SUMMARY
We compared the epidemiological and clinical features of avian influenza A(H7N9) virus infections in the population in Zhejiang province, China, between March and April 2013 (first wave) and October 2013 and February 2014 (second wave). No statistical difference was found for age, sex, occupation, presence of underlying conditions, exposure history, white blood cell count, lymphocyte percentage and illness timeline and duration (all P > 0·05). The virus spread to 30 new counties compared to the first wave. The case-fatality rate was 22% in the first wave and 42% in the second (P = 0·023). Of those infected, 66% in the first wave and 62% in the second wave had underlying conditions. The proportion of those exposed to live poultry markets were 80% and 66%, respectively. We recommend permanent closure of live poultry markets and reformation of poultry supply and sales.
Key words: Avian influenza A(H7N9), epidemiology, clinical features
INTRODUCTION
Human infections with H7N9 virus were first reported in China in February 2013. The infections raised great concern because of the increasing number of cases and affected areas, clinical features, virus properties, and heavy social burden [1]. By 31 July 2013, 132 confirmed cases of human infection with H7N9 virus (including 43 deaths) were reported on mainland China [2, 3]. Of the 132 cases, 45 were residents of Zhejiang province in southeastern China, representing the highest percentage of cases on mainland China. The epidemic in the first half of the year in Zhejiang province ceased in April 2013 with the last human case occurring on 18 April 2013 [4]. However, in October 2013, a new human H7N9 case was identified through a national surveillance system for unexplained pneumonia in Zhejiang province [5]. Since then, the cases have gradually increased to 93 confirmed human cases including 39 deaths, with the last case confirmed on 27 February 2014 [6]. During January 2014, Zhejiang province accounted for half the cases on mainland China.
This study describes and compares epidemiological and clinical profiles of patients in the H7N9 epidemic before and after October 2013 in Zhejiang province, exploring similarities and differences between the two waves of the H7N9 epidemic.
METHODS
Patient definition
Identification of laboratory-confirmed patients infected with H7N9 virus was according to the Chinese Guideline of Diagnosis and Treatment for Human Infections with the Avian Influenza A(H7N9) Virus issued by the National Health and Family Planning Commission of the People's Republic of China [7, 8].
Data collection
We designed a standardized questionnaire to collect patient demographic information, cigarette smoking, underlying conditions (including chronic pulmonary disease, cardiovascular disease, hypertension, diabetes), illness timeline and duration (including days of illness before visiting hospital, days of illness before confirmation, days of illness before hospitalization, days of illness before Tamiflu treatment) and clinical features (including temperature, white blood cell count, lymphocyte percentage). Clinical information collected was the most abnormal reading for each feature during the course of illness. We conducted in-depth interviews of patients regarding their exposure history during the 2 weeks before illness onset. Detailed exposure history included visiting live poultry markets, direct contact with poultry or swine, buying live or freshly slaughtered poultry, killing live or freshly slaughtered poultry and raising poultry at home or around the house. This investigation was in response to a public health emergency. As such, it was exempt from the requirement for ethical approval and informed consent according to the Law of the People's Republic of China on Prevention and Treatment of Infectious diseases.
Laboratory testing
We tested patients' respiratory specimens by real-time reverse transcription–polymerase chain reaction (rRT–PCR) and viral isolation. We extracted RNA from specimens with Qiagen RNeasy mini-kits (Qiagen GmbH, Germany) and performed rRT–PCR with H7N9-specific primers and probes according to the manufacturer's protocol. For viral isolation, we inoculated respiratory specimens in allantoic cavities of pathogen-free embryonated chicken eggs. We performed rRT–PCR in biosafety level (BSL)-2 facilities, and viral isolation in enhanced BSL-3 facilities, all at Zhejiang Provincial Centre for Disease Control and Prevention.
Data analysis
We compared distributions of age, sex, occupation, cigarette smoking, underlying conditions, exposure history, temperature, white blood cell count, lymphocyte percentage, illness timeline and duration for patients between the first and second waves using t test, Wilcoxon test, Pearson's χ2 test, and Fisher's exact test, as appropriate. For exposure history, respondents who answered yes to at least one of: (1) visiting live poultry markets, (2) direct contact with poultry or swine, (3) buying live or freshly slaughtered poultry, (4) killing live or freshly slaughtered poultry, or (5) raising poultry at home or around the house, were considered to have an exposure history related to poultry. Those who answered yes to at least one of: (1) chronic pulmonary disease, (2) cardiovascular disease, (3) hypertension or (4) diabetes, were regarded as having underlying conditions. Statistical analyses were performed using SPSS v. 15·0 (SPSS Inc., USA). A two-sided P value <0·05 was considered as statistically significant.
RESULTS
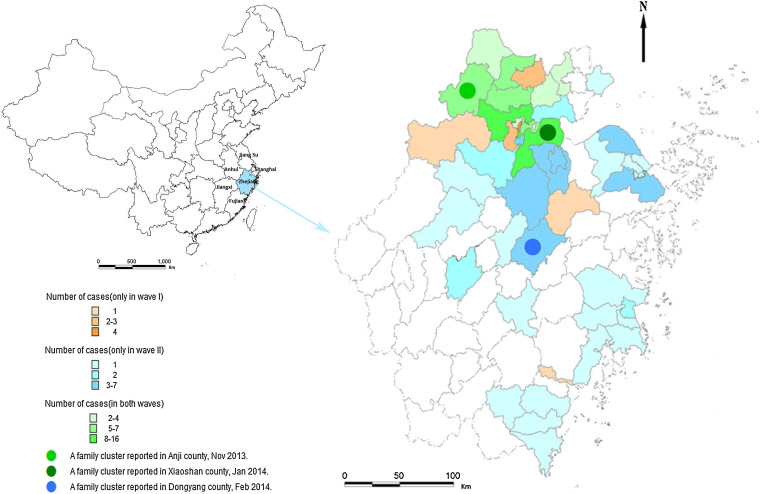
During March–April 2013 (first wave) 45 laboratory-confirmed cases of H7N9 viral infection were identified in 17 counties. From October 2013 to February 2014 (second wave) 93 cases were identified from 41 counties. The case-fatality rate was 22% in the first wave and 42% in the second wave (P = 0·023). Of the 41 counties in the second wave, 30 were affected for the first time by the H7N9 virus (Fig. 1).
Fig. 1.
Geographical distribution of patients infected with H7N9 virus in Zhejiang province, China. Zhejiang province is situated in the southeast of China and borders on Shanghai municipality, Jiangsu, Anhui, Jiangxi and Fujian provinces. Of 90 counties of Zhejiang province, 47 had patients infected with H7N9 virus as of April 2014, of which six reported cases only in the first wave of the epidemic, 30 only in the second wave and 11 in both waves. Three family clusters were reported in the second wave, in the middle and north of the province.
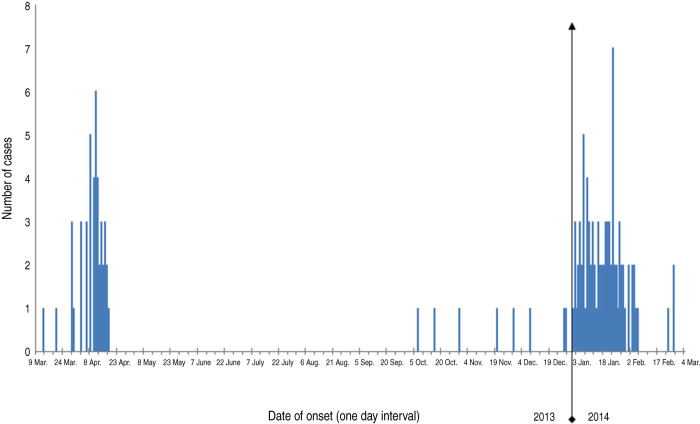
In the second wave of the H7N9 epidemic, the first case occurred on 7 October 2013. During the next 2 months, patients were sporadically identified. Beginning in 2014, the number of cases increased sharply and peaked on 23 January 2014 (Fig. 2).
Fig. 2.
Number of patients infected with H7N9 virus by date of onset in Zhejiang province, China. H7N9 human infections in Zhejiang province occurred in two waves. The first lasted from 13 March to 18 April 2013, peaking on 11 April 2013. The second wave started on 7 October 2013 and ceased on 26 February 2014, peaking on 23 January 2014.
No significant differences were observed in the patients between the two waves regarding age (P = 0·278), sex (P = 0·521), occupation (P = 0·434), underlying conditions (P = 0·668), exposure history (P = 1·000), white blood cell count (P = 0·978), lymphocyte percentage (P = 0·632), days of illness before visiting the hospital (P = 0·142), days of illness before confirmation (P = 0·855), days of illness before hospitalization (P = 0·342) or days of illness before Tamiflu treatment (P = 0·378) (Table 1). In both the first and second waves, farmers (36% first wave, 45% second) and retirees (31% first wave, 22% second) were the majority of people infected with H7N9 virus. Men comprised a higher proportion of patients than women in both waves. Notably, 80% had exposure to a live poultry market in the first wave and 66% in the second.
Table 1.
Characteristics of human infections with H7N9 virus before and after October 2013 in Zhejiang province, China
| Characteristics | Patients before October 2013 | Patients after October 2013 | P value |
|---|---|---|---|
| Deaths, n (%) | 10 (22·2%) | 39 (41·9%) | 0·023* |
| Age (years), median (IQR†, range) | 62 (21, 31–86) | 59 (24, 1–84) | 0·278 |
| Age, years, n (%) | |||
| 0–19 | 0 (0·0) | 3 (3·3) | |
| 20–39 | 7 (15·6) | 14 (15·2) | |
| 40–59 | 14 (31·1) | 30 (32·6) | |
| ⩾60 | 24 (53·3) | 45 (48·9) | |
| Male, n (%) | 28 (62·2) | 63 (67·7) | 0·521 |
| Occupation, n (%) | 0·434 | ||
| Farmer | 16 (35·6) | 42 (45·2) | |
| Home-maker | 4 (8·9) | 3 (3·2) | |
| Retiree | 14 (31·1) | 20 (21·5) | |
| Self-employed | 6 (13·3) | 13 (14·0) | |
| Worker | 5 (11·1) | 12 (12·9) | |
| Child | 0 (0·0) | 3 (3·2) | |
| Cigarette smoking, n (%) | 19 (43·2) | 6 (15·4) | 0·008* |
| Underlying conditions, n (%) | 29 (65·9) | 46 (62·0) | 0·668 |
| Chronic pulmonary disease | 5 (11·4) | 1 (1·4) | 0·031* |
| Cardiovascular disease | 8 (18·2) | 24 (34·3) | 0·062 |
| Hypertension | 20 (45·5) | 34 (43·0) | 0·796 |
| Diabetes | 8 (18·2) | 16 (21·6) | 0·654 |
| Exposure history, n (%) | 42 (93·3) | 85 (92·4) | 1·000 |
| Visiting live poultry markets | 36 (80·0) | 61 (66·3) | 0·098 |
| Direct contact with poultry or swine | 30 (66·7) | 47 (51·1) | 0·084 |
| Buying live or freshly slaughtered poultry | 18 (40·0) | 20 (21·7) | 0·025* |
| Killing live or freshly slaughtered poultry | 5 (11·1) | 15 (16·3) | 0·419 |
| Raising poultry at home or around the house | 14 (31·1) | 16 (17·4) | 0·068 |
| Occupational exposure | 0 (0·0) | 14 (15·2) | — |
| Clinical features | |||
| Temperature (°C), mean (s.d.) | 39·1 (0·6) | 39·4 (0·6) | 0·032* |
| WBC ( × 109/l), median (IQR†, range) | 4·4 (3·1, 2·3–9·2) | 4·6 (2·7, 0·8–17·1) | 0·978 |
| Lymphocyte, %, median (IQR†, range) | 13·6 (15·4, 4·7–69·5) | 16·8 (10·2, 1·2–90·0) | 0·632 |
| Timeline and duration, median (IQR†, range) | |||
| Days of illness before visiting hospital | 1·0 (3·0, 0–19) | 1·0 (2·0, 0–31) | 0·142 |
| Days of illness before confirmation | 7·5 (3·0, 4–22) | 8·0 (4·0, 1–39) | 0·855 |
| Days of illness before hospitalization | 5·0 (3·0, 1–19) | 4·0 (3·0, 0–36) | 0·342 |
| Days of illness before Tamiflu treatment | 6·0 (3·0, 1–19) | 5·0 (4·3, 0–36) | 0·378 |
IQR, Interquartile range; s.d., standard deviation.
IQR is the 1st quartile subtracted from the 3rd quartile.
P < 0·05.
We saw a much smaller proportion of smokers in patients in the second wave (P = 0·008). We found a significant difference in temperature (P = 0·032) that was 0·3 °C higher in the second wave. The proportion of patients with underlying conditions was 66% in the first wave and 62% in the second. When underlying conditions were further analysed as chronic pulmonary disease, cardiovascular disease, hypertension or diabetes, a lower percentage of patients had chronic pulmonary disease in the second wave compared to the first (P = 0·031). In addition, we observed a lower percentage of patients with a history of buying live or freshly slaughtered poultry in the second wave compared to the first (P = 0·025) (Table 1).
DISCUSSION
Based on our analysis of patients infected with H7N9 virus before and after October 2013, no significant differences were observed between the two waves of the epidemic in patients' age, sex, occupation, presence of underlying conditions, exposure history, white blood cell count, lymphocyte percentage or illness timeline and duration. From March 2013 to February 2014, we observed two successive peaks in spring and winter, with no cases in summer. This temporal distribution was in accordance with ordinary influenza. Smoking is a risk factor for human infection with H7N9 virus in several studies [9–11]. Our results showed that 43% of patients in the first wave of the epidemic were smokers. However, the proportion of smokers was only 15% in the second wave, below the current smoking prevalence of 28% in Zhejiang province [12]. The percentage of patients who bought live or freshly slaughtered poultry was much lower in the second wave than the first, which could be attributed to the effective multi-faceted health education campaign conducted by the government and health authorities.
Case-fatality rate from human infection with H7N9 virus was 22% in the first and 42% in the second wave in Zhejiang province. This result indicated that H7N9 virus was much more virulent for humans than previously reported H7 viruses but less virulent than H5N1 virus [13]. Case-fatality rate in the second wave was significantly higher than the first wave. However, the Chinese Centre for Disease Control and Prevention (China CDC) declared that viruses from patients after October 2013 were highly homologous to viruses from patients infected in April 2013. [14]. We observed no significant change in patients' demographic characteristics in the two waves. We have several possible explanations for the higher case-fatality rate in the second wave. First, the proportions of patients who killed live poultry or had occupational exposure were higher in the second wave than in the first (killed poultry: first wave 11·1%, second wave 16·3%; occupational exposure: first wave 0·0%, second wave 15·2%). This result indicated that more patients in the second wave might have been infected by a relatively high viral dose, leading to the higher case-fatality rate. Second, the second wave lasted for 5 months with many cases occurring in January and February. This put pressure on the medical care system, possibly contributing to the elevated case-fatality rate.
We successively identified three family clusters in the second wave in Zhejiang province. The first was in November 2013 with a son-in-law and his father-in-law; the second was in January 2014 with a daughter and her parents, and the third was in February 2014 with two sisters (Fig. 2). Currently no evidence supports variation of virus transmissibility and sustained human-to-human transmission; nonetheless, these family clusters imply possible limited human-to-human transmission, warning of the potentially greater transmission of the virus. Thus, organizations such as CDC should pay attention to the infectious status of close contacts of patients. Active surveillance of H7N9 virus carriers is also required.
Our study showed a wide geographical spread of patients in counties in Zhejiang province, with 30 counties that were first affected by H7N9 virus in the second wave. Many of these counties were distributed in the middle and the south of Zhejiang province. According to nucleic acid testing of H7N9 virus during the first wave in Zhejiang province, positive rates were higher than 43% of environmental samples from live poultry markets that patients had visited before the onset of illness [15]. These results indicated that the H7N9 virus existed widely in live poultry markets. The virus continues to spread within the province, based on our current results. Therefore, strengthening the surveillance of wildfowl and poultry viral infection and environmental contamination of the virus are important.
The proportion of patients with underlying conditions was high in the first (66%) and second (62%) waves of the epidemic in our study. Underlying conditions were risk factors of mortality in human H7N9 infections in an earlier investigation in Zhejiang province [9]. A study in Jiangsu province, which is close to Zhejiang province, showed that underlying conditions were strongly related to H7N9 virus infection [10]. Shanghai also had similar results in human cases, suggesting underlying conditions such as chronic heart and lung disease contribute to H7N9 infection [16]. Evidence is generally consistent that underlying conditions increase risk of infection with H7N9 virus, possibly through the poor immune function of people with these conditions [17]. Thus health education about self-protection and avoidance of risk factors is especially important to people with underlying conditions and particular attention should be paid to this population.
In earlier epidemiological investigations of human infections with H7N9 virus in China, exposure to live poultry markets was high [13, 18]. Previous studies genetically comparing H7N9 virus strains isolated from infected patients with viruses from chickens from live poultry markets showed that the gene sequences of these two groups of viruses were highly homologous [11, 19]. This suggests that a large proportion of human infections with H7N9 virus originate from live poultry markets. Similar evidence on the association between visiting live poultry markets and human infection has been reported in other epidemiological and laboratory studies [20, 21]. In addition, a decline in the number of H7N9 patients was seen after live poultry markets were closed in Shanghai, Jiangsu province and Zhejiang province, with the last case reported on 17 April 2013 [22]. This result further reinforced the hypothesis that live poultry markets are a high-risk location for human infection. In accordance with former reports, our study found high proportions of live poultry market exposure in infected patients in both waves of the epidemic. This result indicates that live poultry markets remained a main place for transmission in Zhejiang province. Farmers, retirees and men were the major groups of infected patients in our investigation. A partial explanation is that these groups frequently visit live poultry markets or are exposed to live poultry, due to Chinese customs and practices. Therefore, we recommend that live poultry markets be permanently closed. This recommendation coincides with a recent editorial suggesting closure of poultry markets to avoid potential reassortment and global pandemics [23]. We further suggest a reformation of poultry supply and sales to reduce risk of human infection with avian influenza viruses.
This study had two main limitations. First, we could not identify risk factors for H7N9 human infections. The study was designed to compare epidemiological and clinical features of H7N9-infected patients before and after October 2013 and could analyse only the differences and similarities in risk factors between the two waves of the epidemic. Therefore, we could not directly identify risk factors. Second, we collected information through questionnaires and interviews, so recall bias could not be excluded and might have affected the results. For example, patients might not clearly recall their exposure histories and this could, to some extent, influence the results of our data analysis.
In summary, we compared the epidemiological and clinical characteristics of laboratory-confirmed cases of human infection with H7N9 virus before and after October 2013 in Zhejiang province. We found no significant changes in most of the epidemiological and clinical features between the two waves of the H7N9 epidemic. Case-fatality rate from human infection with H7N9 virus is still high in Zhejiang province. Human infections are geographically widespread in the province. The proportion of patients who have underlying conditions remains high. In addition, we observed a high proportion of exposure to live poultry markets in infected patients in both waves of the epidemic. We suggest that renewed efforts should be made to enhance surveillance of wildfowl and poultry infection and environmental contamination with H7N9 virus. We further recommend permanent closure of live poultry markets and reformation of the poultry supply and sales to reduce risk of human infection with avian influenza viruses.
ACKNOWLEDGEMENTS
The authors thank Enfu Chen and Yuanyuan Xiao from Zhejiang Provincial Centre for Disease Control and Prevention for help with the preparation of this article.
This study was supported by the programme for Zhejiang leading team of science and technology innovation (2011R50021). None of the funders had any role in the study design and the collection, analysis, and interpretation of data, or in the writing of the article and the decision to submit it for publication.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Liu J, et al. H7N9: a low pathogenic avian influenza A virus infecting humans. Current Opinion in Virology 2014; 5: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health and Family Planning Commission of the People's Republic of China. Briefing on human infection with avian influenza A(H7N9) virus in June, 2013. (http://www.moh.gov.cn/yjb/s3578/201307/75a172fb9cf54ede8f96da5c3f72efd4.shtml). Accessed 20 October 2013.
- 3.World Health Organization. Human infection with avian influenza A(H7N9) virus in China – update (http://www.who.int/csr/don/2013_05_29/en/index.html). Accessed 20 October 2013.
- 4.Chen E, et al. Human infection with avian influenza A(H7N9) virus re-emerges in China in winter 2013. Eurosurveillance 2013; 18: pii = 20616. [DOI] [PubMed] [Google Scholar]
- 5.Zehjiang Health Bureau. New case of human infection with H7N9 virus in Zhejiang province (http://www.zjwst.gov.cn/art/2013/4/28/art_362_229196.html). Accessed 28 April 2013.
- 6.Zehjiang Health Bureau. Two new cases of human infection with H7N9 virus in Zhejiang province (http://www.zjwst.gov.cn/art/2014/2/28/art_362_408859.html). Accessed 7 May 2014.
- 7.National Health and Family Planning Commission of the People's Republic of China. Chinese guideline of diagnosis and treatment for human infections with the avian influenza A(H7N9) virus (2nd edn, 2013) (http://www.nhfpc.gov.cn/yzygj/s3585u/201304/7e2ad4cdf98b4e2285eab1c15ded8370.shtml). Accessed 10 April 2013.
- 8.National Health and Family Planning Commission of the People's Republic of China. Chinese guideline of diagnosis and treatment for human infections with the avian influenza A(H7N9) virus (2014 edition) (http://www.nhfpc.gov.cn/yzygj/s3593g/201401/3f69fe196ecb4cfc8a2d6d96182f8b22.shtml). Accessed 24 January 2014.
- 9.Liu S, et al. Epidemiological, clinical and viral characteristics of fatal cases of human avian influenza A(H7N9) virus in Zhejiang Province, China. Journal of Infection 2013; 67: 595–605. [DOI] [PubMed] [Google Scholar]
- 10.Ai J, et al. Case-control study of risk factors for human infection with influenza A(H7N9) virus in Jiangsu Province, China, 2013. Eurosurveillance 2013; 18: 20510. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 2013; 381: 1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WU QQ, et al. Surveys on tobacco use and related issues among adults in Zhejiang province. Chinese Preventive Medicine 2012; 13: 824–827. [Google Scholar]
- 13.Li Q, et al. Preliminary Report: Epidemiology of the avian influenza A(H7N9) outbreak in China. New England Journal of Medicine. Published online 24 April 2013. doi: 10.1056/NEJMoa1304617. [DOI] [Google Scholar]
- 14.Chinese Centre for Disease Control and Prevention. No change of risk assessment for H7N9 epidemic (http://www.chinacdc.cn/mtdx/crbxx/201401/t20140128_93119.htm). Accessed 30 January 2014.
- 15.Chen E, et al. Epidemiological characteristics and control strategies of human infection with avian influenza A(H7N9) virus in Zhejiang province. Chinese Journal Public Health 2013; 29: 625–627. [Google Scholar]
- 16.Chen X, et al. Clinical features and factors associated with outcomes of patients infected with a Novel Influenza A(H7N9) virus: a preliminary study. PLoS ONE 2013; 8: e73362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan Y, et al. H7N9 Incident, immune status, the elderly and a warning of an influenza pandemic. Journal of Infection in Developing Countries 2013; 7: 302–307. [DOI] [PubMed] [Google Scholar]
- 18.Cowling BJ, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet 2013; 382: 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao C, et al. Live-animal markets and influenza A(H7N9) virus infection. New England Journal of Medicine 2013; 368: 2337–2339. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 2013; 381: 1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J, et al. Epidemiological link between exposure to poultry and all influenza A(H7N9) confirmed cases in Huzhou city, China, March to May 2013. Eurosurveillance 2013; 18. pii = 20481. [PubMed] [Google Scholar]
- 22.Murhekar M, et al. Avian influenza A(H7N9) and the closure of live bird markets. Western Pacific Surveillance Response Journal 2013; 4: 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao GF. Influenza and the live poultry trade. Science 2014; 344: 235. [DOI] [PubMed] [Google Scholar]