SUMMARY
We investigated an outbreak of gastroenteritis following a Christmas buffet served on 4–9 December 2012 to ~1300 hotel guests. More than 300 people were reported ill in initial interviews with hotel guests. To identify possible sources of infection we conducted a cohort investigation through which we identified 214 probable cases. Illness was associated with consumption of scrambled eggs (odds ratio 9·07, 95% confidence interval 5·20–15·84). Imported chives added fresh to the scrambled eggs were the suspected source of the outbreak but were unavailable for testing. Enterotoxigenic Escherichia coli (ETEC) infection was eventually confirmed in 40 hotel guests. This outbreak reinforces that ETEC should be considered in non-endemic countries when the clinical picture is consistent and common gastrointestinal pathogens are not found. Following this outbreak, the Norwegian Food Safety Authority recommended that imported fresh herbs should be heat-treated before use in commercial kitchens.
Key words: Escherichia coli, foodborne infections, outbreaks
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is a frequent cause of traveller's diarrhoea [1] and an emerging cause of outbreaks in developed countries such as the USA [2], Denmark [3, 4], South Korea [5] and Japan [6]. This group of diarrhoeagenic E. coli is characterized by the production of one or both of two enterotoxins, a heat-labile toxin (LT) and a heat-stable toxin (ST) [7]. LT resembles cholera toxin and produces similar symptoms (watery diarrhoea, abdominal pain, fever, headache and, less frequently, vomiting) [2]. Symptoms are generally self-limiting but have been found to have a median duration of up to 7 days in outbreaks [8]. Although all pathogenic E. coli are mandatorily notifiable, ETEC has not previously been implicated in any outbreaks in Norway. Typically, between 10 and 35 cases of ETEC are reported annually, of which more than 90% are related to travel abroad [9]. However, ETEC cases are probably under-ascertained as ETEC is not included in the routine panel of tests for gastrointestinal pathogens. ETEC may therefore be under-recognized as a cause of foodborne illness in Norway.
On 10 December 2012, the local Food Safety Authority (FSA) and Municipal Health Officer received information about an outbreak of gastroenteritis in guests who had visited a hotel in southeastern Norway between 4 and 9 December. The hotel is a venue frequently used for meetings, seminars and professional days, as well as hosting individual guests, and the onsite restaurant caters to large groups. During the Christmas season, the hotel offers a buffet of traditional Christmas food, which includes almost the same food items for lunch and dinner every day. At the time of the alert, 40 guests had reported gastroenteritis after visiting the hotel. During the following 48 h, the number of guests with gastrointestinal symptoms increased to over 300, representing up to 20% of guests that had visited the hotel during that period. On 11 December the Norwegian Institute of Public Health (NIPH) was notified and an outbreak investigation team including the Municipal Health Officer, the local FSA, the local hospital and the NIPH was established in order to identify the aetiological agent, determine the extent of the outbreak and identify possible sources of infection.
METHODS
Epidemiological investigation
Initial investigation and case definition
The hotel estimated that over 1300 people had visited the hotel during the outbreak period and provided the outbreak team with a list of 37 groups that had pre-booked tables for meals at the hotel. Members of the outbreak team called the main contact for each of the groups that had visited the hotel between 4 and 9 December to obtain a description of the typical symptoms and generate hypotheses about the source of the outbreak. The initial reports from the group contacts indicated that symptom duration was relatively long and that the buffet was the most likely source of infection, as several groups had only visited the hotel to attend the buffet. A case was therefore defined as any person who had eaten at least one meal from the hotel's buffet between 4 and 9 December, and had experienced diarrhoea or vomiting after the meal with symptom duration of ⩾3 days.
Cohort investigation
We conducted a retrospective cohort investigation among guests who had visited the hotel between 4 and 9 December 2012. All guests from the 33 groups which we were able to contact were included in the investigation. A web-based questionnaire was developed in order to collect information on demographics, course of illness including time of onset, duration of symptoms, type of symptoms and whether medical attention was sought, and information on consumption of 127 food items included on the buffet menu. As the initial information from the group contacts indicated that many hotel guests who became ill had only eaten either lunch or dinner at the hotel, breakfast items were not included in the questionnaire. Therefore, respondents were included only if they had eaten lunch or dinner from the Christmas buffet provided by the hotel at least once during the outbreak period. Many of the hotel guests ate more than one meal from the buffet. As we assumed guests would not be able to recall which food items they had eaten at specific meals on specific days from the buffet, we asked guests to respond whether they had eaten the food items from the buffet at any time during their stay. Respondents were excluded from the analysis if they reported gastrointestinal symptoms that did not meet the case definition.
The main contact person for each group was asked to forward the web-based questionnaire to the other members of their respective groups. The questionnaire was made available through Questback, an online survey software, from 13 to 30 December 2012. The questionnaire was accompanied by a letter stating that participation was voluntary and the confidentiality of all participants would be maintained. Response to the questionnaire was considered consent for participation. Approval from an ethical review board was not necessary, as the Norwegian Act relating to control of communicable diseases [10] gives NIPH general clearance to conduct public health investigations of acute events.
Human samples
In Norway, stool samples from adults with diarrhoea that are sent to a microbiological laboratory for examination are routinely tested for Salmonella, Campylobacter, Yersinia and Shigella. If bloody diarrhoea is reported, specimens are also tested for Shiga-toxin producing E. coli (STEC). If associated with an outbreak, specimens are also tested for norovirus and to some extent Clostridium difficile toxin and Staphylococcus aureus enterotoxin. As the hotel guests had already returned to their home communities, those seeking medical attention visited their respective local doctors and any stool specimens collected were initially analysed at different primary laboratories throughout the country. On 14 December an alert regarding the outbreak was issued through the closed Norwegian laboratory network communication platform MikInfo, recommending that laboratories extend their routine analyses in patients that could be linked to the outbreak. Some of the faecal samples received were subsequently tested for Clostridium, Listeria and staphylococci. Furthermore, on 18 December, hotel guests were specifically asked by email to supply stool specimens for analysis if they still had symptoms. Specimens from some of the patients were received and examined at local laboratories and then forwarded to the National Reference Laboratory for Enteropathogenic Bacteria (NRL) at the NIPH for reference analyses, while for other patients the samples were received locally but forwarded directly to NIPH. At NIPH all samples were analysed by agglutination with O-antisera and an in-house multiplex PCR detecting virulence genes specific for main groups of diarrhoeagenic E. coli (EHEC, EPEC, ETEC, EAEC, EIEC) Target genes for ETEC were LTI, STIa, STIb, rrs [11, 12]. We used O78:H1 (strain FH-Ba-649) as a positive control and E. coli HS (commensal) (strain FH-Ba-878) as a negative control.
Food and water samples
Between 10 and 18 December, the local FSA collected water samples from several locations in the kitchen, and food samples, including, fish, vegetables, cured meats, and sauces and salads produced at the hotel. All food samples were cultivated and examined for presence of Clostridium perfringens, Bacillus cereus, E. coli O103 and O157 and Salmonella. Fish products and chives were also analysed for Aeromonas spp. and E. coli (general). Samples of fish were tested for Listeria monocytogenes and three meat products were tested for norovirus. Water was tested for C. perfringens, intestinal enterococci, E. coli (general), coliforms and total colony counts at 22°C.
Environmental investigation
The FSA visited the hotel to inspect the premises on 10 December 2012. The kitchen employees were interviewed regarding food preparation, storage, delivery and sanitizing procedures. They were also questioned about gastrointestinal symptoms experienced at any point in December.
Statistical analysis
Data from the questionnaire were extracted from Questback on 3 January 2013. Descriptive univariable and multivariable analyses were performed in Excel (Microsoft, USA) and Stata v. 12 (StataCorp., USA). We determined the number of people exposed to various food items, number of ill people in the exposed and unexposed groups, attack rate (AR) and relative risk (RR) with 95% confidence intervals (95% CI) for all food items. Food items were included in the multivariable analysis if at least 40% of cases had consumed the food item and the P value in the univariable analysis was <0·2. Multivariable analysis was performed using logistic regression with odds ratio (OR) as measure of association, as Poisson regression was not possible due to overdispersion and negative binomial regression did not converge. A stepwise backwards elimination procedure was used to remove the food items with the highest P value until only food items significant at P < 0·05 remained.
RESULTS
The 33 groups included in the investigation varied in size from two to 82 people, making up a total of about 650 people, all of whom resided in Norway. It was estimated that over 300 people were ill. Several guests reported that they had only consumed food items from a table of cold food, which included different types of smoked and cured fish and scrambled eggs. Initial information from the group contacts indicated that guests experienced abdominal pain and diarrhoea starting 24–48 h after attending the hotel. At least seven hotel guests were hospitalized due to their symptoms, including two patients with renal failure characterized by elevated C-reactive protein levels.
Case finding
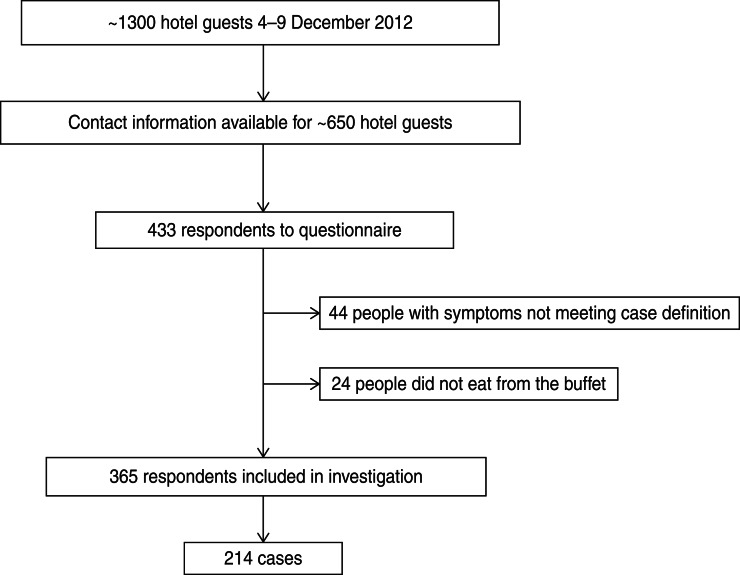
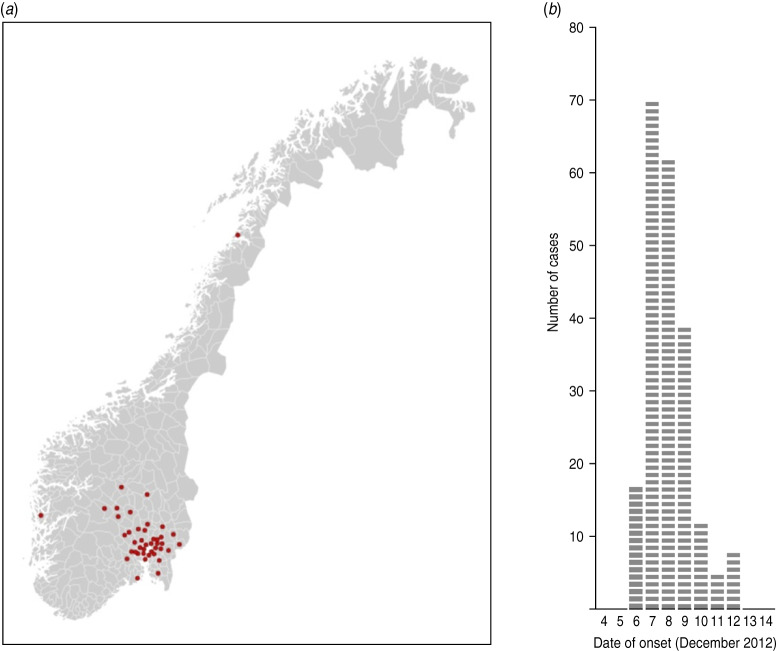
Of the ~650 people who received the web-based questionnaire, 433 responded, giving an estimated response rate of 67% (Fig. 1). Sixty-eight respondents were excluded from the analysis as they either had not eaten from the buffet or had gastrointestinal symptoms that did not meet the case definition. Among the respondents, we identified 214 people matching the case definition, resulting in an AR of 59%. The median age group was 50–55 years (range 5–9 years to 80–84 years) and 116 (54%) were men. Cases came from at least 42 municipalities, mainly in Southeastern Norway (Fig. 2a). Most cases became ill on 7 and 8 December, with onset dates ranging from 6 to 12 December (Fig. 2b). The symptoms reported were diarrhoea and abdominal pain, both reported by 90% of cases, nausea (50%), fever (31%) and vomiting (18%). More than 70% of cases reported symptom duration of >4 days or still being sick at the time they responded to the questionnaire.
Fig. 1.
Overview of hotel guests and cohort study participants following an outbreak at a hotel in Norway, December 2012.
Fig. 2.
(a) Geographical distribution of municipality of residence of cases following an outbreak at a hotel in Norway, December 2012. Each red dot indicates a municipality with at least one case. (b) Distribution of cases by date of symptom onset following an outbreak at a hotel in Norway, December 2012.
Cohort investigation
In the univariable analyses, the relative risk was highest in respondents who had eaten scrambled eggs (RR 2·9, 95% CI 2·0–4·2). Eight food items explained at least 40% of cases and had a P value of <0·2 in the univariable analysis (Table 1), and were therefore included in the multivariable regression analysis (Table 2). Of the four food items that remained significant, scrambled eggs had the highest adjusted OR (9·07, 95% CI 5·2–15·8).
Table 1.
Results of univariable analysis for food items with P value <0·2 and >40% cases exposed following an outbreak at a hotel, Norway, December 2012
| Food item | Exposed | Unexposed | RR | (95% CI) | P value | % cases exposed | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Cases | AR, % | Total | Cases | AR, % | |||||
| Scrambled eggs | 173 | 127 | 73·4 | 87 | 22 | 25·29 | 2·9 | (2·0–4·2) | <0·00 | 85·2 |
| Smoked trout | 114 | 84 | 73·7 | 234 | 115 | 49·15 | 1·5 | (1·3–1·8) | <0·00 | 42·2 |
| Cooked trout | 109 | 79 | 72·5 | 239 | 120 | 50·21 | 1·44 | (1·2–1·7) | <0·00 | 39·7 |
| Cured trout | 118 | 79 | 67 | 230 | 120 | 52·17 | 1·28 | (1·1–1·5) | 0·01 | 39·7 |
| Bread | 150 | 95 | 63·3 | 198 | 104 | 52·53 | 1·21 | (1·0–1·4) | 0·04 | 47·7 |
| Tap water | 245 | 147 | 60 | 96 | 47 | 48·96 | 1·23 | (0·98–1·5) | 0·06 | 75·8 |
| Cooked potatoes | 233 | 140 | 60·1 | 115 | 59 | 51·3 | 1·17 | (0·95–1·4) | 0·12 | 70·4 |
| Mashed rutabaga | 141 | 87 | 61·7 | 207 | 112 | 54·11 | 1·14 | (0·95–1·4) | 0·16 | 43·7 |
AR, Attack rate; RR, relative risk; CI, confidence interval.
Table 2.
Results of multivariable analysis following an outbreak at a hotel, Norway, December 2012
| Food item | aOR | (95% CI) | P value |
|---|---|---|---|
| Scrambled eggs | 9·07 | (5·2–15·8) | <0·001 |
| Smoked trout | 1·99 | (1·1–3·5) | 0·02 |
| Cooked potatoes | 1·73 | (1·0–2·9) | 0·04 |
| Mashed rutabaga | 1·84 | (1·1–3·1) | <0·001 |
aOR, Adjusted odds ratio; CI, confidence interval.
Microbiological investigation
As many guests of the hotel became ill after returning to their home communities, it is unknown how many cases were ultimately tested. Thirty respondents to the questionnaire indicated that they submitted faecal specimens. Many respondents had not yet received the results at the time they completed the questionnaire, but with the exception of one positive test for norovirus in a person with a chronic infection, only negative results were reported in the first 10 days of the investigation. On 19 December, the NRL identified an LT1-positive E. coli O78 (ETEC) in a mixed culture sent from a hospital treating a hospitalized outbreak patient. After the identification of ETEC O78 in this specimen, local laboratories that had collected specimens from patients connected to the outbreak forwarded stored specimens to the NRL for testing. In 40 of the 45 patients with a faecal sample available, ETEC LT1-positive E. coli O78 was detected. Other ETEC serotypes were not detected. Multilocus variable number of tandem repeat analysis [13] with a tenth tandem repeat addition (CCR02), indicated that the isolates were identical or closely related. Four isolates varied from the others in one target (CVN014). These isolates carried three different lengths of this tandem repeat [138 bp (n = 1), 193 bp (n = 1), and 245 bp (n = 2) vs. 270 bp (n = 36)] in the dominant version of this locus. As the hotel guests were not asked to provide their names or contact information when answering the questionnaire, it is not known whether those patients that tested positive for ETEC participated in the cohort investigation.
A total of 22 samples were collected from the hotel kitchen, of which 19 were food samples and three were water samples. Neither ETEC, nor any other pathogen, could be identified in any food or water samples. The hotel is connected to a public water supply, for which no potential contamination episodes were reported.
Environmental investigation
The kitchen was closed for 1 day to be washed and disinfected, and several food items produced by the hotel were temporarily removed from the buffet. Interviews with the staff at the hotel regarding illnesses supported that no employees were ill prior to the outbreak. The hotel used fresh, locally produced eggs, with some milk or cream added, to make scrambled eggs. Chopped fresh chives were added to the scrambled eggs before being placed on the buffet. The chives were washed with running water before being added to the eggs. Neither eggs nor chives from the batches used during the outbreak period were available for sampling. The traceback investigation indicated that the chives used were imported from a country outside the European region where ETEC is endemic.
DISCUSSION
This is the first reported outbreak caused by ETEC, and one of the largest foodborne outbreaks identified in Norway with over 300 suspected cases. For the first 10 days of the investigation, the pathogen was unknown. The relatively small number of cases reporting vomiting and the long duration of symptoms suggested a bacterial aetiology, but tests for routine gastrointestinal pathogens were negative. As most local laboratories in Norway do not routinely test for ETEC, the identification of the aetiology was delayed, although the incubation period of 24–48 h and the clinical picture, including duration of illness >60 h and a diarrhoea-to-vomiting ratio of >2·5 was similar to other reported ETEC outbreaks [8, 14, 15]. Several outbreaks of ETEC in developed countries, including the USA and Denmark were also only identified after routine tests for gastrointestinal pathogens were negative and supplementary analyses were performed [3, 4, 16].
This outbreak presented another unique challenge in terms of collecting microbiological results of specimens from primary laboratories throughout the country. After the hotel guests had returned to their home communities, it was difficult to determine who had been tested, for what pathogens and at which laboratories. This was particularly true in the case of negative results. Positive laboratory tests for common gastrointestinal diseases (salmonellosis, campylobacteriosis, shigellosis and yersiniosis, as well as infections caused by STEC, EPEC and ETEC) are notifiable to MSIS, the Norwegian Communicable Disease Surveillance System, and for most pathogens the strains should be forwarded to the NRL for confirmation and further typing. The Norwegian medical microbiology laboratory network MikInfo proved to be a valuable preparedness tool for increasing awareness regarding the outbreak and for collecting information about known cases. However, although many microbiologists were aware of the ongoing outbreak, they were not always informed that the patient could be part of the outbreak. Improved communication between clinicians and microbiologists appears warranted in order to more quickly identify the aetiology of outbreaks.
The holiday season can be a high-risk period for foodborne illness, due to the high volume of guests, the wide range of foods offered in buffets, and the resulting pressure on often new and short-term staff. In this case, there was no evidence of food safety or hygiene problems at the hotel. Previous ETEC outbreaks in developed countries have been attributed to a variety of food items, including water, seafood, vegetables and imported herbs, as well as through food items prepared by infected food handlers, but not to eggs [2, 4, 8, 16]. Chives were concluded to be the most likely source of infection, as they were imported from an ETEC-endemic country and not heated before consumption. As the scrambled eggs were cooked before the chives were added, the warm eggs provided a good environment for the growth of ETEC introduced with the raw chives. In response to this outbreak, as well as other recent Norwegian outbreaks associated with consumption of fresh herbs [17], the FSA has recommended that commercial kitchens should heat-treat fresh herbs imported from outside Europe prior to serving. This outbreak also reinforces that imported fresh produce, including herbs, sprouts, lettuce and other vegetables, should not be disregarded as possible vehicles for foodborne outbreaks in Norway, as well as other European countries [18–20].
This investigation had several limitations. The Municipal Health Authorities reported a high background level of norovirus in the community at the time of the outbreak. For this reason, we chose to use a restrictive case definition, specifying symptom duration of ⩾3 days, to discriminate true cases of ETEC infection. Out of 258 guests with symptoms, 44 (17%) were excluded on this basis, which may have led to an overestimation of the attack rate compared to what it would have been if they were included in the analysis as non-cases. However, if we had elected to use a more sensitive case definition, such as symptom duration of ⩽2 days instead of ⩽3 days, more of the excluded respondents would have met the case definition, which would have resulted in a higher attack rate.
We had to rely on the contact person for each of the groups to distribute the questionnaires to their respective group members. We were therefore unable to calculate a precise response rate and cannot be sure that the intended recipients had access to the electronic questionnaires. Email addresses were not available for all group members, further reducing the likelihood that all group members received the questionnaire. Despite this, we estimated we had a response rate of at least 67%, which we consider sufficient to make the cohort study results reliable.
Since the guests were asked about what they had eaten from the buffet at any point during their stay, rather than describe the food items they consumed at each mealtime separately, it was not possible to identify a single mealtime where contaminated scrambled eggs may have been served. However, chives were added to scrambled eggs served on the buffet at several meals, which explains the distribution of onset of symptoms shown in Figure 2b, assuming an incubation period for ETEC of between 24 h and 72 h.
CONCLUSIONS AND RECOMMENDATIONS
We have described the first known outbreak of ETEC in Norway. While there were no conclusive microbiological results, the epidemiological and traceback investigations suggested that scrambled eggs with chives are the most likely vehicle of infection, particularly as the chives were imported from an ETEC-endemic country. This supports the recommendation that fresh herbs imported from countries outside the European region should be heat-treated prior to use in commercial kitchens. The challenge for laboratories to discern an uncommon pathogen as the aetiology of an outbreak, especially when then affected group is dispersed at the time the outbreak is reported, may postpone or hinder the identification of the causative agent. This outbreak reinforces that ETEC should be considered a possible aetiological agent in outbreaks when routine gastrointestinal pathogens are not found, particularly when imported products are implicated.
ACKNOWLEDGEMENTS
We thank all hotel guests for participating in the investigation and responding to the questionnaire, and Astri Ham and Laila Jensvoll of the Norwegian Food Safety Authority for providing data related to the traceback investigation. We also thank Alicia Barrasa and Katrine Borgen of the European Programme for Intervention Epidemiology Training (EPIET) for reviewing the manuscript.
This research received no specific grant from any funding agency, commercial or not-for-profit.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Anon. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Weekly Epidemiological Record 2006; 81: 97–104. [PubMed] [Google Scholar]
- 2.Dalton CB, et al. Outbreaks of enterotoxigenic Escherichia coli infection in American adults: a clinical and epidemiologic profile. Epidemiology and Infection 1999; 123: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pakalniskiene J, et al. A foodborne outbreak of enterotoxigenic E. coli and Salmonella Anatum infection after a high-school dinner in Denmark, November 2006. Epidemiology and Infection 2009; 137: 396–401. [DOI] [PubMed] [Google Scholar]
- 4.Ethelberg S, et al. Outbreaks of gastroenteritis linked to lettuce, Denmark, January 2010. Eurosurveillance 2010; 15(6). [PubMed] [Google Scholar]
- 5.Cho SH, et al. Outbreak of enterotoxigenic Escherichia coli O169 enteritis in schoolchildren associated with consumption of kimchi, Republic of Korea, 2012. Epidemiology and Infection 2014: 142: 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konishi N, et al. Bacteriological and epidemiological characteristics of enterotoxigenic Escherichia coli isolated in Tokyo, Japan, between 1966 and 2009. Journal of Clinical Microbiology 2011; 49: 3348–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qadri F, et al. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clinical Microbiology Reviews 2005; 18: 465–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoder JS, et al. Outbreak of enterotoxigenic Escherichia coli infection with an unusually long duration of illness. Clinical Infectious Diseases 2006; 42: 1513–1517. [DOI] [PubMed] [Google Scholar]
- 9.Norwegian Surveillance System for Communicable Diseases (MSIS). (www.msis.no). Accessed 3 December 2013.
- 10.Infectious Disease Control Act (Smittevernloven). (www.lovdata.no/lov/1994-08-05-55). Accessed 3 December 2013.
- 11.Bolin I, et al. Enterotoxigenic Escherichia coli with STh and STp genotypes is associated with diarrhea both in children in areas of endemicity and in travelers. Journal of Clinical Microbiology 2006; 44: 3872–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G, Clark CG, Rodgers FG. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. Journal of Clinical Microbiology 2002; 40: 3613–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naseer U, et al. Multi-locus variable number of tandem repeat analysis for rapid and accurate typing of virulent multidrug resistant Escherichia coli clones. PLoS One 2012; 7: e41232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beatty ME, et al. Enterotoxin-producing Escherichia coli O169:H41, United States. Emerging Infectious Diseases 2004; 10: 518–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beatty ME, et al. Epidemic diarrhea due to enterotoxigenic Escherichia coli. Clinical Infectious Diseases 2006; 42: 329–334. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, et al. An outbreak of enterotoxigenic Escherichia coli associated with sushi restaurants in Nevada, 2004. Clinical infectious diseases 2008; 47: 1–7. [DOI] [PubMed] [Google Scholar]
- 17.Guzman-Herrador B, et al. Outbreak of Shigella sonnei infection in Norway linked to consumption of fresh basil, October 2011. Eurosurveillance 2011; 16(44). [PubMed] [Google Scholar]
- 18.MacDonald E, et al. Yersinia enterocolitica outbreak associated with ready-to-eat salad mix, Norway, 2011. Emerging Infectious Diseases 2012; 18: 1496–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heier BT, et al. Shigella sonnei infections in Norway associated with sugar peas, May-June 2009. Eurosurveillance 2009; 14(24). [DOI] [PubMed] [Google Scholar]
- 20.Emberland KE, et al. Outbreak of Salmonella Weltevreden infections in Norway, Denmark and Finland associated with alfalfa sprouts, July-October 2007. Eurosurveillance 2007; 12(11): E071129071124. [DOI] [PubMed] [Google Scholar]