SUMMARY
Q fever, first described in 1937, is a worldwide zoonosis caused by Coxiella burnetii that has long been considered an under-reported and under-diagnosed illness. In China, the disease was initially reported in 1950 and in the last 25 years (1989–2013) there have been 29 reports on Q fever in China, nearly half of which were in the last 5 years. These publications have largely been in Chinese and in this review we summarize their findings to enable a better understanding of Q fever in China. The overall prevalence of C. burnetii infections in the reports is 10% (1139/11 209) in people, 15% (288/1918) in cattle and 12% (176/1440) in goats. These infections occurred widely in China with positive people and/or animals reported in 64 cities/municipalities from 19 provinces, particularly those in the eastern, western and northern areas. Cattle and goats had the highest seroprevalences of all the domestic animals studied and a wide variety of ticks were found to be infected. Mice were also commonly infected and had high copy numbers of C. burnetii DNA, suggesting they might be important in the epidemiology of Q fever in China.
Key words: China, C. burnetii, distribution, molecular biology, Q fever
INTRODUCTION
Q fever is an important zoonosis with a worldwide distribution that is caused by Coxiella burnetii, a strict intracellular Gram-negative bacterium [1]. The organism infects people and a wide range of wild and domesticated animals including sheep, cattle, goats, dogs, cats, pigeons and rabbits. Although most C. burnetii infections in animals are asymptomatic, they can sometimes result in late-stage abortion, stillbirths, or delivery of weak offspring. Infected animals shed C. burnetii into the environment in milk, colostrum, birth products and urine [2] and thus play an important role in maintaining the organism in the environment [3]. C. burnetii is extremely resistant to desiccation, low and high pH, and ultraviolet radiation so it can remain infective in soil for many months.
Humans are very susceptible to C. burnetii and infections can result from exposure to only a few organisms [4]. Most infections are asymptomatic (60%) but some cause acute signs including influenza-like illness and atypical pneumonia. In chronic cases there can be endocarditis, chronic hepatitis and osteomyelitis with mortalities of up to 11% [1, 5]. Infections are often an occupational hazard in people working with livestock and exposed to highly infectious aerosols from birth products, infectious dust particles or contaminated wool [6]. These include workers in slaughterhouses, meat-packing plants and tanneries as well as veterinarians and farmers.
Q fever often occurs in outbreaks in people, for example in Spain [7], Switzerland [8], Great Britain [9] and Germany [10]. A recent outbreak in The Netherlands affected over 3700 people and lead to the loss of over 50 000 dairy goats [11]. This has led to a renewed interest in Q fever worldwide which seems justified as the prevalence in domestic ruminants around the world has increased from 7·4% to 10% between 2007 and 2008, with the greatest increase in goats, from 9·7% to 15·7% [12].
Although Q fever occurs worldwide and has been classified as a notifiable animal disease by the World Organization for Animal Health (OIE) [13], details on the disease are scant in most countries. This might be due to under-reporting as in many countries the disease is not considered economically significant. In other cases it is because local reports are not in English and may therefore not be incorporated into international publications. As this is the case in China, we reviewed the local Chinese publications on Q fever and also international publications relating to the disease in China. In this report we provide a summary of the more recent data collected on Q fever in China.
OVERVIEW OF Q FEVER IN CHINA
Q fever was first reported in China in the 1950s when patients with atypical pneumonia were diagnosed serologically with the complement fixation test (CFT) [14, 15]. In 1962, the first case of Q fever was definitively confirmed and the first Chinese strain of C. burnetii (Qi Yi) was isolated [16]. Moreover, in the 1960s, five outbreaks of Q fever in abattoir workers, stockyard men and troops from Inner Mongolia, Sichuan, Yunnan and Tibet were investigated using serology and bacterial isolation [17]. Thereafter, there have been many studies and those on animals have indicated C. burnetii is widely distributed in China with several natural foci (Fig. 1). Between 1989 and 2013, there were 29 studies on people and animals (25 seroepidemiological studies, three seroepidemiological/molecular studies and one molecular study) which described C. burnetii infections in 64 cities/municipalities from 19 provinces across China (Tables 1 and 2). The principal animal species studied were cattle, goats, dogs, pigs and mice while horses, deer, marmots, sheep and rabbits were only surveyed once during that period. Nearly half of the studies were performed in the first 20 years while the remainder were conducted in the last 5 years, possibly because of the availability of more simple, sensitive and rapid diagnostic techniques. Initially the CFT was used for serology [18, 19] but this was replaced by the immunofluorescence assay (IFA) test and most recently with the sensitive enzyme-linked immunosorbent assay (ELISA) which requires a single dilution of sera and can be automated [20, 21]. As substrate the ELISA uses immunodominant antigens of C. burnetii produced using proteomic techniques developed by Chinese scientists [22, 23]. These replaced whole-cell antigens previously used in testing which required sophisticated tissue culture facilities for C. burnetii.
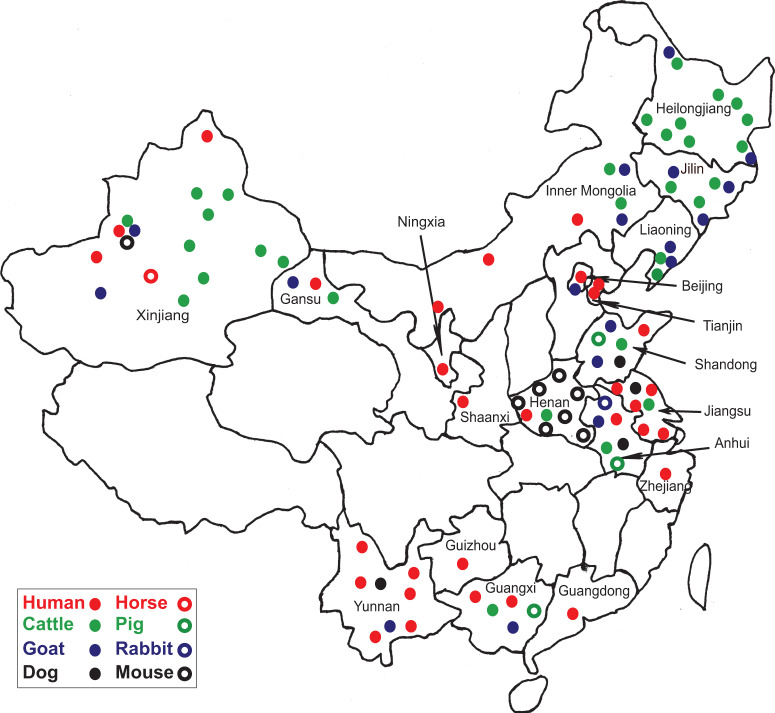
Fig. 1.
Sites in China where samples from people or animals were tested and the percentages found positive for anti-C. burnetii antibody. Dots represent sites where samples obtained from people and various animal species were found to be antibody positive against C. burnetii. Seropositive people were found in 14 provinces, mostly in eastern areas. Prevalences were higher in Inner Mongolia and Beijing than in Anhui and Xinjiang. Seropositive animals were found in 16 provinces, mostly those in the east, northeast and northwest of China. Seropositive cattle were more commonly found in the northeastern provinces, Inner Mongolia, Xinjiang, and Henan. Seropositive goats were mostly found in central Anhui province as well as in northeastern provinces and Inner Mongolia.
Table 1.
Prevalences of people with evidence of Q fever in different provinces in China, 1989–2013
| Province | Positivity: percentage, positive/total number examined (city/county when available) | Test used [reference] |
|---|---|---|
| Anhui | 9·9%, 15/151 (Fuyang) | CFT [37] |
| 54%, 108/200 (Guangde); 56%, 112/200 (Mingguang); 51·2%, 109/213 (Huaiyuan) | IFA [26] | |
| Beijing | 39%, 307 /787 | IFA [20] |
| Gansu | 7·4%, 33/446 (Tianshui) | IFA [29] |
| Guangdong | 3%, 15/493; 3·9%, 24/500 | CFT [47] |
| Guangxi | 3·1%, 10/327; 4·3%, 4/94 (Nanning) | CFT [18] |
| Guizhou | 5%, 3/461 | CFT [30] |
| Henan | 2·5%, 6/244 (Lushixian) | IFA [43] |
| Jiangsu | 0%, 0/643 (Binhai); 0·3%, 1/368 (Lishui); 0·2%, 1/510 (Peixian); 1·2%, 6/501 (Xuyi); 0%, 0/538 (Yixing) | IFA [32] |
| Inner Mongolia | 62·7%, 69/110 (Azuoqi); 70%, 70/100 (Damaoqi); 46·3%, 62/134 (Keshenketengqi) | CFT [24] |
| Ningxia | 5·6%, 1/18 (Guyuan) | IFA [29] |
| Shandong | 30%, 30/100 (Changle) | CFT [25] |
| Shanxi | 6·7%, 13/193 (Longxian) | IFA [29] |
| Tianjin | 0·6%, 6/944 (Shiqu); 1%, 5/518 (Haibinqu); 1·5%, 10/650 (Jiaoxian) | IFA [48] |
| 17·9%, 5/28 (Beichen); 12·5%, 3/24 (Xiqing); 6·3%, 3/48 (Tanggu); 8·3%, 2/24 (Ninghe); 4·2%, 1/24 (Dagang) | IFA [31] | |
| Yunnan | 5·3%, 5/95 (Simao); 14·3%, 15/105 (Xundian); 5·4%, 2/37 (Yulong) | IFA [39] |
| 14%, 7/50 (Kunming); 4·6%, 2/44 (Mengzi); 6·7%, 7/150 (Xiaguan) | CFT [19] | |
| Xinjiang | 33·3%, 8/24 (Yili) | PCR [27] |
| 17·1%, 25/366 | CFT [49] | |
| 25%, 6/24 (Yili) | IFA [50] | |
| 5·9%, 4/68 (Aletai) | IFA [29] | |
| Zhejiang | 5·5%, 32/579 | IFA [51] |
CFT, Complement fixation test; IFA, immunofluorescence assay; PCR, polymerase chain reaction.
Table 2.
Prevalences of animals with evidence of Coxiella burnetii infections in different provinces in China, 1989–2013
| Province | Positivity: percentage, positive/total number examined (city/county when available) | Animal | Test used [reference] |
|---|---|---|---|
| Anhui | 60·6%, 80/132 | Goat | IFA [26] |
| 0%, 0/22 | PCR [27] | ||
| 75%, 9/12 | Dog | IFA [26] | |
| 10%, 10/100 (Fuyang) | Pig | CFT [37] | |
| 7·7%, 1/13 (Fuyang) | Rabbit | CFT [37] | |
| 50%, 3/6 | Cattle | IFA [26] | |
| 18·6%, 8/43 (Fuyang) | CFT [37] | ||
| Beijing | 4·5%, 2/44 | Goat | PCR [27] |
| Gansu | 5·4%, 8/147 | Tick | PCR [29] |
| 3·9%, 2/51 (Diebu) | Cattle | IFA [42] | |
| 5·6%,15/270 (Diebu) | Goat | ||
| Guangxi | 16·6%, 1/6 (Nanning) | Goat | CFT [18] |
| 0%, 0/9 (Nanning) | Pig | ||
| 0%, 0/6 (Nanning) | Cattle | ||
| Heilongjiang | 19%, 17/60 (Dongning); 28·4%, 21/74 (Heihe); 13%, 9/69 (Heihe); 7·1%, 5/57 (Mudanjiang); 7·4%, 4/54 (Harbin); 3%, 2/66 (Qiqihar); 3·9%, 2/61 (Jiamusi); 14·3%, 9/63 (Shuangyashan); 11·1%, 6/54 (Jixi); 14·5%, 11/76 (Daqing); 18%, 9/64 (Suihua) | Cattle | ELISA [38] |
| 4·2%, 1/24 (Dongning); 9·3%, 3/32 (Heihe) | Goat | ||
| Henan | 20%, 3/15 (Jiyuan); 50%, 10/20 (Lingbao); 0%, 0/20 (Runan); 0%, 0/20 (Qixian); 30%, 6/20 (Huiji); 15%, 3/20 (Neixiang); 15%, 3/20 (Wuzhi) | Mice | IFA [44] |
| 11·8%, 6/51 (Lushixian) | Cattle | IFA [43] | |
| 5%, 2/40 (Lushixian) | Sheep | ||
| 0%, 0/6 (Lushixian) | Dog | ||
| Inner Mongolia | 32%, 16/50 (Tongliao); 27·5%, 11/40 (Keyouzhongqi) | Cattle | ELISA [38] |
| 22%, 11/50 (Tongliao); 16%, 8/50 (Keyouzhongqi) | Goat | ||
| Jilin | 36%, 18/50 (Changchun); 25·5%24/84 (Tonghua); 23·5%, 8/34 (Yanbian) | Cattle | ELISA [38] |
| 15%, 6/40 (Changchun); 7·5%,3/40 (Tonghua); 0%, 0/32 (Yanbian) | Goat | ||
| Jiangsu | 3·3%, 4/122 | Dog | IFA [32] |
| 10·8%, 17/158 | Cattle | ||
| 0%, 0/150 | Goat | ||
| Liaoning | 21·6%, 16/74 (Dalian); 24·4%, 11/45 (Pulandian); 4%, 12/30 (Lushun) | Cattle | ELISA [38] |
| 19·4%, 7/36 (Dalian); 29·1%, 9/31 (Pulandian); 0%, 0/20 (Lushun) | Goat | ||
| Ningxia | 3·4%, 7/206 | Tick | PCR [29] |
| Shandong | 8·8%, 5/57 | Dog | CFT [41] |
| 8·9%, 9/101 (Changle); 4·8%, 10/209 | Goat | ||
| 1·7%, 2/120 | Cattle | ||
| 0%, 0/57 | Pig | ||
| Shanxi | 6%, 67/1117 | Tick | PCR [29] |
| Xinjiang | 11·1%, 4/36 (Hutubi); 0%, 0/43 (Changji); 22·2%, 8/36 (Yushugou); 0%, 0/38 (Qitai); 29·9%, 12/41 (Hami); 10%, 4/40 (Kuerle); 20·7%, 6/29 (Yili); 0%, 0/33 (Wulumuqi); 22·7%, 5/22 (Yanqi) | Cattle | ELISA [21] |
| 25%, 4/16 (Yili) | Goat | PCR [27] | |
| 14·1%, 12/85 | IFA [50] | ||
| 22·2%, 4/18 | Horse | PCR [27] | |
| 0%, 0/3 | Marmot | ||
| 0%, 0/1 | Deer | ||
| 11·9%, 118/990 | Tick | PCR [29] | |
| Yunnan | 1·3%, 1/78 | Dog | IFA [40] |
| 0%, 0/60 | Cattle | ||
| 0·8%, 1/132 | Goat | ||
| 26·2% | Mice | IFA [45] | |
| Zhejiang | 0%, 0/7 | Cattle | PCR [27] |
| 0%, 0/15 | Goat |
CFT, Complement fixation test; ELISA, enzyme-linked immunosorbent assay; IFA, immunofluorescence assay; PCR, polymerase chain reaction.
The overall prevalence of anti-Coxiella antibodies in the people studied to date in China is 10·2% (1139/11 209) (Table 3). The sensitivities of the CFT and IFA seem to be similar in people with a prevalence of 10·8% (343/3180) by CFT and 9·9% (796/8029) by IFA. The prevalence in cattle from all the studies in China is 15% (288/1918) while 12% (176/1440) of goats have been found positive. ELISA surveys have produced the highest seroprevalences in cattle (17·6%, 250/1423) and IFA the lowest (8·6%, 28/326). In goats the prevalences in IFA surveys and ELISA are similar, i.e. 14% (108/769) and 13·5% (48/355), respectively.
Table 3.
Prevalences of anti-Coxiella burnetii antibodies in people, goats and cattle detected by different assays
| Species | Positive/total number (%) | ||
|---|---|---|---|
| CFT | IFA | ELISA | |
| Human | 343/3 180 (10·8%) | 796/8 029 (9·9%) | Not available |
| Goat | 20/316 (6·3%)* | 108/769 (14%) | 48/355 (13·5%) |
| Cattle | 10/169 (5·9%) | 28/326 (8·6%) | 250/1 423 (17·6%)* |
CFT, Complement fixation test; ELISA, enzyme-linked immunosorbent assay; IFA, immunofluorescence assay.
Denotes significant difference with other assays in the same row.
Q fever in people
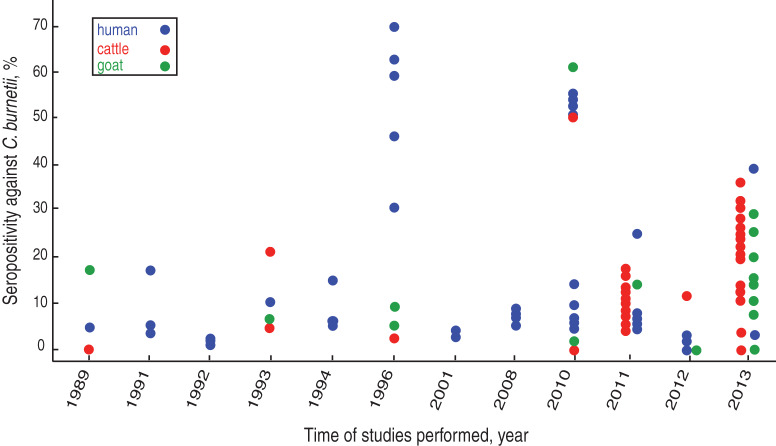
Between 1989 and 2013 there were 19 surveys using serology (CFT or IFA) or polymerase chain reaction (PCR) on almost 10 000 human sera and blood samples from several provinces (Table 1). The majority of these studies were performed between 2010 and 2013. In eight of the 10 seroprevalence studies conducted before 2008, the prevalence rate was under 20%. A 1996 survey showed a 59% seroprevalence in 344 healthy people from three cities in Inner Mongolia (62·7%, in Azuoqi, 70% in Damaoqi, 46·3% in Keshenketengqi) [24] while a study performed a year later showed 30% of middle school students (n = 100) in Shandong were positive [25]. Surveys after 2010 that were conducted in Eastern China also showed generally higher seroprevalences, varying from 56% in Anhui province [26] to 39% in Beijing [20] (Fig. 2). These increased seroprevalence rates could have been due to the use of the more sensitive IFA test replacing the CFT. In a molecular study in Yili region, Xinjiang province, C. burnetii DNA was detected in eight (33·3%) of 24 human blood samples using the loop-mediated isothermal amplification (LAMP) PCR assay [27].
Fig. 2.
Prevalence of antibodies to C. burnetii in China between 1989 and 2013. Dots represent people (blue), cattle (red) and goats (green) that were tested for C. burnetii antibody. Of the 28 studies in the period, 16 were performed in the 20-year period of 1989–2008. Generally, seroprevalence appears to increase throughout the studies with higher detection rates observed during the last 4 years.
Overall, anti-Coxiella antibodies have been detected in people from 32 counties in 15 provinces in China (Table 1). Most infected people had no symptoms or mild febrile disease with influenza-like signs. Seroprevalence varied in different areas but was generally higher in the eastern provinces; Inner Mongolia (58·4%), Anhui (53·7%), Beijing (39%) and Shandong (30%). Lower prevalences were reported from Xinjiang (25%) in the West of China (Table 1, Fig. 1) while the other areas had prevalences of under 10% generally. Possible reasons for these variations in prevalence include geoclimatic differences, numbers of cases examined in different areas, lifestyles in different provinces, prevalence of ticks, socioeconomic status, distribution of domestic animals and their proximity to human dwellings, availability of the diagnostic tests and laboratories, and the sensitivity and specificity of the assay used. The variations seen in the 16S-23S ISR (intergenic spacer region) of seven C. burnetii isolates from China might reflect adaptions to different geographical circumstances [28].
Epidemiological data have shown that most seropositive people in China come from rural areas and have contact with animals [26, 29], such as being livestock breeders, being exposed to animal abortion products and assisting with parturitions. In many studies seropositive people were not found to be significantly different with respect to age, sex or occupation [20, 26, 30, 31]. However, in 2011 an IFA survey of local residents in Northwestern China revealed occupations such as cattle and goat breeders, meat processors and veterinarians were significantly associated with seropositivity [29]. Similarly, other studies have shown females have significantly higher prevalences than males in Shandong (31·4% vs. 29·2%) [25], Anhui (58% vs. 49%) [26] and Jiangsu (0·4% vs. 0·2%) provinces [32]. This is contrary to elsewhere in the world where studies have shown males are more likely to be infected [1]. High prevalences in females in China could be related to common activities of women in rural areas such as tending of livestock and tea picking which increases the chance of contact with ticks and exposure to infected soil and animals [26].
The reported clinical features of Q fever have varied considerably according to geographical location, gender, age, strain, size of inoculum and route of infection. A retrospective study on 500 serum samples of community-acquired pneumonia cases from 12 provincial hospitals collected between November 2001 and June 2002 revealed 44 cases of Q fever pneumonia [33]. On the other hand, hepatitis was considered as the major clinical presentation of acute Q fever cases admitted to E-Da hospital in Kaohsiung County in Southern Taiwan between 2004 and 2007 [34]. As elsewhere, Q fever in China should be suspected in febrile patients with hepatitis or atypical pneumonia who have a history of contact with domestic animals [35].
Q fever is a common cause of fever of unknown origin in the world [1] and in a study of an epidemic of fever of unknown origin in Beijing [36] anti-Coxiella IgG was detected by IFA in 67% of patients. Healthcare workers should be aware of the possibility of Q fever in febrile patients with non-specific signs, especially those with a history of association with domestic livestock.
It is of note that Q fever is not a notifiable disease in China and thus test facilities are not widely available. Most cases are diagnosed in retrospective and epidemiological studies which means acute cases are often misdiagnosed leading to the greater possibility of chronic infections which have a poor prognosis and high mortality.
C. burnetii infections in cattle
There have been 11 seroprevalence studies on cattle since 1989 with four performed prior to 1996 revealing low seroprevalences of under 20% [30, 36, 37]. In 2010, the rate was considerably higher, being 50% in cattle from Anhui province [26] although the sample was very small (n = 6). Increasing seroprevalences were also found in five studies conducted between 2011 and 2013, the highest being 36% in Changchun city, Jilin province [38] (Fig. 2). The highest seroprevalences nationally were found in animals from northern and central provinces. No seropositive cattle were found in studies in Guangxi [18] and Yunnan provinces [39] which are in Southern China (Table 2, Fig. 1). Seropositive animals were associated with abortions and were generally older [21, 29, 40]. In the only molecular study to date on cattle in China, C. burnetii DNA was not detected in any of the seven cattle tested from Zhejiang province [27].
C. burnetii infections in goats
Goats are important sources of C. burnetii infection in people and ten serosurveys were conducted on this species in China between 1989 and 2013. The seroprevalences reported varied from 60·61% to 29·1% in Anhui and Liaoning provinces, to 4·8% and 5·6% in Shandong and Gansu provinces, respectively [26, 38, 41, 42] (Table 2, Fig. 2). Higher goat seropositivity was reported in animals from the northern and southern provinces of China rather than from the central provinces (Table 2, Fig. 1). The variation in prevalence could be due to small sample sizes or could reflect climatic or agricultural differences between provinces in different areas. Moreover, it may be because of differences between sensitivities of the tests used in the different provinces with the CFT being more insensitive than the more recently introduced ELISA and IFA [3]. There is only a single recent molecular study which was conducted on blood from goats from four provinces (Xinjiang, Anhui, Zhejiang, Beijing) using the LAMP PCR assay. Twenty-five percent (4/16) of goats from Xinjiang were positive, as were 5% from Beijing (2/44) [27].
C. burnetii infections in dogs
A total of five seroprevalence studies were performed on dogs between 1996 and 2012 using CFT and IFA (Table 2). Although four of these were performed in closely neighbouring provinces in Eastern China (Anhui, Henan, Shandong, Jiangsu), the prevalences were very different. The highest prevalences were in Anhui (75%), followed by Shandong (9%) [26, 41], with the lowest in Jiangsu (3%) [32], Yunnan (1·28%) [39] and Henan (0/6) [43] provinces.
C. burnetii infections in pigs
Serology using CFT on pigs from Guangxi, Anhui and Shandong provinces [18, 37, 41] revealed that only animals from Anhui were positive (10%, 10/100). Therefore pigs might not play an important role in Q fever in China.
C. burnetii infections in mice
Serosurveillance of mice using IFA has shown positive animals in Henan (19%) in the east of China [44] and in Yuxi city (26%) in Yunnan province in the south of China [45]. Moreover, high copy numbers of C. burnetii DNA were detected using real-time PCR in 32%, 28% and 60% of blood, liver and spleen samples, respectively, of mice in the latter study. The high copy numbers and prevalence of infections suggest mice could play a role in the epidemiology of Q fever in China.
C. burnetii infections in ticks
Ticks are commonly infected with C. burnetii but they are probably not important in the maintenance of infections in livestock and people [3]. It is possible, however, that organisms present in tick faeces might contaminate hides and wool and be a source of infection for people and animals. In China, one study [29] showed C. burnetii DNA in eight tick species (Dermacentor silvarum, D. niveus, D. nuttalli, Hyalomma detritum, Hy. scupense, Haemaphysalis japonica, H. concinna, H. qinghaiensis) from four provinces (Gansu, Ningxia, Shanxi, Xinjiang) in Northwestern China. Overall, 10% (239/2460) of the ticks studied were positive with significantly higher infection levels in Hy. detritum (84/428), H. concinna (48/356) and D. niveus (46/967) (P < 0·01). Ticks from Xinjiang province were most commonly positive (11·9%), in particular Hy. detritum and Hy. scupenses. A later study [38] showed DNA of C. burnetii in D. silvarum, Ixodes persulcatus, H. conicinna, H. japonica, Boophilus microplus and D. nuttalli from three northeastern provinces (Jilin, Liaoning, Heilongjiang) and Inner Mongolia. Overall 2% of the 475 pooled tick samples (n = 10 for each pool) were positive. Although ticks are infected, as in the rest of the world it has been reported there is no good evidence that Q fever is regularly transmitted to humans by tickbites in China [46].
C. burnetii infections in other animals
In 2013, the only study involving sheep in China revealed 5% of animals (2/40) in Lushixian, Henan province in the east of China, were seropositive by IFA [42]. Although a deer and three marmots from Xinjiang province were negative for C. burnetii in a LAMP PCR assay, DNA of the organism has been detected in 22% (4/18) of horses in the only study on this species in China [27]. Further studies are needed to determine the role these other species might have in the epidemiology of Q fever in China.
CONCLUSION
The overall prevalence of Q fever infections in 29 studies to date since 1989 in China has been 10% (1139/11 209) in people, 15% (288/1918) in cattle and 12% (176/1440) in goats. The seroprevalences reported in cattle and goats are higher than those in other domestic animal species indicating they are major sources of human infection in China, as is the case elsewhere in the world [3]. Although human and animal infections are known to occur and be relatively common in China, Q fever is not a reportable disease in the country and clinical cases are probably largely unrecognizable on account of insufficient and inadequate disease surveillance. Of note is the lack of clinical case reports in the country. There is a need for information on the epidemiology of C. burnetii in China as well as many other issues such as distribution, pathogenesis and molecular typing. The data from the studies to date in China provide only a basic picture of Q fever in the country. Active surveillance and further research studies are recommended, particularly with modern and widely applicable molecular methods, to more clearly define the epidemiology and importance of C. burnetii infections in animals and people in China. This will enable the formulation and implementation of locally applicable control methods for Q fever which can be implemented by animal and human healthcare workers.
ACKNOWLEDGEMENTS
This project was supported by grants from the National Natural Science Foundation of China (no. 31272575), by the Jiangsu Co-innovation Center for the Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, Jiangsu, P.R. China, and by the Priority Academic Programme Development of Jiangsu Higher Education Institutions.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Maurin M, Raoult D. Q fever. Clinical Microbiological Review 1999; 12: 518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodolakis A, et al. Comparison of Coxiella burnetii shedding in milk of dairy bovine caprine and ovine herds. Journal of Dairy Science 2007; 90: 5352–5360. [DOI] [PubMed] [Google Scholar]
- 3.Kelly PJ. Q fever. In: Coetzer JAW, Thompson GR, Tustin RC, eds. Infectious Diseases of Livestock with Special Reference to Southern Africa, Volume 1, 2nd edn. USA: Oxford University Press, 2005, pp. 565–572. [Google Scholar]
- 4.Beslagic ES, et al. Public health problem of zoonoses with emphasis on Q fever. Annals New York Academy of Science 2006; 1078: 203–205. [DOI] [PubMed] [Google Scholar]
- 5.Delgado Naranjo J, et al. Study and management of a Q fever outbreak among machine tool workers in the Basque Country (Spain). Epidemiology Research International 2011; 2011: doi: 10.1155/2011/136946. [DOI] [Google Scholar]
- 6.Marrie TJ, et al. Truckin’ pneumonia: an outbreak of Q fever in a truck repair plant probably due to aerosols from clothing contaminated by contact with newborn kittens. Epidemiology and Infection 1989; 102: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Errasti CA, et al. An outbreak of Q fever in the Basque country. Canadian Medical Association Journal 1984; 131: 48–49. [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis G, et al. An important outbreak of human Q fever in a Swiss alpine valley. International Journal of Epidemiology 1987; 16: 282–287. [DOI] [PubMed] [Google Scholar]
- 9.Guigno D, et al. Primary humoral antibody response to Coxiella burnetii, the causative agent of Q fever. Journal of Clinical Microbiology 1992; 30: 1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider T, et al. An epidemic of Q fever in Berlin: epidemiological and clinical aspects. Deutsche Medizinische Wochenschrift 1993; 118: 689–695. [DOI] [PubMed] [Google Scholar]
- 11.Schimmer B, et al. Sustained intensive transmission of Q fever in the south of the Netherlands. Euro Surveillence 2009; 14: 3. [DOI] [PubMed] [Google Scholar]
- 12.EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion on Q fever. EFSA Journal 2010; 8: 1595–1708. [Google Scholar]
- 13.OIE. Q fever: chapter 2·1·12. In: Manual of Standard Diagnostic Tests and Vaccines for Terrestrial Animals, 7th edn, 2012; 2: 1–13. [Google Scholar]
- 14.Zhang NC, et al. Preliminary study on the problem of Q fever in Beijing [in Chinese]. Chinese Medical Journal 1951; 37: 235. [Google Scholar]
- 15.Zhai SC, Liu SH. Q fever: report of a case [in Chinese]. Chinese Journal of International Medicine 1957; 5: 316. [Google Scholar]
- 16.Yu SR, et al. Isolation and identification of Q fever rickettsia in Sichuan in monograph on Rickettsiaceae and Chlamydiaceae infections [in Chinese]. Chinese Journal of Epidemiology Press Beijing 1981; 18. [Google Scholar]
- 17.Yu S. Coxiella burnetii in China. In: Williams JC and Thompson HA, eds. Q Fever: The Biology of Coxiella burnetii. USA: CRC Press, 1991, pp. 327–339. [Google Scholar]
- 18.Cui JZ, et al. The first report of the seroepidemiology of Q fever in Guangxi [in Chinese]. Chinese Journal of Public Health 1989; 5: 56–57. [Google Scholar]
- 19.Yang FL, Chen MH, Dou HF. Study on the infection of Q fever in patients infected respiratory disease in some regions of Yunnan [in Chinese]. Chinese Journal of Zoonoses 1994; 10: 51–52. [Google Scholar]
- 20.Sun Y, et al. Serological investigation on Q fever in stockbreeding-related population in Beijing area. Occupation and Health 2013; 29: 2967–2971. [Google Scholar]
- 21.Zhang J, et al. Detection of Q fever antibodies and the result analysis in Xinjiang region [in Chinese]. Chinese Journal of Animal Health Inspection 2013; 30: 43–44. [Google Scholar]
- 22.Chao CC, et al. Identification cloning and expression of potential diagnostic markers for Q fever. Annals New York Academy of Science 2005; 1063: 76–78. [DOI] [PubMed] [Google Scholar]
- 23.Xiong X, et al. Potential serodiagnostic markers for Q fever identified in by immunoproteomic and protein microarray approaches [in Chinese]. BMC Microbiology 2012; 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu LC, et al. Study on the antibody levels of Q fever in some healthy people in Inner Mongolia [in Chinese]. Inner Mongolia Medical Journal 1996; 16: 38–39. [Google Scholar]
- 25.Sun T, et al. Study on the infection of Q fever and spotted fever among Students [in Chinese]. Chinese Journal of School Doctor 1996; 10: 355–356. [Google Scholar]
- 26.Zhang Y, et al. Seroepidemiological Investigation on Q fever of people and livestock in different regions of Anhui province [in Chinese]. Anhui Journal of Preventive Medicine 2010; 16: 87–89. [Google Scholar]
- 27.Pan L, et al. Rapid simple and sensitive detection of Q fever by loop-mediated isothermal amplification of the htpAB gene. PLoS Neglected Tropical Diseases 2013; 7 (Suppl. 5): e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu T, et al. Detection of Coxiella burnetii by semi-nested PCR and DNA probe [in Chinese]. Acta Academiae Medicine Militaris Tertiae 2001; 23: 1104–1106. [Google Scholar]
- 29.Zhang F, Liu Z. Molecular epidemiological studies on Coxiella burnetii from Northwestern China [in Chinese]. Journal of Pathogen Biology 2011; 6: 183–185. [Google Scholar]
- 30.Yang XP, Shi Y, Guan BW. Serum-epidemiology investigation on rickettsia disease among people of planning longtan reservoir environment [in Chinese]. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong 1991; 20: 277–278. [Google Scholar]
- 31.Zhang L, et al. Rickettsial Seroepidemiology among farm workers Tianjin People's Republic of China. Emerging Infectious Diseases 2008; 14: 938–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan Z, Li L, Zhang L. Cross-sectional survey on the prevalence of antibodies to several types of Rickettsia in human and livestock in Jiangsu province [in Chinese]. Journal of Soochow University Medical Science Edition 2012; 32: 445–450. [Google Scholar]
- 33.Shi-hu C, et al. 44 cases were misdiagnosed as ordinary pneumonia of Q fever cox pneumonia patients were retrospectively analyzed [in Chinese]. Science Technology and Engineering 2007; 17: 2335–2339. [Google Scholar]
- 34.Lai CH, et al. Acute Q fever: an emerging and endemic disease in Southern Taiwan. Scandinavian Journal of Infectious Diseases 2008; 40: 105–110. [DOI] [PubMed] [Google Scholar]
- 35.Bellazreg F, et al. Acute Q fever in hospitalised patients in Central Tunisia: report of 21 cases. Clinical Microbiology and Infection 2009; 15: 138–139. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, et al. Detection of antibody against Q fever from sera provided by patients in an epidemic of fever of unknown origin [in Chinese]. Chinese Journal of Health Laboratory Technology 2008; 18: 1130–1131. [Google Scholar]
- 37.Wang SQ, et al. Study on the infection of Q fever in Huaibei [in Chinese]. Chinese Journal of Zoonoses 1993; 9: 19–20. [Google Scholar]
- 38.Guo Y, Tick-borne disease Q fever in thermal detection methods to establish and North and Northeast Inner Mongolia disease investigation (dissertation) [in Chinese]. Jilin, China: Jilin Agricultural University, 2013. 56 pp. [Google Scholar]
- 39.Chang L, et al. Sero-epidemiologic investigation on rickettsiosis of humans and domestic animals in Yunnan province [in Chinese]. Chinese Journal of Zoonoses 2010; 2: 189–192. [Google Scholar]
- 40.Hong-Bo N, et al. Sero-prevalence of Q fever in dairy cows in Northeastern China. African Journal of Microbiology Research 2011; 5: 3964–3967. [Google Scholar]
- 41.Li Z, Chen T, Huang JT. Preliminary investigation on the seroepidemiology of Q fever among animals in Shandong Province [in Chinese]. Chinese Journal of Zoonoses 1996; 12 (Suppl. 1): 12. [Google Scholar]
- 42.Yang YS, et al. Sero-epidemiology investigation on Q fever among cattle and goat [in Chinese]. Chinese Journal of Veterinary Science and Technology 1993; 23: 18–19. [Google Scholar]
- 43.Zhang D, LV J. Sero-epidemiologic investigation on rickettsiosis of humans and livestock in Lushi County [in Chinese]. Henan Journal of Preventive Medicine 2013; 24: 418–421. [Google Scholar]
- 44.Wu ZW, et al. Study on the seroepidemiology of Rickettsia infection in Henan province, 2004 [in Chinese]. Chinese Journal of Epidemiology 2006; 27: 366–367. [PubMed] [Google Scholar]
- 45.Ya H, et al. Establishment of real-time PCR in detecting of Coxiella burnetii and its application to testing mouse samples collected from Yunnan province [in Chinese]. Infectious Disease Information 2009; 22: 345–350. [Google Scholar]
- 46.Wu X, et al. Distribution of tick-borne diseases in China. Parasites and Vectors 2013; 6: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He JF, et al. The infection of Q fever infection in Guangdong Province [in Chinese]. Chinese Journal of Zoonoses 2001; 17: 97–98. [Google Scholar]
- 48.Zhang ZL, et al. Study on the infection of rickettsia disease among people in Tianjin [in Chinese]. Chinese Journal of Public Health 1992; 8: 253–254. [Google Scholar]
- 49.Kong ZM, Zhou XR. Seroepidemiology investigation and isolation of Q fever among people in Xinjiang [in Chinese]. Medical Journal of National Defending Forces in Northwest China 1991; 12: 43–44. [Google Scholar]
- 50.Fan DS, et al. Seroepidemiology of Q fever in Yili region of Xinjiang [in Chinese]. Disease Surveillance 2011; 26: 15–17. [Google Scholar]
- 51.Chai CL, et al. Sero-epidemiologic investigation on tick-borne diseases of humans and domestic animals in Zhejiang province [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 2010; 31: 1144–1147. [PubMed] [Google Scholar]