Abstract
In the long-chain n-alkane degrader Acinetobacter sp. strain M-1, two alkane hydroxylase complexes are switched by controlling the expression of two n-alkane hydroxylase-encoding genes in response to the chain length of n-alkanes, while rubredoxin and rubredoxin ruductase are encoded by a single gene and expressed constitutively.
Several strains in the genus Acinetobacter are known as n-alkane utilizers (4, 10). Among them, our isolate, Acinetobacter sp. strain M-1, is characterized by its ability to degrade a variety of n-alkanes, including very long chain n-alkanes (or paraffin wax) with carbon chain lengths of C20 to C44 that are in a solid state at ambient temperature (18).
Several pathways have been proposed for the initial reaction of n-alkane degradation by Acinetobacter strains (1, 2, 4, 5). Previously, we demonstrated three n-alkane dioxygenase activities in Acinetobacter sp. strain M-1, which had been postulated by Finnerty (5). We assume that these enzymes are involved in the oxidation of n-alkanes that are slightly dissolved in the cytosol or oil inclusion of the cell, because the enzymes were found in the soluble fraction of the cell extract of strain M-1. Recently, the genes encoding alkane hydroxylase (alkM) (15), rubredoxin (rubA), and rubredoxin reductase (rubB) (8) in Acinetobacter calcoaceticus strain ADP1 were found, and each of the genes was shown to be indispensable for n-alkane degradation. These results suggest that a three-component alkane hydroxylase complex participates in n-alkane degradation in strain ADP1, which is similar to that in a medium-chain (C6 to C12) n-alkane degrader, Pseudomonas oleovorans (24). The difference in the organization of the genes involved in n-alkane degradation between P. oleovorans and A. calcoaceticus strain ADP1 is that these genes are dispersed over the chromosomal DNA in strain ADP1, while they form an operon on a large OCT plasmid in P. oleovorans.
We describe here the isolation and characterization of genes in strain M-1 that are homologous to alkM, rubA, and rubB of strain ADP1. The most characteristic feature of strain M-1 was that two genes encoded alkane hydroxylases and they were differentially induced in response to the chain length of n-alkanes.
Cloning of two alkane hydroxylase genes, alkMa and alkMb, from Acinetobacter sp. strain M-1.
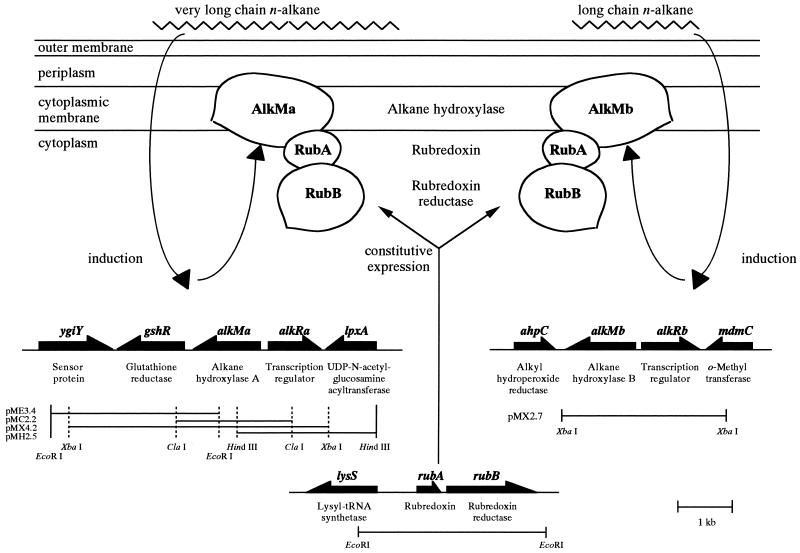
We intended to clone the alkane hydroxylase-encoding gene from Acinetobacter sp. strain M-1 to study the molecular basis of the alkane hydroxylase complex in this organism. We designed the PCR primers mono-N and mono-C (Table 1) based on the highly conserved regions between alkM of A. calcoaceticus strain ADP1 (15) and alkB of P. oleovorans (11) and used the chromosomal DNA of strain M-1 as a template. This PCR yielded a 790-bp DNA fragment, and the sequence of the fragment was identical to a part of the alkMa gene (see below). Southern blot analysis using the fragment as the probe revealed that the probe hybridized to at least two bands in the genomic DNA of strain M-1 that had been digested with various restriction enzymes (data not shown). The hybridization was performed under low-stringency conditions at 37°C in the buffer from AlkPhosDirect (Amersham Pharmacia Biotech UK Ltd., Buckinghamshire, England). From these results, we cloned two alkane hydroxylase genes, alkMa and alkMb, into pBluescript II SK+ (Stratagene, La Jolla, Calif.) through colony hybridization as described previously (17). The four fragments in pMX4.2, pMC2.2, pMH2.5, and pME3.4 (Table 2) overlapped each other, and the span contained four complete open reading frames (ORFs) and a partial ORF, covering a total of 6,089 bp. The 2.7-kb XbaI fragment in pMX2.7 (Table 2) contained two ORFs and a partial ORF over a span of 2,868 bp (Fig. 1). Inverse PCR (13) was performed to amplify the downstream region of alkMb. The BclI-digested chromosomal DNA of strain M-1 was self-ligated and used as a template. The primers used were IPA2up and IPA2dn (Table 1). The resulting PCR-amplified 4-kb fragment was cloned and sequenced. Since the two cloned fragments harboring alkMa and alkMb could explain all of the hybridizing bands that appeared in the Southern blot analysis, we concluded that strain M-1 has two alkane hydroxylase genes.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| pMX4.2 | Apr; contains 4.2-kb XbaI fragment of the gene library including alkMa and alkRa (Fig. 1) | This study |
| pME3.4 | Apr; contains 3.4-kb EcoRI fragment of the gene library (Fig. 1) | This study |
| pMC2.2 | Apr; contains 2.2-kb ClaI fragment of the gene library (Fig. 1) | This study |
| pMH2.5 | Apr; contains 2.5-kb HindIII fragment of the gene library (Fig. 1) | This study |
| pMX2.7 | Apr; contains 2.7-kb XbaI fragment of the gene library including alkMb and alkRb (Fig. 1) | This study |
| pKT231 | Kmr, Smr | 3 |
| pMFY31 | Apr, Tcr, Cmr | 6 |
| pMFYalkMa | Apr, Tcr, Cmr; contains PstI-digested pMX4.2 in the PstI site of pMFY31 | This study |
| pMFYalkMb | Apr, Tcr, Cmr; contains PstI-digested pMX2.7 in the PstI site of pMFY31 | This study |
TABLE 2.
Sequences of primers used in this study
| Primer | Sequencea (5′→3′) |
|---|---|
| Mono-N | TTCCGGTGATTGATACGATTATTGG |
| Mono-C | ACGCGTCGGATAAGCGTGATGATC |
| IPA2up | AACTATACCTGGTTTGCAGG |
| IPA2dn | GAAATACAGAGTCGTTTAAAATGC |
| alkMUp | CGGGGTAAGCATGAATAGCT |
| alkMDn | CGTACAGCTACTTGGTGGAC |
| Km-NBam | GGATCCGGACCAGTTGGTGATTTT |
| Km-CBam | GGATCCTTAGAAAAACTCATCGAGC |
| RBDXN | ATGAAAAAGTATCAATGTATCG |
| RBDXC | TTAAACTTCGATCATTTCAAA |
| IRBDXN | TCGTAAATCCAACCACAAAC |
| IRBDXC | TTGCGGCGTTTCAAAAGCTG |
The BamHI site is italicized.
FIG. 1.
Gene organization and restriction maps of the cloned regions, including alkMa, alkMb, and rubAB operon, and a proposed model for regulation of the n-alkane hydroxylase complex by n-alkanes in Acinetobacter sp. strain M-1. Thick arrows indicate the orientation of each gene. The downstream region of alkMb was sequenced by inverse PCR (see text). The putative products of the genes are indicated below the thick arrows. Very long chain n-alkanes and long-chain n-alkanes induce alkMa and alkMb, respectively, and each of the alkane hydroxylase components forms a complex with the constitutively expressed rubredoxin and rubredoxin reductase.
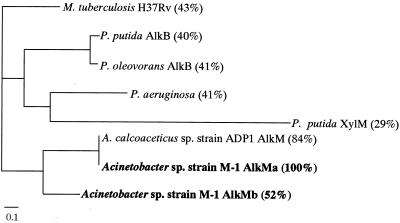
The deduced amino acid sequences of alkMa and alkMb (AlkMa and AlkMb, respectively) showed 52% identity with each other. The eight-histidine motif was conserved in both peptide sequences (20). The hydropathy plots of AlkMa and AlkMb were similar to that of AlkB from P. oleovorans (23), suggesting that these proteins are membrane bound (data not shown). A phylogenetic tree of AlkMa and AlkMb with the hydroxylases of other microbes generated by ClustalW is shown in Fig. 2. AlkMa was more similar to AlkM of strain ADP1 (84% identity) than AlkMb was.
FIG. 2.
Phylogenetic tree of alkane hydroxylases. Complete protein sequences of the alkane hydroxylases of several species were selected from the database and aligned using the ClustalW program. The species and proteins are Mycobacterium tuberculosis Rv3252c (accession number F70593); Pseudomonas putida AlkB (AJ233397); P. oleovorans AlkB (X65936); P. aeruginosa AE004581; P. punda xylene monooxygenase (XylM, A37316); A. calcoaceticus strain ADP1 AlkM (AJ002316); and Acinetobacter sp. strain M-1 AlkMa and AlkMb (AB049410 and AB049411, respectively). The percent identity values of the deduced amino acid sequences to AlkMa are shown in parentheses.
In the upstream regions of alkMa and alkMb, putative transcriptional regulator genes (alkRa and alkRb, respectively) were found. AlkRa showed high similarities with the AraC-XylS type transcription regulators (7), including AlkR of A. calcoaceticus ADP1 (15). On the other hand, alkRb showed higher similarity with a different type of transcription regulator, OruR of Pseudomonas aeruginosa. In the downstream regions of alkMa and alkMb, the glutathione reductase gene (gshR) and alkyl-hydroperoxide reductase gene (ahpC, which was found in the inverse PCR-amplified DNA fragment), respectively, were found. These genes encode proteins involved in scavenging reactive oxygen species that may be generated from n-alkane oxidation.
Both alkMa and alkMb function in A. calcoaceticus strain ADP1.
We attempted to show that alkMa and alkMb indeed encode functional n-alkane hydroxylases. Since several biochemical experiments were not successful, we took advantage of the genetics in A. calcoaceticus strain ADP1 (ATCC 33305; synonymous with strain BD413) (9, 21), which has both high transformation frequency and site-specific recombination efficiency.
We constructed the alkM disruptant (alkMΔ) of A. calcoaceticus ADP1 by inserting the kanamycin-resistance (Kmr) cassette. The ca. 3.0-kb DNA fragment containing the alkane hydroxylase gene alkM (15) was PCR amplified and cloned from the chromosomal DNA of strain ADP1. The primers used were alkMUp and alkMDn (Table 1). In the BclI site of alkM, the Kmr cassette, which was PCR amplified using pKT231 (3) as a template (Table 2), was inserted, and the resulting plasmid was used to transform strain ADP1. The primers used for amplification were KmNBam and KmCBam (Table 1). Transformation was performed as described by Palmen et al. (14). That the proper gene disruption had occurred was confirmed by Southern blot analysis (data not shown). The alkMΔ strain could grow on Luria-Bertani (LB) broth (19) medium containing kanamycin (50 μg/ml), but could not grow on hexadecane.
On the other hand, pMX4.2 and pMX2.7 (Table 2 and Fig. 1) were each digested by PstI (which has a unique site in the multicloning site of the plasmids) and ligated with PstI-digested pMFY31 (6), and the resulting plasmids were pMFYalkMa and pMFYalkMb, respectively. These plasmids had the alkane hydroxylase gene and the corresponding regulator gene from strain M-1, respectively, and each of them was introduced into the alkMΔ strain. Transformants were selected in the presence of both ampicillin (50 μg/ml) and kanamycin (50 μg/ml).
When the alkMΔ strain was transformed by pMFYalkMa or pMFYalkMb, the ability to grow on solidified M9 medium supplemented with hexadecane vapor in the presence of ampicillin and kanamycin was restored. This was not achieved with the control plasmid pMFY31. All of the transformed plasmids could be recovered from the transformants (data not shown), suggesting that these plasmids were maintained but not incorporated into the chromosomal DNA of strain ADP1. These results show that alkMa and alkMb could each complement the inability of the alkMΔ strain to grow on hexadecane. Therefore, alkMa and alkMb both encode a functional alkane hydroxylase. However, growth on n-alkanes of various lengths did not show a detectable difference between the alkMΔ strain carrying pMFYalkMa and pMFYalkMb. Therefore, these in vivo assays using strain ADP1 gave no information on the difference in substrate specificity between the products of the two alkane hydroxylase genes.
Regulation of alkMa and alkMb expression by n-alkanes.
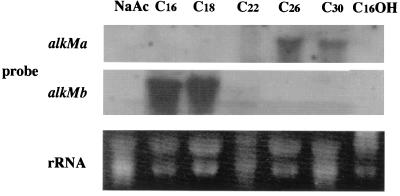
The amino acid sequence identity between AlkRa and AlkRb was only 5.0%, while that between AlkRa and AlkR of the heterologous strain ADP1 is 53%. These results raised the possibility that alkMa and alkMb are regulated in a different manner in strain M-1. Strain M-1 cells were grown on n-alkanes of various lengths as a carbon source, and Northern blot analysis was performed using total RNA extracted from these cells and alkMa- and alkMb-specific probes. Figure 3 clearly demonstrates that (i) neither alkMa nor alkMb expression was induced when strain M-1 was grown on sodium acetate or on hexadecanol, which induces the alk operon in P. oleovorans (13) and (ii) alkMa and alkMb were induced by n-alkanes, although in a different manner. alkMa expression was induced by solid, very long chain alkanes (>C22), and alkMb expression was preferentially induced by liquid long-chain alkanes (C16 to C22).
FIG. 3.
Northern blot analysis of alkMa and alkMb in strain M-1 cells grown on various substrates. Total RNA (15 μg) was loaded in each lane, and alkMa and alkMb transcripts were detected with labeled alkMa or alkMb fragment, respectively, as a probe. Total RNA was prepared from strain M-1 cells that had been grown on a salt medium containing 1% (wt/vol) sodium acetate (NaAc); 0.5% (vol/vol) n-alkane, with the carbon chain length indicated; or 0.5% (wt/vol) hexadecanol (C16OH). rRNA was used as a standard and was visualized by ethidium bromide.
Structure of the rubredoxin and rubredoxin reductase genes (rubA and rubB) in Acinetobacter sp. strain M-1.
To gain further insight into the molecular structure of the n-alkane hydroxylase complex and its regulation in strain M-1, we cloned the genes rubA and rubB, encoding rubredoxin and rubredoxin reductase, respectively, using PCR and inverse PCR techniques and chromosomal DNA from strain M-1 as a template. The primers used were RBDXN and RBDXC (Table 1), which had identical sequences to the 5′ and complementary 3′ termini, respectively, of the rubA gene of strain ADP1 (8). Inverse PCR was performed to amplify the region surrounding rubA using the sequence information. The primers used were IRBDXN and IRBDXC (Table 1). EcoRI-digested and self-ligated chromosomal DNA of strain M-1 was used as the template. The cloning experiment yielded one 3.0-kb EcoRI fragment harboring two ORFs for rubA and rubB and one incomplete ORF (Fig. 1). The rubAB operon was overexpressed under the tac promoter in Escherichia coli, and the recombinant rubredoxin and rubredoxin reductase were purified to apparent homogeneity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). The specific activity of the purified recombinant rubredoxin reductase was 1,200 and 1,750 U mg−1 toward potassium ferricyanide and rubredoxin, respectively. One unit of activity was defined as the amount of the enzyme that catalyzes the NADH-dependent reduction of 1 μmol of ferricyanide or cytochrome c (in the presence of purified rubredoxin) per min.
Genomic Southern analyses of strain M-1 under low-stringency conditions using rubA and rubAB as probes showed only one hybridizing band in various restriction digests (data not shown). This result suggests that in strain M-1, rubredoxin and rubredoxin reductase are each encoded by a single gene, rubA and rubB, respectively.
Regulation of the rubAB operon.
The rubAB operon is constitutively expressed in strain ADP1 (12). We performed Northern blot analysis with total RNA extracted from strain M-1 using rubA and rubB as probes. A hybridizing band was not detectable in strain M-1 that had been grown on any carbon source, including the n-alkanes tested, showing that the rubAB operon was expressed at a very low level. On the other hand, when the cells were grown on glycerol, hexadecane, or triacontane, the specific activity (toward ferricyanide) of the rubredoxin reductase in the cell extract of parent strain M-1 was 0.43, 0.60, and 0.51 U/mg of protein, respectively. In addition, in the intergenic region of rubAB, no transcriptional terminator-like sequence such as an inverted repeat was found. These results suggest that the rubAB operon is constitutively expressed in strain M-1.
Two alkane hydroxylase complexes in response to chain length of n-alkanes in Acinetobacter sp. strain M-1.
From these results, we propose a mechanism for the regulation of the n-alkane hydroxylase complex by n-alkanes in Acinetobacter sp. strain M-1 (Fig. 1). According to this model, the organism controls alkane hydroxylase activity in response to the chain length of the substrate by switching the alkane hydroxylase component, AlkMa or AlkMb, without changing other components of the complex, rubredoxin and rubredoxin reductase, which are constitutively expressed. The low sequence similarity between alkRa and alkRb, which are the putative transcriptional regulators of alkMa and alkMb, respectively, may also suggest distinct regulatory mechanisms for alkMa and alkMb expression by n-alkanes.
Unfortunately, we have not been able to detect the enzyme activity of the alkane hydroxylase complex in cell extracts of Acinetobacter spp. or in the in vitro reconstitution experiment using the recombinant proteins from E. coli (data not shown), while the enzyme activity was reported to be detectable in P. oleovorans (22). Possible reasons for the failure to detect the activity are (i) poor solubility of the substrate (such as tridecane or longer-chain alkanes) in the reaction mixture in comparison with that used in the assay of P. oleovorans alkane hydroxylase (22); (ii) unstable nature of the hydroxylase component (12, 16); and (iii) an unknown factor(s) in the alkane hydroxylase complex of Acinetobacter species. An alternative approach to examining the physiological role and substrate specificity of AlkMa and AlkMb may be the use of genetic analyses, such as gene disruption in Acinetobacter sp. strain M-1. However, we have not succeeded in deriving disruptants of alkMa and alkMb due to the very low efficiency of site-specific recombination in this organism.
n-Alkane metabolism in Acinetobacter sp. strain M-1 is very complicated due to the diversity and overlapping functions of the enzymes. Genetic and biochemical characterization of n-alkane-metabolizing enzymes in Acinetobacter spp. will shed light on the poorly understood mechanism of the metabolism of very long chain n-alkanes in microorganisms and its regulation.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession numbers AB049411(alkMa), AB049412(alkMb), and AB049413(rubAB).
REFERENCES
- 1.Asperger O, Kleber H-P. Metabolism of alkanes by Acinetobacter. In: Towner K J, Bergogne-Berezin E, Fewson C A, editors. The biology of Acinetobacter. New York, N.Y: Plenum Press; 1991. pp. 323–350. [Google Scholar]
- 2.Asperger O, Naumann A, Kleber H-P. Occurrence of cytochrome P-450 in Acinetobacter strains after growth on n-hexadecane. FEMS Microbiol Lett. 1981;11:309–312. [Google Scholar]
- 3.Bagdasarian M, Lurz R, Rücket B, Franklin F C H, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 4.Bajapai U, Kuhad R C, Khanna S. Mineralization of [14C]octadecane by Acinetobacter calcoaceticus S19. Can J Microbiol. 1998;44:681–686. [Google Scholar]
- 5.Finnerty W R. Lipids of Acinetobacter. In: Applewhite A H, editor. Proceedings of the World Conference on Biotechnology for the Fats and Oils Industry III. Champaign, Ill: American Oil Chemical Society; 1988. pp. 184–188. [Google Scholar]
- 6.Fukuda M, Yano K. Construction of broad host range cloning vectors for Gram-negative bacteria. Agric Biol Chem. 1985;49:2719–2724. [Google Scholar]
- 7.Gallegos M-T, Schleif R, Bairoch A, Hofmann K, Ramos J L. AraC/XylS family of transcriptional regulators. Microbiol Mol Biol Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissdörfer W, Frosch S C, Haspel G, Ehrt S, Hillen W. Two genes encoding proteins with similarities to rubredoxin and rubredoxin reductase are required for conversion of dodecane to lauric acid in Acinetobacter calcoaceticus ADP1. Microbiology. 1995;141:1425–1432. doi: 10.1099/13500872-141-6-1425. [DOI] [PubMed] [Google Scholar]
- 9.Juni E, Janik A. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy R S, Finnerty W R. Microbial assimilation of hydrocarbons. I. The fine structure of a hydrocarbon oxidizing Acinetobacter sp. Arch Microbiol. 1975;102:75–83. doi: 10.1007/BF00428349. [DOI] [PubMed] [Google Scholar]
- 11.Kok M, Oldenhuis R, van der Linden M P G, Raatjes P, Kingma J, van Lelyveld P H, Witholt B. The Pseudomonas oleovorans alkane hydroxylase gene. J Biol Chem. 1989;264:5435–5441. [PubMed] [Google Scholar]
- 12.McKenna E J, Coon M J. Enzymatic ω-oxidation. IV. Purification and properties of the ω-hydroxylase of Pseudomonas oleovorans. J Biol Chem. 1970;245:3882–3889. [PubMed] [Google Scholar]
- 13.Ochman H, Gerber A S, Hartl D L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmen R, Vosman B, Buijsman P, Breek C K D, Hellingwerf K J. Physiological characterization of natural transformation in Acinetobacter calcoaceticus. J Gen Microbiol. 1993;139:295–305. doi: 10.1099/00221287-139-2-295. [DOI] [PubMed] [Google Scholar]
- 15.Ratajczak A, Geisdörfer W, Hillen W. Alkane hydroxylase from Acinetobacter sp. strain ADP1 is encoded by alkM and belongs to a new family of bacterial integral-membrane hydrocarbon hydroxylases. Appl Environ Microbiol. 1998;64:1175–1179. doi: 10.1128/aem.64.4.1175-1179.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruettinger R T, Olson S T, Boyer R F, Coon M J. Identification of the ω-hydroxylase of Pseudomonas oleovorans as a nonheme iron protein requiring phospholipid for catalytic activity. Biochem Biophys Res Commun. 1974;57:1011–1017. doi: 10.1016/0006-291x(74)90797-9. [DOI] [PubMed] [Google Scholar]
- 17.Sakai Y, Ishikawa J, Fukasaka S, Yurimoto H, Mitsui R, Yanase H, Kato N. A new carboxylesterase from Brevibacterium linens IFO12171 responsible for the conversion of 1,4-butanediol diacrylate to 4-hydroxybutyl acrylate: purification, characterization, gene cloning, and gene expression in Escherichia coli. Biosci Biotechnol Biochem. 1999;63:688–697. doi: 10.1271/bbb.63.688. [DOI] [PubMed] [Google Scholar]
- 18.Sakai Y, Maeng J H, Tani Y, Kato N. Use of long-chain n-alkanes (C13-C44) by an isolate, Acinetobacter sp. M-1. Biosci Biotechnol Biochem. 1994;58:2128–2130. [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 20.Shanklin J, Whittle E, Fox B G. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry. 1994;33:12787–12794. doi: 10.1021/bi00209a009. [DOI] [PubMed] [Google Scholar]
- 21.Strätz M, Mau M, Timmis K N. System to study horizontal gene exchange among microorganisms without cultivation of recipients. Mol Microbiol. 1996;22:207–215. doi: 10.1046/j.1365-2958.1996.00099.x. [DOI] [PubMed] [Google Scholar]
- 22.van Beilen J B, Kingma J, Witholt B. Substrate specificity of the alkane hydroxylase system of Pseudomonas oleovorans GPol. Enzyme Microb Technol. 1994;16:904–911. [Google Scholar]
- 23.van Beilen J B, Penninga D, Witholt B. Topology of the membrane-bound alkane hydroxylase of Pseudomonas oleovorans. J Biol Chem. 1992;267:9194–9201. [PubMed] [Google Scholar]
- 24.van Beilen J B, Wubbolts M G, Witholt B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation. 1994;5:161–174. doi: 10.1007/BF00696457. [DOI] [PubMed] [Google Scholar]