SUMMARY
Meningitis with a negative cerebrospinal fluid Gram stain (CSF-GS) poses a diagnostic challenge as more than 50% of patients remain without an aetiology. The introduction of polymerase chain reaction (PCR) and arboviral serologies have increased diagnostic capabilities, yet large scale epidemiological studies evaluating their use in clinical practice are lacking. We conducted a prospective observational study in New Orleans between November 1999 and September 2008 (early era) when PCR was not widely available, and in Houston between November 2008 and June 2013 (modern era), when PCR was commonly used. Patients presenting with meningitis and negative CSF-GS were followed for 4 weeks. All investigations, PCR used, and results were recorded as they became available. In 323 patients enrolled, PCR provided the highest diagnostic yield (24·2%) but was ordered for 128 (39·6%) patients; followed by serology for arboviruses (15%) that was ordered for 100 (31%) of all patients. The yield of blood cultures was (10·3%) and that of CSF cultures was 4%; the yield for all other tests was <10%. Overall, 65% of the patients remained without a diagnosis at 4 weeks: 72·1% in early era vs. 53·4% (P < 0·01) in modern era; this change was attributed to diagnosing more viral pathogens, 8·3% and 26·3% (P < 0·01), respectively. The introduction of PCR and arboviral serologies has improved the yield of diagnosing patients with meningitis and a negative CSF-GS, but both tests are being under-utilized.
Key words: Aseptic meningitis, epidemiology, Gram stain, meningitis, polymerase chain reaction, viral meningitis
INTRODUCTION
A patient who presents with signs and symptoms of meningitis may suffer from one of many different infectious or non-infectious causes. One of the initial deliberations facing the physician is whether the aetiological agent is bacterial or if the presentation is what used to be termed ‘aseptic’. Bacterial meningitis and certain viral pathogens such as herpes simplex encephalitis must be treated early and aggressively with intravenous antimicrobial medications in order to reduce morbidity and mortality, while other causes of meningitis usually present a more benign course, and/or require only supportive care [1–3].
When clinical findings suggest meningitis, a lumbar puncture (LP) with cerebrospinal fluid (CSF) analysis including Gram stain and culture are considered standard of care [4]. A Gram stain has the advantage of suggesting the bacterial aetiology ⩾1 day before culture results are available and may offer guidance for initial approach and therapy [5, 6]. The reported sensitivity of Gram stain for bacterial meningitis in adults varies from 60% to 90%; however, its specificity approaches 100% [1, 6, 7]. On the other hand, a negative Gram stain is seen in about 90% of all patients presenting with community-acquired meningitis in the emergency department and poses a serious diagnostic dilemma, not well explored in the molecular diagnostic era [2]. Lack of clinical models and evidence-based guidelines for patients with meningitis and a negative Gram stain have fostered hospitalization and usage of empirical antimicrobial therapy for the majority of patients, including for those eventually found to have viral meningitis or other conditions that do not warrant treatment [2, 3]. This practice represents a significant economic burden and accounts for about 434000 hospitalizations per year with an annual cost of US$310 million in the USA [8].
Polymerase chain reaction (PCR) assays to diagnose viral central nervous system infections have now become standard and have largely replaced viral culture [9]. Large retrospective studies of meningitis with a negative Gram stain have documented that about 70% of causes are unknown [10–12]. The aim of this study was to evaluate the current diagnostic use of available tests including the PCR and arboviral serologies in the evaluation of patients that present with meningitis and a negative Gram stain.
METHODS
This was a prospective observational study conducted over 14 years between November 1999 and June 2013. The patients were grouped as the ‘early era cohort’ generated at the Tulane University Hospital and Clinic, and the Medical Center of Louisiana, New Orleans between November 1999 and September 2008. The study was interrupted between August 2005 and September 2006 due to Hurricane Katrina. This cohort was considered the ‘early era cohort’, employing minimal molecular diagnostics as they became available. The second group, the ‘modern era cohort’, was collected at Memorial Herman Hospital between November 2008 and June 2013, at Lyndon B. Johnson Hospital between December 2009 and June 2013 and St Luke's Episcopal Hospital between February 2010 and February 2013. The latter cohort was considered the ‘modern era cohort’, utilizing molecular diagnostics widely available at the discretion of the treating physicians. The study was approved by the Institutional Review Board of each hospital and written informed consent was obtained from enrollees. Potential patients were identified through the laboratory records of each hospital which included all patients who underwent a LP within the first 24 h of hospitalization. All baseline CSF and serum samples were obtained within 24 h of admission to the hospital. Patients were eligible for inclusion if they were aged >16 years, presented to the emergency department with symptoms of community-acquired meningitis (fever, headache, meningismus, altered sensorium, and/or focal neurological deficits), underwent a diagnostic LP which showed ⩾5 white cells/mm3, and had a negative CSF Gram stain. Patients presenting with a ventricular peritoneal (VP) shunt or those who were post-craniotomy were excluded from the study [12].
This was an observational study, thus the diagnostic evaluation, work-up and treatment were at the discretion of the treating physician. Socio-demographic data, comorbid conditions (measured by the Charlson comorbidity scale), immune competence, exposures, clinical features (including the modified NIH stroke scale) were obtained at a specified ‘zero time’ (the time that the patient was in the emergency department) [9, 10]. Following enrolment, patients were followed for at least 4 weeks in order to ascertain the final diagnosis and outcomes through follow-up visits, and when unavailable to attend for a clinic visit, by a phone call, visit or patient records.
Laboratory testing and diagnostic criteria
All tests were performed at each hospital's clinical microbiology laboratory using commercially available tests, real-time PCR assays were used for the last 4 years of the early era (as they became available for commercial use) and for the entire modern era. CSF from all patients was tested for glucose, protein, cell count, and was cultured for bacterial pathogens. Diagnosing aetiologies were established according to the following criteria: for bacterial meningitis, a positive blood culture or CSF culture or a positive rapid immunochromatographic CSF assay (ICT) such as BinaxNOW (Alere, USA) for S. pneumoniae; fungal meningitis on a positive CSF culture or antigen detection; enterovirus or herpes simplex virus (HSV) meningitis on either positive CSF viral culture or PCR; varicella zoster virus (VZV) was based on either a positive culture or PCR in the CSF or isolation of VZV (viral culture or a positive VZV direct fluorescent antibody) from a co-existing vesicular skin lesion; West Nile virus on positive serology on CSF or serum; St Louis encephalitis on serology increase over a 4-week period; acute HIV on a positive viral load using PCR on serum sample with negative HIV serology; cytomegalovirus or Epstein–Barr virus diagnosis on either a positive PCR on CSF or positive IgM serology in the presence of negative IgG serology; Rickettsia disease on evidence of specific serum antibody or demonstration or Rickettsia in a skin biopsy; bacteraemia on isolation of the pathogen from at least two blood culture samples; syphilis on positive Venereal Disease Research Laboratory test in CSF with a positive rapid plasma reagin (RPR) test; lymphocytic choriomeningitis, leptospirosis, Rocky Mountain spotted fever and Mycoplasma on positive serology; parameningeal and intracranial mass lesions and haemorrhages on documentation by either cranial or spinal imaging.
Statistical analysis
After preliminary evaluation of data for accuracy and consistency, we summarized the baseline demographic data which included means, medians, and ranges for continuous variables and frequency for categorical variables. When appropriate, one-way ANOVA, χ2 and Fisher's exact tests were used to assess differences between the groups of patients with respect to demographic and clinical characteristics. Two-tailed analysis with alpha <0·05 was used for statistical significance. All statistical analyses were performed using SPSS software v. 21 (IBM, USA).
Ethical statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by The University of Texas Health Science Center Institutional review board (IRB) HSC-MS-08-0417; the local boards of all participating institutions approved the same protocol.
RESULTS
Cohort assembly
During the period of the study 496 patients were screened, of these 323 met the inclusion criteria and consented to participate in the study; 205 in the early era cohort and 118 in the modern era cohort. Some 176 cases were excluded for refusal to provide consent, having a VP shunt, or having history of recurrent meningitis or previous craniotomy.
Clinical and laboratory characteristics
The early era cohort included a higher percentage of African Americans (73% vs. 23·7%), uninsured patients (51·7% vs. 17·8%), HIV-positive patients (28·7% vs. 5·8%), history of intravenous drug use (11·2% vs. 0·8%), and comorbidities (34·1% vs. 17·8%) than the modern era cohort (P < 0·05 each) (Table 1). Twenty-two percent of the entire population received antibiotics within the last 7 days prior to LP. Headache was the most common presenting feature (82%), with fever and nausea being common as well at 67% and 54%, respectively. About half (52%) of all patients complained of a stiff neck. The modern era cohort had higher rates of altered mental status, seizures and CSF pleocytosis (P < 0·05).
Table 1.
Clinical and laboratory characteristics of 323 adults with meningitis and a negative Gram stain of cerebrospinal fluid
| New Orleans cohort (N = 205) n (%) |
Houston cohort (N = 118) n (%) |
Both cohorts (N = 323) n (%) |
P value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 111 (54·1) | 59 (50) | 170 (52·6) | 0·49 |
| Age, years | ||||
| Mean (range) | 39·4 ± 13·6 (17–96) | 42·9 ± 16·4 (17 -82) | 41 ± 14·8 (17–96) | 0·04 |
| Ethnicity | ||||
| White | 46 (22·4) | 39 (33) | 85 (26·3) | 0·05 |
| African American | 151 (73·6) | 28 (23·7) | 179 (55·4) | <0·01 |
| Hispanic and others* | 8 (3·9) | 51 (43·2) | 59 (18·2) | <0·01 |
| Insurance | ||||
| Medicare/Medicaid | 56 (27·3) | 30 (25·4) | 86 (26·6) | 0·79 |
| Private | 35 (17·1) | 63 (53·3) | 98 (30·3) | <0·01 |
| Uninsured | 106 (51·7) | 21 (17·8) | 127 (39·3) | <0·01 |
| Unknown | 8 (3·9) | 4 (3·4) | 12 (3·7) | 1·10 |
| Co-existing medical conditions | ||||
| Charlson comorbidity index score >1 | 70 (34·1) | 21 (17·8) | 91 (28·2) | <0·01 |
| HIV positive | 59 (28·7) | 13 (11) | 72 (22·3) | <0·01 |
| Intravenous drug use | 23 (11·2) | 1 (0·8) | 24 (7·4) | <0·01 |
| Immune status | ||||
| Normal host | 137 (66·8) | 98 (83) | 235 (72·7) | <0·01 |
| AIDS | 37 (18) | 6 (5·1) | 43 (13·3) | <0·01 |
| Chronic steroids† | 6 (2·9) | 4 (3·4) | 10 (3·1) | 1·10 |
| Transplant patient‡ | 3 (1·4) | 3 (2·5) | 6 (1·8) | 0·67 |
| Recent antibiotic use§ | ||||
| Any antibiotic | 37 (18) | 37 (31·3) | 74 (22·9) | <0·01 |
| Risk factors | ||||
| Recent mosquito bites§ | 44 (21·4) | 22 (18·6) | 66 (20·4) | 0·57 |
| Recent ill contact§ | 33 (16·1) | 16 (13·5) | 49 (15·2) | 0·63 |
| History of sinusitis | 22 (10·7) | 16 (13·5) | 38 (11·8) | 0·63 |
| TB exposures | 18 (8·8) | 8 (6·8) | 26 (8·6) | 0·67 |
| History of otitis | 7 (3·4) | 8 (6·7) | 15 (4·6) | 0·18 |
| History of travel | 33 (16) | 14 (11·8) | 47 (14·5) | 0·33 |
| History tick bites | 5 (2·4) | 1 (0·8) | 6 (1·8) | 0·43 |
| Season | ||||
| Summer (June–August) | 67 (32·7) | 28 (23·7) | 95 (29·4) | 0·1 |
| Spring (March–May) | 51 (24·9) | 20 (16·9) | 71 (22) | 0·12 |
| Fall (September–November) | 44 (21·5) | 35 (29·6) | 79 (24·4) | 0·11 |
| Winter (December–February) | 43 (20·9) | 35 (29·6) | 78 (24·1) | 0·08 |
| Presenting history | ||||
| Headache | 172 (83·9) | 93 (78·8) | 265 (82) | 0·29 |
| Fever | 147 (71·7) | 72 (61) | 219 (67·8) | 0·06 |
| Nausea | 114 (55·6) | 61 (51·7) | 175 (54·2) | 0·56 |
| Stiff neck | 113 (55·1) | 57 (48·3) | 170 (52·6) | 0·24 |
| Photophobia | 100 (48·7) | 42 (35·6) | 142 (44) | 0·02 |
| Malaise | 88 (42·9) | 40 (33·9) | 128 (39·6) | 0·12 |
| Respiratory symptoms | 54 (26·3) | 23 (19·5) | 77 (23·8) | 0·14 |
| Seizures <7 days | 15 (7·3) | 20 (16·9) | 35 (10·8) | <0·01 |
| Presenting signs | ||||
| Temperature >38·4 °C | 100 (48·8) | 72 (61) | 172 (53·2) | 0·04 |
| Decreased level of consciousness (GCS <15) | 42 (20·5) | 40 (33·9) | 82 (25·4) | 0·01 |
| Nuchal rigidity | 53 (25·9) | 24 (20·3) | 77 (23·8) | 0·28 |
| Focal neurological deficits¶ | 26 (12·7) | 21 (17·8) | 47 (14·5) | 0·25 |
| Vesicular or petechial rash | 12 (5·9) | 5 (4·2) | 17 (5·3) | 0·61 |
| Serum WBC count, cells/μl, median (range) | 8200 (2300–31110) | 9200 (2400–64500) | 8600 (2300–64500) | 0·14 |
| CSF WBC count, cells/μl, median (range) | 51 (6–53790) | 131 (7–20421) | 79 (6–20421) | 0·03 |
| CSF protein, mg/dl, median (range) | 62 (6–1438) | 78 (16–7300) | 65 (6–7300) | 0·08 |
| CSF glucose, mg/dl, median (range) | 54 (0–316) | 52 (0–193) | 54 (0–316) | 0·46 |
| CSF ALC, cells/μl, median (range) | 80 (0–100) | 81 (2–100) | 80 (0–100) | 0·78 |
| CSF ANC > 500 | 2 (1) | 11 (9·3) | 13 (4) | <0·01 |
| CSF WBC > 1000 | 23 (11·2) | 15 (12·7) | 38 (11·8) | 0·72 |
HIV, Human immunodeficiency virus; AIDS, acquired immune deficiency syndrome; TB, Mycobacterium tuberculosis;
GCS, Glasgow Coma Scale; CSF, cerebral spinal fluid; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; WBC, white blood cell.
Values given are n (%) unless stated otherwise.
P value by Fisher's exact test or two-way ANOVA
Includes Asian, Native Indian and unknown/declined to answer.
More than 0·5 mg/kg of methyl-prednisone or equivalent in the last 14 days,
After a solid organ or haematopoietic transplant and currently taking immunosuppression on a chronic basis.
Within the last 7 days,
Focal neurological deficits includes aphasia, palsy.
Laboratory diagnosis and aetiologies of meningitis with a negative Gram stain
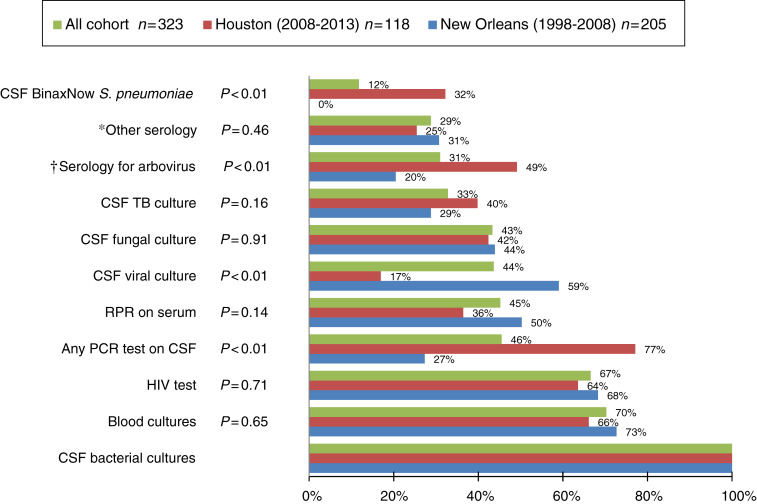
Figure 1 depicts the investigations performed on the different cohorts in order to establish the diagnosis. All patients underwent bacterial CSF cultures while blood cultures were only obtained from 70% of the patients. CSF viral culture, which was performed on 60% of the patients in early era and on only 17% of modern era patients, was mostly replaced by molecular diagnostics with at least one CSF PCR ordered (77% in modern vs. 27% in early) (P < 0·05). HIV serology and serum RPR were performed in 67% and in 45% of the total cohort, respectively. Arboviral serology was performed in only one-third of the total cohort (49% in modern, 20% in early).
Fig. 1.
Investigations performed to establish diagnosis by period/location, on patients with meningitis and a negative Gram stain. P value was calculated using Fisher's exact test comparing the number of tests performed in each category between patients in Houston and New Orleans. Patients with more than one test were counted only once in each category. CSF BinaxNow (Alere, USA) is a rapid immunochromatographic CSF assay. * Serology for cytomegalovirus, Mycoplasma pneumonia, Bartonella, leptospirosis, Rocky Mountain spotted fever, Ehrlichia. † Serology on CSF and serum for West Nile virus and other arboviruses (St Louis encephalitis, western and eastern equine encephalitis, California encephalitis).
The yield of the diagnostic tests performed on both cohorts is shown in Table 2. The tests that offered the highest yield for diagnosis were molecular diagnostics (24·2%), followed by serology for arboviruses (15%), and blood cultures in the modern era cohort (10·3%); the yield for all other tests was below 10% but included important aetiologies such as bacterial, fungal, and mycobacterial meningitis. CSF ICT assay was performed in 38 patients in the modern era cohort; however, it was positive in only one case. This patient had negative CSF and blood cultures and no previous antibiotic therapy; he received a full course of intravenous antibiotic therapy.
Table 2.
Yield of Investigations performed on patients with meningitis and a negative Gram stain
| New Orleans cohort (N = 205) n (%) |
Houston cohort (N = 118) n (%) |
All cohorts (N = 323) n (%) |
|
|---|---|---|---|
| Viral causes tested | |||
| Positive serum HIV test | 61/140 (43·6) | 14/75 (18·7) | 75/215 (34·9) |
| Any positive viral PCR on CSF* | 14/37 (37·8) | 17/91 (18·7) | 31/128 (24·2) |
| Positive CSF viral culture | 1/121 (0·8) | 0/20 (0) | 1/141 (0·8) |
| Positive arbovirus test in CSF or serum† | 2/42 (4·8) | 13/58 (22·4) | 15/100 (15) |
| Positive CMV IGM serum and negative IgG | 3/63 (4·8) | 1/30 (3·3) | 4/93 (4·3) |
| Positive for lymphocytic choriomeningitis IgM serum | 2/54 (3·7) | 1/22 (4·5) | 3/76 (3·9) |
| Positive EBV IgM serum | 1/29 (3·4) | 2/28 (7·1) | 3/57 (5·3) |
| Bacterial, fungal and other causes tested | |||
| Growth on CSF bacterial culture | 6/205 (2·9) | 7/118 (5·9) | 13/323 (4) |
| Positive blood cultures | 7/149 (4·7) | 8/78 (10·3) | 15/227 (6·6) |
| Positive RPR serum | 1/103 (0·9) | 2/43 (4·6) | 3/146 (2·1) |
| Positive cryptococcal antigen on CSF | 6/88 (6·8) | 2/55 (3·6) | 8/143 (5·6) |
| Positive VDRL on CSF | 1/91 (1·1) | 2/50 (4) | 3/141 (2·1) |
| Growth on CSF fungal culture | 6/90 (6·7) | 2/50 (4) | 8/140 (5·7) |
| Growth on CSF TB cultures | 2/59 (3·4) | 0/47 (0) | 2/106 (1·9) |
| Positive Mycoplasma IgM serum | 2/51 (3·9) | 2/36 (5·5) | 4/87 (4·6) |
| Positive cryptococcal antigen serum | 2/64 (3·1) | 1/23 (4·3) | 3/87 (3·4) |
| Positive for Bartonella serum | 1/45 (2·2) | 1/30 (3·3) | 2/75 (2·6) |
| Positive leptospirosis antibody serum | 0/45 (4·4) | 0/23 (0) | 0/68 (0) |
| Positive Rocky Mountain spotted fever IgM serum | 3/25 (12) | 1/22 (4·5) | 4/47 (8·5) |
| Positive for Ehrlichia (by IFA) serum | 1/28 (3·6) | 0/18 (0) | 1/46 (2·2) |
| Positive CSF BinaxNOW S. pneumoniae | 0/0 (0) | 1/38 (2·6) | 1/38 (2·6) |
PCR, Polymerase chain reaction; CSF, cerebral spinal fluid; CMV, cytomegalovirus; EBV, Epstein–Barr virus; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory; IFA, immunofluorescence assay.
IFA, Immunofluorescence assay (CSF BinaxNow S. pneumonia; Alere, USA) is a rapid immunochromatographic CSF assay.
Refers to having at least one PCR for either herpes simplex 1 and 2, varicella zoster virus or enterovirus (not all four were checked for every patient).
Included enzyme immunoassay serology for California encephalitis, western and eastern equine encephalitis, St Louis encephalitis, West Nile virus (not all five were tested for every patient).
Table 3 describes the aetiologies of the two cohorts. Overall 65% of the patients remained without a diagnosis at 4 weeks following assessment; this was a significant improvement from 72·1% in the earlier era, to 53·4% in the modern era cohorts. This change was attributed to diagnosing additional viral entities by either CSF PCR or by arboviral serology, i.e. only 8·3% of the patients had a viral entity diagnosed in early era cohort compared to 26·3% in the modern era cohort.
Table 3.
Atiologies of 323 patients with meningitis and a negative cerebrospinal fluid Gram stain
| Aetiology | Method of detection | New Orleans cohort (N = 205) n/N (%) |
Houston cohort (N = 118) n/N (%) |
All cohorts (N = 323) n/N (%) |
P value |
|---|---|---|---|---|---|
| Unknown | 148/205 (72·1%) | 63/118 (53·4%) | 211/323 (65·3%) | <0·01 | |
| Confirmed aetiology | 42/205 (20·5%) | 47/118 (39·8%) | 89/323 (27·5%) | <0·01 | |
| Viral | 17/205 (8·3%) | 31/118 (26·3%) | 48/323 (14·9%) | <0·01 | |
| Enterovirus | By PCR on CSF By viral culture |
7 1 |
6 0 |
13 1 |
|
| Herpes simplex 1 and 2 | PCR on CSF | 4 | 8 | 12 | |
| Varicella zoster virus | PCR on CSF | 3 | 3 | 6 | |
| St Louis encephalitis | Serology on CSF or serum | 1 | 6 | 7 | |
| West Nile virus | Serology on CSF or serum | 1 | 7 | 8 | |
| Acute HIV | PCR on serum with negative serology | 0 | 1 | 1 | |
| Bacterial | 12/205 (5·8%) | 10/118 (8·5%) | 22/323 (6·8%) | 0·36 | |
| Streptococcus pneumoniae | CSF cultures, blood cultures and BinaxNow | 3 | 4 | 7 | |
| Staphylococcus aureus | CSF cultures and blood cultures | 5 | 2 | 7 | |
| Group b streptococcus | CSF cultures and blood cultures | 1 | 3 | 4 | |
| Listeria monocytogenes | CSF culture | 1 | 0 | 1 | |
| Escherichia coli | blood cultures | 2 | 1 | 3 | |
| Other | 13/205 (6·3%) | 6/118 (5·1%) | 19/323 (5·9%) | 0·8 | |
| Cryptococcus neoformans | Cryptococcal antigen on CSF/serum or growth CSF culture | 8 | 2 | 10 | |
| Treponema pallidum | VDRL on CSF | 1 | 2 | 3 | |
| Mycobacterium tuberculosis | By CSF cultures or respiratory cultures | 2 | 1 | 3 | |
| CNS bleed | Imaging | 2 | 0 | 2 | |
| Histoplasma capsulatum | CSF Fungal cultures | 0 | 1 | 1 | |
| Presumed aetiology | 15/205 (7·3%) | 8/118 (6·8%) | 23/323 (7·1%) | 0·99 | |
| Mycoplasma pneumonia | Serology (+IgM and −IgG) | 2 | 2 | 4 | |
| Rocky mountain spotted fever | Serology (+IgM and −IgG) | 3 | 1 | 4 | |
| Cytomegalovirus | Serology (+IgM and −IgG) | 3 | 1 | 4 | |
| Epstein–Barr virus | Serology (+IgM and −IgG) | 1 | 2 | 3 | |
| Lymphocytic choriomeningitis virus | Serology | 2 | 1 | 3 | |
| Bartonella henselae | Serology | 1 | 1 | 2 | |
| Toxoplasma gondii | Positive serology with radiological findings in AIDS patient | 1 | 0 | 1 | |
| Ehrlichia chaffeensis | By IFA on serum | 1 | 0 | 1 | |
| CNS vasculitis | Imaging | 1 | 0 | 1 |
PCR, Polymerase chain reaction; CSF, cerebrospinal fluid; VDRL, Venereal Disease Research Laboratory; CNS, central nervous system; HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome; IFA, indirect fluorescent antibody.
Comparing the New Orleans cohort and Houston cohort using Fisher's exact test.
DISCUSSION
In this observational prospective study we describe the epidemiology and aetiologies that cause meningitis in patients who presents with signs and symptoms of meningitis and a negative Gram stain. Since most investigations into the causes of meningitis require 24–48 h or longer, and the majority of patients that present to the emergency department with community-acquired meningitis have a negative Gram stain, the physician is faced with a diagnostic and therapeutic dilemma. This dilemma is magnified as no epidemiological data or diagnostic algorithms are available that incorporate modern molecular diagnostic methods to help guide the physician.
In recent years, molecular and nucleic acid amplification tests have become common practice in evaluating patients with infections, particularly for the diagnosis of CNS infections. Recovery of any pathogen from a typically sterile site such as the CSF most likely represents an infection, typically mono-microbial in immunocompetent patients [11]. Furthermore, the chance of a false-positive PCR assay result in CSF is reduced compared to other body sites due to the lack of common PCR inhibitors such as haem, endonucleases, and exonucleases [9].
The lack of data has led to considerable concern regarding potential urgent treatable conditions and many physicians are compelled to admit patients for observation, for further work-up and treatment with the combination of antibacterial, antiviral or even antifungal therapies. Our results show that PCR and arboviral serologies assisted in identifying causes of meningitis within a large group of patients who previously were undiagnosed. This subgroup of patients were shown to be afflicted by different viral entities, some of which may be treatable, such as, VZV, HSV and acute HIV, whereas for others there still is no treatment options, e.g. West Nile virus and enterovirus.
Addressing the results in Figure 1 and Tables 2 and 3, it becomes clear that there was no uniform method in investigation of the cause of meningitis when a patient presented with a negative Gram stain. Each clinician selected his own set of assays based on personal experience, resulting in a certain variance between patients tested, influenced on numerous occasions by personal bias in prioritizing certain tests over others. Furthermore, it was apparent that on many occasions, physicians would order large series of tests but the quantity of CSF available for evaluation was insufficient, leading to inconsistent approaches.
However, even with this unsystematic approach to diagnosis, performed on two different populations, which may have been suffering from different causes of meningitis, the diagnostic yield improved by more than 20% between the two periods. We believe that in incorporating a unified approach, the prioritization of clinically meaningful tests would become established, and the diagnostic yield would increase even more.
Another difference to note between the two cohorts relates to the number of immunocompromised patients, specifically those with AIDS. The introduction and widespread use of highly active antiretroviral therapy (HAART) coincided with our modern era cohort. Thus, we observe that in the early era the number of patients that were immunocompromised, specifically with AIDS, was much higher and these patients were at a higher risk for opportunistic infections involving the central nervous system. Whereas in the modern era when the use of HAART became widespread, the number of immunocompromised patients, specifically those with AIDS, was greatly reduced.
Despite its methodological advantages, our study has some limitations. First, most notably was that this was an observational study performed on populations from different cities, institutions, and different risks, relying on the physicians' preferred assays. This obviously led to large variance in the diagnostic evaluation performed on the patients, and more importantly, led to incomplete data and hence, limited our ability to compare the yield of the different assays in a more rigorous way other than descriptively. Second, the diagnostic assays between institutions varied and this could affect the sensitivity of the assays. Third, because the sensitivity and specificity are different between the conventional PCR which was used in the first 4 years in the early era and the real-time PCR which was used in the remainder of the study years, the yield of investigations could be affected. Finally, the difference in testing for arboviruses could represent the difference in the time periods for both sites. West Nile virus was introduced to both Louisiana and Texas in 2002 [13]. The relative lack of testing in the New Orleans institutions could be because there was no outbreak between 1999 and 2001, and as the epidemic progressed arboviral testing increased. Despite increased awareness, arboviral serologies in the Houston site in recent years are being ordered for <50% of patients.
One of the study's strengths is its prospective, non-interventional nature, demonstrating how clinicians that were not compelled to follow guidelines or study structure utilized the assays available to them. It also demonstrated that when newer assays became available and were incorporated into practice, the diagnostic yield improved, providing data to help guide the clinicians.
In conclusion, the introduction of PCR and arboviral serologies is helpful in diagnosing patients with meningitis and a negative Gram stain. However, certain challenges remain as the majority of patients do not present with the aetiology and the diagnostic work-ups are not standardized, leading to over-treatment.
ACKNOWLEDGEMENTS
Support for this study was provided by the National Center for Research Resources (NIH-1 K23 RR018929-01A2) (PI: Hasbun) and the Grant A. Starr Foundation (PI: Wootton).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.van de Beek D, et al. Clinical features and prognostic factors in adults with bacterial meningitis. New England Journal of Medicine 2004; 351: 1849–1859. [DOI] [PubMed] [Google Scholar]
- 2.Scheld WM, et al. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. Journal of Infectious Disease 2002; 186 (Suppl. 2): S225–233. [DOI] [PubMed] [Google Scholar]
- 3.Hasbun R. The acute aseptic meningitis syndrome. Currrent Infectious Disease Reports 2000; 2: 345–351. [DOI] [PubMed] [Google Scholar]
- 4.Tunkel AR, et al. Practice guidelines for the management of bacterial meningitis. Clinincal Infectious Disease 2004; 39: 1267–1284. [DOI] [PubMed] [Google Scholar]
- 5.Geiseler PJ, et al. Community-acquired purulent meningitis: a review of 1,316 cases during the antibiotic era, 1954–1976. Review of Infectious Disease 1980; 2: 725–745. [DOI] [PubMed] [Google Scholar]
- 6.Spanos A, Harrell FE, Durack DT. Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. Journal of the American Medical Assosciation 1989; 262: 2700–2707. [PubMed] [Google Scholar]
- 7.Fitch MT, van de Beek D. Emergency diagnosis and treatment of adult meningitis. Lancet Infectious Disease 2007; 7: 191–200. [DOI] [PubMed] [Google Scholar]
- 8.Khetsuriani N, et al. Viral meningitis-associated hospitalizations in the United States, 1988–1999. Neuroepidemiology 2003; 22: 345–352. [DOI] [PubMed] [Google Scholar]
- 9.Debiasi RL, Tyler KL. Molecular methods for diagnosis of viral encephalitis. Clinical Microbiology Reviews 2004; 17: 903–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasbun R, et al. Risk score for identifying adults with CSF pleocytosis and negative CSF Gram stain at low risk for an urgent treatable cause. Journal of Infection 2013; 67: 102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoury NT, et al. Meningitis with a negative cerebrospinal fluid Gram stain in adults: risk classification for an adverse clinical outcome. Mayo Clinic Proceedings 2012; 87: 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negrini B, Kelleher KJ, Wald ER. Cerebrospinal fluid findings in aseptic versus bacterial meningitis. Pediatrics 2000; 105: 316–319. [DOI] [PubMed] [Google Scholar]
- 13.Petersen LR, et al. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiology and Infection 2013; 141: 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]