SUMMARY
The objective of this study was to estimate the direct financial costs of hospital care for management of invasive group A streptococcal (GAS) infections using hospital records for cases diagnosed in England. We linked laboratory-confirmed cases (n = 3696) identified through national surveillance to hospital episode statistics and reimbursement codes. From these codes we estimated the direct hospital costs of admissions. Almost all notified invasive GAS cases (92% of 3696) were successfully matched to a primary hospital admission. Of these, secondary admissions (within 30 days of primary admission) were further identified for 593 (17%). After exclusion of nosocomial cases (12%), the median costs of primary and secondary hospital admissions were estimated by subgroup analysis as £1984–£2212 per case, totalling £4·43–£6·34 million per year in England. With adjustment for unmatched cases this equated to £4·84–£6·93 million per year. Adults aged 16–64 years accounted for 48% of costs but only 40% of cases, largely due to an increased number of surgical procedures. The direct costs of hospital admissions for invasive GAS infection are substantial. These estimated costs will contribute to a full assessment of the total economic burden of invasive GAS infection as a means to assess potential savings through prevention measures.
Key words: Cost of disease, hospital costs, streptococcal infections, Streptococcus pyogenes, treatment costs
INTRODUCTION
Group A streptococcal (GAS) infection causes a diverse range of diseases from common, mild complaints such as pharyngitis, to severe conditions, such as necrotizing fasciitis [1]. While invasive GAS infections (Streptococcus pyogenes isolated from a normally sterile site) are uncommon at around 3/100 000 persons in the UK and other developed countries [2, 3], most result in hospitalization [4–6] and around one fifth will require critical care [2, 6]. Mortality is very high, estimated at 16% at 7 days post-diagnosis [7]. Although risk factors for invasive GAS infection are well established [8], in about a quarter of cases no risk factors are identified [2]. Asymptomatic carriers may be sources of GAS infection [1] and given that most cases occur sporadically, opportunities for prevention are limited [8]. As such, there is a clear need to develop a safe and effective GAS vaccine [9]. However, the diversity in serotypes [10], cross-reaction of antibodies with human tissues [11] and the lack of suitable animal models [12] have all restricted vaccine development.
Invasive GAS infection accounts for only part of the global burden of GAS infections [1, 13] yet given the complex care needed for management of invasive GAS infections the financial costs of hospitalization may be considerable. An assessment of the full economic burden will be required before considering the cost-effectiveness of any vaccination programme. The societal costs of GAS pharyngitis in an area of the USA have been estimated to be $205 per case [13] but the cost of hospital admissions associated with invasive GAS infection are currently unknown. Methods to estimate the cost of hospital admissions for infections can either utilize the actual billing cost [14–16] or use unit costs based on either the type of admission [17, 18] or a diagnosis resource group [19–25].
As a means to assess the economic burden associated with hospital care for the management of invasive GAS infection, we estimated the direct costs of hospital resources associated with admissions for a 3-year period in England (2008–2010). We used a case-based methodology with healthcare resource group codes derived from a national database of hospital admissions (Hospital Episode Statistics; HES). These codes are used to reimburse costs for care provided to patients admitted to hospital in England.
METHODS
Case definitions and ascertainment
Cases were defined by the isolation of GAS from a normally sterile site from clinical specimens taken between 1 January 2008 and 31 December 2010. Cases were identified through a national database integrating routine laboratory surveillance reports and records of isolates submitted to the national reference laboratory. For individuals with more than one specimen, the date of the earliest GAS-positive specimen was used and the individual included once in this study. A nosocomial case was defined as one where the date of the positive specimen was ⩾2 days after hospital admission.
Cases were linked to hospital admissions using their unique National Health Service (NHS) number. A primary admission was defined as one where the GAS-positive specimen was taken during the admission or up to 14 days prior to the admission date (in order to include cases with a delayed admission, for example patients initially managed in community healthcare facilities). A secondary admission was defined as a subsequent admission ⩽30 days after the primary admission discharge.
Healthcare resource groups
In order to cost admissions, the ICD-10 primary diagnosis codes and the interventions and procedures (Office of Population Censuses and Surveys classification of interventions and procedures, version 4; OPCS-4) recorded for each case within HES were used to generate a healthcare resource group casemix classification code (version 4, HRG4) using the 2010/2011 NHS Local Payment Grouper (LPG; http://www.hscic.gov.uk). The LPG uses codes recorded for a hospital admission and produces a single HRG4 code for that admission. Where an admission was followed by transfer(s) to another hospital, these admissions were combined to generate a single HRG4 code. HRG4 codes were translated into reimbursement costs by referring to the NHS national tariff for 2010/2011 [26]. Extended hospital stays not covered by the standard tariff (long stay trim-point) were identified and costs adjusted using the per diem for each code.
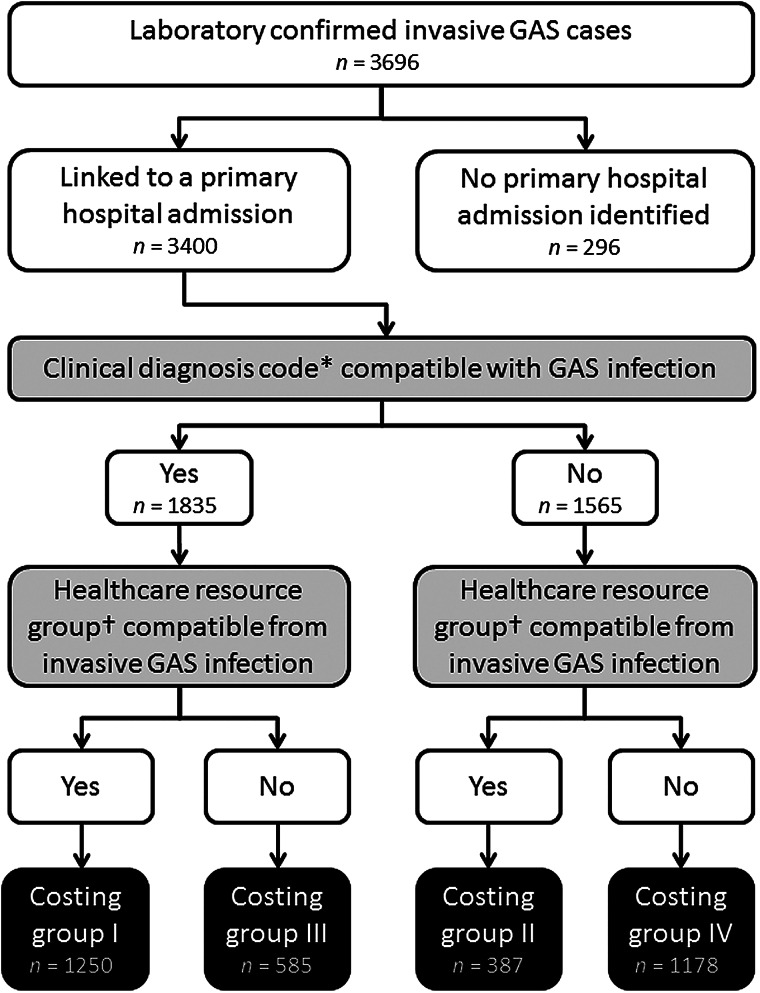
Cases with a primary hospital admission were divided into four groups (I–IV) to account for the differing degree of certainty in attributing their hospital costs directly to invasive GAS infection. This group allocation was based on the presence or absence of (1) diagnosis codes for invasive GAS infection combined with the presence or absence of (2) a resource group code compatible with treatment for an invasive GAS infection (creating four groups in total; Fig. 1). For (1), ICD-10 diagnosis codes were used based on those recorded which included mention of: abscess, cellulitis, empyema, endocarditis, endometriosis, fasciitis, gangrene, infective arthritis, meningitis, myositis, osteomyelitis, peritonitis, pneumonia, puerperal sepsis, sepsis, septicaemia, toxic shock syndrome, unspecified streptococcal infection. We excluded ICD-10 codes that specified non-GAS organisms. For (2), we derived a list of HRG4 codes that were associated with ICD-10 codes from (1) in the 2010/2011 NHS HRG4 Code to Group workbook v. 1·0 (http://www.hscic.gov.uk).
Fig. 1.
Schematic outlining the attribution of cases to costing groups of invasive group A streptococcal (GAS) cases. * ICD-10 primary diagnosis code. † Healthcare resource group code, version 4.
As a new LPG is released each year, we tested the suitability of using a single LPG for the 3 years of study data by assessing the consistency of coding for primary diagnosis (ICD-10), main operative procedures (OPCS-4) and resource group (HRG4) codes for all primary admissions. For this purpose we compared only the distribution of codes that accounted for ⩾1% of primary admissions during a study year and where the code had a hierarchy level ⩾3 (thus excluding low resource expenditure procedures). Study groups were also created for three character OPCS-4 codes for the main operation, excluding subsidiary classification codes (from OPCS-4, chapters Y and Z) from all primary admissions not defined as nosocomial infections.
Costing critical care
As payments for critical care in the NHS are covered by separate arrangements to other costs for admitted care, these are not reliably identified through HES. In recognition of this, the expected frequency of critical care was determined using the available enhanced surveillance data for invasive GAS in England, for 2003/2004 and 2009 [2, 27]. HES data for Augmented Care Periods (ACPs) were available for cases with a hospital admission between April 2008 and March 2009 and used to determine the number of organs supported during critical care and the length of stay on intensive-care or high-dependency units. These were then used to cost critical care episodes according to NHS benchmark costs for 2010/2011 [28].
Sensitivity and age group analysis
A subgroup sensitivity analysis was undertaken in order to account for the inability to determine those admissions with costs directly as a result of invasive GAS infection and uncertainty as to which secondary admissions were truly a consequence of invasive GAS infection. This sensitivity analysis calculated costs with and without costing group IV (Fig. 1).
Analysis of costs was undertaken according to the following age groups: children (⩽15 years), working age adults (16–64 years), older adults (⩾65 years).
Statistical analysis
Comparison of continuous variables for independent groups was performed using the Mann–Whitney U test.
RESULTS
Linkage of cases to hospital admissions
Of the 3696 cases of invasive GAS infection identified through national surveillance in England during 2008–2010, 3555 (96%) were linked to ⩾1 hospital admission. Of these, 3400 (92% of total cases) represented primary admissions, of which 227 involved transfers between hospitals and 593 (17%) had ⩾1 secondary admission. The median interval between primary discharge and secondary admission was 9 days [interquartile (IQR) range 3–20]. Of the 3383 primary admissions, 403 (11·9%) represented nosocomial infections.
Clinical diagnoses
A wide array of ICD-10 diagnostic codes was associated with primary admissions, reflecting both the spectrum of clinical presentations for invasive GAS infection and comorbidities (Supplementary Fig. S1). Up to six primary diagnosis codes were indicated, although the majority of cases had just one code (2761/3400, 81·2%). Over half (1835/3400, 54%) of primary admissions were associated with an invasive GAS diagnosis code although only one code specifically mentioned GAS as the causative organism (A40·0, ‘Sepsis due to streptococcus, group A’), recorded for 12% (422/3400) of primary admissions. The four highest frequency diagnosis groups were septicaemia (638, 15·2%), skin and soft tissue infection (540, 12·9%), pneumonia (373, 8·9%), and infective arthritis (212, 5·1%). Of the 1756 different ICD-10 diagnosis codes, 117 included a named condition consistent with invasive GAS infection and of these 93 were used as indicators of admission for invasive GAS infection (Supplementary Table S1) and to identify 48 corresponding resource group codes (Supplementary Table S2) to define costing groups (Table 1).
Table 1.
Costing groups for primary admissions of invasive GAS infection cases based on diagnosis and healthcare resource group codes, England 2008–2010
| Costing group | Invasive GAS diagnosis code* | Invasive GAS hospital costing code† | Cases (%)‡ |
|---|---|---|---|
| I | Yes | Yes | 1250 (33·8) |
| II | No | Yes | 387 (10·5) |
| III | Yes | No | 585 (15·8) |
| IV | No | No | 1178 (31·9) |
| All primary admissions | Yes/No | Yes/no | 3400 (92·0) |
GAS, Group A streptococcal.
One of 93 ICD-10 codes selected as indicative of invasive GAS infection.
One of 48 healthcare resource group, version 4 codes associated with the 93 invasive GAS ICD-10 diagnosis codes.
Cases included from the full dataset (n = 3696) were those with a linked primary hospital admission (n = 3400).
Although the diversity of diagnosis codes for secondary admissions was substantial (Supplementary Fig. S2), 288 (28·9%) of these 994 diagnoses (after excluding those associated with nosocomial cases) were from ICD-10 groups compatible with diagnosis of invasive GAS infection: septicaemia (42, 4·2%), skin and subcutaneous tissue infections (75, 7·5%), pneumonia (42, 4·2%), infective arthritis (73, 7·3%), other groups (56, 5·6%).
Healthcare resource groups
All primary admissions were successfully assigned a resource group code. Diagnosis, procedure and resultant resource group codes showed a general consistency of use over the study period (Supplementary Fig. S3). HRG4 resource group codes for primary admissions were predominantly those for infectious diseases (502, 14·8%), skin disorders (424, 12·5%), thoracic procedures and disorders (406, 11·9%), and paediatric medicine (404, 11·9%) (Supplementary Fig. S4). The paediatric medicine subchapter contains clinical codes for care of all children ⩽18 years, of the 404 primary admissions with resource group codes within this subchapter >75% were associated with codes for either infections (288, 71·3%) or skin disorders (20, 5·0%).
Costs of hospital admissions
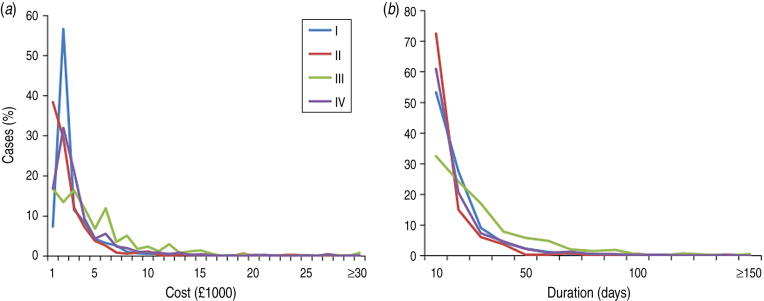
The costing groups used in this study separate cases based on diagnosis and treatment codes (Table 1), with clear differences in hospital admission costs and length of stay after removing nosocomial cases (Fig. 2). Of the nosocomial infections which could be costed (n = 391), 118 (30%) had an HRG4 resource group code not included in our list of invasive GAS infection codes, suggesting costs dominated by management of other conditions.
Fig. 2.
Distribution of (a) primary admission costs (Healthcare resource group code version 4 resource groups) and (b) duration of primary admission for cases in costing groups (I–IV). Nosocomial cases have been excluded.
The highest proportion of cases fell within costing group I (1250, 34% of all cases; Table 2), characterized by both diagnosis and treatment compatible with invasive GAS infection. Median costs of hospital care (entire stay, excluding critical care) were £1707 for these patients. Costing group II, cases without invasive GAS clinical diagnosis codes but whose care was compatible with management of invasive GAS infection, had shorter admissions (median 5 days, Fig. 2) and lower in-hospital mortality rates (7%, Table 1). Conversely, costing group III, patients with invasive GAS clinical diagnosis codes but without resource codes directly attributable to invasive GAS infection (e.g. non-trauma-related procedures), had substantially longer admissions (median 17 days, Fig. 2) and high costs (median £3108, excluding critical care; Fig. 2). Costing group IV represents cases with neither an invasive GAS diagnosis code nor an invasive GAS resource group code and contained the highest proportion of nosocomial cases for any costing group (Table 2).
Table 2.
Costs of primary hospital admissions for cases of invasive GAS infection, England 2008–2010
| Costing group | n | Median duration of primary admission (IQR) | Hospital care (HRG4) costs (£) | Critical care costs | Combined annual HRG4 and critical care cost (million £) | Nosocomial GAS infections | Annual costs adjusted for nosocomial GAS infections (million £) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Annual* (millions) | n (%) | Median (£, IQR) | Annual (million £) | Adjusted† annual (million £) | Cases (%) | Costs of admissions (million £) | |||||
| I | 1250 | 9 (4 –17) | 1707 (1324–3092) | 1·20 | 65 (5·2) | 5544 (1386– 12470) | 0·18 | 0·74 | 1·94 | 77 (6·2) | 0·13 | 1·81 |
| II | 387 | 5 (3 –13) | 1527 (842–2872) | 0·34 | 12 (3·1) | 4851 (2772– 12820) | 0·04 | 0·17 | 0·51 | 41 (10·6) | 0·10 | 0·41 |
| III | 585 | 17 (8 –32) | 3108 (1653–5599) | 0·87 | 61 (10·4) | 8316 (1386– 29110) | 0·35 | 1·38 | 2·26 | 53 (9·1) | 0·24 | 2·01 |
| IV | 1161 | 9 (3 –20) | 2141 (1132– 4086) | 1·39 | 46 (3·9) | 4158 (1386– 9702) | 0·14 | 0·58 | 1·96 | 220 (18·9) | 0·49 | 1·47 |
| All primary admissions‡ | 3383 | 9 (3 –20) | 1980 (1292–3872) | 3·80 | 184 (5·4) | 5544 (1386– 14210) | 0·72 | 2·87 | 6·67 | 391 (11·6) | 0·96 | 5·71 |
GAS, group A streptococcal; HRG4, Healthcare resource group, version 4; IQR, interquartile range.
Annual costs are an average for the three years of the study.
Costs adjusted on basis of expected overall critical care frequency.
Cases from the full dataset (n = 3696) were those with a linked primary hospital admission (n = 3400) after removal of cases which could not be costed (n = 17).
Invasive GAS cases which died in hospital had reduced lengths of admission and costs, although this was not statistically significant for costing groups II and IV (Table 3). The proportion of nosocomial cases was significantly higher for costing groups where diagnosis of invasive GAS infection could not be determined from HES [costing groups I and III: 7·8% (130/1835); costing groups II and IV: 17·4% (273/1565); χ2 = 86·76, P < 0·0001]. The median length of critical care stay for the small proportion of cases where this could be determined (184, 5·4%) was 4 days (IQR 1–10). ACP data identified a median of three organs supported during critical care (pragmatically assumed to be constant during a critical care stay), translating to £1386 per bed-day. For total cost estimates, we adjusted HES costs using external validation of the likely frequency of critical care (20% of cases).
Table 3.
Duration of primary admission and hospital care costs for cases of invasive GAS infection which died in hospital, England 2008–2010
| Costing group | n* | Cases who died in hospital (%) | Median duration of primary admission in days (IQR) | Median hospital care (HRG4) costs (IQR) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-fatal cases | Fatal cases | Comparison† | Non-fatal cases | Fatal cases | Comparison† | |||||
| Z score | P value | Z score | P value | |||||||
| I | 1173 | 219 (18·7) | 10 (5–18) | 2 (1–6) | 13·04 | <0·0001 | 1729 (1324–3128) | 1707 (1324–1707) | 5·06 | <0·0001 |
| II | 346 | 19 (5·5) | 5 (2–11) | 0 (0–3) | 5·02 | <0·0001 | 1425 (842–2597) | 1324 (751–1707) | 1·55 | 0·0123 |
| III | 532 | 83 (15·6) | 18 (9–33) | 2 (1–14) | 8·28 | <0·0001 | 3359 (1836–5940) | 1999 (1092–3906) | 3·72 | 0·0002 |
| IV | 941 | 161 (17·1) | 8 (3–16) | 1 (1–6) | 8·70 | <0·0001 | 2051 (1076–3568) | 1921 (1130–2900) | 0·47 | 0·6400 |
| All primary admissions | 2992 | 482 (16·1) | 10 (1–19) | 2 (1–7) | 17·60 | <0·0001 | 1944 (1175–3666) | 1707 (1324–2294) | 4·42 | <0·0001 |
HRG4, Healthcare resource group, version 4; IQR, interquartile range.
Cases included from the full dataset (n = 3696) were those with a linked primary hospital admission (n = 3400) after removal of nosocomial cases (n = 403) and/or those that could not be costed (n = 17, of which 12 were also nosocomial cases).
Mann–Whitney U test.
After producing cost estimates for secondary admissions (Table 4), subgroup sensitivity analysis estimated the cost of hospital resources for invasive GAS infections to be £4·43–£6·34 million per year in England (Table 5). After adjusting for cases which could not be linked to a hospital admission, these costs are £4·84–£6·93 million per year in England. The median cost per case was £1984–£2212, with substantial variation in the cost of individual admissions (£247–£358 100). Costs by age group were largely consistent with the distribution of cases, although the expected fraction of costs for working age adults (16–64 years) was slightly higher, while that for children (⩽15 years) and older adults (⩾65 years) reduced (Table 6). This would appear to be due to an increased number of operations (particularly abdominal, chest, skin and soft tissue surgeries), procedures related to pregnancy, and higher numbers of cases requiring cardiovascular and respiratory care (Table 7). It was not accounted for by an increased length of stay for working age adults (median 9 days, IQR 4–18), as length of stay was significantly longer for older adults (median 12 days, IQR 4–28, Z = −5·17, P < 0·0001).
Table 4.
Costs of secondary hospital admissions within 30 days of primary admission for cases of invasive GAS infection, England 2008–10
| Costing group | n* | Secondary admissions per case (no. of cases) | Total number of secondary admissions | Hospital care (HRG4) costs (£) | Combined annual cost‡ (million £) | Invasive GAS infection admissions§ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ⩾5 | ⩾1 | Median (IQR) | Annual† (millions) | n (%) | Annual cost (million £) | ||||
| I | 1173 | 957 | 173 | 25 | 7 | 3 | 8 | 216 | 315 | 1568 (935–2293) | 0·21 | 0·22 | 125 (39·7) | 0·10 |
| II | 346 | 286 | 43 | 10 | 3 | 3 | 1 | 60 | 92 | 1449 (742–2031) | 0·06 | 0·06 | 15 (16·3) | 0·01 |
| III | 532 | 415 | 94 | 15 | 3 | 4 | 1 | 117 | 159 | 1648 (1024–2886) | 0·14 | 0·14 | 78 (49·1) | 0·08 |
| IV | 941 | 741 | 151 | 29 | 3 | 3 | 14 | 200 | 377 | 1190 (630–1774) | 0·22 | 0·22 | 66 (17·5) | 0·05 |
| All primary admissions | 2992 | 2399 | 461 | 79 | 16 | 13 | 24 | 593 | 943 | 1449 (796–2061) | 0·63 | 0·63 | 284 (30·1) | 0·25 |
GAS, Group A streptococcal; HRG4, healthcare resource group, version 4; IQR, interquartile range.
Cases included from the full dataset (n = 3696) were those with a linked primary hospital admission (n = 3400) after removal of nosocomial cases (n = 403) and/or those that could not be costed (n = 17, of which 12 were also nosocomial cases).
Annual costs are an average for the 3 years of the study.
Includes costs for critical care.
Admissions defined by the recording of an invasive GAS infection ICD-10 diagnosis code.
Table 5.
Subgroup sensitivity analysis of hospital care costs for cases of invasive GAS infections, England 2008–2010
| Estimate level | Primary hospital admission costing groups (n*) | Percentage of total cases† | Total annual‡ costs of primary hospital admissions (million £) | Secondary hospital admissions included | Total costs of secondary hospital admissions (million £) | Combined costs of primary and secondary admissions (million £) | Median total costs (£) per costed case (IQR) |
|---|---|---|---|---|---|---|---|
| Lower | I–III (2 051) | 55·5 | 4·23 | Invasive GAS diagnosis§ | 0·20 | 4·43 | 1984 (1324–4458) |
| Upper | I–IV (2992) | 81·0 | 5·71 | All | 0·63 | 6·34 | 2212 (1324–4952) |
GAS, Group A streptococcal; IQR, interquartile range.
Cases included from the full dataset (n = 3696) were those with a linked primary hospital admission (n = 3400) after removal of nosocomial cases (n = 403) and/or those that could not be costed (n = 17, of which 12 were also nosocomial cases), for the lower estimate cases from costing group IV (n = 941) were also excluded.
For the entire study (n = 3696).
Annual costs are an average for the 3 years of the study.
Includes only secondary hospital admissions with a diagnosis code for invasive GAS infection.
Table 6.
Distribution of cases and total annual hospital care costs by age group for invasive GAS infections, England 2008–2010
| Age group | No. of cases (%) | Total annual costs (million £) | |
|---|---|---|---|
| Lower estimate (%) | Upper estimate (%) | ||
| All ages | 3383* (100·0) | 4·43 (100·0) | 6·34 (100·0) |
| 0–15 years | 539 (15·9) | 0·53 (12·0) | 0·72 (11·4) |
| 16–64 years | 1346 (39·8) | 2·11 (47·6) | 3·03 (47·8) |
| ⩾65 years | 1498 (44·2) | 1·80 (40·6) | 2·58 (40·7) |
GAS, Group A streptococcal.
Cases included from the full dataset (n = 3696) were those with a linked primary hospital admission (n = 3400) after removal of cases which could not be costed (n = 17).
Table 7.
Types of main operative procedures recorded for primary hospital admissions of cases of invasive GAS infection by age group and by number of main procedures, England 2008–2010
| OPCS-4 study group* | OPCS-4 study subgroup | No. of primary admissions undergoing ⩾1 main procedure (%†) | No. of primary admissions according to the number of main procedures recorded‡ | Total no. of procedures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All ages (n = 2997) | 0–15 yr (n = 511) | 16–64 yr (n = 1207) | ⩾65 yr (n = 1279) | 0 | 1 | 2 | 3 | 4 | ⩾5 | |||
| Cardiovascular care | Cardiac interventions | 15 (0·5) | 1 (0·2) | 8 (0·7) | 6 (0·5) | 2982 | 12 | 3 | 18 | |||
| Echocardiography | 229 (7·6) | 15 (2·9) | 129 (10·7) | 85 (6·6) | 2768 | 182 | 35 | 6 | 6 | 294 | ||
| Intravenous and intra-arterial procedures | 407 (13·6) | 58 (11·4) | 197 (16·3) | 152 (11·9) | 2590 | 273 | 101 | 18 | 6 | 9 | 612 | |
| Diagnostics | Biopsies | 28 (0·9) | 3 (0·6) | 14 (1·2) | 11 (0·9) | 2969 | 27 | 1 | 29 | |||
| Diagnostic imaging and tests | 882 (29·4) | 116 (22·7) | 410 (34·0) | 356 (27·8) | 2115 | 131 | 267 | 214 | 111 | 159 | 2807 | |
| Gastrointestinal care | Endoscopy or intubation of gastrointestinal tract | 105 (3·5) | 1 (0·2) | 36 (3·0) | 68 (5·3) | 2892 | 76 | 26 | 3 | 137 | ||
| Gynaecology and obstetric care | Female genital tract procedures | 10 (0·3) | 0 (0·0) | 8 (0·7) | 2 (0·2) | 2987 | 8 | 2 | 12 | |||
| Pregnancy and delivery (excluding surgical delivery) | 29 (1·0) | 0 (0·0) | 29 (2·4) | 0 (0·0) | 2968 | 21 | 7 | 1 | 38 | |||
| Renal care | Catheterization and endoscopic examination of bladder | 40 (1·3) | 1 (0·2) | 9 (0·7) | 30 (2·3) | 2957 | 33 | 4 | 2 | 1 | 51 | |
| Dialysis for renal failure | 121 (4·0) | 4 (0·8) | 66 (5·5) | 51 (4·0) | 2876 | 86 | 16 | 6 | 4 | 9 | 230 | |
| Respiratory care | Endoscopy of respiratory tract | 74 (2·5) | 8 (1·6) | 44 (3·6) | 22 (1·7) | 2923 | 57 | 13 | 2 | 2 | 97 | |
| Invasive ventilations and associated procedures | 109 (3·6) | 0 (0·0) | 65 (5·4) | 44 (3·4) | 2888 | 80 | 25 | 1 | 2 | 1 | 146 | |
| Non-invasive ventilations and associated procedures | 274 (9·1) | 38 (7·4) | 135 (11·2) | 101 (7·9) | 2723 | 215 | 48 | 7 | 3 | 1 | 352 | |
| Pleural drainage and respiratory tests | 128 (4·3) | 22 (4·3) | 86 (7·1) | 20 (1·6) | 2869 | 79 | 33 | 10 | 5 | 1 | 201 | |
| Surgery and associated procedures | Abdominal surgery | 72 (2·4) | 3 (0·6) | 55 (4·6) | 14 (1·1) | 2925 | 49 | 15 | 7 | 1 | 104 | |
| Amputations | 55 (1·8) | 1 (0·2) | 27 (2·2) | 27 (2·1) | 2942 | 38 | 11 | 4 | 1 | 1 | 88 | |
| Chest surgery including heart and lung | 21 (0·7) | 5 (1·0) | 13 (1·1) | 3 (0·2) | 2976 | 13 | 5 | 1 | 1 | 1 | 35 | |
| Female genital tract and pregnancy-related operations | 36 (1·2) | 1 (0·2) | 34 (2·8) | 1 (0·1) | 2961 | 29 | 5 | 1 | 1 | 46 | ||
| Gastrointestinal tract surgery | 41 (1·4) | 5 (1·0) | 21 (1·7) | 15 (1·2) | 2956 | 31 | 9 | 1 | 52 | |||
| Head and neck surgery | 64 (2·1) | 17 (3·3) | 29 (2·4) | 18 (1·4) | 2933 | 35 | 11 | 6 | 10 | 2 | 125 | |
| Skin surgery | 191 (6·4) | 11 (2·2) | 115 (9·5) | 65 (5·1) | 2806 | 80 | 44 | 22 | 22 | 23 | 469 | |
| Soft tissue and musculoskeletal surgery | 339 (11·3) | 55 (10·8) | 164 (13·6) | 120 (9·4) | 2658 | 171 | 85 | 44 | 19 | 20 | 670 | |
| Unclassified operative procedures | 170 (5·7) | 16 (3·1) | 102 (8·5) | 52 (4·1) | 2827 | 130 | 34 | 3 | 2 | 1 | 221 | |
| Treatment and rehabilitation | High cost drugs | 35 (1·2) | 4 (0·8) | 21 (1·7) | 10 (0·8) | 2962 | 26 | 8 | 1 | 46 | ||
| Injections, infusions and transfusions | 47 (1·6) | 11 (2·2) | 20 (1·7) | 16 (1·3) | 2950 | 40 | 5 | 1 | 1 | 62 | ||
| Other treatments (including anaesthetic drugs) | 54 (1·8) | 9 (0·3) | 30 (1·0) | 15 (0·5) | 2943 | 39 | 14 | 1 | 70 | |||
| Rehabilitation | 35 (1·2) | 0 (0·0) | 6 (0·5) | 29 (2·3) | 2962 | 31 | 3 | 1 | 40 | |||
| Resuscitation | 30 (1·0) | 1 (0·2) | 10 (0·8) | 19 (1·5) | 2967 | 28 | 2 | 32 | ||||
| Any costing group | Any study subgroup | 1688 (56·3) | 231 (45·2) | 794 (65·8) | 663 (51·8) | 1309 | 370 | 333 | 243 | 187 | 555 | 7084 |
GAS, Group A streptococcal; OPCS-4, Office of Population Censuses and Surveys classification of interventions and procedures, version 4.
Grouping according to Supplementary Table S3.
Cases included from the full dataset (n = 3696) were those with a linked primary hospital admission (n = 3400) after removal of nosocomial cases (n = 403) (n = 3400–403 = 2997).
Zeroes have been omitted for ease of presentation.
DISCUSSION
This study provides the first estimate of the cost of hospital care for invasive GAS infection. The generation of these costs has included careful consideration of both the clinical nature of the infection and the methodological approach to costing admissions using routinely collected hospital admission data. With a study of this kind, a single point estimate of costs will be unlikely to satisfactorily represent the full complexity of the analysis undertaken and the accompanying uncertainty. With this in mind, we have estimated the cost of hospital resources associated with admissions for treatment of invasive GAS infection to be £4·43–£6·34 million per year in England. This reflects uncertainty in directly attributing costs to treatment of invasive GAS infection for both primary and secondary hospital admissions. Conservatively we have excluded all nosocomial infections from our costing estimates. However, clinical management of invasive GAS infection may have dominated the treatment costs for some of these cases and their removal will represent an underestimate. We have also likely excluded some community-acquired cases where there was late diagnosis. Our estimate is for cases that we have been able to successfully link to a primary hospital admission and could be 8% higher (£4·84–£6·93 million per year) if we failed to identify corresponding hospital records for cases that were in fact treated within a hospital setting. Published percentages of cases requiring hospitalization range from 98–99% in Australia and New Zealand [4, 6] to 91% in the USA [5].
The lower range of our estimated costs is based on cases with invasive GAS infection diagnosis codes and/or invasive GAS infection healthcare resource group codes (for the associated primary hospital admission). The exclusion of other cases was made to remove admissions which may have been dominated by condition(s) independent to invasive GAS infection. However, this excludes a substantial proportion of cases (32%) with laboratory-confirmed invasive GAS infection, some of which will undoubtedly have been admitted to hospital because of that invasive GAS infection. Although exclusion of these cases is logical when wishing to reduce the risk of overestimating costs, such a step is reliant on accurate and relevant coding within HES and the a priori selection of appropriate invasive GAS infection defining codes. Although we have been thorough in our reduction of error from the latter, we have not determined the influence of inaccurate HES codes [29] or the dominance of other conditions with regard to diagnosis. Ultimately, these cases either reflect those where treatment for invasive GAS infection truly did not dominant hospital care or where the nature of HES coding has obscured direct recognition of that care.
We are not aware of any published studies which have applied this case level methodology to comparative invasive bacterial infections in England. A small study published over 20 years ago for invasive Haemophilus influenzae infection found costs for hospital admissions using base reference costs (£2300 without critical care, £4300 with) [30] to be not dissimilar to those found here, but this comparison does not consider inflation. Costs of hospital admissions for invasive pneumococcal infection (£903–£1547, depending on clinical presentation) [20] and invasive meningococcal infection (£2337) [31] have been produced for cost-effectiveness estimates and are also not widely different from our estimates for invasive GAS. However, the lack of a case list independent to hospital admission data prevents such aggregate studies accounting for variance in costs at an individual level. Moreover, the clinical diversity and distribution of comorbidities for invasive GAS infection restricts the application of definitions on the basis of diagnostic coding. Diagnoses made on admission to hospital may involve recording of underlying conditions and risk factors for invasive GAS infection such as cancer and chronic diseases [8] which do not preclude the presence (recognized or not) of a concomitant invasive GAS infection. This situation is further complicated for secondary hospital admissions where there is the combination of invasive GAS infection, post-operative care, post-infection care and post-infection sequelae. For this reason, we adopted a pragmatic approach to defining secondary admissions, applying a simple definition with the acceptance that both the inclusion of unrelated admissions and censoring of invasive GAS-related ones were likely. Nevertheless, we feel that the majority of re-admissions as a direct consequence of a previous invasive GAS infection will occur within 30 days of the end of the primary admission.
Critical care resources made up a substantial contribution to our estimates of costs, upon which we have made three assumptions. First, we have made a fixed adjustment based on the assumption that the HES-based methodology has under-ascertained critical care cases by a factor of 4 (due to the use of a dedicated separate data stream for critical care admissions). This assumption is based on published data for England [2, 27], Australia [4], Denmark [32] and New Zealand [6]. While the principle and magnitude of the adjustment are reasonable, we were unable to assess the level of adjustment separately for each costing group. As such, these subgroup-specific critical care estimates are not likely to be robust. Second, we have assumed that the duration of critical care episodes when recorded in HES is an accurate duration of critical care provided generally for invasive GAS patients. As the median duration of these episodes (4 days) appears to be broadly consistent with that reported in Canada [33] and New Zealand [6], this would appear to be sensible. Third, we have based costs on support for three organs during critical care, which is validated by limited data from our study and elsewhere [33]. After the discontinuation of the ACP dataset, critical care episodes are not routinely or consistently recorded in HES and a separate dataset now exists for that purpose [34].
The total economic burden of invasive GAS infections will of course include general practice consultations/other primary care costs and further costs associated with lost working days and life years. The latter will be substantial for invasive GAS infection given the associated high mortality, although for these cases healthcare costs will ultimately be reduced as death tends to occur rapidly after diagnosis [7]. We have limited our study to invasive infections caused by GAS and have not attempted to estimate the financial costs associated with the management of less severe GAS infections by primary care services or those costs associated with public health actions, such as the management of outbreaks and close contacts of cases. All of these costs are to our knowledge unknown yet vital should a full estimate of cost-effectiveness for vaccination be required. Invasive GAS infection has a high mortality rate and affects all ages, including the otherwise healthy [2], and the burden is likely to be considerable. Despite the modest progress in vaccine development for GAS infection [12], it seems likely that a commitment to development of a vaccine for GAS will continue [35]. The costing estimates from this study will contribute towards a full cost-effectiveness estimate as and when it is undertaken, but for now provides valuable knowledge of the financial costs of invasive GAS infections to secondary healthcare in England.
ACKNOWLEDGEMENTS
We thank Sam Bracebridge, Androulla Efstratiou and Russell Gorton for their contributions to development of this study. We also thank Suzanne Elgohari and Nick Hinton for performing data linkage, John Schofield from the NHS Information Centre for advice on costing, and Claudia Geue for commenting on an earlier version of this manuscript.
This work was supported by Public Health England. S.S. acknowledges the support of the National Institute for Health Research Biomedical Research Centre at Imperial College London.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814002489.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Ralph AP, Carapetis JR. Group A streptococcal diseases and their global burden. Current Topics in Microbiology and Immunology 2013; 368: 1–27. [DOI] [PubMed] [Google Scholar]
- 2.Lamagni TL, et al. Severe Streptococcus pyogenes infections, United Kingdom, 2003–2004 Epidemiology and Infection 2008; 14: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steer AC, et al. Invasive group A streptococcal disease. Drugs 2012; 72: 1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Grady K-AF, et al. The epidemiology of invasive group A streptococcal disease in Victoria, Australia. Medical Journal of Australia 2007; 186: 565–569. [DOI] [PubMed] [Google Scholar]
- 5.O'Loughlin RE, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clinical Infectious Diseases 2007; 45: 853–862. [DOI] [PubMed] [Google Scholar]
- 6.Safar A, et al. Invasive group A streptococcal infection and vaccine implications, Auckland, New Zealand. Emerging Infectious Diseases 2011; 17: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamagni TL, et al. Predictors of death after severe Streptococcus pyogenes infection. Emerging Infectious Diseases 2009; 15: 1304–1307. [DOI] [PubMed] [Google Scholar]
- 8.Smith A, et al. Invasive group A streptococcal disease: should close contacts routinely receive antibiotic prophylaxis? Lancet Infectious Diseases 2005; 5: 494–500. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organisation. Group A streptococcal vaccine development: current status and issues of relevance to less developed countries. 2005 (http://www.who.int/maternal_child_adolescent/documents/ivb_05_14/en). Accessed 4 July 2014.
- 10.Steer AC, et al. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infectious Diseases 2009; 9: 611–616. [DOI] [PubMed] [Google Scholar]
- 11.Ellis NMJ, et al. Priming the immune system for heart disease: a perspective on group A streptococci. Journal of Infectious Diseases 2010; 202: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 12.Henningham A, Gillen CM, Walker MJ. Group A streptococcal vaccine candidates: potential for the development of a human vaccine. Current Topics in Microbiology and Immunology 2013; 368: 207–242. [DOI] [PubMed] [Google Scholar]
- 13.Pfoh E, et al. Burden and economic cost of group A streptococcal pharyngitis. Pediatrics 2008; 121: 229–234. [DOI] [PubMed] [Google Scholar]
- 14.Kitchin OP, et al. Costs of admission for paediatric pneumonia in a setting of human immunodeficiency virus infection. International Journal of Tuberculosis and Lung Disease 2012; 15: 1702–1706. [DOI] [PubMed] [Google Scholar]
- 15.Dinleyici EC, et al. The epidemiology and economic impact of varicella-related hospitalizations in Turkey from 2008 to 2010: a nationwide survey during the pre-vaccine era (VARICOMP study). European Journal of Pediatrics 2012; 171: 817–825. [DOI] [PubMed] [Google Scholar]
- 16.Kim MK, et al. Factors associated with length of stay and hospital charges for patients hospitalized with mouth cellulitis. Journal of Oral and Maxillofacial Surgery 2012; 113: 21–28. [DOI] [PubMed] [Google Scholar]
- 17.Clark SJ, et al. Respiratory syncytial virus infection in high risk infants and the potential impact of prophylaxis in a United Kingdom cohort. Archives of Diseases in Childhood 2000; 83: 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen K-T, et al. Hospital-based study of the economic burden associated with rotavirus diarrhea in Taiwan. Vaccine 2007; 25: 4266–4272. [DOI] [PubMed] [Google Scholar]
- 19.Scott G, et al. Economic cost of community-acquired pneumonia in New Zealand adults. New Zealand Medical Journal 2004; 117: U933. [PubMed] [Google Scholar]
- 20.McIntosh EDG, et al. Pneumococcal pneumonia in the UK – how herd immunity affects the cost-effectiveness of 7-valent pneumococcal conjugate vaccine (PCV). Vaccine 2005; 23: 1739–1745. [DOI] [PubMed] [Google Scholar]
- 21.López-de-Andrés A, et al. Hospitalizations associated with rotavirus gastroenteritis in Spain, 2001–2005. BMC Public Health 2008; 8: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes ML, et al. Comparative costs of hospitalisation among infants at high risk for respiratory syncytial virus lower respiratory tract infection during the first year of life. Journal of Medical Economics 2010; 13: 136–141. [DOI] [PubMed] [Google Scholar]
- 23.Morgan C, et al. Burden on UK secondary care of rotavirus disease and seasonal infections in children. Current Medical Research and Opinion 2010; 26: 2449–2455. [DOI] [PubMed] [Google Scholar]
- 24.Desai S, et al. Genital warts and cost of care in England. Sexually Transmitted Infections 2011; 87: 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pockett RD, et al. A comparison of healthcare resource use for rotavirus and RSV between vulnerable children with co-morbidities and healthy children: a case control study. Journal of Medical Economics 2013; 16: 560–565. [DOI] [PubMed] [Google Scholar]
- 26.Department of Health. 2010–11 National Tariff Information. 2010 (http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_112284). Accessed 15 January 2013.
- 27.Health Protection Agency. Cessation of enhanced data collection for invasive group A streptococcal disease. Health Protection Report 2010; 4 (http://www.hpa.org.uk/hpr/archives/2010/news0310.htm). Accessed 4 April 2013.
- 28.Department of Health. Payment by results guidance for 2010–11. 2010 (http://collections.europarchive.org/tna/20100509080731/http:/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_112970.pdf). Accessed 7 January 2014.
- 29.Burns EM, et al. Systematic review of discharge coding accuracy. Journal of Public Health (Oxford) 2011; 34: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quigley C, et al. Haemophilus influenza type b disease in north-west England. Journal of Infection 1993; 26: 215–220. [DOI] [PubMed] [Google Scholar]
- 31.Trotter CL, Edmunds WJ. Modelling cost effectiveness of meningococcal serogroup C conjugate vaccination campaign in England and Wales. British Medical Journal 2005; 324: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekelund K, et al. Invasive group A, B, C and G streptococcal infections in Denmark, 1999–2002: epidemiological and clinical aspects. Clinical Microbiology and Infection 2005; 11: 569–576. [DOI] [PubMed] [Google Scholar]
- 33.Mehta SM, et al. Morbidity and mortality of patients with invasive group A streptococcal infections admitted to the ICU. Chest 2006; 130: 1679–1686. [DOI] [PubMed] [Google Scholar]
- 34.NHS Information Centre for Health and Social Care. Adult critical care in England–April 2010 to March 2011: Experimental statistics (http://www.ic.nhs.uk/catalogue/PUB06193/adul-crit-care-data-eng-apr-10-mar-11-rep.pdf). Accessed 29 April 2013.
- 35.Kaplan EL. Clinical management of the most common group A β-hemolytic streptococcal infections. Current Topics in Microbiology and Immunology 2013; 368: 243–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814002489.
click here to view supplementary material