Abstract
The transcription start sites for the tatABCD and tatE loci, encoding components of the Tat (twin-arginine translocase) protein export pathway, have been identified. Expression studies indicate that the tatABCD and tatE transcription units are expressed constitutively. Translational fusion experiments suggest that TatA is synthesized at a much higher level than the other Tat proteins.
The Tat (twin-arginine translocation) export pathway is a recently discovered protein transport system found in the cytoplasmic membranes of most bacteria and in the energy-transducing membranes of plant organelles (2, 17). Proteins are targeted to the Tat apparatus by N-terminal signal sequences that harbor the (S/T)RRxFLK twin-arginine motif (1). Analysis of the Escherichia coli genome sequence has identified in excess of 20 gene products that are likely to be exported by the Tat system (2). Unlike the well-characterized Sec export pathway, which translocates substrates in an extended conformation, the Tat pathway appears to be capable of exporting prefolded proteins, often containing redox cofactors (13). Thus, the Tat system plays a critical role in the biogenesis of electron transfer chains. Many of the most highly expressed substrates of the Tat system in E. coli, including the uptake hydrogenases, the trimethylamine-N-oxide and dimethyl sulfoxide reductases, the periplasmic nitrate reductase, and formate dehydrogenase-N, are key components of anaerobic respiratory chains (7). Therefore, it was anticipated that the highest demand for protein export by the Tat pathway might be under anoxic respiratory conditions. We demonstrate here that the tat genes are expressed constitutively, indicating a requirement for the Tat export machinery under all growth regimens.
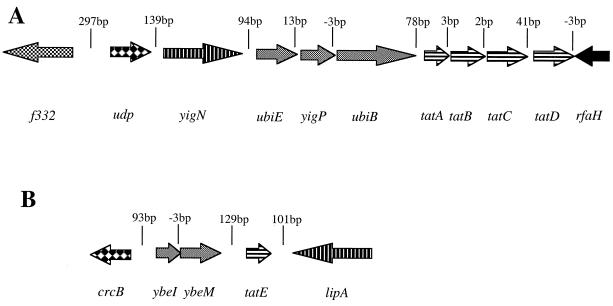
Studies of E. coli have identified two genetic loci coding for components of the Tat pathway. The tatA transcription unit (Fig. 1A), located at min 86 on the E. coli chromosome, comprises four genes, tatA to tatD (14). Of these, tatB and tatC encode essential Tat components (3, 5, 15, 19). The tatD gene is not required for Tat-dependent protein export, and Northern blot analysis suggests that although tatD is cotranscribed with tatABC, the presence of a putative stem-loop structure in the tatC-tatD intergenic region serves to control the level of TatD (20). The tatA gene encodes a protein exhibiting greater than 50% amino acid sequence identity with TatE. These two proteins have overlapping roles in protein export by the Tat pathway such that only a strain lacking both of the genes is completely blocked in protein export (14). The tatE gene (Fig. 1B) is located at 14 min on the E. coli chromosome and is apparently monocistronic. In this report, we probe the regulatory features of the two genetic loci required for Tat-dependent protein export.
FIG. 1.
Genetic organization of the E. coli chromosome at 86 min (A) and 14 min (B). The intergenic distances in base pairs are indicated. Groups of genes which may form transcriptional units are shaded similarly.
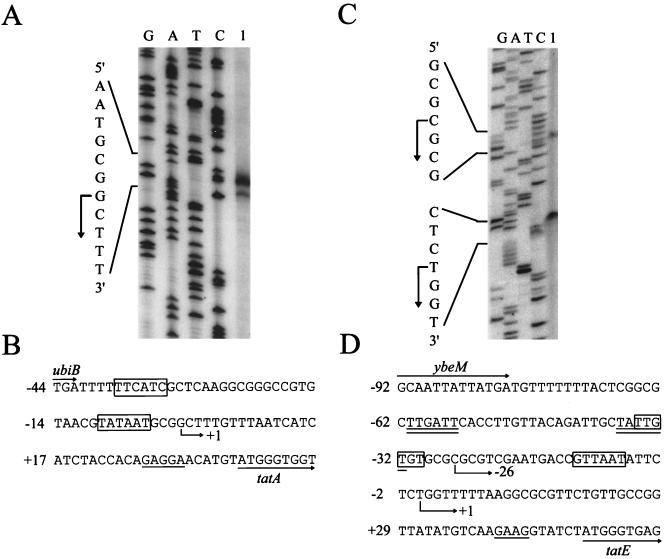
Using primer extension analysis of total RNA isolated from cells grown aerobically in CR-Hyd medium (6), we identified the transcription start site of the tatABCD locus (Fig. 2A). The transcription start site lies 37 bp upstream of the tatA start codon, which we have identified by N-terminal sequence analysis of the isolated TatA protein (F. Sargent, T. Palmer, and B. C. Berks, unpublished data). Only one major start site was identified regardless of whether cells were grown aerobically, anaerobically in the presence of glycerol with either nitrate or fumarate, or fermentatively (data not shown). The putative promoter region of tatA is highlighted in Fig. 2B. Reasonable matches for the consensus −10 and −35 recognition sequences of ς70-dependent promoters are apparent. Despite the relatively short intergenic region between the tatA transcription unit and the cluster of upstream genes involved in quinone biosynthesis, there was no evidence from either primer extension or reverse transcriptase PCR analysis of any transcriptional readthrough into the tat genes (data not shown).
FIG. 2.
Primer extension mapping of the tatABCD operon and tatE gene transcription initiation sites. (A) To map the tatABCD operon initiation site, total RNA was isolated from MC4100 (F− ΔlacU169 araD139 rpsL150 relA1 ptsF rbs flbB5301 [4]) grown aerobically to mid-exponential phase in CR-Hyd minimal medium [CR-Hyd contains, per liter, 5.29 g of K2HPO4, 8.24 g of KH2PO4, 5 g of peptone, and 2 g of (NH4)2SO4 (6)], with 0.4% (wt/vol) glucose as the carbon source. RNA isolation and primer extension (using 20 μg of total RNA) were performed as described previously (16). The primer sequence was 5′-TGGTTCATCATCGCTCATT-3′, and the sequencing ladder was generated using plasmid pFAT415 (15). The angled arrow at the left indicates the location of the mapped 5′ end of the transcript. (B) DNA sequence of the tatABCD promoter region. The angled arrow indicates the location of the mapped transcription start site. The 3′ end of ubiB and the 5′ end of tatA are designated by horizontal arrows. The putative ribosome binding site of tatA is underlined, and possible −10 and −35 sequences for ς70 recognition are boxed. Numbering is from the transcriptional start site, which is taken as +1. (C) To map the tatE gene transcription initiation site, total RNA was isolated from MC4100 grown anaerobically to mid-exponential phase in CR-Hyd medium supplemented with 0.5% (wt/vol) glycerol and 0.4% (wt/vol) fumarate. Primer extension performed with labeled oligonucleotide 5′-CCAAACAGCAGAACGACCAG-3′, and the sequencing ladder was generated using plasmid pFAT45 (15). The angled arrows at the left indicate locations of the mapped 5′ ends of the transcripts. (D) DNA sequence of the tatE promoter region. The angled arrows indicate locations of the mapped transcription start sites. The 3′ end of ybeM and the 5′ end of tatE are designated by horizontal arrows. The putative ribosome binding site of tatE is underlined. The possible −10 and −35 sequences for ς70 recognition of the lower transcription start site are boxed, and those of the upper start site are double underlined. Numbering is with respect to the more 3′ transcriptional start site, which is taken as +1.
To investigate the expression levels of the tatA transcription unit, we constructed both transcriptional and translational fusions of tatA to the lacZ gene. Each fusion was constructed using the same fragment of the tatA upstream region. DNA from 532 bp upstream to the first codon of tatA was amplified by PCR and cloned into either plasmid pRS551 (transcriptional fusion) or plasmid pRS552 (translational fusion) (18). The tatA-lacZ transcriptional and translational fusions were delivered into the lambda attachment (att) site on the chromosome of MC4100 as described elsewhere (18). High levels of expression from the tatA promoter could be detected under both aerobic and anaerobic growth conditions (Table 1), confirming the observations made by monitoring transcript levels. The transcriptional tatA-lacZ fusion strain respiring with either glucose plus oxygen or glycerol plus nitrate produced in the region of 900 to 1,000 Miller units. The same strain growing fermentatively showed approximately half the level of β-galactosidase activity, suggesting there may be some down-regulation under these conditions.
TABLE 1.
Expression of single-copy lacZ transcriptional and translational fusions to tatA and tatE present at the att sitea
| Relevant genotype | β-Galactosidase sp act (Miller units)
|
||
|---|---|---|---|
| Glucose + oxygen | Glucose + formate | Glycerol + nitrate | |
| MC4100 φ(tatA-lacZ) | 860 (1,090) | 430 (520) | 990 (770) |
| MC4100ΔtatA φ(tatA-lacZ) | 810 (970) | 550 (370) | 1,040 (795) |
| MC4100 φ(tatE-lacZ) | 630 (29) | 500 (42) | 710 (65) |
| MC4100ΔtatE φ(tatE-lacZ) | 560 (14) | 570 (19) | 1,430 (35) |
Translational fusion data are presented in parentheses. The tat-lacZ fusions, constructed as described in the text, were delivered onto the chromosome of either MC4100, ELV16 (MC4100ΔtatA [15]) or J1M1 (MC4100ΔtatE [14]). Strains were cultured in CR-Hyd minimal medium (6) supplemented with 0.4% (wt/vol) glucose, 0.2% (wt/vol) formate, 0.5% (wt/vol) glycerol, or 0.4% (wt/vol) nitrate. β-Galactosidase enzyme activity was determined as described by Miller (9). All assays were performed in triplicate and repeated at least three times, with results varying by no more than 15%.
High levels of β-galactosidase activity could also be detected in MC4100 harboring the tatA-lacZ translational fusion, indicating that tatA is translated with high efficiency (Table 1). This is consistent with the presence of a region rich in A and G residues between 6 and 11 nucleotides upstream of the tatA translational start site (Fig. 2B), which approximates a good Shine-Dalgarno sequence. The highest level of translational activity was observed with strains growing aerobically, with slightly lower levels for strains respiring anaerobically. Again the lowest activity was seen for cells fermenting glucose. Taken together, the results from the tatA transcriptional and translational fusions indicate that there is no aerobic/anaerobic regulation of tatABCD operon expression, but that there may be some down-regulation (approximately twofold) under fermentative growth conditions. These observations imply that there should be Tat substrate proteins synthesized and exported under all growth conditions since there is a high and constitutive level of expression of genes encoding the core Tat components.
The tatE gene encodes a protein with high sequence similarity to TatA, and the two proteins share overlapping functions with respect to Tat-dependent protein export. However, an in-frame deletion in tatA apparently has a more severe protein transport defect than a similar mutation in tatE. To investigate the interrelationship between tatA and tatE further, we investigated the transcription of tatE. Primer extension analysis of total cellular RNA, using a primer antisense to the tatE coding region, identified the presence of two major transcripts (Fig. 2C). As shown in Fig. 2D, the start sites of these transcripts are located at 49 and 75 bp, respectively, upstream of the tatE translational start site. The same transcriptional start sites were apparent using RNA isolated from cells grown aerobically and anaerobically (data not shown). Both of the start sites are preceded by putative ς70-dependent −10 and −35 promoter sequences (Fig. 2D). In addition to the two major transcripts, a number of minor transcripts further upstream were also detected. No transcriptional readthrough from the ybeH promoter (Fig. 1) occurred, since reverse transcriptase PCR experiments using primers to tatE and ybeM yielded no product (data not shown).
To investigate the regulation of tatE and compare it with that of tatA, we constructed transcriptional and translational fusions of tatE to the lacZ gene in manner identical to that used to create the tatA fusions described above. Each fusion was directly after the first codon of tatE and carried 707 bp of upstream DNA. The transcriptional and translational fusions were recombined into the lambda att site of strain MC4100. Expression of β-galactosidase activity from the tatE-lacZ transcriptional fusion was almost as high as that for the tatA-lacZ fusion, with activities of 500 to 700 Miller units detected (Table 1). There was no significant effect of growth conditions on the level of expression, and the fusion was expressed at comparable levels both aerobically and anaerobically. In contrast, the tatE-lacZ translational fusion was expressed at significantly lower levels, up to 50-fold lower than the corresponding tatA-lacZ translational fusion (Table 1). This difference may be due to in the fact that the tatE gene has a poor ribosome binding site.
Our regulatory studies suggest that the genes encoding the known components of the Tat translocase are expressed at almost constitutive levels in all growth conditions tested. This is broadly similar to the expression reported for the genes encoding the core membrane components of the Sec translocon, where the secYEG genes appear not to be regulated, regardless of the demand for protein secretion (10). The exception to this is secA, which encodes the ATPase required to drive translocation of preproteins across the membrane. The translation of secA is repressed under conditions of excess protein secretion capacity and is induced 10- to 20-fold when protein secretion is blocked (11). This effect has been demonstrated to be autoregulatory, in that SecA is able to bind specifically to the translational initiation region on its mRNA, presumably preventing further translation (12).
To investigate whether there was any evidence for autoregulation of tatA and/or tatE expression, we recombined the φ(tatA-lacZ) and φ(tatE-lacZ) transcriptional and translational fusions into the chromosome of the tatA deletion mutant ELV16 (15) and of the ΔtatE strain J1M1 (14), respectively, and measured the level of β-galactosidase activity. There appeared to be no significant increase in the level of expression of tatA transcriptional or translational fusions in strains where tatA has been deleted (Table 1). Comparison of β-galactosidase activities of tatE-lacZ fusions in a strain lacking tatE (Table 1) suggests a modest (maximum twofold) decrease in expression. The reciprocal experiments in which tatA-lacZ expression was examined in a tatE mutant, and vice versa, also did not give any indication of regulation (data not shown).
The translational fusion experiments described above suggest that the cellular production of TatA is much greater than the production of TatE. To compare expression levels of tatA and tatE translational fusions with levels of the other tat genes, we constructed chromosomal in-frame fusions of lacZ to each of tatA, tatB, tatC, tatD, and tatE. The fusions were constructed in an analogous manner. The entire coding region of each tat gene with the exception of the first two and either last two (tatE), last three (tatD), last four (tatC), last eight (tatB), or last six (tatA) codons was deleted. The deleted regions were replaced by the entire coding region of lacZ lacking the native start and stop codons. Each construct was subcloned into plasmid pMAK705 and transferred to the chromosome of MC4100 by the method of Hamilton et al. (8). From the results in Table 2, it is apparent that tatA is the most highly expressed of the tat genes, and that the level of expression of the tatA-lacZ translation fusion at the native site is comparable to that at the att site (Table 1). Curiously, the expression of tatE from its native site appears to be significantly lower than expression of the tatE-lacZ translational fusion present at the att site. This may be accounted for by the slight difference in position of the fusion junction relative to lacZ; at the native site, the start codon of tatE is fused to codon 2 of lacZ, whereas the fusion in the att site is to lacZ codon 8. The difference in chromosomal location of the two fusions may also be an important factor in accounting for the remaining difference. This discrepancy notwithstanding, the results presented here clearly indicate that tatA expression is between 50- and 200-fold higher than tatE expression. Assuming that this difference is reflected at the level of TatA and TatE protein synthesis, it might account for the observation that a deletion of tatA shows a more severe Tat export defect than a deletion of tatE (14).
TABLE 2.
Expression of gene replacement lacZ translational fusions to each of the tat genesa
| Strain | Relevant genotype | β-Galactosidase sp act (Miller units)
|
|
|---|---|---|---|
| Glucose + oxygen | Glycerol + nitrate | ||
| ELV16Z | MC4100 φ(ΔtatA-lacZ) | 1,170 | 925 |
| BO/DZ | MC4100 φ(ΔtatB-lacZ) | 45 | 35 |
| B1LK0Z | MC4100 φ(ΔtatC-lacZ) | 18 | 21 |
| S1DZ | MC4100 φ(ΔtatD-lacZ) | 6 | 4 |
| J1M1Z | MC4100 φ(ΔtatE-lacZ) | 5 | 8 |
The fusions were constructed as described in the text. Strains were cultured in CR-Hyd medium supplemented with 0.4% (wt/vol) glucose, 0.5% (wt/vol) glycerol, or 0.4% (wt/vol) nitrate.
Comparison of the β-galactosidase levels from strains ELV16Z and BO/DZ (Table 2) indicates that the tatB-lacZ translational fusion appears to be expressed at a level approximately 26-fold lower than that of tatA. This may be accounted for by the rather poor ribosome binding site of tatB and the GUG initiation codon. The tatC gene was expressed at a level approximately twofold lower than that of tatB. In general, these observations are consistent previous experiments in which we produced radiolabeled tatABCD gene products in vivo under control of the phage T7 φ10 promoter. Under these conditions, the amount of TatA produced was much greater than that of TatB, which in turn was greater than that of TatC (14, 15, 20). The synthesis of β-galactosidase from the ΔtatD-lacZ fusion is extremely low and strongly suggests that the proposed stem-loop structure in the tatC-tatD intergenic region plays a significant role in controlling the synthesis of TatD, possibly through enhanced mRNA degradation (20). Assuming that the rates of turnover of the Tat proteins are similar, these results indicate that TatA forms the major component of the Tat protein export system. Future studies will aim to corroborate these findings at the level of Tat protein synthesis.
Acknowledgments
This work was supported by BBSRC grant 83/P11832 to T.P. and by CEC grant QLRT-1999-00917 to ExporteRRs. T.P. is funded by a Royal Society University Research Fellowship.
REFERENCES
- 1.Berks B C. A common export pathway for proteins binding complex redox cofactors? Mol Microbiol. 1996;22:393–404. doi: 10.1046/j.1365-2958.1996.00114.x. [DOI] [PubMed] [Google Scholar]
- 2.Berks B C, Sargent F, Palmer T. The Tat protein export pathway. Mol Microbiol. 2000;35:260–274. doi: 10.1046/j.1365-2958.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- 3.Bogsch E G, Sargent F, Stanley N R, Berks B C, Robinson C, Palmer T. An essential component of a novel bacterial export system with homologues in plastids and mitochondria. J Biol Chem. 1998;273:18003–18006. doi: 10.1074/jbc.273.29.18003. [DOI] [PubMed] [Google Scholar]
- 4.Casadaban M J, Cohen S N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci USA. 1979;76:4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanal A, Santini C-L, Wu L-F. Potential receptor function of three homologous components, TatA, TatB and TatE, of the twin-arginine signal sequence-dependent metalloenzyme translocation pathway in Escherichia coli. Mol Microbiol. 1998;30:673–676. doi: 10.1046/j.1365-2958.1998.01095.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen G N, Rickenberg W H. Concentration specifique reversible des amino acids chez Escherichia coli. Ann Inst Pasteur (Paris) 1956;91:693–720. [PubMed] [Google Scholar]
- 7.Gennis R B, Stewart V. Respiration. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 217–261. [Google Scholar]
- 8.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 10.Oliver D B. SecA protein: autoregulated ATPase catalysing preprotein insertion and translocation across the Escherichia coli inner membrane. Mol Microbiol. 1993;7:159–165. doi: 10.1111/j.1365-2958.1993.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 11.Oliver D B, Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982;30:311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- 12.Salavati R, Oliver D B. Competition between ribosome and SecA binding promotes Escherichia coli secA translational autoregulation. RNA. 1995;1:735–753. [PMC free article] [PubMed] [Google Scholar]
- 13.Santini C-L, Ize B, Chanal A, Müller M, Giordano G, Wu L-F. A novel Sec-independent periplasmic translocation pathway in Escherichia coli. EMBO J. 1998;17:101–112. doi: 10.1093/emboj/17.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargent F, Bogsch E G, Stanley N R, Wexler M, Robinson C, Berks B C, Palmer T. Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J. 1998;17:3640–3650. doi: 10.1093/emboj/17.13.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sargent F, Stanley N R, Berks B C, Palmer T. Sec-independent protein translocation in Escherichia coli: a distinct and pivotal role for the TatB protein. J Biol Chem. 1999;274:36073–36083. doi: 10.1074/jbc.274.51.36073. [DOI] [PubMed] [Google Scholar]
- 16.Sawers G, Böck A. Novel transcriptional control of the pyruvate formate-lyase gene: upstream regulatory sequences and multiple promoters regulate anaerobic expression. J Bacteriol. 1989;171:2485–2498. doi: 10.1128/jb.171.5.2485-2498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settles A M, Martienssen R. Old and new pathways of protein export in chloroplasts and bacteria. Trends Cell Biol. 1998;8:494–501. doi: 10.1016/s0962-8924(98)01387-7. [DOI] [PubMed] [Google Scholar]
- 18.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based protein and operon fusion cloning tools. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 19.Weiner J H, Bilous P T, Shaw G M, Lubitz S P, Frost L, Thomas G H, Cole J A, Turner R J. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell. 1998;93:93–101. doi: 10.1016/s0092-8674(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 20.Wexler M, Sargent F, Jack R L, Stanley N R, Bogsch E G, Robinson C, Berks B C, Palmer T. TatD is a cytoplasmic protein with DNase activity. No requirement for TatD-family proteins in Sec-independent protein export. J Biol Chem. 2000;275:16717–16722. doi: 10.1074/jbc.M000800200. [DOI] [PubMed] [Google Scholar]