Abstract
The analysis of Serratia liquefaciens MG1 ′luxAB insertion mutants that are responsive to N-butanoyl-l-homoserine lactone revealed that expression of lipB is controlled by the swr quorum-sensing system. LipB is part of the Lip exporter, a type I secretion system, which is responsible for the secretion of extracellular lipase, metalloprotease, and S-layer protein.
Serratia liquefaciens MG1 employs a quorum-sensing system, the swr system, to control swarming motility. Swarming of S. liquefaciens MG1 is characterized by differentiation of short motile rods at the periphery of a colony into elongated, polyploid, and hyperflagellated swarm cells that migrate coordinately in rafts atop the agar surface (7, 9). The swr system relies on two proteins, SwrI, which directs the synthesis of the diffusable signal molecules N-butanoyl-l-homoserine lactone (C4-HSL) and N-hexanoyl-l-homoserine lactone in a molar ratio of 10 to 1, and SwrR, which, after binding of the signal molecules, is thought to activate or repress transcription of target genes (8, 9, 12). A global analysis by two-dimensional polyacrylamide gel electrophoresis (PAGE) revealed that at least 28 genes are under control of the swr regulatory system (12).
By transposon mutagenesis, we have recently isolated 19 ′luxAB insertion mutants of S. liquefaciens MG44 (swrI) that are responsive to the presence of C4-HSL (16). Only one of these mutants, S. liquefaciens PL10, was unable to form a swarming colony when C4-HSL was provided in the medium. This mutant was demonstrated to bear the insertion in a gene, swrA, which encodes an enzyme that catalyzes the synthesis of the biosurfactant serrawettin W2. This compound, a cyclic lipodepsipentapeptide carrying a 3-hydroxy C10 fatty acid side chain, lowers the surface tension of the surrounding medium, a process that is indispensable for the development of an S. liquefaciens MG1 swarming colony. The swrA gene appears to be the only gene of the swr regulon that is required for swarming motility, since addition of purified serrawettin W2 to swarm plates fully restores swarming of the swrI mutant MG44 in the absence of C4-HSL. Thus, most of the quorum-sensing-regulated genes in S. liquefaciens MG1 are involved in functions not related to swarming motility.
In this report we show that one of the ′luxAB insertion mutants which is responsive to the addition of C4-HSL bears the transposon insertion within the lipB gene, which encodes a component of the Lip exporter. This type I protein secretion system is responsible for the transport of the S. liquefaciens lipase, metalloprotease, and S-layer protein. As a consequence of the involvement of the swr system in the regulation of lipB expression, the levels of extracellular metalloprotease and S-layer protein are significantly lowered in a swrI mutant. However, the amount of secreted lipase is unchanged.
Expression of the lipB gene is quorum sensing regulated.
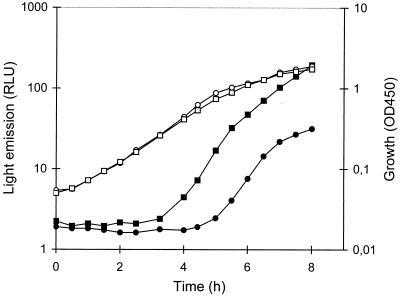
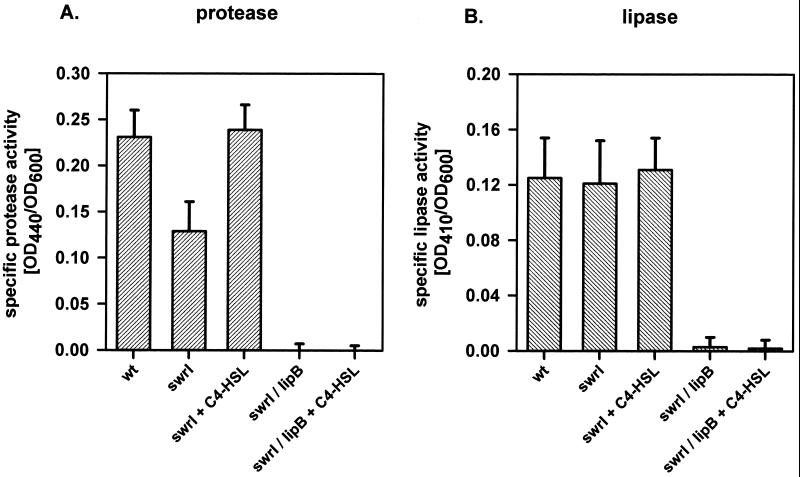
We previously noticed that the S. liquefaciens swrI mutant MG44 exhibits lower proteolytic activity than the wild-type MG1 (8). This suggests that either the expression or the secretion of the S. liquefaciens MG1 protease(s) is under control of the swr system. To address this issue, we tested the 19 previously isolated mutants that bear ′luxAB insertions in quorum-sensing-regulated genes for proteolytic activity on 2% (wt/vol) skim milk plates (14). One mutant, MG3645, was found to be completely protease negative on our test plates, and this mutant was further investigated. When grown in liquid AB medium (6) supplemented with 0.2% glucose and 0.2% Casamino Acids, this mutant showed an approximately fivefold stimulation of bioluminescence in response to addition of 200 nM C4-HSL (Fig. 1). The proteolytic activity of the supernatants of cultures of MG1, MG44, and MG3645 grown in the presence or absence of 200 nM C4-HSL was measured as described previously (4). The extracellular proteolytic activity of the swrI mutant MG44 was about twofold reduced compared with that of the wild type (Fig. 2A). Addition of C4-HSL to the culture medium completely restored protease activity. Supernatants of MG3645 cultures showed virtually no protease activity irrespective of the presence or absence of C4-HSL.
FIG. 1.
Induction of bioluminescence in the ′luxAB insertion mutant S. liquefaciens MG3645 in the presence of C4-HSL. Bacterial cultures were grown in AB medium containing 0.2% glucose, 0.2% Casamino Acids, and either no (circles) or 200 nM (squares) C4-HSL. Optical densities at 450 nm (OD450) (open symbols) and bioluminescence (measured in relative light units [RLU]) (closed symbols) were determined along the growth curve. The data presented are representative of those from at least three separate experiments.
FIG. 2.
Enzymatic activities of culture supernatants of S. liquefaciens MG1 and different mutant strains. Cultures were grown in LB medium in the presence or absence of 200 nM C4-HSL for 16 h. Sterile filtered supernatants were used for enzymatic measurements of proteolytic (A) and lipolytic (B) activities. Each bar represents the average from at least three independent experiments. Error bars represent the standard errors of the means. OD440, optical density at 440 nm.
To characterize the nature of the transposon insertion in strain MG3645, the mutated locus was cloned. This was accomplished by digesting chromosomal DNA of MG3645 with PmlI, a restriction enzyme that cuts downstream of the ′luxAB genes of the mini-Tn5 luxAB res-npt-xylE-res element, and ligating the DNA fragments into the cloning vector pUC18Not cut with SmaI. Following transformation of Escherichia coli MT102, a bioluminescent clone was isolated. The recombinant plasmid contains part of the transposon and approximately 10 kb of chromosomal DNA upstream of the transposon insertion point. Nucleotide sequence analysis of the flanking DNA revealed that the transposon had inserted into the lipB gene of S. liquefaciens MG1 (the N-terminal 67 amino acid residues show 100% amino acid identity with the corresponding region of LipB from S. marcescens). LipB belongs to the well-characterized ATP-binding cassette protein superfamily of transporters, which are involved in the import and export of a wide variety of substrates, including antibiotics, sugars, amino acids, peptides and proteins (13). Together with two other proteins, LipC, a membrane fusion protein, and LipD, an outer membrane protein, it constitutes the Lip exporter of Serratia marcescens (1). This type I secretion apparatus has been demonstrated to utilize C-terminal signal sequences to mediate the secretion of metalloprotease, lipase, and S-layer protein in S. marcescens (1, 2, 15, 22).
Sequence analysis of the lipB upstream region revealed the presence of the slaA gene, which codes for the S-layer protein (GenBank accession no. AY007218). Interestingly, the same gene organization has been described not only for S. marcescens (15) but also for Caulobacter crescentus (3) and Campylobacter fetus (20).
Secretion of extracellular metalloprotease is quorum sensing regulated.
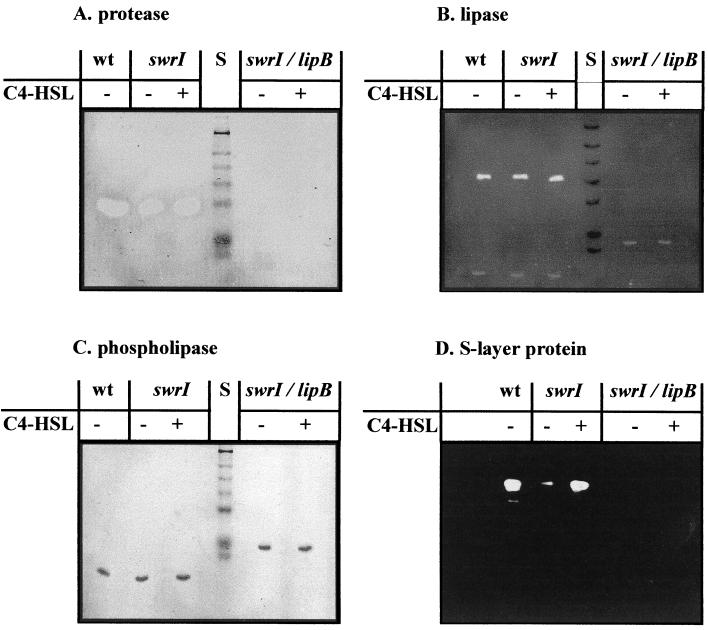
To further investigate the role of quorum sensing in the regulation of extracellular proteolytic activity, we analyzed supernatants with the aid of zymograms. For that, sodium dodecyl sulfate (SDS)-PAGE of the culture supernatants with 0.2% (wt/vol) azocasein incorporated in the gel matrix (10% polyacrylamide) was performed. The enzymatic activity was visualized by washing the gel two times with 50 mM Tris-HCl (pH 7.5) containing 25% (vol/vol) isopropanol for 15 min, followed by incubation in 50 mM Tris-HCl (pH 7.5) for 1 h at 45°C and destaining with 1 M NaOH. Proteolytic enzyme bands were detected as colorless zones in an orange background. In these zymograms one major band with proteolytic activity was detected with the wild type, and this band was completely missing in the lipB mutant (Fig. 3A). However, these zymograms do not allow quantitative measurements. In fact, the twofold reduction of protease activity of the swrI mutant was not clearly visible in the zymograms.
FIG. 3.
Analysis of extracellular proteins produced by S. liquefaciens MG1 and different mutant strains. Cultures were grown in LB medium in the presence or absence of 200 nM C4-HSL for 16 h as indicated. The protein molecular mass standard (lanes S) consists of proteins of 196, 118, 90, 70, 55, 38, and 33 kDa. Enzymatic activities of sterile filtered supernatants were visualized with the aid of zymograms as described in the text. The SlaA protein was visualized by immunoblotting with antisera against SlaA. wt, wild type.
To ascertain the identity of the protease, we purified the enzyme from S. liquefaciens MG1 culture supernatant (grown to the stationary phase in Luria-Bertani [LB] medium) by ultrafiltration (Minisette tangential-flow system, 10 K omega membrane; Pall-Filtron), followed by hydrophobic interaction chromatography (Phenyl-Resource column; Pharmacia), and gel filtration (Superdex 200 prep grade; Pharmacia) via Fast protein liquid chromatography. SDS-PAGE analysis of the final pool indicated the presence of a single protein band with a molecular mass of approximately 52 kDa (data not shown). The N-terminal amino acid sequence of the protease was determined with an Applied Biosystems Procise sequencer. The following N-terminal amino acid sequence was obtained: AAT TGYDAVDDLLHYHERGNGIQINGKDSFSNEQAGLFIT. This sequence is identical to the N-terminal sequence of the PrtA metalloprotease of S. marcescens (17).
These data support previous results which suggested that in S. liquefaciens MG1 the production of extracellular metalloprotease is controlled by the swr regulatory system (8) and are consistent with the finding that the PrtA metalloprotease is secreted by the Lip exporter in S. marcescens (15, 17).
Role of the swr system in the secretion of extracellular lipase and S-layer protein.
Since the Lip exporter of S. marcescens is responsible not only for the secretion of metalloprotease but also for the secretion of lipase and S-layer protein (15), we next investigated whether the production of the latter proteins is also controlled by the swr system in S. liquefaciens MG1.
Lipase activity was determined by incubating 100 μl of culture supernatant with 1 ml of a substrate mixture containing 1 volume of 0.3% (wt/vol) p-nitrophenylpalmitate in isopropanol and 9 volumes of 0.2% (wt/vol) sodium deoxycholate plus 0.1% (wt/vol) gummi arabicum in 50 mM sodium phosphate buffer (pH 8.0) for 30 min at 37°C (19). Enzymatic activity was measured photometrically at 410 nm. As shown in Fig. 2B, lipase activities of culture supernatants of the swrI mutant and the wild type were indistinguishable, and the presence or absence of C4-HSL had no effect on the result. As expected, no lipase activity was detected in the culture supernatants of the lipB mutant. We also overlaid renaturated SDS-polyacrylamide gels with the fluorescent substrate methylumbelliferylbutyrate (0.01 M in N,N-dimethylformamide [DMF]) in order to visualize lipase activity (Fig. 3B). These zymograms confirmed the results of the enzymatic measurements and clearly showed that the S. liquefaciens MG1 lipase is secreted by the Lip exporter. However, inactivation of the swr regulatory system had no effect on the amounts of extracellular lipase.
To determine the effect of the quorum-sensing system on the production of S-layer protein, Western blotting using antisera against SlaA (15) were performed (Fig. 3D). Cell surface proteins were prepared as described by Kawai et al. (15) and were separated by SDS-PAGE. The proteins were then transferred to an Immobilon P membrane (Qiagen, Hilden, Germany) by electroblotting, and the membrane was overlaid with 1:1,000-diluted SlaA antibodies. S-layer protein bands were detected via horseradisch peroxidase-conjugated anti-rabbit immunoglobulin G and the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Uppsala, Sweden). The swrI mutant was found to produce about 10-fold less S-layer protein than the wild type, a defect that could be fully restored by the addition of 200 nM C4-HSL to the culture medium. The mutant MG3645 did not produce any detectable SlaA protein independently of whether C4-HSL was present or not. Thus, production of S-layer protein but not of extracellular lipase is regulated by the swr system in S. liquefaciens MG1.
Processing of the phospholipase by the metalloprotease.
The zymograms used for the detection of the lipase showed an additional band with a molecular mass of about 30 kDa. Interestingly, this band was found to be shifted to a higher molecular mass (38 kDa) in the lipB mutant. Since S. liquefaciens MG1 produces an extracellular phospholipase (PhlA) with a molecular mass of about 38 kDa which is known to show some activity with Tween 20 as a substrate (11), we speculated that this band could represent this enzyme. In fact, when polyacrylamide gels were overlaid with egg yolk agar, a substrate that is specific for the phospholipase, the same pattern of bands was detected (Fig. 3C). In S. liquefaciens MG1 expression and secretion of the phospholipase are coupled to synthesis and export of flagella via the flhDC master regulator (10). In agreement with phlA being part of the flagellar regulon, we observed that a mutation in neither swrI nor lipB affected the amount of extracellular phospholipase. However, the phospholipase was apparently processed in the wild type and the swrI mutant but not in the lipB mutant. Since the lipB mutant does not produce metalloprotease, we tested whether PhlA is proteolytically processed by this enzyme. When supernatants of the lipB mutant were incubated at 30°C for 1 h with purified metalloprotease, the phospholipase was indeed converted to its processed form (data not shown). The swrI mutant apparently produces sufficient amounts of metalloprotease to process all extracellular phospholipase (Fig. 3C). Given that both forms of the enzyme exhibit phospholipolytic activity in our assay, the biological relevance of the PhlA processing remains unclear.
Expression of slaA is independent of the swr regulatory system.
While our results show that expression of the Lip exporter is under control of the swr system, these data do not exclude the possibility that expression of metalloprotease or S-layer protein itself is swr regulated. Moreover, since slaA is located upstream of the lip operon and is transcribed in the same direction, it is possible that the lip operon is, at least in part, cotranscribed with slaA. To address this issue, we constructed transcriptional fusions of the slaA and lipB promoter regions to the promoterless luxAB genes. The two promoter regions were PCR amplified using the primer pair LipB1 (5′-CGCGAAGGATCCACCCGGTCG-3′) and LipB2 (5′-GTGTGGGATCCGTTGTATTCAGC-3′) (BamHI restriction sites are underlined) for PlipB and the primer pair SlaA1 (5′-GCTGGATCCGATAAGCTTTACGTC-3′) and SlaA2 (5′-AAAGGATCCCGGCAACTTCCTTCTGG-3′) for PslaA. Following restriction with BamHI, the two DNA fragments (685 bp for PlipB and 733 bp for PslaA) were inserted into the promoter probe vector pGA-L9 cut with the same enzyme. Plasmids which contain the inserts in the orientation placing the promoters upstream of the promoterless luxAB genes of the vector were chosen, and these constructs were designated pTO1 (PlipB::luxAB) and pTO21 (PslaA::luxAB). The plasmids were then transferred to the S. liquefaciens wild-type strain MG1 and the swrI mutant MG44. Measurements of bioluminescence revealed that transcription of slaA is entirely independent of the swr system (Table 1). In contrast, transcription of lipB was found to be about threefold reduced in the swrI mutant compared with the wild type, and addition of 200 nM C4-HSL to the growth medium completely restored bioluminescence levels. These data clearly show that expression of lipB but not of slaA is subject to control by the swr system.
TABLE 1.
Induction of PslaA and PlipB transcriptional luxAB gene fusions in S. liquefaciens MG1 and MG44a
| Plasmid | Specific bioluminescence (RLU/OD600 unit)b
|
||
|---|---|---|---|
| MG1 (wild type) | MG44(swrI)
|
||
| Without C4-HSL | With C4-HSL | ||
| pTO1 (PlipB::luxAB) | 11,860 ± 250 | 4,700 ± 102 | 13,650 ± 260 |
| pTO21 (PslaA::luxAB) | 4,992 ± 127 | 4,814 ± 158 | 4,962 ± 112 |
Bacterial cultures were grown exponentially in LB medium. Cell densities and bioluminescence were monitored along the growth curve. C4-HSL was added at a final concentration of 200 nM.
Results are means and standard deviations for multiple samples taken during exponential growth from at least two independent experiments. Following addition of 1 μl of n-decanal (Sigma) to 200 μl of sample, bioluminescence was quantified in a Lumat LB 9506 luminometer (EG & Berthold, Bad Wildbad, Germany). RLU, relative light units; OD600, optical density at 600 nm.
Conclusions.
The genetic and physiological characterization of a ′luxAB insertion mutant of S. liquefaciens MG44, which responds to the presence of C4-HSL with increased bioluminescence, revealed that the strain bears the transposon in the lipB gene. This gene encodes an ATP-binding cassette transporter protein that is part of the Lip exporter, a type I secretion system, which is required for the secretion of lipase, metalloprotease, and S-layer protein in both S. marcescens (15) and S. liquefaciens MG1 (this study). Our results are reminiscent of the situation found with Pseudomonas aeruginosa, in which expression of the xcp secretion system was shown to be regulated by the two quorum-sensing systems (the las and rhl systems) that operate in this opportunistic pathogen (5). Various virulence factors, including exotoxin A, lipase, the LasA and LasB proteases, and phospholipase C, are secreted with the aid of amino-terminal signal sequences of precursor proteins across the inner membrane and then from the periplasmic space via the Xcp translocation machinery across the outer membrane (21). This two-step secretion mechanism is known as the general or type II secretory pathway (18).
The results presented here demonstrate that the swr quorum-sensing system of S. liquefaciens MG1 is involved in the regulation of lipBCD expression, whereas it does not affect expression of the S-layer protein SlaA, which is transported by the Lip exporter. We therefore suggest that in the swrI mutant MG44, production of extracellular SlaA and metalloprotease is limited at the secretion step rather than at the level of expression. However, it is puzzling that the amount of extracellular lipase, which is also transported by the Lip exporter (as indicated by the fact that the lipB mutant MG3645 is completely lipase negative), is unchanged in the swrI mutant. At present we hypothesize that the Lip exporter has a higher affinity for the lipase than for the metalloprotease and the S-layer protein and thus that limiting exporter capacity will affect secretion of the latter two proteins but not of the lipase. This model is strongly supported by the findings of Akatsuka et al. (2), who demonstrated that overexpression of the LipA lipase in S. marcescens greatly reduces production of extracellular metalloprotease, indicating that secretion of the two proteins is competitive.
Acknowledgments
We thank L. Stabell and B. Schumacher for excellent technical assistance.
This work was supported by grants from the BMBF (no. 0311948) and the DFG (EB 2051/1-2).
REFERENCES
- 1.Akatsuka H, Kawai E, Omori K, Shibatani T. The three genes lipB, lipC, and lipD involved in the extracellular secretion of the Serratia marcescens lipase which lacks an N-terminal signal peptide. J Bacteriol. 1995;177:6381–6389. doi: 10.1128/jb.177.22.6381-6389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akatsuka H, Kawai E, Omori K, Komatsubara S, Shibatani T. Overproduction of the extracellular lipase is closely related to that of metalloprotease in Serratia marcescens. J Ferment Bioeng. 1996;81:115–120. [Google Scholar]
- 3.Awram P, Smit J. The Caulobacter cresentus paracrystalline S-layer protein is secreted by an ABC transporter (type I) secretion apparatus. J Bacteriol. 1998;180:3062–3069. doi: 10.1128/jb.180.12.3062-3069.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayora S, Götz F. Genetic and biochemical properties of an extracellular neutral metalloprotease from Staphylococcus hyicus subsp. hyicus. Mol Gen Genet. 1994;242:421–430. doi: 10.1007/BF00281792. [DOI] [PubMed] [Google Scholar]
- 5.Chapon-Hervé V, Akrim M, Latifi A, Williams P, Lazdunski A, Bally M. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol. 1997;24:1169–1178. doi: 10.1046/j.1365-2958.1997.4271794.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark J D, Maaløe O. DNA replication and the cell division cycle in Escherichia coli. J Mol Biol. 1967;23:99–112. [Google Scholar]
- 7.Eberl L, Christiansen G, Molin S, Givskov M. Differentiation of Serratia liquefaciens into swarm cells is controlled by the expression of the flhD master operon. J Bacteriol. 1996;178:554–559. doi: 10.1128/jb.178.2.554-559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberl L, Winson M K, Sternberg C, Christiansen G, Chhabra S R, Bycroft B W, Stewart G S A B, Williams P, Molin S, Givskov M. Involvement of N-acyl-l-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 9.Eberl L, Molin S, Givskov M. Surface motility of Serratia liquefaciens MG1. J Bacteriol. 1999;181:1703–1712. doi: 10.1128/jb.181.6.1703-1712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Givskov M, Eberl L, Christiansen G, Benedik M J, Molin S. Induction of phospholipase- and flagellar synthesis is controlled by expression of the flagellar master operon flhD. Mol Microbiol. 1995;15:445–454. doi: 10.1111/j.1365-2958.1995.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 11.Givskov M, Olsen L, Molin S. Cloning and expression in Escherichia coli of the gene for an extracellular phospholipase from Serratia liquefaciens. J Bacteriol. 1988;170:5855–5862. doi: 10.1128/jb.170.12.5855-5862.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Givskov M, Östling J, Eberl L, Lindum P W, Christensen A B, Christiansen G, Molin S, Kjelleberg S. Two separate regulatory systems participate in the control of swarming motility in Serratia liquefaciens. J Bacteriol. 1998;180:742–745. doi: 10.1128/jb.180.3.742-745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins C F. ABC-transporters—from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 14.Hines D A, Saurugger P N, Ihler G M, Benedik M J. Genetic analysis of extracellular proteins of Serratia marcescens. J Bacteriol. 1988;170:4141–4146. doi: 10.1128/jb.170.9.4141-4146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai E, Akatsuka H, Idei A, Shibatani T, Omori K. Serratia marcescens S-layer protein is secreted extracellularly via an ATP-binding cassette exporter, the Lip system. Mol Microbiol. 1998;27:941–952. doi: 10.1046/j.1365-2958.1998.00739.x. [DOI] [PubMed] [Google Scholar]
- 16.Lindum P W, Anthoni U, Christophersen C, Eberl L, Molin S, Givskov M. N-Acyl-l-homoserine lactone autoinducers control production of an extracellular lipopeptide biosurfactant required for swarming motility of Serratia liquefaciens MG1. J Bacteriol. 1998;180:6384–6388. doi: 10.1128/jb.180.23.6384-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakahama K, Yoshimura K, Marumoto R, Kikuchi M, Lee I S, Hase T, Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acids Res. 1986;14:5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pugsley A P. The complete secretion pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuer W, Jaeger K E, Winkler U K. Purification of extracellular lipase from Pseudomonas aeruginosa. J Bacteriol. 1986;168:1070–1074. doi: 10.1128/jb.168.3.1070-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson S A, Shedd O L, Ray K C, Beins M H, Jorgensen J P, Blaser M J. Campylobacter fetus surface layer proteins are transported by a type I secretion system. J Bacteriol. 1998;180:6450–6458. doi: 10.1128/jb.180.24.6450-6458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tommassen J, Filloux A, Bally M, Murgier M, Lazdunski A. Protein secretion in Pseudomonas aeruginosa. FEMS Microbiol Rev. 1992;103:1320–1327. doi: 10.1016/0378-1097(92)90336-m. [DOI] [PubMed] [Google Scholar]
- 22.Wandersman C. Secretion across the outer membrane. Trends Genet. 1992;8:317–321. doi: 10.1016/0168-9525(92)90264-5. [DOI] [PubMed] [Google Scholar]