SUMMARY
We investigated the effect of climatic, demographic factors and intra-country geographical variations on the incidence of invasive meningococcal disease (IMD) in Italy. For this purpose, incidence rates of IMD cases reported in Italy between 1994 and 2012 were calculated, and a cluster analysis was performed. A geographical gradient was determined, with lower incidence rates in central and southern Italy, compared to the northern parts, where most clusters were observed. IMD rates were higher in medium-sized towns than in villages. Adults were at lower risk of IMD than children aged ⩽4 years. IMD incidence tended to decrease with increasing monthly mean temperatures (incidence rate ratio 0·94, 95% confidence interval 0·90–0·99). In conclusion, geographical variations in IMD incidence were found, where age and temperature were associated with disease occurrence. Whether geographical variations should be considered in national intervention plans is still a matter for discussion.
Key words: Climate, demographic factors, IMD incidence
INTRODUCTION
Invasive meningococcal disease (IMD) is a major public health threat in terms of global distribution, epidemic potential, predominant disease burden in children and adolescents, with severe clinical manifestations. The majority of cases of IMD are caused by meningococci belonging to five different serogroups (A, B, C, Y, W135), but ‘new’ strains of serogroup X have recently emerged in the so-called ‘African meningitis belt’ [1].
At the global level, there is wide geographical variation in the incidence of IMD and prevalence of Neisseria meningitidis cases, with highest rates observed in the African belt [1]. Variations in IMD incidence have also been reported among, and within, countries with temperate climates. In Europe, for example, large differences have been observed, with higher IMD rates in the UK compared to Italy and other Mediterranean countries [2]. With regard to IMD identified risk factors in European studies, age, overcrowding, and smoking were the most frequently reported [3–8].
Climatic factors and associated human behaviour may explain, at least in part, the variation in IMD epidemic dynamics. Increased IMD incidence appears to be associated with dry and hot climate in the African meningitis belt, and with wet and cold climate in temperate areas of the world [5, 9–13]. In Italy, IMD has a seasonal trend, and co-occurring factors, such as cold weather and human behaviour are responsible in playing a role in determining the higher incidence observed during winter [5].
Moreover, there is some evidence supporting a causal relationship between low air humidity and IMD occurrence [13]. A dry climate could increase the risk of IMD through the damage of the pharyngeal mucosa, causing either a higher probability of meningococcal transmission [14], or an increased risk of invasive disease after colonization of the pharyngeal mucosa [15]. Nevertheless, the role played by climatic factors in IMD occurrence warrants further in-depth investigation.
In order to identify relevant demographic and climatic factors associated with IMD incidence, we investigated the temporal and spatial distribution of cases that occurred over two decades in Italy, a country with low IMD incidence (0·2–0·3/100 000 inhabitants per year), characterized by large geographical variations (http://www.simi.iss.it/meningite_batterica.htm). The possible spatio-temporal clusters of IMD was also assessed, using a discrete Poisson probability model. Finally, the relationship of climatic factors and IMD was studied using random-effects Poisson regression models.
METHODS
Source of data and descriptive analyses
All cases of IMD (sepsis and meningitis) reported to the National Surveillance System in Italy, between 1994 and 2012 were included in the study. Data on circulating serogroups were also collected for all IMD cases by the Istituto Superiore di Sanità (Rome, Italy), which is responsible for the coordination of national surveillance of invasive bacterial diseases.
Due to the lack of information on the exact location of the cases, each case was assigned the coordinates of the centroid of the local municipality.
Crude age, gender-specific, and age-adjusted incidence rates were calculated. Annual and monthly trends of IMD were examined. As a reference population, data from the 2001 Italian census was used, disaggregated at the municipality level and stratified by age group and gender, provided by the Italian National Institute of Statistics (ISTAT; http://istat.it). Information on altitude was also obtained from ISTAT. Data on the monthly mean land surface ambient temperature (°F) and water vapour were obtained from NASA Earth Observations (NEO) datasets (http://neo.sci.gsfc.nasa.gov). These data represent bands of radiances measured by NOAA-AVHRR orbiting satellites, which provide well-calibrated datasets at a resolution of 0·1°F previously corrected for atmospheric and orbital disturbances. Both temperature and water vapour values are calculated by NEO as the monthly mean of daily maximum values. Province-averaged monthly values of temperature, water vapour and altitude were calculated for each month and year of the study period and standardized.
Urbanization level was measured at the municipality level and, according to ISTAT, was divided into three categories: ‘high’, for municipalities with more than 500 inhabitants/km2 and at least 50 000 inhabitants; ‘medium’, for municipalities with 100–500 inhabitants/km2 or at least 50 000 inhabitants; ‘low’, for the remaining municipalities.
In accordance with ISTAT, Italy may be subdivided in five macro areas: North-West, North-East, Central, Southern, and Islands. For the purpose of the study, three macro areas were defined: North-West and North-East were aggregated and categorized as ‘North’, South and Islands were categorized as ‘South’, and ‘Central’ was analysed as a separate category.
Spatio-temporal cluster analysis
Detection of spatio-temporal clusters of IMD in Italy for the period 1994–2012 was performed using SaTScan software v. 9·1 [16, 17]. Cases were aggregated at the local municipality level, and assessed for geo-temporal clustering, using retrospective space–time analysis with a discrete Poisson probability model [16, 17]. The temporal reference unit was ‘day’, since temporal aggregation was obtained using the exact date of the event.
In the Poisson model, a circular moving window scans the area under study. Each sub-area is named as a zone. Each zone pertains to a number of events (cases) conditioned on a certain population, which represents the sum of the values of the spatial entities (in our case, municipality centroids) contained in the scanning window. When events and population are calculated for a zone, a likelihood function (LF) under the Poisson hypothesis is computed [16, 17], comparing the events within and outside the zone. Using the extended version of the Kulldorff scan method permitted the location and detection of spatial aggregates over time, while taking into account variations of the underlying population at risk. Under the null hypothesis, the number of expected cases is proportional to the population size, while the alternative hypothesis is that the number of cases is higher ‘within’ than ‘outside’ the defined spatio-temporal window. The maximum temporal window was set at 60 days, to take into account a period of time that permits transmission of the infection, development of the disease, and detection of the cases, while the maximum spatial window was set to consider 50% of the population (standard parameter of the SatScan software). The dataset for cluster analysis was formulated to have, for each day (for the entire study period), the cumulative number of cases that occurred in each municipality.
A cluster was considered ‘true’ if, after performing the spatio-temporal analysis, all the cases included had the same serotype.
For the evaluation of spatial cluster dimensions, the cluster radius was increased by 20%, to take into account cases that were artificially cumulated in the municipality centroid, in order to reduce their dispersion.
Climatic and other risk factors analysis
Month and province were selected as temporal and geographical units, respectively, for this analysis. Since climate data were not available before 2000, the analysis was restricted to the period 2000–2012. Age (years) was categorized as follows: 0–4 = 0, 5–9 = 1, 10–14 = 2, 15–19 = 3, 20–24 = 4, 25–29 = 5, 30–34 = 6, 35–39 = 7, 40–44 = 8, 45–49 = 9, 50–54 = 10, 55–59 = 11, 60–64 = 12, 65–69 = 13, 70–74 = 14, 75–79 = 15, 80–84 = 16, ⩾85 = 17. Number of cases and population size were aggregated according to province, age group, urbanization level, and month. The data contained evidence of zero-inflation and overdispersion. However, the Vuong test statistics were close to zero, suggesting that the zero inflated-Poisson model was not a significant improvement over a standard Poisson model (P = 0·500). The likelihood ratio test found that alpha was equal to zero (P = 0·940).
One-level random-effect multilevel models were fitted with number of cases as the dependent variable and population size as an offset to determine the association between climate variables and IMD. Mean temperature, and water vapour were considered as exposure variables; age group, urbanization level, macro area, altitude, and year were assumed to be potential confounders. A model without explanatory variables was first performed. This included the fixed intercept term and one random term associated with the intercept, which reflects the variation at the province level. Second, climate variables were added and a univariate analysis for each of them was performed. Third, the same terms of the latter model were included, but adjusting the period. Fourth, additional adjustment for age group was done. Finally, fully adjusted models were performed, which included additional adjustment for macro areas, altitude and urbanization level. Climate variables, time periods (as cubic spline parameters), and age group were a priori included in the final model, while the other factors were evaluated as potential confounders for climate variables. In the final model, age in four categories was recorded as follows: 0–4 = 0, 5–14 = 1, 15–24 = 2 and ⩾25 = 3.
Restricted cubic spline methodology was used to fit potential nonlinear relationships between year and number of cases; knots were placed at fixed percentiles of ‘year’ marginal distribution: knots were set in 2001, 2006, and 2011. Log likelihood ratio test was used to compare the fit of the model, using age group as a categorical variable (0–4 = 0, 5–14 = 1, 15–24 = 2, ⩾25 = 3), or treated as a ‘continuous’ variable (0–4 = 0, 5–9 = 1, 10–14 = 2, 15–19 = 3, 20–24 = 4, 25–29 = 5, 30–34 = 6, 35–39 = 7, 40–44 = 8, 45–49 = 9, 50–54 = 10, 55–59 = 11, 60–64 = 12, 65–69 = 13, 70–74 = 14, 75–79 = 15, 80–84 = 16, ⩾85 = 17). Age group was then used as categorical variable (0–4 = 0, 5–14 = 1, 15–24 = 2, ⩾25 = 3), as it fitted the model better.
The significance of the random components was tested by checking if the estimates were greater than twice the standard error. The analysis, including water vapour, was restricted to the years when this information was available (2005–2012). The statistical analysis was performed using Stata v. 13 (StataCorp LP, USA) [18], and the significance level was set at P = 0·05.
Meningitis incidence and description of climatic parameters
Overall, 3894 cases were reported between 1994 and 2012. For 28 cases the age was missing, hence analysis was carried out on 3866 cases. The median age of the patients was 16 years (range 0–92 years).
The crude annual incidence was 0·38/100 000 per year [95% confidence interval (CI) 0·36–0·39) for the period 1994–2012. IMD incidence tended to decrease with increasing age, being more than tenfold higher for children aged 0–4 years than for adults aged ⩾25 years (Table 1). The age-adjusted incidence/100 000 per year (1994–2012) was virtually the same as the crude incidence (0·38, 95% CI 0·36–0·39). The age-adjusted incidence was 0·35 (95% CI 0·35–0·38) in females and 0·39 (95% CI 0·37–0·40) in males, differences between males and females were not statistically significant as was apparent from the overlapping confidence intervals [incidence rate ratio (IRR) = 1·0695% CI 0·99–1·13, P = 0·53].
Table 1.
Mean incidence of meningitis per 100 000 population by age group in Italy during the period 1994–2012
| Age group (years) | No. of cases | Population | Incidence | 95% CI |
|---|---|---|---|---|
| 0–4 | 1187 | 2 618 794 | 2·51 | (2·38–2·67) |
| 5–9 | 388 | 2 679 104 | 0·80 | (0·73–0·89) |
| 10–14 | 257 | 2 805 287 | 0·51 | (0·45–0·57) |
| 15–19 | 532 | 2 963 629 | 1·00 | (0·92–1·09) |
| 20–24 | 287 | 3 424 350 | 0·47 | (0·41–0·52) |
| ⩾25 | 1215 | 42 504 580 | 0·16 | (0·15–0·17) |
CI, Confidence interval.
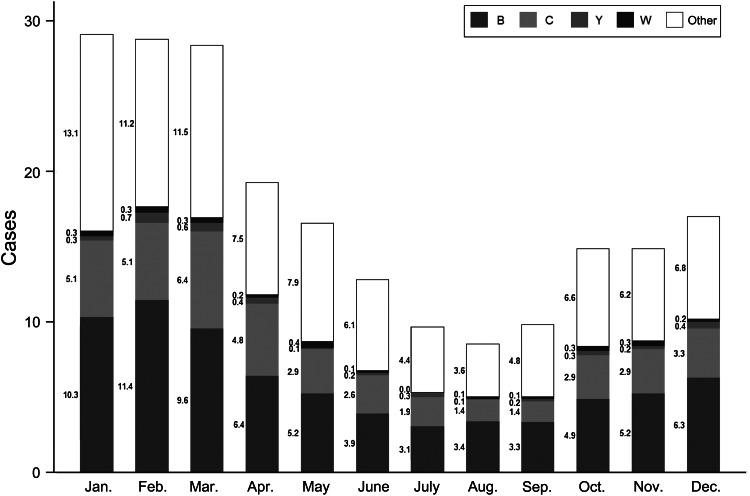
Age-adjusted rates are given in Table 2. The highest number of cases was reported from January to March (Fig. 1).
Table 2.
Crude and age adjusted incidence of meningitis and 95% confidence intervals/100 000 population per year, 1994–2012
| Year | No. of cases | Crude rates | Adjusted rates | 95% CI |
|---|---|---|---|---|
| 1994 | 164 | 0·31 | 0·28 | (0·23–0·32) |
| 1995 | 200 | 0·36 | 0·35 | (0·30–0·40) |
| 1996 | 169 | 0·31 | 0·30 | (0·25–0·34) |
| 1997 | 182 | 0·34 | 0·32 | (0·27–0·36) |
| 1998 | 155 | 0·28 | 0·27 | (0·23–0·32) |
| 1999 | 275 | 0·48 | 0·48 | (0·43–0·54) |
| 2000 | 250 | 0·46 | 0·44 | (0·38–0·49) |
| 2001 | 203 | 0·36 | 0·36 | (0·31–0·41) |
| 2002 | 217 | 0·39 | 0·38 | (0·33–0·43) |
| 2003 | 277 | 0·48 | 0·48 | (0·42–0·54) |
| 2004 | 343 | 0·61 | 0·60 | (0·53–0·66) |
| 2005 | 324 | 0·57 | 0·57 | (0·51–0·63) |
| 2006 | 179 | 0·33 | 0·31 | (0·26–0·35) |
| 2007 | 186 | 0·32 | 0·32 | (0·27–0·36) |
| 2008 | 183 | 0·33 | 0·32 | (0·28–0·37) |
| 2009 | 188 | 0·35 | 0·33 | (0·28–0·38) |
| 2010 | 150 | 0·27 | 0·26 | (0·22–0·31) |
| 2011 | 152 | 0·27 | 0·27 | (0·22–0·31) |
| 2012 | 97 | 0·17 | 0·17 | (0·14–0·20) |
| Total | 3894 | 0·38 | 0·38 | (0·37–0·39) |
CI, Confidence interval.
Fig. 1.
Average number of invasive meningococcal disease cases by month, and distribution of the main serogroups in Italy, 1994–2012.
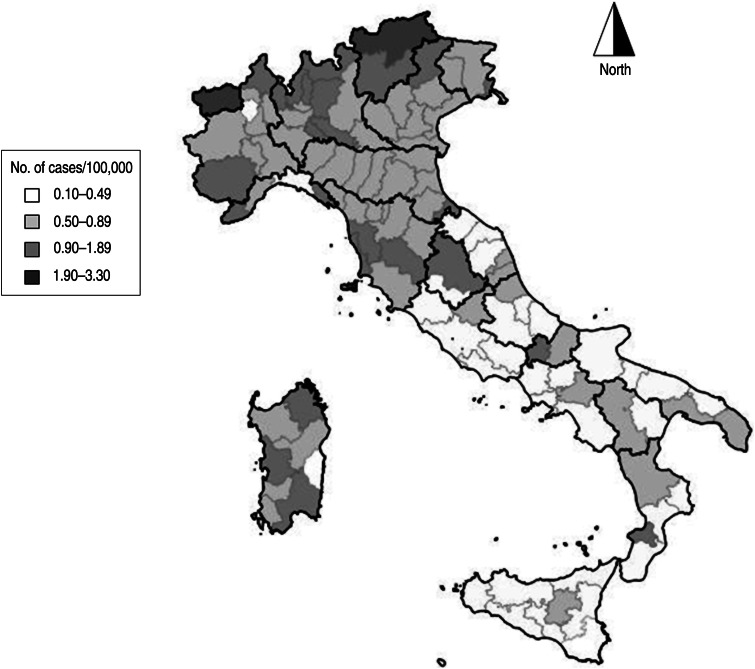
There was a large variation in age-adjusted incidence rates among the provinces (Fig. 2); age-adjusted incidence ranged from 0·08 (95% CI 0·00–0·20) to 3·33 (95% CI 0·06–6·61) per 100 000 inhabitants.
Fig. 2.
Mean annual incidence of meningitis per 100 000 population by province in Italy, 1994–2012.
Cluster analysis
For the cluster analysis, information on the residence and the date of diagnosis was available for 3572 out of 3894 cases. These cases occurred in 1497 of the 8118 municipalities (total number of municipalities in Italy). During the whole study period (1 January 1994 to 31 December 2012), 33 (0·92%) cases in 11 clusters were identified, most of which were located in Northern Italy (Fig. 2). The number of cases included in the clusters ranged from two to five, and the maximum cluster radius was 5·5 km. The largest cluster occurred in Bari, (Apulia, Southern Italy) in January 2007; with a duration of 7 days, and involved five cases. Of the 11 clusters, four had N. meningitidis serogroup B, and three serogroup C, while for the remaining cases the serogroup was unknown. With regard to age distribution, the cases included in the clusters had a median age of 18·5 years (range 0–79 years), without any significant differences compared to the other cases. To evaluate the sensitivity of the parameters for the detection of the spatio-temporal clusters, models with temporal windows of 30 and 120 days were run without any significant difference in cluster detection. The effect of changing the spatial windows to 10% and 25% of the population was also tested; these changes increased the size of some clusters including cases with different serogroups, thus the most conservative approach of using 50% of the population was adopted.
Association between climate and incidence of meningitis
In the univariate analysis, incidence of meningitis was significantly associated with monthly mean temperatures z scores (IRR 0·94, 95% CI 0·90–0·99). After adjusting for macro area (North, Central, South), urbanization level, year of occurrence, altitude, and age group, the rate for monthly mean temperature decreased by a factor of 0·94 (IRR 0. 94 95% CI 0·90–0·98) if monthly mean temperatures increased by 1 °C (temperature standard deviation = 33·82 °F which is about 1 °C) which means that IMD cases decreased by ~6% for every 1 °C increase in temperature (Table 3). There was evidence of nonlinearity for age (likelihood ratio test χ2 = 15·77, P < 0·001). The rates in the 15–24 and ⩾25 years age groups were 81% and 55% of children aged 0–4 years (reference category), respectively. Altitude had a positive effect on IMD risk, although it was not significant. A geographical gradient was found in relation to the macro area of residence, with statistically significant lower rates in Central and Southern Italy compared to Northern Italy (reference category) (Table 3). The rate for large towns was not statistically different from that of villages (reference category) whereas medium-sized towns had a rate 39% lower than that of villages. When the analysis was restricted to those years in which water vapour information was available (2005–2012), the incidence of IMD was not significantly associated with water vapour (IRR 0·96, 95% CI 0·90–1·02). There was no reversal of the relationship between any independent variable and the outcome variable, when other covariates were included in the model, which indicates stability in the model estimates. As shown in the Supplementary online material (Figs S1–S3), the multilevel random-effect Poisson model fitted well. It is possible that unobserved heterogeneity between provinces was the explanation for both zero-inflation and overdispersion.
Table 3.
Incidence rate ratios (IRR), 95% confidence intervals (CI) and P values from the random effect Poisson model of meningitis cases for monthly mean temperature, 2000–2012. Random-effect variance (province level) was 0·87 (s.e. = 0·07)
| IRR | 95% CI | P | |
|---|---|---|---|
| Monthly mean temperature – standardized* | 0·94 | (0·90–0·98) | 0·01 |
| Urbanization level | |||
| Low | 1 | ||
| Medium | 0·39 | (0·27–0·57) | <0·01 |
| High | 0·44 | (0·08–2·58) | 0·37 |
| Mean altitude province-standardized† | 1·20 | (0·99–1·45) | 0·07 |
| Macro area | |||
| North | 1 | ||
| Central | 0·51 | (0·33–0·78) | <0·01 |
| South | 0·50 | (0·32–0·78) | <0·01 |
| Age group (years) | |||
| 0–4 | 1 | ||
| 5–14 | 0·92 | (0·81–1·04) | 0·19 |
| 15–24 | 0·81 | (0·71–0·91) | <0·01 |
| ⩾25 | 0·55 | (0·49–0·62) | <0·01 |
| Restricted cubic splines for calendar year | |||
| beta1 (2001–2005)‡ | 1·04 | (0·98–1·12) | 0·16 |
| beta2 (2006–2011)‡ | 0·95 | (0·89–1·01) | 0·07 |
An increase of 1 zeta score of monthly mean temperature corresponds to ~1 °C.
An increase of 1 z score of altitude corresponds to ~5 mm.
Exponentiated coefficients of restricted cubic splines for calendar year.
DISCUSSION
Our analysis of a large dataset of cases reported in Italy over two decades confirmed the large geographical variations in IMD incidence within the country, with significantly lower rates in the Central and Southern parts compared to Northern Italy. Geographical variations in IMD incidence were also observed in Europe, with the highest rates in England and the lowest rates in Mediterranean countries, such as Italy [2]. Several clusters of IMD cases were identified, with the largest one including five cases. The majority of these clusters were due to serogroup C meningococci. As expected, adults aged ⩾25 years were at lower risk of IMD than children aged 0–4 years, while no gender difference was found.
In order to explain variations in IMD incidence, we investigated the possible role played by climatic variables, such as temperature and humidity, along with other environmental and demographic factors. Consistent with the seasonal trend of IMD in temperate climatic areas, a peak of cases occurred during winter, between January and March, when the climate is colder and dry. Although there is evidence of a north–south gradient, which may be considered a proxy of an association between higher incidence rates and lower temperatures in the European continent, whether this is due to a direct effect of low temperatures or to an indirect effect through seasonal differences in human behaviours or co-infections remains unknown.
Studies conducted in Northern Europe found an association between IMD and influenza and/or human respiratory syncytial virus (hRSV) activity [19]. However, it is not easy to disentangle the role of factors which may influence the simultaneous occurrence of acute respiratory infections (ARI) and IMD during the cold season. Whether the co-occurrence of different infections is due to common seasonal factors (cold weather and human behaviour), or to an effect of ARI on the development of IMD remains, at least, in part, undefined. An association between IMD and respiratory infections was also found in studies conducted in the African meningitis belt [12], where dust and respiratory infections may have an additive or even multi-perspective effect on IMD through mucosa damage [20].
The in-depth relationship between influenza and IMD was not investigated. The monthly distribution of IMD cases in 2009 (the influenza pandemic year) did not differ from that of non-pandemic years, although in 2009 the pandemic influenza virus subtype H1N1 peaked in mid-November instead of the end of January/beginning of February, when the seasonal influenza peak usually occurs (data not shown). However, no conclusions can be derived from a single year's observation, especially if we consider that other possible confounders, such as behavioural change, due to people being worried about the pandemic flu, may have influenced transmission dynamics.
No association between humidity and IMD incidence was found. This is in conflict with studies conducted in North America, which show that humid climate may predict the occurrence of IMD [21]. Different results have been obtained also from studies on the association with hyperendemicity during the dry season in the African meningitis belt [22]. Moreover, the relationship between climate and year-to-year variability in IMD outbreaks remains, to some extent, undefined [23]. However, epidemic dynamics in Italy and in the African meningitis belt are different, and caution should be exercised when comparing the impact of climatic and social factors [2, 24].
In our analysis, even though the absolute number of cases was highest in areas with an elevated level of urbanization, the adjusted risk of IMD was highest in medium-sized areas (100–500 inhabitants). To what extent this is due to behavioural factors, such as different modalities of social aggregation, should be explored. With regard to the effect of altitude there was some indication of a direct effect, although there was a lack of statistical significance in the fully adjusted multilevel model. As to which factors are influencing such disease distribution patterns requires further investigation.
A tendency towards a decline in the number of IMD cases, resulting in a number of cases similar to those reported before 1999 was observed since 2006, coinciding with the introduction of meningococcal C vaccination. This vaccination is recommended in Italy, with a current schedule of a single dose at the age of 13 months. Vaccine strategies in Italy are based on regional plans in accordance with national recommendations; in some regions, meningococcal C vaccination is offered free to all infants as well as to specific risk groups, such as patients with splenic dysfunction or immunodeficiency. In 2012, the conjugate meningococcal C vaccine was introduced in the National Immunization Plan. Between 2008 and 2012, the estimated coverage of meningococcal C vaccination in the country was about 72% (data not shown). Unfortunately, the population effect of meningococcal serogroup C conjugate vaccines was not evaluated since the study period was too short to draw definitive conclusions. Furthermore, it should be considered that other serogroups, such as serogroup Y, are emerging in the country [25], contributing to the dynamics of the overall meningococcal burden of disease.
This study has some limitations. First, differential underreporting between regions can not be ruled out. However, this does not explain entirely the north–south trend in IMD, since IMD is a severe disease with high epidemic potential, with a low likelihood of being underreported. Studies conducted in Central Italy showed negligible underreporting [26]. Second, differences in vaccine coverage were not verified in the multivariate analysis. However, they were not likely to explain geographical variations, which pre-existed the introduction of vaccination. Moreover, there was no significant difference in vaccine coverage between the North and the Central/South of the Italy, with vaccine coverage ranging between 59·9% and 92·5% and between 53·4% and 90·5%, respectively (data not shown). Third, our analysis was based on invasive disease and did not take into account the prevalence of carriage. However, there is no reason to suppose that differences in carriage:disease ratio may occur in different areas of the country. Studies aimed at investigating the influence of climatic and behavioural factors on carriage and transmission dynamics could contribute to bridging important knowledge gaps. Fourth, we did not find a significant effect of water vapour (a proxy for humidity) on IMD incidence; yet, this does not exclude completely the possible role played by other factors affecting humidity (air pressure or evaporation, precipitation intensity, etc.), which was found to be associated with IMD in Africa [12, 13). Finally, the role of the environmental factors was not explored, such as air pollution levels, which were found to be associated with IMD as reported by Magoni et al. [5]. Moreover, the increase that these authors described in 2003 as a cluster was not detected; however, interestingly, a cluster (cluster ID 5, Fig. 3) in the same period (early 2003) very close to the area that these authors investigated was detected in our study. This could indirectly support to the role of the variables that Magoni et al. took into consideration as ‘risk modulators’ for acute meningitis.
Fig. 3.
Distribution of invasive meningococcal disease cases by municipality. Clusters are shown on the map and numbered according to the time of occurrence from the beginning of the study period.
In conclusion, large geographical variations in IMD incidence in Italy, with a marked north–south gradient, were found. Several clusters were also identified. Low mean temperature and young age increased the risk of IMD. Whether intervention strategies should be backed by the knowledge of epidemic dynamics at the local level remains a matter for discussion.
ACKNOWLEDGEMENTS
The authors thank Fortunato P. D'Ancona and Gianluca Cavallaro for database support. This study was supported by the Italian Ministry of Health as CCM project ‘Sorveglianza delle malattie invasive da Neisseria meningitidis, Streptococcus pneumoniae ed Haemophilus influenzae’ 2012–2013.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814002659.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Trotter CL, Greenwood BM. Meningococcal carriage in the African meningitis belt. Lancet Infectious Diseases 2007; 7: 797–803. [DOI] [PubMed] [Google Scholar]
- 2.ECDC. Annual Epidemiological Report 2013. (http://www.ecdc.europa.eu/en/publications/Publications/annual-epidemiological-report-2013.pdf). 2014.
- 3.Davies A, et al. Risk factors for Neisseria meningitidis carriage in a school during a community outbreak of meningococcal infection. Epidemiology & Infection 1996. October; 117: 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuart JM, et al. Effect of smoking on meningococcal carriage. Lancet 1989. September 23; 2: 723–5. [DOI] [PubMed] [Google Scholar]
- 5.Magoni M, et al. Do environmental factors influence the occurrence of acute meningitis in industrialized countries? An epidemic of varying aetiology in Northern Italy. European Journal of Epidemiology 2006; 21: 465–468. [DOI] [PubMed] [Google Scholar]
- 6.Pereiro I, et al. Risk factors for invasive disease among children in Spain. Journal of Infection 2004; 48: 320–329. [DOI] [PubMed] [Google Scholar]
- 7.Stanwell-Smith RE, et al. Smoking, the environment and meningococcal disease: a case control study. Epidemiology & Infection 1994; 112: 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tully J, et al. Risk and protective factors for meningococcal disease in adolescents: matched cohort study. British Medical Journal 2006; 332: 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Block C, et al. Forty years of meningococcal disease in Israel: 1951–1990. Clinical Infectious Diseases 1993; 17: 126–132. [DOI] [PubMed] [Google Scholar]
- 10.Collier CG. Weather conditions prior to major outbreaks of meningococcal meningitis in the United Kingdom. International Journal of Biometeorology 1992; 36: 18–29. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay AP, et al. Meningococcal disease and meteorological conditions in Auckland, New Zealand. Australian and New Zealand Journal of Public Health 2002; 26: 212–218. [DOI] [PubMed] [Google Scholar]
- 12.Mueller JE, et al. Association of respiratory tract infection symptoms and air humidity with meningococcal carriage in Burkina Faso. Tropical Medicine and International Health 2008; 13: 1543–1552. [DOI] [PubMed] [Google Scholar]
- 13.Thomson MC, et al. Potential of environmental models to predict meningitis epidemics in Africa. Tropical Medicine & International Health 2006; 11: 781–788. [DOI] [PubMed] [Google Scholar]
- 14.Ghipponi P, et al. Study of bacterial air pollution in an arid region of Africa affected by cerebrospinal meningitis. Bulletin of the World Health Organization 1971; 45: 95–101. [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwood BM, Bradley AK, Wall RA. Meningococcal disease and season in sub-Saharan Africa. Lancet 1985; 2: 829–830. [DOI] [PubMed] [Google Scholar]
- 16.Kulldorff M. A spatial scan statistic. Communications in Statistics: Theory and Methods 1997; 26: 1481–1496. [Google Scholar]
- 17.SaTScan Inc. SaTScan™ v8·0 software for the spatial and space-time scan statistics [computer program]. 2009.
- 18.StataCorp. Stata statistical software: release 13. College Station, TX: StataCorp LP [computer program]. 2013.
- 19.Jansen AG, et al. Invasive pneumococcal and meningococcal disease: association with influenza virus and respiratory syncytial virus activity? Epidemiology & Infection 2008; 136: 1448–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agier L, et al. Seasonality of meningitis in Africa and climate forcing: aerosols stand out. Journal of the Royal Society Interface 2013; 10: 20120814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinlin LM, et al. Environmental exposures and invasive meningococcal disease: an evaluation of effects on varying time scales. American Journal of Epidemiology 2009; 169: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller JE, Gessner BD. A hypothetical explanatory model for meningococcal meningitis in the African meningitis belt. International Journal of Infectious Diseases 2010; 14: e553–e559. [DOI] [PubMed] [Google Scholar]
- 23.Yaka P, et al. Relationships between climate and year-to-year variability in meningitis outbreaks: a case study in Burkina Faso and Niger. International Journal of Health Geographics 2008; 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindgren E, Ebi KL. Climate change and communicable diseases in the EU Member States. ECDC 2014 (http://www.ecdc.europa.eu/en/publications/).
- 25.Fazio C, et al. Characterization of invasive serogroup Y meningococci in Italy: prevalence of ST-23 complex/cluster A3. New Microbiologica 2008; 31: 467–472. [PubMed] [Google Scholar]
- 26.Faustini A, et al. Estimating incidence of bacterial meningitis with capture-recapture method, Lazio Region, Italy. European Journal of Epidemiology 2000; 16: 843–848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814002659.
click here to view supplementary material