SUMMARY
The prison setting has been often cited as a possible reservoir of tuberculosis (TB) including multidrug-resistant (MDR)-TB. This is particularly true in low-income, high TB prevalence countries in Sub-Saharan Africa. A systemic literature review was done to assess the prevalence, drug resistance and risk factors for acquiring TB in the prison population. Our review indicated a high prevalence of TB in prisons which is reported to be 3- to 1000-fold higher than that found in the civilian population, indicating evidence and the need for public health policy formulation. In addition, high levels of MDR and extensively drug-resistant (XDR)-TB have been reported from prisons, which is a warning call to review prison TB control strategy. Multiple risk factors such as overcrowding, poor ventilation, malnutrition, human immunodeficiency virus (HIV), and others have fuelled the spread of TB in prisons. Furthermore, the impact extends beyond the prison walls; it affects the civilian population, because family visits, prison staff, and members of the judiciary system could be potential portals of exit for TB transmission. The health of prisoners is a neglected political and scientific issue. Within these background conditions, it is suggested that political leaders and scientific communities should work together and give special attention to the control of TB and MDR-TB in prisons. If not, TB in prisons will remain a neglected global problem and threatens national and international TB control programmes. Further researches are required on the prevalence and drug resistance of smear-negative TB in prisons. In addition, evidence of the circulating strains and transmission dynamics inside prisons is also warranted.
Key words: Drug-resistant tuberculosis, incidence, prison, prisoners, risk factors, tuberculosis
INTRODUCTION
Tuberculosis (TB) in the prison setting poses a major public health problem globally, especially in countries of the former Soviet Union [1] and in Sub-Saharan Africa (SSA) [2, 3], where TB in prisons remains a neglected plague and a human rights issue [1, 3–6]. Prisons are settings in which TB transmission occurs and high rates of active TB have been reported worldwide [1–6]. The situation in SSA makes TB a major public health problem due to human immunodeficiency virus (HIV) infection, overcrowding, insufficient ventilation, poor hygiene, low socioeconomic status, poor nutrition, prolonged duration in prison, and poor general health of inmates [3, 7, 8]. Moreover, delayed diagnosis and inappropriate case management increases the problems associated with TB [9]. Owing to incomplete, interrupted, inadequate treatment, or poor healthcare management, prisoners harbour drug-resistant TB [10, 11], which is increasingly being reported from SSA prisons [2, 3, 5] as well as from Eastern European prisons [1, 11–13]. Prison settings are important, but often neglected, reservoirs for drug-resistant TB transmission and pose a threat to individuals in the outside community [1, 2, 10–13]. According to World Health Organization (WHO) estimates, the world's prisons hold 8–10 million prisoners each day, with 4–6 times this number passing through prison system each year. Studies in prevalence rates of TB in prison populations are among the highest documented in any population. While the bulk of studies have focused on the TB situation in prisons in Eastern Europe and the USA, data on the prevalence and incidence of TB in prisons in SSA countries are limited. Based on active screening studies from SSA prisons [2, 5, 14–20], TB prevalence rates were reported several fold higher than the national rates, indicating TB prevention and control in prisons is not well addressed nor integrated into most national TB programmes.
Given the high risk of TB in prison settings, there is an urgent need for policy makers, programme managers, and scientific communities to revise their efforts and implement effective control programmes to protect not only the health of people that are imprisoned but also the health of the wider community. Here we review and summarize the available literature on prevalence, drug resistance, and risk factors for acquiring TB in the prison population, as well as transmission and control of TB in prisons and discuss the importance of our findings for informing TB control policies in prison settings.
METHODS
A literature search was conducted for articles published between 1992 and 2014 from 1 January 2014 to 28 February 2014 using the online databases PubMed/Medline and Google scholar. To fined articles related to TB in prison settings, we considered one of the following key words such as: ‘Tuberculosis’ and ‘prisons’, ‘risk factors’, ‘drug resistance’, ‘prevalence rate’, and ‘control’. Each term was searched separately with the name of the regions and/or countries. Only English-language papers and WHO websites were included in the search and the searches were focused on studies of prevalence, drug resistance, and major risk factors associated with TB in prisons. Only study reports that used original research papers and case-control studies were reviewed. Literature that did not report on a study of TB in prisons was excluded. The three authors independently reviewed all of the studies found and WHO websites. A structured form for data extraction was used and included such points as country where the study was conducted, year of publication, TB prevalence rate in prison(s), total number of prisoners investigated for TB or TB in a comparison to the general population, risk factor for the prevalence, and drug-resistant TB in prison(s). We assessed and tallied the frequency of prevalence, drug resistance of TB and risk factors across all studies reported. In this review we report the risk factors and recommendations most commonly reported in the literature.
RESULTS
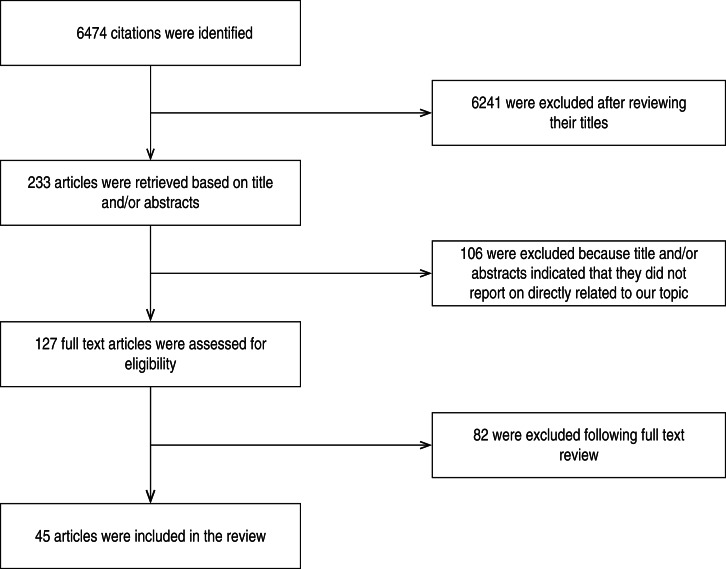
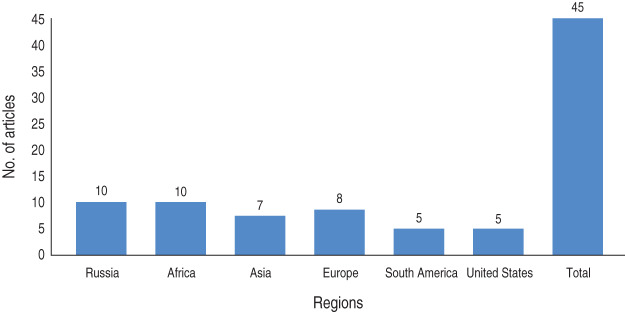
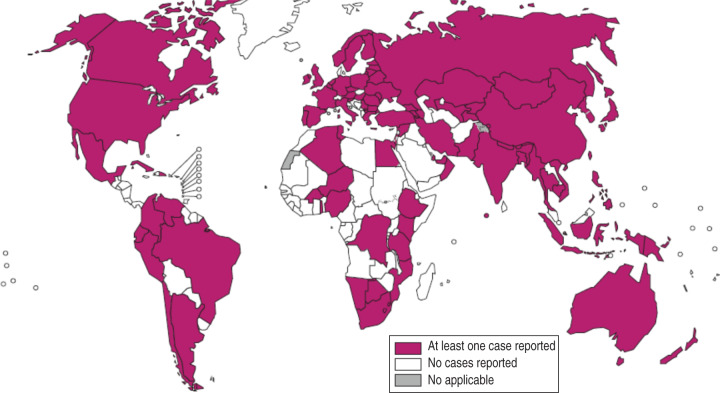
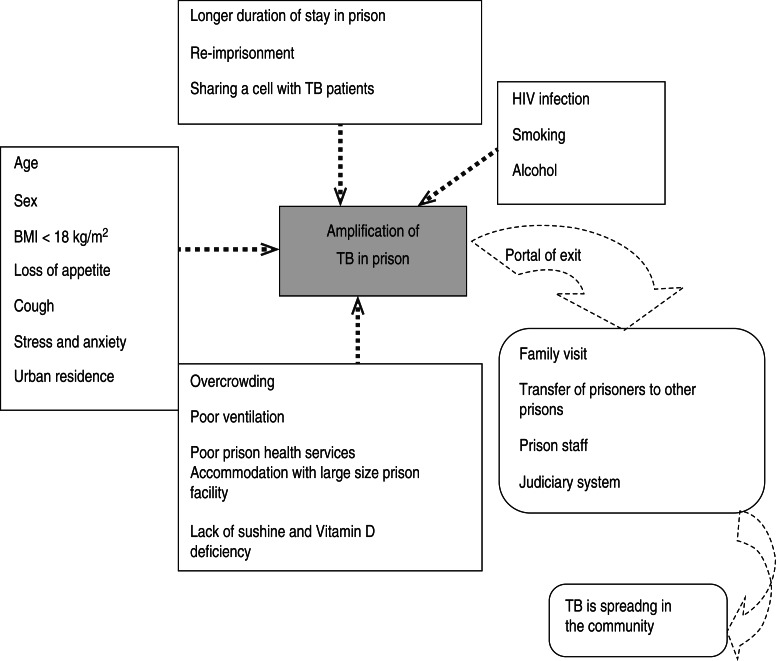
A research paper published between 1992 and 2014 shows the included studies were varied in terms of research methods and study site. As summarized in Figure 1, our electronic search resulted in 6474 citations. Finally, 127 articles were retrieved for full text review and 45 papers were determined to meet eligibility criteria and included in this review. Of the 45 relevant articles, 10 each were from Russia and Africa, seven from Asia, eight from Europe and five each from South America and the USA (Fig. 2). The prevalence of TB in prisons has been reported to be 3- to 1000-fold higher than that found in the civilian population (Table 1). In addition, high levels of multidrug-resistant (MDR)-TB at 54·8% and extensively drug-resistant (XDR)-TB at 11·1% have been reported from prison settings (Table 2). According to a WHO report in 2013, the notified XDR-TB cases among countries are shown in Figure 3. Multiple risk factors such as overcrowding, poor ventilation, malnutrition, HIV, and others have fuelled the spread of TB in prisons (Fig. 4). The most common recommendations by investigators to prevent and control the spread of TB in prison setting were active case-finding, followed by implementation of directly observed treatment, short course (DOTS)strategy and the need for improved mycobacteriological laboratory services (Table 3).
Fig. 1.
Flow diagram showing literature review.
Fig. 2.
Frequency of included articles for review by region.
Table 1.
A summary of prevalence studies reporting TB in prisons
| Country, reference | Year(s) of study | Study type | Prevalence rate in prisons, n/N (%)* | Prevalence rate compared with general population during the study period*† |
|---|---|---|---|---|
| Russia Aerts et al. [1] |
1997–1998 | TB screening study | 448/7 473 (5·9) | 200 |
| Zambia Habeenzu et al. [2] |
2000–2001 | Active case-finding study | 245/6 118 (4) | 10 |
| Cameroon Noeske et al. [5] |
2003–2004 | Active case-finding survey | 87/2 474 (3·5) | 35 |
| WHO European zone Aerts et al. [6] |
2002 | Questionnaire survey | 232/100 000 (n.a.) | 83·6 |
| Botswana CDC [14] |
2002 | Active case-finding survey | 39/1 027 (3·8) | 10 |
| Ivory coast Koffi [15] |
1990–1992 | Case control study | 108/1 862 (5·8) | 14·5 |
| Ethiopia Abebe et al. [16] |
2008 | TB screening study | 33/37 (18·9) | 7 |
| Ethiopia Moges et al. [17] |
2011 | Active case-finding survey | 26/250 (10·4) | 9 |
| Malawi Yerokhin et al. [21] |
1996 | Case-finding survey | 47/914 (5·1) | 10 |
| USA MacNeil et al. [24] |
1993–2003 | National survey | 7 820/210 976 (3·8) | 4 |
| Pakistan Rao [29] |
2002 | Observational and chest-X ray survey | 79/4 870 (1·6) | 3·75 |
| USA Hutton et al. [35] |
1984–1985 | National survey | 177/9 664 (1·8) | 3·9 |
| Brazil Abrahão et al. [36] |
2000–2001 | Tuberculin survey | 601/932 (64·5) | 70 |
| Brazil Lemos et al. [37] |
2003–2004 | Tuberculin and chest X-ray survey | 6/237 (2·5) | 42 |
| Turkey Öngen et al. [38] |
2006–2007 | Radiography screening study | 108/100 000 (n.a.) | 4 |
| Colombia Ruda et al. [39] |
2010–2012 | Prospective cohort study | 72/1 305 (5·5) | 20 |
| Spain March et al. [40] |
1994–1996 | Epidemiological and molecular study | 2 775/100 000 (n.a.) | 50 |
| London Story et al. [42] |
2003 | Cohort study | 208/100 000 (n.a.) | 15 |
| Mexico Hernández-León et al. [43] |
2010–2011 | Cohort study | 28/172 (n.a.) | 1 000 |
| Taiwan Chiang et al. [44] |
1998–1999 | Mass radiography screening study | 107/51 496 (0·21) | 4 |
| Thailand Sretrirutchai et al. [45] |
1998 | TB screening and molecular study | 304/4 751 (6·4) | 8 |
| Bangladesh Banu et al. [47] |
2005–2007 | Active case-finding study | 245/1 781 (13·8) | 20 |
| Azerbaijan Pfyffer et al. [41] |
2001 | Genotypic characterization | 4 667/100 000 (n.a.) | 50 |
n, Number of cases; N, total number of cases; n.a., not available.
All values are given as reported in the cited studies.
Fold higher.
Table 2.
Studies reporting drug-resistant TB in prisons
| Country, reference | Main findings* |
|---|---|
| Russia Aerts et al. [1] |
Multiphasic screening of all smear positive tuberculosis in prisons in Georgia, the ex-USSR state, was done between July 1997 and August 1998. A total of 215 (77·9%) isolates were resistant to at least one drug and 37 (13%) were found to be MDR. |
| Zambia Habeenzu et al. [2] |
TB screening in 13 prisons was carried out between 2000 and 2001. Resistance to at least one anti-TB drug was observed in 40 (23·8%) and 16 (9·5%) were MDR-TB. |
| Azerbaijan Conix et al. [9] |
Assessment of a tuberculosis control programme in a prison setting in Baku, Azerbaijan was performed between 1995 and 1998. Drug resistance data on admission was available for 131 patients. 72 (55%), 33 (25%), 34 (26%), and 98 (75%) patients had resistance to INH, RMP, EMB, and SM, respectively. |
| Thailand Pleumpanupat et al. [10] |
A total of 154 TB patients between 1 May and 31 October 2000 were enrolled in the study. Resistance to at least one drug and MDR-TB was found in 50·6% and 19·5% of the subjects, respectively. |
| Russia Ruddy et al. [11] |
A cross-sectional survey was carried out of 600 patients (309 civilians and 291 prisoners) between 2001 and 2002. Prevalence of INH, RMP, SM, EMB, and PZA resistance in new cases was (civilian and prison patients) 38·0%, 25·2%, 34·6%, 14·7%, 7·2% and MDR-TB observed in prison new cases was 37·3%. |
| Russia Toungoussova et al. [12] |
A total of 114 M. tuberculosis isolates recovered from patients in the Archangel region were studied for drug susceptibility in 2001. MDR-TB was high in new (34·0%) and previously treated (55·0%) cases. Resistance to EMB, INH, RMP, and SM was observed, respectively, at 36 (31·6%), 53 (46·5%), 43 (37·7%), and 79 (69·3%). |
| Russia Ignatova et al. [13] |
Eighty-seven culture-positive patients with TB who were diagnosed in the Ozerki prison hospital (Tula region) from 2001to 2002 were included. Drug resistance to SM, INH, KAN, and RMP was 77 (88·5%), 67 (77·0%), 58 (66·7%), and 66 (75·9%), respectively. MDR-TB was seen in 62 (71·2%) cases. |
| Brazil Abrahão et al. [36] |
TB status screening in country jail prisoners in the western sector of the city of São Paulo, Brazil was carried out between 2000 and 2001. In the 21 M. tuberculosis strains identified 2 (9·5%) were resistant to INH and RMP, while 1 (4·5%) was resistant to INH, RMP, and PZA. |
| Colombia Rueda et al. [39] |
Prospective cohort study from 2010 to 2012 for TB screening in four Colombian prisons indicated three cases were INH resistant and two were SM resistant. |
| Spain March et al. [40] |
IS6110 and polymorphic GC rich repeat sequence-based restriction fragment length polymorphism were combined with epidemiological studies to assess the relatedness of isolates from all patients with confirmed TB at five prisons. The study was performed from July 1994 to December 1996. Low rates of both initial (2·9%) and acquired (5·8%) drug resistance were identified. |
| Azerbaijan Pfyffer et al. [41] |
Genotypic characterization of 65 isolates of M. tuberculosis in a prison of Azerbaijan was carried out in 2001. MDR-TB was seen in 34 (52·3%) cases. |
| Thailand Sretrirutchai et al. [45] |
Cross-sectional, descriptive, clinical and molecular studies at four prisons in Southern Thailand were carried out between 1997 and 1998, 39% of the M. tuberculosis isolates were resistant to INH; three of these isolates were also borderline to RMP. |
| China Wong et al. [46] |
A prospective nationwide TB surveillance was conducted in prisoners in 24 correctional institutions. Eleven of the 314 culture-positive cases showed resistance to INH and RMP (MDR-TB). |
| Bangladesh Banu et al. [47] |
Active screening of a total number of 11 001 inmates over a period of 2 years from October 2005 to September 2007 was done. Resistance of INH, RMP, SM, and EMB was 11·4%, 0·8%, 22·4%, and 6·5%, respectively. No multidrug resistance was observed. |
| Russia Drobniewski et al. [51] |
A molecular study in 2001 on 140 patients showed 58·2%, 51·6%, and 44·7% of cultures were resistant to RMP, INH, and MDR-TB, respectively; 80% of prisoners were RMP resistant. |
| Russia Balabanova et al. [52] |
A long-term observational study was conducted from 2002 to 2008 and MDR-TB and XDR-TB rates of 54·8% and 11·1% were indentified in the region. Mutation conferring resistance to RMP, INH, and fluroquinolone was observed at 81/92 (88·8%), 91/92 (98·9%), and 68/92 (73·5%), respectively. |
| USA (New York state) Valway et al. [54] |
A total of 171 TB patients from 1990 to 1991 were screened and 37 (32%) of 116, with drug susceptibility determined, had MDR-TB. |
| UK Anderson et al. [55] |
A retrospective study between 2004 and 2007 on 205 UK prisoners showed that 48/139 (34·5%) were resistant to INH. |
EMB, Ethambutol; INH, isoniazid; KAN, kanamycin; MDR, multidrug resistant; PZA, pyrazinamide; RMP, rifampicin; SM, streptomycin.
The value of drug resistance and MDR-TB is reported in the cited studies.
Fig. 3.
Notified extensively drug-resistant (XDR)-TB cases in countries, 2013 (Source: WHO Global tuberculosis report, 2013.)
Fig. 4.
Risk factors that fuel TB infection in prisons and potential portals of exit for TB from prison to the community. (Sources: references [1–3, 5, 6, 16–18, 20, 23–25, 37, 47, 56–62].)
Table 3.
A summary of recommended TB control measures in prison setting
| Active case-finding Implement DOTS strategy in prison Active surveillance and TB screening Adequate case-finding and containment strategies Integration of national TB programmes with that of prison health services Entry examinations and latent TB infection screening Isolation of prisoners with TB |
Improve socio-sanitary conditions for prison inmates Prison reform Health education of inmates Strengthening of TB control programme Improve the nutritional status of prisoners Intervention measures targeting inmates Implementation of WHO guidelines Support the need for improved screening |
DISCUSSION
Evidence of prevalence of TB in prisons
In this review, we observed that, globally, the level of TB in prisons has been reported much higher than that of TB in the general population [1, 2, 5, 14–29]. The WHO estimated a prevalence of TB in prisons about 10- to 100-fold higher than the prevalence in the general population [30]. According to our review of published studies, this ranged from 3·75- to 1000-fold higher than in the general population, both in high- and low-income countries (Table 1). In the studies from Russian prisons, the prevalence of TB was 5995/100 000 in Georgian (448 cases from 7473 prisoners) prison inmates, almost 200 times more than the prevalence rate of TB in the general population [1]. The observation was confirmed for other Russian prison populations by study results from Slavukij et al. in 2002 [31] and Lobacheva et al. in 2005 [32]. According to studies in prisons from the WHO European region, the median TB detection rate was 393/100 000 inmates, which is 83·6 times more TB than in the general population [6]. Based on this report, TB incidence rates in prisons of the surveyed countries were 8–35 times higher than in the general population. A study conducted in France showed a high prevalence rate of TB at 215/100 000 prisoners [33] and in Turkey the rate was 341/100 000 prisoners [34]. Reports in other prison settings confirmed the overall trend [35–43]. In the USA the rate was 3–11 times higher than in the general population and in Brazil it was 2065/100 000 and 2500/100 000 inmates, which is 70- and 42-fold higher than in the general population. Studies conducted in prisons in some Asian countries confirmed TB prevalence rates several times higher than in the general population [29, 44–47]. In Pakistan, Taiwan, Thailand, and Bangladesh the rate was 79/4870, 258·7/100000, 304/4751, and 2227/100 000, respectively, which is 3·75, 4, 8, and 20 times higher than in the general population, respectively. A TB surveillance study in correctional institutions over the period from 1999 to 2005 in Hong Kong found a very high TB prevalence rate in prisoners.
TB in African prisons poses a particularly challenging public health, economic, and social problem mainly in SSA, which also has a high prevalence of HIV infection [30]. In SSA, where poverty, HIV/AIDS, and chronic malnutrition are prevalent, the prison population probably has a high burden of TB [3, 30]. TB in prisons in SSA lacks the attention of both programme managers and scientific communities. It is one of the challenge areas in national TB prevention and control strategies. TB in prisons is prevalent, but data are scarce due to the lack of an active monitoring programme in SSA. This review yielded alarming information for understanding the disease burden as well as to motivate planning and implementing TB control and prevention measures in prisons. To our knowledge, there are ten published datasets that describe TB in prisons in SSA. These data reported high prevalence and incidence rates of TB compared to the general population [2, 5, 14–20, 28]. For instance, in an active case-finding survey in Madagascar [28], the prevalence of TB in Antananarivo prison was 16 times higher and Zambian prisons [2] had about 10-fold higher incidence than in the general population. A cross-sectional, cell-to-cell survey in 18 prisons in Malawi reported that 47 (5142/100 000) inmates had pulmonary TB [18]. In 2009, Banda et al. [19] reported a period prevalence of 0·7% (54/7661). Cross-sectional studies from the Ivory Coast [15], Tanzania [20], Botswana [14], and Cameroon [5], all confirm high TB prevalence rates in prisons compared to the civilian population.
In Ethiopia, TB in prisons is not well documented despite the presence of multiple physical, individual, and social factors that favour TB epidemics. The observed point prevalence in eastern Ethiopian prisons was 1913/100 000 inmates [95% confidence interval (CI) 1410–2580], seven times higher than that of the general population [16] and in 2012 Moges et al. [17] reported a point prevalence of TB at 1482·3/100 000 in the prison population, which was 9·1 times higher than in the general population. These data suggest that inadequate TB control measures in prison settings have a considerable impact on communities inside and outside prisons in Ethiopia and with comparable situations in other SSA countries; it is arguably conceivable to be the case in SSA, generally. A review of all relevant English publications on TB in prisons in SSA performed by O'Grady et al. [3] in 2011 also suggested that there is evidence of an increasing prevalence of active TB in prisons in SSA with drug-resistant TB increasingly being detected. The survey of studies on TB prevalence and incidence rates shows disturbingly that TB in prisons is increasing and will threaten national TB programmes due to delayed case detection, incomplete treatment of TB, and release and recidivism without screening [16].
Drug-resistant TB in prison
The WHO's 2010 Global MDR-TB report estimated that there were 440 000 MDR-TB cases (3·6%, 95% CI 3·0–4·4) and 150 000 deaths were due to MDR-TB worldwide in 2008 [48]. China and India accounted for 50% of MDR-TB worldwide. According to a WHO/International Union against Tuberculosis and Lung Disease (IUATLD) survey of 20 countries with the highest rates of MDR-TB in previously treated cases, 14 are in the European Region [49]. In Africa 69 000 MDR-TB cases were reported in 2008 [48]. Published studies on prevalence and incidence rates are increasingly available, but accurate data on drug-resistant TB in prisons is limited.
In prisons, high levels of MDR-TB and XDR-TB have been reported (Table 2). Prisons pool and serve the spread of drug resistance and MDR-TB [11], which is increasingly being reported worldwide [1–3, 6, 7, 9, 11, 12, 14, 30, 36, 47, 48, 50–55]. A TB surveillance study in WHO European Region prisons in 2001 showed high levels of MDR-TB: 50% in Estonia, 18% in Azerbaijan, 14·0% in Latvia, and 0·6% in Spain [6]. Other studies showed high levels of MDR-TB from prisons with up to 24% of all TB patients [30]. In a Russian prison 11·1% XDR-TB was reported [52]. Data on countries reporting at least one XDR-TB is presented in Figure 3. Re-imprisonment, poor healthcare, inadequate treatment, pressure on prisoners to self-treat, access to uncontrolled anti-TB drugs through the prison black market, staff and visitors, release and recidivism without screening, and failure to complete treatment were identified as prison factors contributing to the development of MDR-TB [30, 47]. Obviously, MDR-TB is more difficult to treat than normal TB and consumes more resources. Preferably, all suspected TB cases should undergo drug susceptibility testing; however, the efficacy of this policy depends on local feasibility and skilled staff. At least, prison inmates must have access to the same facilities and healthcare services as the general population. Poor health service management, inadequate health professionals for prisons, poverty, lack of continuous monitoring and evaluation, inadequate discharge planning, contact tracing and follow-up after discharge, and general low attention given to prisoners are barriers to the implementation of TB control in prisons [3].
Effect of risk factors on TB infection and disease
Several potential environmental, social, and host-related risk factors that promote transmission and development of active TB in prisons were investigated in this review (Fig. 4). In prisons the most identified risk factors are HIV infection, poor ventilation, cough, overcrowding, malnutrition, stress and anxiety, poor prison health services, smoking, alcohol, addictive drugs, lack of sunshine and vitamin D deficiency, longer duration of stay in prison, young age, and urban residence [1, 3, 5, 6, 16, 17, 20, 47, 56–60]. Studies have shown that malnutrition and/or a body mass index (BMI) <18·5 kg/m2 were associated with increased risk of developing TB in prisons [17, 20, 47, 60]. For instance, a study in Zambia found that nutritional status and food intake was universally poor in all surveyed prisons. Similarly, studies in the Ivory Coast, Ethiopia, Tanzania, Cameroon, and Russia reported a BMI < 18·5 kg/m2 as a significant predictor of TB.
HIV infection has emerged as the most important risk factor for development of TB in persons infected with Mycobacterium tuberculosis [3, 5, 17, 56–58, 60]. For example, those with HIV co-infection have an increased risk of reactivation of TB at 10% during each year of infection [59]. There is evidence that TB infection in HIV-infected patients progresses to TB more rapidly than in those without HIV infection [56–59]. Being an urban resident, an inmate with a cough lasting >4 weeks, or sharing a cell with a confirmed TB case increased the risk of TB by nearly 4-, 3-, and 3·4-fold, respectively [16]. In 2009 Lemos et al. [37] found cough as a determinant risk factor for active TB [prevalence rate (PR) 8·8, 95% CI 1·04–73·9, P = 0·025].
In addition to several risk factors, people with low socioeconomic status have a higher likelihood of being exposed to crowded and less ventilated places [56]. Crowded living conditions increase transmission of tubercle bacilli, resulting in a higher prevalence of TB infection with subsequent increased incidence of disease [61]. Consequent to low socioeconomic status, individuals may have poor access to healthcare that could increase the risk and prolong the period of infectiousness.
A case-control study in Russia reported that an overcrowded cell (more than two people per bed) and spending less time outdoors were independent risk factors for developing TB in the prison [62]. The Georgian study also indicated that risk was also three times higher in inmates of a large size prison facility. Large prisons are notorious for having poor hygienic standards and lacking adequate ventilation [1]. The length of imprisonment is one of the commonly identified risk factors for TB. The Georgian study showed that the risk of acquiring TB for those imprisoned ⩾2 years was twofold greater than for those who were imprisoned for <1 year [1]. The longer prison stay may contribute to infection through lengthier exposure in addition to deterioration of immunological function as a result of poor living conditions and physical and emotional stress [3, 61]. On the other hand, the length of stay was not a significant risk factor for TB in a study of Zambian prisons [2]. A history of previously being in a prison [5, 27, 63, 64] was found to increase the risk of TB. Overall, the studies explicitly stated that the prison-related factors contribute to a high TB burden both inside as well as outside of prisons and thus need to be addressed in TB control strategies.
Age and sex differences in the prevalence of TB infection and disease have been reported worldwide [2, 17, 18, 20, 23, 24, 56, 60]. Children are at higher risk of contracting TB infection and disease [56]. Studies have shown that prisoners aged 15–44 years had a higher risk compared to other age groups of acquiring TB and developing the disease [16]. However, Winetsky et al. [60] reported in 2014 that old age >50 years (PR 5·79, 95% CI 3·07–10·91) increased the risk of acquiring TB in prison. Prison studies indicate a significant difference between male and female prisoners [2, 18, 23, 24]. High incidence rates in male prisoners were reported in Zambia and Malawi [2, 18]. Moreover, in 2005 Sanchez et al. reported high incidence rates of TB in female prisoners [25]. The epidemiological difference could be due to poorer access to healthcare facilities, higher exposure to infection, and increased susceptibility rather than biological difference [61].
Risk of infection and progression to TB
The development of TB disease requires a two-stage process in which a susceptible person exposed to an infectious TB case first becomes infected and may later develop the disease, depending on various factors influencing the risk of exposure, the risk of infection, the risk of developing disease, and the risk of death [65]. The first step is to have contact with an infectious TB patient, who expectorates bacilli in the surrounding environment. The probability of contact between a susceptible person and a source case depends on the prevalence of active pulmonary TB in a given population (in this case, the prison setting) and is influenced by several factors, the most important being crowding and poor ventilation [65]. Droplet nuclei are produced and spread when patients with pulmonary or laryngeal TB cough, sneeze, speak, or sing. Of persons exposed to an infectious TB case, the risk of becoming infected is due to the joint effect of three factors: (1) the infectivity of the source case, (2) the frequency of exposure to the source case, and (3) susceptibility of the person to infection [65]. The probability of infectivity of the case to tubercle bacilli depends on the frequency of coughing, the number of bacilli in the sputum [66], and the microbial ‘virulence’ [67]. In patients with TB of the respiratory tract not all are equally efficient in transmitting. Patients whose sputum smears are positive for acid-fast bacilli have ⩾5000 organisms/ml of sputum [68] and infect many of their close contacts. The close contact between a susceptible person and the infector determines the degree of exposure [65]. In 2003, Beggs et al. identified small room volume, high resident density, and poor ventilation rate as crucial factors for TB transmission in confined spaces [69]. The degree of vulnerability of the case is a function of health status of the person and genetic factors [70, 71]. In 2003 Tufariello and colleagues [72] stated that progress of TB from infection to the disease involves the tubercle bacilli overcoming the immune system's defences, and in primary TB, which constitutes around 10% of all cases, the progression of the infection to TB disease occurs soon after infection. In many individuals, the disease may remain dormant within the body with the immune system capable of containing the infection (latent TB) [72]. When the immune system weakens due to host-related factors, the infection is reactivated. The risk of this reactivation rises when immunity is suppressed [56, 72]. Any factor influencing the risk of infection and/or the risk of breakdown after infection affects the incidence of TB disease in a given population [59]. Environmental factors such as overcrowding, poor ventilation, urban residence, or low socioeconomic status affect the risk of infection [73]. These factors may have an impact on the incidence of TB in a given population as the result of their effect on both the risk of infection and the risk of disease once a person is infected [65].
Transmission of TB inside and outside prison cells
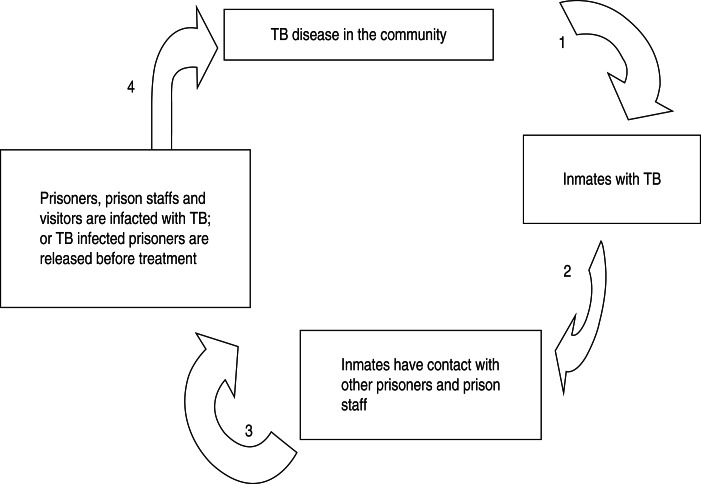
Prisoners usually come from communities with higher TB prevalence rates and bring with them an unhealthy lifestyle [74]. Because of ignorance, neglect, or lack of means for screening, infected prisoners may enter prisons [3]. Prisons concentrate individuals usually in overcrowded and unhygienic environments and limit access to healthcare services [74]. Overcrowded prisons facilitate the spread of TB, because prisoners are in close contact with others without access to outside spaces. Living together in cramped quarters with no ventilation is another major factor for contracting TB [74]. Another aspect facilitating TB communication is that prisoners are often highly mobile within the system; inside the prison, between different prisons and institutions of the judiciary system, as well as healthcare institutions. Prison staff and visitors come and go as well. This pattern clearly indicates TB in prisons is not only of concern for prisoners, but also for the wider community [75]. In 2010 Baussano et al. [76] reported the fraction of TB in the general population attributable to transmission from prisons to be 8·5% in high-income countries and 6·3% in middle- or low-income countries. The prison setting is therefore becoming the place for concentrating, disseminating, and worsening TB, including MDR-TB, and even for exporting it into the general population. The question at present is what to do about what was until very recently ‘a forgotten and neglected disease’ [77].
Prevention and control of TB in prison
A simple model of TB infection control measures in prison is given in Figure 5. The model encompasses several infection prevention and control measures as indicated by the numbers next to the arrows [78]. The numbers by the arrows indicate the following practical TB prevention and control measures that could be implemented to inhibit the TB transmission chain in prisons and the community: (1) prisoners are screened for active and latent TB infection upon entry and during incarceration and isolation, rooms (for occupation for up to 2–4 weeks) should be provided for suspected prisoners; (2) conducting contact case-finding, TB screening for transferred prisoners, and health education; (3) examining prisoners before release and prison staff regularly; (4) providing early TB case detection and successful treatment. Furthermore, special emphasis should be given to establish and improve laboratory facilities for prompt and efficient diagnosis and treatment for control of TB in prison settings [1, 39, 44, 79]. A DOTS strategy for patients with TB was recommended by the WHO [80]. The WHO Stop TB strategy applies to the general population and to TB control in prisons. The WHO/EURO prison health project implemented in 1995 is one of the initiatives addressing and integrating the Stop TB strategy in prison settings [6]. However, these components are rarely implemented in developing countries. Unlike other African countries, Malawi has published guidelines on the implementation of specific interventions for TB in prisons [81]. The national TB programme (NTP) and the prison health services have effectively worked together to improve TB infection control [81]. Successes in TB infection control measures from programmes in Malawi were reported in comparison with other African countries [19].
Fig. 5.
A simplified model of TB infection control methods in prisons [78]. (1, Screening for active and latent TB infection upon entry and during incarceration; 2, conducting contact case-finding, TB screening, and health education; 3, inspecting prisoners before release and prison staff regularly; 4, providing early TB case detection and successful treatment.)
The recommendations by investigators to prevent and control the spread of TB in the prison setting are presented in this review. Thus, according to the investigators, active case-finding and TB screening, adequate case-finding and containment strategies, implementation of DOTS strategy in prison, integration of a national TB control strategy with that of prison health services, entry examination for active and latent TB infection screening, intervention measures targeting inmates, the need for improved mycobacteriological laboratory services, and the need for improved screening and isolation of prisoners with TB were the reported recommendations to tackle TB in prisons [1, 2, 6, 14–17, 21, 29, 38–40, 44, 45, 47]. According to our review the most common recommendations by investigators to control TB in the prison setting were active case-finding followed by implementation of DOTS strategy and the need for improved mycobacteriological laboratory services. These control measures published in different journals provide much needed evidence for responsible agencies to renovate their effort to prevent the spread of TB in prison. In addition, each recommendation including the WHO guideline are available on how best to control and prevent TB in prisons [30].
Role of research in TB infection control
We are confident that research will play a vital role in the fight against TB. Promoting research is a key component of the Stop TB strategy [80], which includes programme-based operational research and research on introducing new tools into practice. There are questions that remain unanswered about TB in African prisons, mainly in SSA regions, suggesting an urgent need for research in addition to the efforts currently being made to improve programmes. The neglected issue of TB in prisons requires attention from scientific communities and political leaders. Political support and appropriate financing of research work is critical for TB infection control, since politicians have a responsibility to protect prisoners from harm from the disease [3]. Priority research questions should be identified and developed to assess new interventions and to improve TB infection control performance in prisons. This will provide an evidence base that can help governments allocate appropriate and adequate funding for TB infection control in prisons.
CONCLUSION
The incidence of TB in prisons was found to be several fold higher compared to the general population, indicating evidence for public health policy formulation. In addition, our review revealed that MDR and XDR-TB is widespread in prisons. Multiple factors such as overcrowding, poor ventilation, malnutrition, HIV, and others increases the transmission of TB within prisons. It is suggested that political leaders and scientific communities should work together and give special attention to the control of TB and MDR-TB in prisons. If not, TB in prisons will remain a neglected global problem and threaten national and international TB control programmes. Further research is required on the prevalence and drug resistance of smear-negative TB in prisons. In addition, evidence of the circulating strains and transmission dynamics inside the prison setting is also warranted.
ACKNOWLEDGEMENTS
We thank the German Federal Ministry of Education and Research (BMBF 1315883), the Institute of Medical Microbiology and Epidemiology of Infectious Diseases, Clinical Immunology, University Hospital Leipzig, Germany, Translational Centre for Regenerative Medicine (TRM)-Leipzig, University of Leipzig, Germany, the German Academic Exchange Service (DAAD), and University of Bahir Dar, Ethiopia for supporting and funding the research.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Aerts A, et al. Pulmonary tuberculosis in prisons of the ex-USSR state Georgia: results of a nationwide prevalence survey among sentenced inmates. International Journal of Tuberculosis and Lung Disease 2000; 4: 1104–1110. [PubMed] [Google Scholar]
- 2.Habeenzu C, et al. Tuberculosis and multidrug resistance in Zambian prisons, 2000–2001. International Journal of Tuberculosis and Lung Disease 2007; 11: 1216–1220. [PubMed] [Google Scholar]
- 3.O'Grady J, et al. Tuberculosis in prisons in sub-Saharan Africa – the need for improved health services, surveillance and control. Tuberculosis (Edinburgh) 2011; 91: 173–178. [DOI] [PubMed] [Google Scholar]
- 4.Drobniewski F. Tuberculosis in prisons-forgotten plague. Lancent 1995; 345: 948–949. [DOI] [PubMed] [Google Scholar]
- 5.Noeske J, et al. Pulmonary tuberculosis in the Central Prison of Douala, Cameroon. East African Medical Journal 2006, 83: 25–30. [DOI] [PubMed] [Google Scholar]
- 6.Aerts A, et al. Tuberculosis and tuberculosis control in European prisons. International Journal of Tuberculosis and Lung Disease 2006; 10: 1215–1223. [PubMed] [Google Scholar]
- 7.Braun MM, et al. Increasing incidence of tuberculosis in a prison inmate population. Journal of the American Medical Association 1989; 261: 393–397. [PubMed] [Google Scholar]
- 8.Snider DE Jr., Hutton MD Tuberculosis in correctional institutions. Journal of the American Medical Association 1989; 261: 436–437. [PubMed] [Google Scholar]
- 9.Coninx R, et al. First-line tuberculosis therapy and drug resistant M. tuberculosis in prisons. Lancent 1999; 353: 969–973. [DOI] [PubMed] [Google Scholar]
- 10.Pleumpanupat W, et al. Resistance to anti-tuberculosis drugs among smear positive cases in Thai prisons 2 years after the implementation of the DOTS strategy. International Journal of Tuberculosis and Lung Disease 2003; 7: 472–477. [PubMed] [Google Scholar]
- 11.Ruddy M, et al. Rates of drug resistance and risk factor analysis in civilian and prison patients with tuberculosis in Samara Region, Russia. Thorax 2005, 60: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toungoussova OS, et al. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates in the Archangel prison in Russia: predominance of the W-Beijing clone family. Clinical Infectious Diseases 2003; 37: 665–672. [DOI] [PubMed] [Google Scholar]
- 13.Ignatova A, et al. Predominance of multi-drug-resistant LAM and Beijing family strains among Mycobacterium tuberculosis isolates recovered from prison inmates in Tula Region, Russia. Journal of Medical Microbiology 2006, 55: 1413–1418. [DOI] [PubMed] [Google Scholar]
- 14.CDC. Rapid assessment of tuberculosis in a large prison system – Botswana, 2002. Morbidity and Mortality Weekly Report 2003; 52: 250–252. [PubMed] [Google Scholar]
- 15.Koffi N, et al. Smear positive pulmonary tuberculosis in a prison setting: experience in the penal camp of Bouake, Ivory Coast. International Journal of Tuberculosis and Lung Disease 1997; 1: 250–253. [PubMed] [Google Scholar]
- 16.Abebe DS, et al. Prevalence of pulmonary tuberculosis and associated risk factors in Eastern Ethiopian prisons. International Journal of Tuberculosis and Lung Disease 2010; 15: 668–673. [DOI] [PubMed] [Google Scholar]
- 17.Moges B, et al. Prevalence of smear positive pulmonary tuberculosis among prisoners in North Gondar Zone Prison, northwest Ethiopia. BMC Infectious Diseases 2012; 12: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyangulu DS, et al. Tuberculosis in a prison population in Malawi. Lancet 1997; 350: 1284–1287. [DOI] [PubMed] [Google Scholar]
- 19.Banda HT, et al. Prevalence of smear-positive pulmonary tuberculosis among prisoners in Malawi: a national survey. International Journal of Tuberculosis and Lung Disease 2009; 13: 1557–1559. [PubMed] [Google Scholar]
- 20.Rutta E, et al. Tuberculosis in a prison population in Mwanza, Tanzania. International Journal of Tuberculosis and Lung Disease 1994. –1997; 2001: 703–706. [PubMed] [Google Scholar]
- 21.Yerokhin VV, Punga VV, Rybka LN. Tuberculosis in Russia and the problem of multiple drug resistance. Annals of the New York Academy of Sciences 2001; 953: 133–137. [DOI] [PubMed] [Google Scholar]
- 22.Stern V. Problems in prisons worldwide, with a particular focus on Russia. Annals of the New York Academy of Sciences 2001; 953: 113–119. [DOI] [PubMed] [Google Scholar]
- 23.Jittimanee SX, et al. A prevalence survey for smear positive tuberculosis in Thai prisons. International Journal of Tuberculosis and Lung Disease 2007; 11: 556–561. [PubMed] [Google Scholar]
- 24.MacNeil JR, Lobato MN, Moore M. An unanswered health disparity: tuberculosis among correctional inmates, 1993 through 2003. American Journal of Public Health 2005; 95: 1800–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez A, et al. Prevalence of pulmonary tuberculosis and comparative evaluation of screening strategies in a Brazilian prison. International Journal of Tuberculosis and Lung Disease 2005; 9: 633–639. [PubMed] [Google Scholar]
- 26.Bergmire-Sweat D, et al. Tuberculosis outbreak in a Texas prison, 1994. Epidemiology and Infection 1996; 117: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin SV et al. Predictive factors of M. tuberculosis infection and pulmonary tuberculosis in prisoners. International Journal of Epidemiology 1995; 24: 630–636. [DOI] [PubMed] [Google Scholar]
- 28.Rasolofo-Razanamparany V, et al. Transmission of tuberculosis in the prison of Antananarivo (Madagascar). Research in Microbiology 2000; 151: 785–795. [DOI] [PubMed] [Google Scholar]
- 29.Rao NA. Prevalence of pulmonary tuberculosis in Karachi central prison. Journal of Pakistan Medical Association 2004; 54: 413–415. [PubMed] [Google Scholar]
- 30.WHO, ICRC. Tuberculosis control in prison: a manual for program managers, 2000. Report No.: WHO/CDS/TB/2000·281.
- 31.Slavuckij A, et al. Decentralization of the DOTS programme within a Russian penitentiary system. How to ensure the continuity of tuberculosis treatment in pre-trial detention centres. European Journal of Public Health 2002; 12: 94–98. [DOI] [PubMed] [Google Scholar]
- 32.Lobacheva T, et al. Pulmonary tuberculosis in two remand prisons (SIZOs) in St Petersburg, Russia. Eurosurveillance. 2005; 10: 93–96. [PubMed] [Google Scholar]
- 33.Hanau-Berçot B et al. A one-year prospective study (1994–1995) for a first evaluation of tuberculosis transmission in French prisons. International Journal of Tuberculosis and Lung Disease 2000; 4: 853–859. [PubMed] [Google Scholar]
- 34.Kiter G, et al. Tuberculosis in Nazilli District Prison, Turkey, 1997–2001. International Journal of Tuberculosis and Lung Disease 2003; 7: 153–158. [PubMed] [Google Scholar]
- 35.Hutton MD, Cauthen GM, Bloch AB. Results of a 29-state survey of tuberculosis in nursing homes and correctional facilities. Public Health Reports 1993; 108: 305–314. [PMC free article] [PubMed] [Google Scholar]
- 36.Abrahão RM, Nogueira PA, Malucelli MI. Tuberculosis in county jail prisoners in the western sector of the city of São Paulo, Brazil. International Journal of Tuberculosis and Lung Disease 2006; 10: 203–208. [PubMed] [Google Scholar]
- 37.Lemos AC, Matos ED, Bittencourt CN. Prevalence of active and latent TB among inmates in a prison hospital in Bahia, Brazil. Jornal Brasileiro de Pneumologia 2009; 35: 63–68. [DOI] [PubMed] [Google Scholar]
- 38.Öngen G, et al. Pulmonary tuberculosis incidence in Turkish prisons: importance of screening and case finding strategies. Tuberculosis and Thorax 2013; 61: 21–27. [DOI] [PubMed] [Google Scholar]
- 39.Rueda ZV, et al. High incidence of tuberculosis, low sensitivity of current diagnostic scheme and prolonged culture positivity in four Colombian prisons. A cohort study. PLoS ONE 2013; 8: e80592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.March F, et al. Predictors of tuberculosis transmission in prisons: an analysis using conventional and molecular methods. AIDS 2000; 14: 525–535 [DOI] [PubMed] [Google Scholar]
- 41.Pfyffer GE, et al. Multidrug-resistant tuberculosis in prison inmates, Azerbaijan. Emerging Infectious Diseases 2001; 7: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Story A, et al. Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax 2007; 62: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernández-León C, et al. Active tuberculosis in a cohort of HIV-infected inmates in a prison in Mexico City: clinical and epidemiological characteristics. Salud Pública de México 2012; 54: 571–578. [DOI] [PubMed] [Google Scholar]
- 44.Chiang CY, et al. Pulmonary tuberculosis in the Taiwanese prison population. Journal of the Formosan Medical Association 2002; 101: 537–541. [PubMed] [Google Scholar]
- 45.Sretrirutchai S, et al. Tuberculosis in Thai prisons: magnitude, transmission and drug susceptibility. International Journal of Tuberculosis and Lung Disease 2002; 6: 208–214. [PubMed] [Google Scholar]
- 46.Wong MY, et al. TB surveillance in correctional institutions in Hong Kong 1999–2005. International Journal of Tuberculosis and Lung Disease 2008; 12: 93–98. [PubMed] [Google Scholar]
- 47.Banu S, et al. Prevalence Tuberculosis and drug resistance in Dhaka Central Jail, the largest prison in Bangladesh. PLoS ONE 2010; 5: e10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO. Multidrug and extensively drug-resistant TB (M/XDR-TB) – 2010 global report on surveillance and Response. Geneva: World Health Organization, 2010. [Google Scholar]
- 49.WHO. Anti-tuberculosis drug resistance in the world. Third global report. TheWHO/IUATLD Global Project on Antituberculosis Drug Resistance Surveillance, 1999–2002. Geneva: World Health Organization, 2004. [Google Scholar]
- 50.Kimerling ME, et al. Inadequacy of the current WHO re-treatment regimen in a central Siberian prison: treatment failure and MDR-TB. International Journal of Tuberculosis and Lung Disease 1999; 3: 451–453. [PubMed] [Google Scholar]
- 51.Drobniewski F, et al. Rifampin- and multidrug-resistant tuberculosis in Russian civilians and prison inmates: dominance of the Beijing strain family. Emerging Infectious Diseases 2002; 8: 1320–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balabanova Y, et al. Survival of civilian and prisoner drug-sensitive, multi and extensive drug- resistant tuberculosis cohorts prospectively followed in Russia. PLoS ONE 2011; 6: e20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.CDC. Probable transmission of multidrug resistant tuberculosis in a correctional facility-Calfornia. Morbidity and Mortality Weekly Report 1993; 42: 48–51. [PubMed] [Google Scholar]
- 54.Valway SE, et al. Multidrug-resistant tuberculosis in the New York State prison system, 1990–1991. Journal of Infectious Diseases 1994; 170: 151–156. [DOI] [PubMed] [Google Scholar]
- 55.Anderson C, et al. Tuberculosis in UK prisoners: a challenge for control. Journal of Epidemiology and Community Health 2010; 64: 373–376. [DOI] [PubMed] [Google Scholar]
- 56.Narasimhan P, et al. Risk factors for tuberculosis. Pulmonary Medicine 2013; 2013: 828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bloom B, Murray CJL. Tuberculosis .Commentary on a re emergent killer. Science 1992, 257: 1055–1064. [DOI] [PubMed] [Google Scholar]
- 58.Smith PG. Epidemiology of tuberculosis. In: Bloom B, ed. Tuberculosis, Pathogenesis, Protection and Control. Washington, DC: American Society for Microiology, 1994. [Google Scholar]
- 59.Noeske J. Tuberculosis control in prisons. Health/AIDS Programme Cameroon (http://www.intechopen.com/download/get/type/pdfs/id/36957).
- 60.Winetsky DE, et al. Prevalence, risk factors and social context of active pulmonary tuberculosis among prison Inmates in Tajikistan. PLoS ONE 2014; 9: e86046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rieder HL. Epidemiologic Basis of Tuberculosis Control, 1st edn. Paris, IUATLD, 1999 [Google Scholar]
- 62.Lobacheva T, Asikainen T, Giesecke J. Risk factors for developing tuberculosis in remand prisons in St. Petersburg, Russia – a case-control study. European Journal of Epidemiology 2007; 22: 121–127. [DOI] [PubMed] [Google Scholar]
- 63.Coker R, et al. Risk factors for pulmonary tuberculosis in Russia: case-control study. British Medical Journal 2006; 14: 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacNeil JR, et al. Jails, a neglected opportunity for tuberculosis prevention. American Journal of Preventive Medicine 2005; 28: 225–228. [DOI] [PubMed] [Google Scholar]
- 65.Lienhardt C. From exposure to disease: The role of environmental factors in susceptibility to and development of tuberculosis. Epidemiologic Reviews 2001; 23: 288–301. [DOI] [PubMed] [Google Scholar]
- 66.Shaw JB, Wynn-Williams N. Infectivity of pulmonary tuberculosis in relation to sputum status. American Review of Tuberculosis 1954; 69: 724–732. [DOI] [PubMed] [Google Scholar]
- 67.North RJ, Izzo AA. Mycobacterial virulence. Virulence strains of Mycobacteria tuberculosis have faster in vivo doubling times and are better equipped to resist growth inhibiting functions of macrophages in the presence and absence of specific immunity. Journal of Experimental Medicine 1993; 177: 1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeager H Jr., et al. Quantitative studies of Mycobacterial populations in sputum and saliva. American Review of Respiratory Disease 1967, 95: 998–1004. [DOI] [PubMed] [Google Scholar]
- 69.Beggs CB, et al. The transmission of tuberculosis in confined places: an analytical review of alternative epidemiological models. International Journal of Tuberculosis and Lung Disease 2003; 7: 1015–1026. [PubMed] [Google Scholar]
- 70.Di Perri G, et al. Nosocomial epidemic of active tuberculosis in HIV infected patients. Lancet 1989; 2: 1502–1504. [PubMed] [Google Scholar]
- 71.Newport MJ, et al. A mutation in the IFNγ receptor gene and susceptibility to Mycobacterial infection. New England Journal of Medcine 1996; 26: 1941–1949. [DOI] [PubMed] [Google Scholar]
- 72.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infectious Diseases 2003; 3: 578–590 [DOI] [PubMed] [Google Scholar]
- 73.Comstock GW. Epidemiology of tuberculosis. American Review of Respiratory Disease 1982; 125 (Suppl.): 8–15. [DOI] [PubMed] [Google Scholar]
- 74.Reyes H. Pitfalls of TB management in prisons, revisited. International Journal of Prison Health 2007, 3: 43–67. [Google Scholar]
- 75.Greifinger RB, Heywood NJ, Glaser JB. Tuberculosis in prison: balancing justice and public health. Journal of Law, Medicine and Ethics 1993; 21: 332–341. [DOI] [PubMed] [Google Scholar]
- 76.Baussano I, et al. Tuberculosis incidence in prisons: a systematic review. PLoS Medicine 2010; 7: e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coninx R, et al. Tuberculosis in prisons in countries with high prevalence. British Medical Journal (Clinical Research Edition) 2000; 12: 320, 440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dara M, et al. Guidelines for control of tuberculosis in prisons. Tuberculosis Coalition for Technical Assistance and International Committee of the Red Cross, 2009.
- 79.Vinkeles Melchers NV, et al. State of affairs of tuberculosis in prison facilities: a systematic review of screening practices and recommendations for best TB control. PLoS ONE 2013; 8: e53644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.WHO. The stop TB strategy. The global plan to stop TB 2011–2015 (http://www.stoptb.org/assets/documents/global/plan/IP_factsheet_strategy_screen.pdf).
- 81.Harries AD, et al. Tuberculosis control in Malawian prisons: from research to policy and practice. International Journal of Tuberculosis and Lung Disease 2004; 8: 614–617. [PubMed] [Google Scholar]