD of 0·3.was used as cut-off for positivity. Results of the hantavirus IgM ELISA readings were expressed as Q values, where Q = OD of the sample/mean OD of the cut-off control delivered in the Progen kit. A Q value ⩾2 was considered as positive, whereas Q values <2 and >1 were considered in the grey (equivocal) zone. A Q value <1 was considered negative. An acute sample was taken shortly after admission, i.e. at a mean 10 days (range 5–21 days) post-onset of symptoms (POS), and controlled by a convalescent sample, taken as a mean 22 days (range 17–30) days POS. Concurrent IgG ELISA was not performed, due to the local shortage of diagnostic kits, as mentioned above.
Retrospective study
Twenty-three patients (age 13–78 years, mean 30 years) admitted 1 year before the prospective study with a clinical suspicion of leptospirosis, were selected because they were found positive after screening by Leptospira IgG ELISA (Panbio) using anti-rabbit IgG-peroxidase antibody (A0545, Sigma-Aldrich, USA) as anti-human IgG detector antibody. Results were measured at 450 nm OD. One leptospirosis-negative case was used as control. As for the prospective study, patients were also screened for a prior hantavirus infection by the Puumala IgG ELISA and Hantaan IgG ELISA tests (Progen). Results were expressed as Q values, where Q = OD of the sample/mean OD of the cut-off control delivered in the Progen kit. A Q value >1·5 was considered positive, whereas Q values <1·5 and >1 were considered as being in the grey (equivocal) zone. A Q value <1 was considered negative.
RT–PCR confirmation
RT–PCR was performed on all eight ELISA IgM samples positive for PUUV and/or HTNV (P.M., NRC for Hantaviruses, Belgium). In a first step, primers specific for PUUV and HTNV were used [37]. In a second step, so-called ‘pan-hanta’ primers were also used. For this purpose, a nested RT–PCR assay, as developed by Klempa et al. [38], was used to detect currently known and possible novel members of the genus Hantavirus. This assay is based on so-called degenerated primers, designed from an alignment of all nucleotide sequences known so far of the genomic L segment, highly conserved in all hantavirus species. More elaborative testing, including PRNT on a battery of hantaviruses including Thailand virus (THAIV) [39] and the Indian autochthonous Thottapalyam virus (TPMV) [25], was impossible due to the limited remaining aliquot sizes.
RESULTS
Prospective (IgM) study
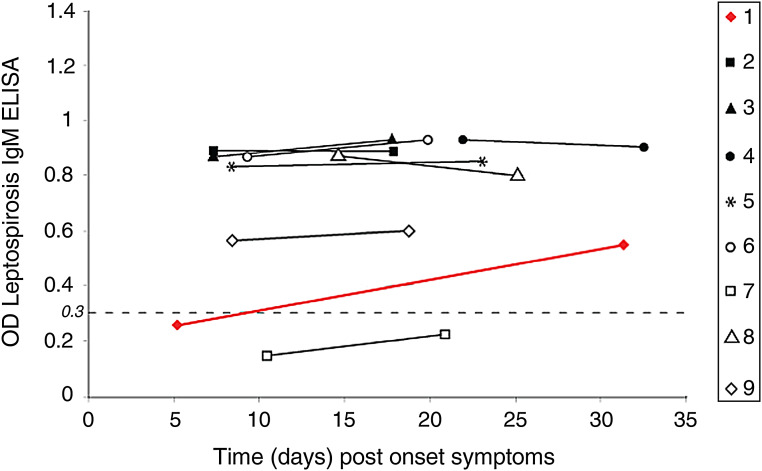
IgM ELISA in the admittedly highly selective prospective study group was in the acute phase positive for leptospirosis in 16/31 (51·62%) cases. Convalescent testing, taken at a mean 22 days POS in the same cohort of 31 cases, picked up nine additional leptospirosis IgM-positive cases, resulting in a total of 25/31 (80·6%) acute leptospirosis cases (data not shown). Moreover, in this original battery of 31 examined sera, only one (3·2%) ELISA IgM positivity was found exclusively for hantavirus (case 7 in Figs 1–3), and one (3·2%) for hantavirus equivocally (case 1, red line in Figs 1–3), whereas 13 (41·9%) were negative for both (data not shown), but seven (22·5%) appeared clearly positive for both pathogens (cases 2–6, 8, 9 in Figs 1–3). Thus in summary, in an acute leptospirosis-suspected cohort of patients, we found evidence of confirmed recent or current hantavirus infection in 8/31 (25·8%). IgM ELISA ratios were rapidly falling or rising for PUUV (Fig. 1), and to a lesser degree for HTNV (Fig. 2), confirming mostly a recent acute hantavirus infection. However, this rapid change of Q values was less pronounced for leptospirosis in the nine cases with dual IgM positivity (Fig. 3), except again in case 1. Here, the Q value rose significantly from 0·25 to 0·54 within 25 days (red line, Fig. 3). Thus, case 1 is probably a truly acute leptospirosis case with a seroconversion, whereas the static PUUV Q values of the same case in the equivocal zone of ELISA IgM positivity (Fig. 1) are suggestive rather for a recent (but no longer acute) hantavirus infection. However, since they both remained well beneath the cut-off baseline of 2, and since for reasons of scarcity of assays we had no control ELISA IgG values, we decided to categorize this borderline case 1 as hantavirus-negative thus resulting in a total of 8/31 hantavirus IgM-positive cases.
Fig. 1.
Prospective study. ELISA IgM results in acute and convalescent sampling for Puumala virus (PUUV) in eight cases of seroconfirmed (ELISA IgM positive) leptospirosis; and one leptospirosis-negative case (case 7). Results are expressed as Q values (observed OD/control cut-off OD), where Q values ⩾2 (- - - -) are considered as positives. Case 1 (red line) remained just under the positivity level, whereas case 2 (■) and case 5 (*) remained PUUV-negative. Five other cases are clearly PUUV-positive in at least one sampling.
Fig. 2.
Prospective study. ELISA IgM results in acute and convalescent sampling for Hantaan virus (HTNV) in eight cases of seroconfirmed (ELISA IgM positive) leptospirosis and one leptospirosis-negative case (case 7). Results are expressed as Q values (observed OD/control cut-off OD), where Q values ⩾2 (- - - -) are considered as positives. Case 1 (red line) is confirmed as hantavirus-negative, whereas case 9 (◊) and case 6 (○) are confirmed as PUUV-positive. Case 2 (■) and case 5 (*) are confirmed as borderline HTNV-positive, and consequently as only PUUV positive.
Fig. 3.
Prospective study. ELISA IgM in acute and convalescent sampling for leptospirosis in nine cases with equivocal or confirmed hantavirus infection. An OD of 0·3 (- - - -) is the cut-off for positivity. Case 1 (red line) is the only case with a seroconversion within 25 days, thus very likely an acute leptospirosis case. All other cases have more stable titres, suggesting recent but not acute leptospirosis. Case 7 (□) is confirmed as leptospirosis-negative, thus as an exclusive hantavirus (PUUV)-only infection.
Markedly, IgM seropositivity was for PUUV exclusively in 2/8 cases (cases 6 and 9), for HTNV exclusively, but only with low Q values, in 2/8 cases (cases 2 and 5), and for PUUV predominantly in the other 6/8 cases (Figs 1 and 2). However, all obtained IgM OD values of PUUV vs. HTNV, acute and/or convalescent, were matched against each other, but no statistically significant difference was found for any matching in a non-parametric Kruskal–Wallis test with Dunn's correction for multiple comparisons (level of significance 0·05).
RT–PCR testing on all eight hantavirus ELISA IgM-positive samples remained negative. We also found clinical differences for patients co-infected with hantaviruses, although these data are too small to draw valid conclusions as to clinical severity of co-infections. Five out of the nine hantavirus+leptospirosis-positive patients had renal involvement before enrolment in the study, whereas this was the case in only three of the leptospirosis-only-positive patients, and in none of the cases negative for both pathogens. In these three categories of patients, mean blood urea nitrogen was 115·8 vs. only 34·3 and 43·3 mg/dl, respectively (normal values 8–25 mg/dl). Markedly, clinical jaundice was present in 7/9 (78%) of hantavirus+leptospirosis-positive patients, but only in three leptospirosis-only-positive patients, and in one patient negative for both pathogens. This was reflected in the mean levels of measured serum total bilirubin of these three groups, being 6·9 vs. only 3·8 and 1·03 mg/dl, respectively (normal value <2·1 mg/dl). Of note, three of the nine hantavirus + leptospirosis-positive cases also complained of cough, suggesting a concomitant degree of pulmonary involvement. It is noteworthy that in fatal Indian hantavirus cases, likewise with a serological PUUV predominance [25], lung as well as renal involvement was also noted, a characteristic so far considered specific for New World hantavirus infections only [17, 19].
Retrospective (IgG) study
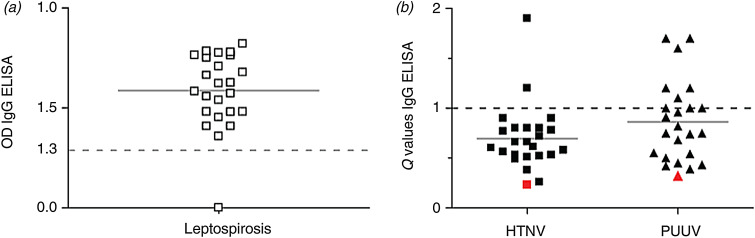
Of the 23 IgG-confirmed leptospirosis patients (Fig. 4a), 2/23 (8·6%) were equivocally positive (Q value for IgG ELISA between 1 and 1·5) for PUUV only, 2/23 (8·6%) were clearly IgG positive (Q values of 1·7 in both) for PUUV only, and two other patients had mixed PUUV and HTNV reactions, of which only 1/23 (4·3%) reacted predominantly against HTNV (Q value 1·9) (Fig. 4b). Of note, exclusive HTNV seroreactivity was not found. Thus, of a retrospective series of 23 patients admitted and serologically confirmed for leptospirosis, 6/23 (26·0%) also showed signs of a past hantavirus infection, again with a net predominance (4/6, 66%) for PUUV. Here again, however, there was no statistically significant difference between measured IgG OD levels of PUUV vs. HTNV in a two-tailed Mann–Whitney test: P = 0·1212, level of significance 0·05. The only leptospirosis-negative case was also hantavirus-negative (open symbol on the baseline of OD = 0.0 of Fig. 4a).
Fig. 4.
Retrospective study. (a) ELISA Ig G results for leptosirosis. The solid line (––) is the mean optical density (OD), the dotted line (- - - -) the cut-off level for positivity. Only one patient was completely IgG-negative (OD = 0·0), shown as an open symbol on the baseline of OD = 0. (b) ELISA IgG results for hantaviruses, Hantaan virus (HTNV) and Puumala virus (PUUV). Results are expressed as Q values (observed OD/control cut-off OD), where Q values >1 (- - - -) are considered as positives. Six patients had IgG levels positive for PUUV, where two cross-reacted also with HTNV. No case had exclusive HTNV seroreactivity. The only patient seronegative for leptospirosis is shown in red.
DISCUSSION
This is the first report of concomitant or recent dual infection with both leptospirosis and hantavirus in a total of 14 (8 prospective, 6 retrospective) cases out of 54 (31 prospectively, 23 retrospectively) screened patients, or 25·9%. The fact that almost all these cases were found in one single province of Sri Lanka (Fig. 5), suggests that this phenomenon probably occurs nationwide. Two caveats in interpreting these results should be noted. First, since leptospirosis ELISA IgM can remain positive for months, and sometimes even for years after the infection, all leptospirosis IgM-positive cases cannot be labelled automatically as ‘acute leptospirosis’. However, at least one case can be considered as truly acute leptospirosis, given the clear ELISA IgM seroconversion (patient 1, red line in Figs 1–3). Hence, and according to WHO definitions, all leptospirosis cases but one (patient 1) should be considered as ‘probable’ cases, because no clear seroconversion and no significant sero-ascension was evidenced. Second, patients finally enrolled in this limited study were highly selected, most of them presenting with severe multi-organ involvement, for which all other known infectious causes had already been excluded prior to the study (see Material and Methods section). On the other hand, and with the current hantavirus IgM results, acute symptoms prompting a hospitalization could be explained in at least 8/31 (25·8%) of the subjects of the prospective study by a recent hantavirus infection. That a double (separate or even concomitant) leptospirosis-hantavirus infection is a rarity in Sri Lanka is furthermore confirmed again in the retrospective study, giving 6/23 or 26·0% incidence. This markedly higher prevalence of co-infection compared to a similar retrospective study in Belgium, resulting in only 9% of co-infected cases [22], may result again from the high degree of pre-study selection of the leptospirosis-suspected cohort in Sri Lanka. Moreover, nothing is known so far of the overall hantavirus seroprevalence in a control Sri Lankan population.
Fig. 5.
Map of Sri Lanka with the localization of habitats of the different categories of patients described.
Indeed, trying to determine the real species of the infecting hantavirus remains a challenge. The finding in both IgM and IgG ELISA results of a clear predominance of an arvicoline agent (PUUV) was unexpected, since the European PUUV arvicoline rodent carrier Myodes glareolus (bank vole) is absent in Sri Lanka, as it is in the whole Indian subcontinent [25]. In particular cases 7 and 3, with positive Q values for PUUV IgM ELISA around Q = 4, confirmed in both acute and convalescent samples (Fig. 1), are too high to be considered just cross-reactions. Given the known presence of both wild murine rats and bandicoot rats, we expected to find a trace of the hantaviruses they are spreading in these regions, i.e. SEOV [17, 25] and/or THAIV [39]. However, both these pathogens should preferentially be picked up by a murine screening antigen such as HTNV, but not by a predominantly arvicoline seroreaction (i.e. PUUV positivity), as found now in Sri Lanka. RT–PCR as confirmation test (see Methods section) offered no further insights, since all were negative. RT–PCR results in hantavirology can only be interpreted if positive, since negative findings may result from too late sampling (given the transient and often low viraemia in most hantavirus infections), equivocal quality of the serum samples, or simply absence of the targeted virus species [37, 38]. On the other hand, repeatedly positive ELISA results with rapidly changing titres in patients with an acute suggestive symptomatology cannot be dismissed as simply false-positives. Since phylogeny of hantaviruses is almost always closely mirrored by the phylogeny of their respective rodent carriers [13], and since no other rodent species of the Arvicolinae subfamily is present on the island, our predominantly PUUV-positive results should be considered as cross-reactions to another, as yet unknown local hantavirus. It should be remembered that so far no mammalogical study has been performed in Sri Lanka, using biomolecular techniques to detect the presence/or not of novel or well-known hantaviruses in the local rodent population, or in other small mammals. Of note, in neighbouring India, we found similarly unexplained PUUV-like predominance in similar and even in fatal leptospirosis-like cases, not only in an ELISA format, but in a more specific immunoblot format as well [25]. Moreover, retrospective analysis of a stored serum sample of a young farmer with a classical clinical picture of leptospirosis during the post-flood outbreak in Sri Lanka, 2008, revealed a high PUUV IgM ELISA titre, whereas dengue infection and leptospirosis were excluded. The hantavirus diagnostic kit used in this case was another commercial ELISA [11]. Finding the same surprising result with three different screening formats in three different studies in the same area cannot be ascribed to coincidence.
The only hantavirus species with a proven presence so far in neighbouring India, is TPMV, which was isolated in 1971 from an insectivore, Suncus murinus (Asian house shrew). Since Sri Lanka has almost exactly the same mammalian fauna as India, including house shrews, local TPMV occurrence is not excluded. However, human pathogenicity from TPMV or from any other newer insectivore-borne hantavirus has not been proven so far [13, 19, 25]. Interestingly, in Belgium and since the 1980s, 3/50 (6%) moles (Talpa europea) and two different shrew species were found to contain PUUV-like antigen in their lungs, detected with a double-sandwich ELISA capture technique [40]. At that time and up to now, insectivores like moles and shrews were not incriminated as vectors for human hantavirus disease. So far, all hantaviruses characterized from insectivores are genetically clearly distinct from rodent-borne hantaviruses, and are consequently not supposed to contain PUUV-like antigens in their organs [38, 40]. However, in two Belgian cases with prior intense mole contact (one having mole's blood splashed in his face), a clinical picture perfectly resembling PUUV-induced HFRS (fever, AKI, thrombocytopenia), was detected and even serologically confirmed as apparently caused by a PUUV-like agent. A third PUUV-like case, likewise with a predominant PUUV-like seroconfirmation, occurred after a bite by a water shrew (Neomys fodiens) [40].
Interestingly, the only similar study so far documenting both leptospirosis infection and hantavirus infection in a major tropical leptospirosis cohort, was similarly performed in Sri Lanka, where Gamage et al. retrospectively documented eight IgG hantavirus positives in 103 leptospirosis-suspected patients during the local 2008 outbreak [39]. A majority of these eight cases appeared seropositive for THAIV, rather than for the ‘classical’ rat-transmitted SEOV. Of note, THAIV was isolated more recently from Bandicota indica (Asian house rat), another rat species belonging to the same Murinae subfamily as the common and globally present wild brown or black rat (Rattus norvegicus and R. rattus), but found only in SW Asia, including India and Sri Lanka. However, in these regions, the Asian house rat is widely spread, often even trapped for consumption of its meat, and is present in and around the human environment. Moreover, this Asian Rattus species is also known as a reservoir for pathogenic Leptospira, mainly serovar Autumnalis [41]. Thus, Bandicota indica could serve as a source of dual separate or even concomitant Leptospira-hantavirus infections in India and Sri Lanka (and in fact throughout SW Asia), as is already the case for its better known cousin, the wild brown or black rat. Since all murine hantavirus serotypes are heavily cross-reacting with each other in all serology formats, a distinction between these two rat-transmitted pathogens is only possible by highly specialized confirmation techniques, such as PRNT, as performed by Gamage et al. [39]. Retrospectively, human THAIV, instead of ‘classical’ SEOV infections, could explain the original but puzzling serological findings by H. W. Lee et al. almost 25 years earlier in leptospirosis-suspected cases in Sri Lanka [29], and part of our inconclusive prior results in neighbouring India, where unexpectedly, only rare and low ELISA and immunoblot titres were found for prototypic SEOV [25].
Of note, leptospirosis is also considered in China as one of the most common zoonoses, with from 1991 to 2010 an average annual incidence of 0·70/100000 population, three major outbreaks having occurred after heavy rainfall and floods. Leptospira interrogans serogroup Icterohaemorrhagiae serovar Lai is the predominant Leptospira responsible for at least 60% of Chinese cases, and the oriental form of Apodemus agrarius (the common striped field mouse) serves as the major animal host [42]. It is intriguing that exactly the same rodent species was found since decades (1978) of being the source of tens of thousands of HTNV-induced HFRS cases in China and in the whole Far East [16, 17, 20]. Moreover, the same subspecies A. agrarius is also the well-known reservoir for scrub typhus, as confirmed in a recent 5-year rodent capture study in Korea, the country where hantaviruses were first discovered in the same local rodent [43]. Finally, the Western form of A. agrarius is responsible for spreading more severe forms of HFRS in Central and Eastern Europe and the Caucasus. Thus, in theory, the same very common rodent could cause double concomitant but clinically indistinguishable zoonoses in the West (HFRS and leptospirosis), and even triple concomitant but similarly indistinguishable zoonoses in the Far East (HFRS, leptospirosis, and scrub typhus). However, and to our knowledge, no systematic prospective or even retrospective studies of such dual or triple human infections have been published. Likewise, after the first documentation in the literature of dual co-infection of a hantavirus (PUUV) and Leptospira in a common European rodent reservoir host (Myodes glareolus or bank vole) [44], no European study is available to demonstrate dual leptospirosis-PUUV infection in a human cohort. In contrast to the Far East situation, however, it has to be admitted that when the bank vole is the main, if not the only, rodent reservoir for PUUV infections in Europe, it is certainly not primordial for spreading leptospirosis in Europe.
Thus, after prior serological and retrospective evidence of non-acute HFRS co-existent with leptospiral antibodies by Gamage et al. in three study subjects in 2011 [39], this is the first prospective study to confirm the existence of a pathogenic hantavirus in Sri Lanka, and the first clinical series of dual acute or recent leptospirosis and HFRS. In both reports, no convincing evidence was found that brown rat-transmitted SEOV is the prime agent causing HFRS in this region, despite the fact that this feral rodent is omnipresent in Sri Lanka, as it is in India.
CONCLUSIONS
-
(1)
Dual hantavirus and leptospirosis infections occur probably much more frequently than hitherto reported, as shown recently in a similar Belgian study [22]. In a series of 31 leptospirosis-suspected acute cases in Sri Lanka, we found evidence of a confirmed recent hantavirus infection in eight (25·8%) of the cases. Given the proven high yield of additional seroconfirmation for both pathogens in a second convalescent serum sample, systematic successive sampling is mandatory, particularly if gold standard techniques for confirmation of both pathogens (MAT, RT–PCR, PRNT, etc.) are not available. Finding signs of another viral emerging infection in more than 1/4 cases hospitalized in a country highly endemic for leptospirosis, definitely merits more attention in the future.
-
(2)
A pathogenic rodent-(or insectivore?)-borne hantavirus is probably present in Sri Lanka, but seems to belong to an unknown hantavirus species, giving apparently cross-reactions with European PUUV. The existence of such a putatively novel hantavirus in both Sri Lanka and in India awaits further confirmation.
-
(3)
Since HFRS is a great mimicker of leptospirosis, and since both zoonoses are often transmitted worldwide by the same rodent reservoir, the wild rat, suspected patients should always whenever possible be screened for SEOV (or other murine) hantaviruses, even when as a first step, serology for leptospirosis already appears positive. Indeed, dual infections are possible (see Introduction), aggravating or at least complicating the clinical picture. In fact, and in the current but limited Sri Lankan study, leptospirosis-confirmed cases co-infected with hantavirus appeared clinically more severely affected (see Material and methods, and Prospective study sections). One interesting cost-benefit analysis of antibiotic treatment (or not) in leptospirosis-suspected cases in Thailand came to the conclusion that a 7-day course of empirical doxcycline therapy was the most efficient strategy if no prior testing of the involved pathogen was possible [45]. This approach is eminently defendable, since not only Leptospira, but also the agents of scrub typhus, murine typhus, other rickettsioses, Q fever and brucellosis might benefit from a simple doxcycline treatment, and since omissison of such treatment can have serious consequences in ‘true’ leptospirosis [45]. However, so-called failures of timely and appropriate antibiotic treatment in leptospirosis-suspected but not confirmed cases can be due to an infection of murine hantaviruses, causing a fatality rate of up to 10% [6]. Indeed, leptospirosis-suspected patients, although in fact infected with a hantavirus, can obviously not benefit from antibiotics, but might well have been treated in the past as such, resulting in a so-called complete recovery within 2–3 weeks to the great satisfaction of both patients and doctors. Since spontaneous and complete restoration to normal is the rule in most HFRS cases [9, 10], for which supportive but cheap measures (e.g. fluid balance control) often suffice, such a minimal approach might be considered if cost-effective and quick hantavirus screening (e.g. with an IgM ELISA) turns out to be positive as the only seroresult. In this context, it is relevant to note that the first author of this 2010 cost-benefit analysis in Thailand, is also the scientist to document the first (2005) seroproven hantavirus cases in his country [46]. Regrettably, but perhaps not surprisingly, the amount of Thai hantavirus cases were not considered, nor discussed, in his cost-benefit analysis 5 years later. Thus, and although rarely applied today as a standard procedure, immediate and repeated screening for both pathogens remains mandatory, as advocated 25 years ago by the two founding fathers of hantavirology [6].
ACKNOWLEDGEMENTS
We thank Dr R. Premaratne, and Dr D. A. D. H. Somasiri of the Faculty of Medicine, University of Kelaniya for their assistance in specimen collection and data recording. Generous supplies of detection kits and reference reagents by Dr David Brown, Director Respiratory and Enteric Research, PHLS, Colindale, UK, Dr Roy Hall, Department of Microbiology, University of Queensland, Australia and Dr P. Chandrasiri, Medical Research Institute, Colombo, Sri Lanka is gratefully acknowledged.
REFERENCES
- 1.Luber G, Prudent N. Climate change and human health. Transactions of the American Clinical and Climatological Association 2009; 120: 113–117. [PMC free article] [PubMed] [Google Scholar]
- 2.Clement J, et al. Relating increasing hantavirus incidences to the changing climate: the mast connection. International Journal of Health Geography 2009; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clement J, et al. Global warming and epidemic trends of an emerging viral disease in Western-Europe: the nephropathia epidemica case. In: Casalegno S, ed. Global Warming impacts: Case Studies on the Economy, Human Health, and on Urban and Natural Environments. Rijeka, Croatia: InTech Open Access Publications, 2011, pp. 39–52. [Google Scholar]
- 4.Pappas G, et al. The globalization of leptospirosis: worldwide incidence trends. International Journal of Infectious Diseases 2008; 12: 351–357. [DOI] [PubMed] [Google Scholar]
- 5.Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clinical Microbiology and Infection 2011; 17: 494–450. [DOI] [PubMed] [Google Scholar]
- 6.Lee HW, van der Groen G. Hemorrhagic fever with renal syndrome. Progress in Medical Virology 1989; 36: 62–102. [PubMed] [Google Scholar]
- 7.Niklasson B, LeDuc J, Nyström K. Nephropathia epidemica: incidence of clinical cases and antibody prevalence in an endemic area of Sweden. Epidemiology and Infection 1987; 99: 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Leptospirosis. Communicable Disease Epidemiological Profile. Geneva: World Health Organization, 2010, pp. 102–108. [Google Scholar]
- 9.Clement J, Maes P, Van Ranst M. Acute kidney injury in emerging, non-tropical infections. Acta Clinica Belgica 2007; 62: 387–395. [DOI] [PubMed] [Google Scholar]
- 10.Clement J, Maes P. Acute kidney injury and hantavirus disease. In: Turner N, Goldsmith D, Winearls C, Lameire N, Himmelfarb J, Remuzzi G, eds. Oxford Textbook of Clinical Nephrology, 4th edn. Oxford: Oxford University Press; (in press). [Google Scholar]
- 11.Dahanayaka NJ, et al. Hantavirus infection mimicking leptospirosis: how long are we going to rely on clinical suspicion? Journal of Infection in Developing Countries 2014; 8: 1072–1075. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, et al. Scrub typhus is an under-recognized cause of acute febrile illness with acute kidney injury in India. PLoS Neglected Tropical Diseases 2014; 8: e2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maes P, et al. Hantaviruses: Immunology, treatment and prevention. Viral Immunology 2004; 17: 481–497. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, et al. Comparison between three rare cases of co-infection with dengue, leptospira and hepatitis E: is early endothelial involvement the culprit in mortality? Annals of Medical & Health Sciences Research 2014; 4 (Suppl. 1): 32–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Report of the First Meeting of the Leptospirosis Burden Epidemiology Reference Group. Geneva: World Health Organization, 2010, pp. 1–34 [Google Scholar]
- 16.World Health Organization. Haemorrhagic fever with renal syndrome: memorandum from a WHO meeting. Bulletin of the World Health Organization 1983; 61: 269–275. [PMC free article] [PubMed] [Google Scholar]
- 17.Clement J, Maes P, Van Ranst M. Hemorrhagic fever with renal syndrome in the new, and hantavirus pulmonary syndrome in the old world: paradi(se)gm lost or regained? Virus Research 2014; 187: 55–58. [DOI] [PubMed] [Google Scholar]
- 18.Lee HW, et al. Isolation of the etiologic agent of Korean hemorrhagic fever. Journal of Infectious Diseases, 1978; 137: 298–308. [DOI] [PubMed] [Google Scholar]
- 19.Lee HW, Vaheri A, Schmaljohn CS. Discovery of hantaviruses and of the Hantavirus genus: personal and historical perspectives of the Presidents of the International Society of Hantaviruses. Virus Research 2014; 187: 2–5. [DOI] [PubMed] [Google Scholar]
- 20.Liu QY, Meng FX, Fan JC. Vector surveillance and control in emergencies in China: proceedings and perspectives [in Chinese]. Chinese Journal of Vector Biology and Control 2011; 22: 1–4. [Google Scholar]
- 21.van der Groen G, et al. Seroepidemiology of Hantaan-related virus infections in Belgian populations. Lancet 1983; 2:1493–1494. [DOI] [PubMed] [Google Scholar]
- 22.Clement J, et al. Leptospirosis versus hantavirus infections in the Netherlands and in Belgium, 2000 to 2014. Eurosurveillance 2014; 19: pii = 20912. [DOI] [PubMed] [Google Scholar]
- 23.Hinrichsen S, et al. Evidence of hantavirus infection in Brazilian patients from Recife with suspected leptospirosis. Lancet 1993; 341: 50. [DOI] [PubMed] [Google Scholar]
- 24.McKenna P, et al. Serological evidence of Hantavirus disease in Northern Ireland. Journal of Medical Virology 1994; 43: 33–38. [DOI] [PubMed] [Google Scholar]
- 25.Clement J, et al. First evidence of fatal hantavirus nephropathy in India, mimicking leptospirosis. Nephrology, Dialysis and Transplantation 2006; 21: 826–827. [DOI] [PubMed] [Google Scholar]
- 26.Kudesia G, et al. Dual infection with leptospira and hantavirus. Lancet 1988; 1, 1397. [DOI] [PubMed] [Google Scholar]
- 27.Nemes Z, Peterfi Z. Simultaneous leptospirosis and hantavirus infection in the same patient. Orvosi Hetilap 2000; 141: 499–502. [PubMed] [Google Scholar]
- 28.Markotić A, et al. Double trouble: hemorrhagic fever with renal syndrome and leptospirosis. Scandinavian Journal of Infectious Diseases 2002; 34: 221–224. [DOI] [PubMed] [Google Scholar]
- 29.Vitarana T, et al. Human hantavirus disease in Sri Lanka. Lancet, 1988; 2: 1263. [PubMed] [Google Scholar]
- 30.Vitarana T. Hantavirus disease. Ceylon Medical Journal 1994; 39: 63–66. [PubMed] [Google Scholar]
- 31.Agampodi SB, et al. Leptospirosis outbreak in Sri Lanka in 2008: lessons for assessing the global burden of disease. American Journal of Tropical Medicine and Hygiene 2011; 85: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yersin C, et al. Pulmonary haemorrhage as a predominant cause of death in leptospirosis in Seychelles. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000; 94: 71–76. [DOI] [PubMed] [Google Scholar]
- 33.Agampodi S, Peacock SJ, Thevanesam V. The potential emergence of leptospirosis in Sri Lanka. Lancet Infectious Diseases 2009; 9: 524–526. [DOI] [PubMed] [Google Scholar]
- 34.Reller ME, et al. Leptospirosis as frequent cause of acute febrile illness in southern Sri Lanka. Emerging Infectious Diseases 2011; 17: 1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamage CD, et al. Analysis of hospital-based sentinel surveillance data on leptospirosis in Sri Lanka, 2005–2008. Japanese Journal of Infectious Diseases 2012; 65: 157–161. [PubMed] [Google Scholar]
- 36.Agampodi SB, et al. Regional differences of leptospirosis in Sri Lanka: observations from a flood-associated outbreak in 2011. PLoS Neglected Tropical Diseases 2014; 8: e2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maes P, et al. Puumala virus. In: Dongyou L ed. Molecular Detection of Human Viral Pathogens. CRC Press, Boca Raton, Florida, USA: 2010, pp. 667–676. [Google Scholar]
- 38.Klempa B, et al. Hantavirus in African wood mouse, Guinea. Emerging Infectious Diseases 2006; 12: 838–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamage CD, et al. Serological evidence of Thailand virus-related hantavirus infection among suspected leptospirosis patients in Kandy, Sri Lanka. Japanese Journal of Infectious Diseases 2011; 64: 72–75. [PubMed] [Google Scholar]
- 40.Clement J, et al. Hantavirus infections in rodents. In: Horzinek M, ed. Virus Infections of Rodents and Lagomorphs, 5th volume in series ‘Virus infections in Vertebrates’ (Osterhaus A, ed.). Amsterdam: Elsevier Science BV, 1994, pp. 295–316. [Google Scholar]
- 41.Saravanan R, et al. Leptospira autumnalis isolated from a human case from Avadi, India, and the serovar's predominance in local rat and bandicoot populations. Annals of Tropical Medicine and Parasitology 2000; 94: 503–506. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Wang H, Yan J. Leptospirosis prevalence in Chinese populations in the last two decades. Microbes and Infection 2012; 14: 317–323. [DOI] [PubMed] [Google Scholar]
- 43.O'Guinn ML, et al. Serological surveillance of scrub typhus, murine typhus, and leptospirosis in small mammals captured at firing points 10 and 60, Gyeonggi province, Republic of Korea, 2001–2005. Vector Borne Zoonotic Diseases 2010; 10: 125–133. [DOI] [PubMed] [Google Scholar]
- 44.Cvetko L, et al. Short report: dual infections with Puumala virus and Leptospira interrogans serovar lora in a bank vole (Clethrionomys glareolus). American Journal of Tropical Medicine and Hygiene 2006; 74: 612–614. [PubMed] [Google Scholar]
- 45.Suputtamongkol Y, et al. Strategies for diagnosis and treatment of suspected leptospirosis: a cost-benefit analysis. PLoS Neglected Tropical Diseases 2010; 4: e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suputtamongkol Y, et al. Hantavirus infection in Thailand: first clinical case report. Southeast Asian Journal of Tropical Medicine and Public Health 2005; 36:700–703. [PubMed] [Google Scholar]