Abstract
Purpose
Brucellosis is an ongoing zoonotic disease in China, but there are few data in Beijing. This study was designed to illustrate clinical characteristics of patients with brucellosis in Beijing, China and explore the risk factors for focal brucellosis.
Patients and Methods
Data of patients with brucellosis were retrospectively collected from the patients’ electronic medical records in Beijing Youan Hospital during 2010 to 2021, including epidemiological, demographic and clinical features. Risk factors for focal brucellosis were identified by multivariable logistic regression models.
Results
A total of 197 patients were included in the study, with 165 (83.8%) cases in acute phase and 32 (16.2%) cases in chronic phase. Patients in acute phase were more likely to have splenomegaly (24.2% vs 3.1%, p=0.007) than those in chronic phase, but had less arthralgia (62.4% vs 81.3%, p=0.040). The median level of alanine aminotransferase (36.9 vs 20.7, p=0.001) was higher in patients at acute stage than those at chronic stage. Of all the patients, 76 (38.6%) were reported with focal complications, including 16 (8.1%) peripheral arthritis, 36 (18.3%) spondylitis, 17 (8.6%) epididymoorchitis, 8 (4.1%) meningitis and 3 (1.3%) endocarditis. Additionally, male (OR 2.76, 95% CI 1.15–6.64, p = 0.023), arthralgia (OR 6.23, 95% CI 2.36–16.43, p < 0.001) and higher level of platelets (OR 1.01, 95% CI 1.00–1.01, p < 0.001) were the independent risk factors for focal brucellosis.
Conclusion
The control of human brucellosis still cannot be ignored due to the re-emerging cases in Beijing, which are more likely to present splenomegaly and abnormal liver function in acute phase. Moreover, male, arthralgia and high level of platelets were the independent risk factors for focal brucellosis.
Keywords: brucellosis, clinical characteristics, epidemiology, focal complications, risk factors
Introduction
Brucellosis is a global zoonotic disease caused by Brucella spp, facultative intracellular gram-negative pathogens.1 Brucellosis is an ancient notifiable disease in China, and the annual incidence rate has been fluctuating since 1905, when brucellosis was first recorded in Shanghai. Although prevention and control measures for brucellosis have made great progress in China with the number of cases from 47,139 (3.4/100,000) in 2016 to 37,947 (2.7/100,000) in 2018, the brucellosis had a resurgence to 44,036 (3.2/100,000) in 2019, mainly in northern China,2,3 including Inner Mongolia, Shanxi, Heilongjiang, Hebei and Jilin. However, epidemiology, laboratory tests, clinical manifestations of patients with brucellosis in northern China, especially in Beijing, a central city geographically surrounded by these epidemic areas of brucellosis, have not been fully explained.
The symptoms of patients with brucellosis are generally nonspecific, such as fever, night sweats, arthralgia, headache, fatigue, anorexia, myalgia and weight loss.4 Brucella infection can be hematogenous or focal, which may cause differences in clinical manifestations and treatments. Usually, brucellosis in human is not lethal;1 whereas if treated improperly, persistent intracellular infection may result in chronic and severe complications including arthritis and spondylitis, which subsequently leads to high rate of disability.3,5,6 It was reported that 10–30% of patients developed to chronic brucellosis and suffered from persistent complications, despite early diagnosis and treatment.7 However, little is known about the differences in epidemiology and clinical characteristics between patients with acute and chronic brucellosis, as well as those with and without focal complications. Alternatively, it is of great importance for the early detection of risk factors for focal brucellosis. The retrospective study was designed to illustrate the clinical characteristics of brucellosis patients in acute and chronic phase, as well as those with and without focal complications in a tertiary hospital in Beijing, and to explore the risk factors for focal brucellosis.
Materials and Methods
Patients
For this retrospective, single-center study, we recruited inpatients with brucellosis from 2010 to 2021 in Beijing Youan Hospital, the Capital Medical University. All patients had a diagnosis of brucellosis confirmed by positive results of blood culture or the standard tube agglutination test (SAT) based on the national guideline of “Updated Guidelines for the Diagnosis of Human Brucellosis — China, 2019”.8 Patients with unclear diagnosis of brucellosis and incomplete medical records were excluded. This study was approved by the ethics committee of the Youan Hospital, Capital Medical University; written informed consent was waived owing to the use of retrospective data from the hospital’s electronic medical record system.
Data Collection and Definitions
We obtained information about demographic, epidemiological and the clinical characteristics of all enrolled patients from electronic medical system. Demographic and epidemiological variables collected in this study included gender, age, region, place of residence (rural or urban), ethnicity, occupation and exposure to animal. Clinical features included clinical signs and symptoms (fever, arthralgia, fatigue, hyperhidrosis, weight loss, myalgia, chills, headache, splenomegaly, enlarged lymph nodes, rashes, digestive system disorders and respiratory disorders), laboratory findings (leukocyte, lymphocyte, neutrophils, monocyte and platelet counts, and levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), creatinine (Cr), hemoglobin, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), albumin) and focal complications (peripheral arthritis, spondylitis, epididymoorchitis, meningitis and endocarditis). Based on the duration of the manifestation and symptom before admission to the hospital, the patients were classified into two groups − acute brucellosis (less than 6 months) and chronic brucellosis (more than 6 months) − according to expert consensus on diagnosis and treatment of brucellosis (2017).9 Focal brucellosis was determined as any clear infection in anatomic regions, such as arthritis, spondylodiscitis, meningitis or infection in other sites other than hematogenous infection.10,11 We assessed the clinical characteristics of patients with brucellosis, and then we explored the risk factors for focal brucellosis. A team of experienced infectious clinicians reviewed, abstracted and cross-checked the data. Each record was checked independently by 2 clinicians.
Statistical Analysis
Statistical analysis was conducted with SPSS version 24.0. Continuous variables assumed normal distribution were expressed as mean (standard deviation (SD)), otherwise, were provided as median [interquartile range (IQR)]. Categorical data were expressed as frequencies (percentages). Variables comparison between patients in acute phase and chronic phase, as well as between patients with and without focal brucellosis used χ2 test or the Fisher’s exact test for categorical variables, and Student’s t-test or Mann–Whitney U-test, as appropriate, for continuous variables. Univariable and multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CI) of potential risk factors. Receiver operating characteristic (ROC) curves were constructed for measurement data to determine the optimal cutoff values for predicting the risk of focal brucellosis. Additionally, the sensitivity and specificity of each parameter and the area under the curve (AUC) was calculated. For all the statistical analyses, p below 0.05 was considered statistically significant.
Results
Epidemiological Characteristics of Patients with Brucellosis in Beijing
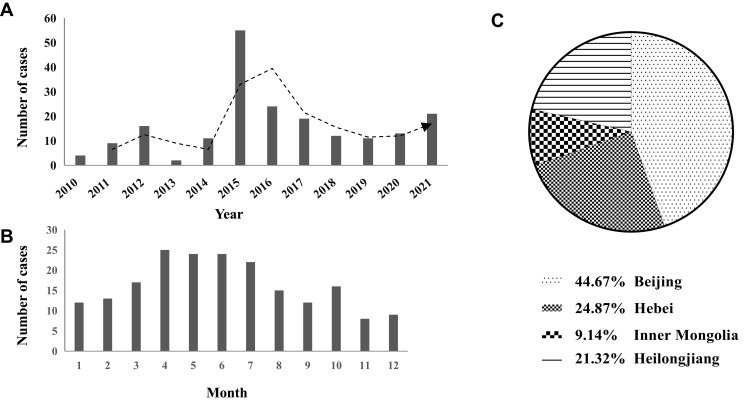
A total of 200 inpatients with brucellosis were admitted to Beijing Youan Hospital during 2010 to 2021, and 3 patients were excluded due to incomplete medical data. Finally, 197 patients were enrolled in this study. The cases of brucellosis reported in this study increased gradually from 4 in 2010 to a peak of 55 in 2017, and decreased to 11 in 2019 without significant downward trend since then (Figure 1A). Brucellosis has seasonal variations, and the number of reported cases peaked during April to July in this study, counting for nearly a half (48.2%) of all the reported cases in the whole calendar year (Figure 1B). Most of the patients came from Beijing (44.7%), Hebei (24.9%) and Inner Mongolia Autonomous Region (9.1%) (Figure 1C). Additionally, 88.8% patients with brucellosis had a contact history of infected sheep or cattle. In terms of occupations, 72 (36.5) patients were farmers or herdsmen, 6 (3%) patients were animal and dairy processing workers. The proportion of farmers and herdsmen in chronic brucellosis was higher than that in acute brucellosis. The distribution of regions, place of residence (rural or urban), ethnicity and animal exposure were not different statistically between acute and chronic brucellosis, similar results can be seen in patients with and without focal complications (p > 0.05) (Table 1, Supplementary Table 1).
Figure 1.
Time and regional distribution of 197 brucellosis cases in Beijing Youan Hospital during 2010–2021. (A) Annual reported cases of brucellosis; (B) monthly reported cases of brucellosis; (C) the proportion of brucellosis from different regions.
Table 1.
Demographic and Epidemiological Characteristics Between Acute and Chronic Brucellosis Cases in Beijing Youan Hospital Between 2010 and 2021
| Variable | Acute (n =165) | Chronic (n =32) | p value |
|---|---|---|---|
| Male, n (%) | 116 (70.3%) | 23 (71.9%) | 1.000 |
| Age, years, median (IQR) | 48 (26.5–58.0) | 54 (48.0–64.0) | 0.002 |
| Regions, n (%) | 0.149 | ||
| Beijing | 78 (47.3%) | 10 (31.3%) | NA |
| Hebei | 42 (25.5%) | 7 (21.9%) | NA |
| Inner Mongolia | 14 (8.5%) | 4 (12.5%) | NA |
| Others | 31 (18.8%) | 11 (34.4%) | NA |
| Place of residence, n (%) | 0.080 | ||
| Rural | 90 (54.5%) | 23 (71.9%) | NA |
| Urban | 75 (45.5%) | 9 (28.1%) | NA |
| Ethnicity, Han, n (%) | 153 (92.7%) | 31 (96.9%) | 0.483 |
| Occupations, n (%) | 0.030 | ||
| Farmer & herdsman | 55 (33.3%) | 17 (53.1%) | NA |
| Personnel in animal and dairy processing | 5 (3.0%) | 1 (3.1%) | NA |
| Staff | 26 (15.8%) | 1 (3.1%) | NA |
| Student | 18 (10.9%) | 0 (0.0%) | NA |
| Other occupations | 61 (37.0%) | 13 (40.6%) | NA |
| Animals exposure, n (%) | 0.715 | ||
| Cattle | 51 (30.9%) | 7 (21.9%) | NA |
| Sheep | 139 (84.2%) | 26 (81.2%) | NA |
| Other animals | 17 (10.3%) | 5 (15.6%) | NA |
| Hospitalization time, days, mean ± S D | 13 (7.0–20.0) | 12 (7.3–20.0) | 0.972 |
Note: Bold values denote statistically significant differences (p < 0.05).
Abbreviation: SD, Standard deviation.
Comparisons Between Patients with Acute Brucellosis and Chronic Brucellosis
Brucellosis can be clinically classified as acute and chronic brucellosis based on the time from the first symptom onset to the time of consultation. In our study, 165 (83.8%) patients were in acute phase and 32 (16.2%) were in chronic phase.
We analyzed the clinical features of brucellosis between acute and chronic phases (Table 2). In this study, the most common symptoms were fever (82.7%), arthralgia (65.5%), hyperhidrosis (44.2%), fatigue (40.6%) and weight loss (26.9%). Patients in acute phase were more likely to have chill (26.1% vs 9.4%, p=0.040) and splenomegaly (24.2% vs 3.1%, p=0.007) than chronic phase, while less likely to present arthralgia (62.4% vs 81.3%, p=0.040). Other nonspecific symptoms and signs were similar between the two groups.
Table 2.
Clinical Characteristics Between Acute and Chronic Brucellosis in Beijing Youan Hospital Between 2010 and 2021
| Variable | Acute (n=165) | Chronic (n=32) | p value |
|---|---|---|---|
| Symptoms and signs, n (%) | |||
| Fever | 140 (84.8%) | 23 (71.9%) | 0.076 |
| Arthralgia | 103 (62.4%) | 26 (81.3%) | 0.040 |
| Hyperhidrosis | 71 (43.0%) | 16 (50.0%) | 0.467 |
| Fatigue | 65 (39.4%) | 15 (46.9%) | 0.430 |
| Weight loss | 45 (27.3%) | 8 (25.0%) | 0.791 |
| Myalgia | 33 (20.0%) | 6 (18.8%) | 0.087 |
| Chills | 43 (26.1%) | 3 (9.4%) | 0.041 |
| Headache | 21 (12.7%) | 3 (9.4%) | 0.772 |
| Splenomegaly | 40 (24.2%) | 1 (3.1%) | 0.007 |
| Enlarged lymph nodes | 9 (5.5%) | 1 (3.1%) | 1.000 |
| Rashes | 8 (4.8%) | 2 (6.3%) | 0.667 |
| Digestive system disordersa | 36 (21.8%) | 4 (12.5%) | 0.230 |
| Respiratory disordersb | 29 (18.8%) | 2 (6.5%) | 0.107 |
| Laboratory findings | |||
| ESR (mm/h), median (IQR) | 25.0 (10.0–45) | 21.5 (6.0–37.5) | 0.254 |
| CRP (mg/L), median (IQR) | 20.0 (6.9–40.8) | 10.8 (1.0–31.3) | 0.084 |
| Leukocyte (×109/L), median (IQR) | 5.7 (4.2–7.4) | 6.8 (4.3–8.3) | 0.190 |
| Lymphocyte (×109/L), median (IQR) | 2.0 (1.4–2.8) | 2.0 (1.6–2.7) | 0.895 |
| Neutrophils (×109/L), median (IQR) | 2.6 (2.0–4.1) | 3.7 (2.3–5.0) | 0.060 |
| Monocyte (×109/L), median (IQR) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.688 |
| Hemoglobin (g/L), mean ± SD | 119.8 ± 19.3 | 127.3 ± 19.1 | 0.047 |
| Albumin (g/L) | 36.4 ± 4.8 | 38.4 ± 4.5 | 0.019 |
| Platelet count (×109/L), median (IQR) | 211.5 (150.0–281.0) | 218 (181.2–278.2) | 0.462 |
| ALT (U/L), median (IQR) | 37.1 (23.5–65.2) | 20.3 (12.8–35.0) | <0.001 |
| AST (U/L), median (IQR) | 37.2 (23.9–63.6) | 20.8 (16.8–31.4) | <0.001 |
| TBIL (μmol/L), median (IQR) | 12.6 (8.8–19.7) | 11.0 (7.7–16.9) | 0.366 |
| Creatinine (μmol/L), median (IQR) | 57.8 (45.0–67.6) | 59.6 (53.9–67.6) | 0.237 |
| Focal involvement, n (%) | 64 (38.8%) | 12 (37.5%) | 1.000 |
| Peripheral arthritis | 14 (8.5%) | 2 (6.3%) | 1.000 |
| Spondylitis | 28 (17.0%) | 8 (25.0%) | 0.282 |
| Epididymoorchitis | 16 (9.7%) | 1 (3.1%) | 0.317 |
| Meningitis | 8 (4.8%) | 0 (0.0%) | 0.358 |
| Endocarditis | 2 (1.2%) | 1 (3.1%) | 0.414 |
Notes: Bold values denote statistically significant differences (p < 0.05). aDigestive system disorders include anorexia, vomiting and abdominal pain. bRespiratory disorders include pneumonia and pleural effusions.
Abbreviations: SD, standard deviation; IQR, interquartile range; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin.
Laboratory findings were assessed in all patients on admission. The CRP level (20.0 vs 10.8, p = 0.084) was numerically higher in acute phase compared with those in chronic phase, though it had no significant difference between them. Moreover, patients in acute phase had higher level of alanine aminotransferase (ALT) (37.1 vs 20.3, p < 0.001), aspartate aminotransferase (AST) (37.2 vs 20.2, p < 0.001), hemoglobin (119.9 vs 127.3, p = 0.047) and albumin (36.4 vs 38.4, p = 0.019) than those in chronic phase, while the level of leukocyte, platelet, total bilirubin, creatinine were similar between patients in acute and chronic phases (p > 0.05) (Table 2). Focal complications were also compared between the two groups, and epididymoorchitis and meningitis seemed more likely to present acutely; however, there were no significant differences in complications between the two groups (Table 2).
Comparisons Between Patients with and without Focal Brucellosis
Of all the 197 patients, 76 (38.6%) were reported with focal complications, including 16 (8.1%) peripheral arthritis, 36 (18.3%) spondylitis, 17 (8.6%) epididymoorchitis, 8 (4.1%) meningitis and 3 (1.3%) endocarditis. Patients with focal involvements were older than those without focal complications (53.5 vs 48.0, p = 0.025). And there were more male patients in focal brucellosis (+) group than focal brucellosis (-) group (81.6% vs 63.6%, p = 0.007).
Fever, arthralgia, fatigue, hyperhidrosis and loss of weight were the main symptoms in patients with brucellosis regardless of focal involvements (Table 3). Of note, the symptoms and signs between patients with and without focal brucellosis were similar, except that arthralgia occurred more frequently in the patients with focal complications (89.5% vs 50.4%, p < 0.001). In addition, the median level of ESR in focal brucellosis was higher than those without focal brucellosis (35.5 vs 21.0, p = 0.016). Similarly, the median levels of neutrophils (3.6 vs 2.1, p = 0.013), monocytes (0.45 vs 0.36, p = 0.011) and platelets (231.5 vs 195.5, p < 0.001) were higher in patients with focal involvements than those without focal involvements. However, the levels of ALT (24.7 vs 39.2, p < 0.001) and AST (25.1 vs 40.3, p < 0.001) were lower in patients with focal brucellosis than those without focal brucellosis.
Table 3.
Clinical Characteristics Between Patients with or Without Focal Brucellosis in Beijing Youan Hospital Between 2010 and 2021
| Variable | Focal Brucellosis (+) (n=76) | Focal Brucellosis (-) (n=121) | p value |
|---|---|---|---|
| Symptoms and signs, n (%) | |||
| Fever | 64 (84.2%) | 99 (81.8%) | 0.665 |
| Arthralgia | 68 (89.5%) | 61 (50.4%) | <0.001 |
| Fatigue | 31 (40.8%) | 49 (40.5%) | 0.967 |
| Hyperhidrosis | 31 (40.8%) | 56 (46.3%) | 0.450 |
| Myalgia | 15 (19.7%) | 24 (19.8%) | 0.987 |
| Chills | 20 (26.3%) | 26 (21.5%) | 0.436 |
| Headache | 11 (14.5%) | 13 (10.7%) | 0.436 |
| Weight loss | 23 (30.3%) | 30 (24.8%) | 0.399 |
| Cough | 4 (5.3%) | 16 (13.2%) | 0.072 |
| Pleural effusion | 7 (9.2%) | 10 (8.3%) | 0.818 |
| Nausea | 11 (14.5%) | 10 (8.3%) | 0.169 |
| Vomiting | 7 (9.2%) | 8 (6.6%) | 0.503 |
| Anorexia | 6 (7.9%) | 21 (17.4%) | 0.060 |
| Stomach ache | 5 (6.6%) | 2 (1.7%) | 0.110 |
| Rashes | 2 (2.6%) | 8 (6.6%) | 0.322 |
| Splenomegaly | 17 (22.4%) | 24 (19.8%) | 0.670 |
| Enlarged lymph nodes | 1 (1.3%) | 9 (7.4%) | 0.092 |
| Clinical course, n (%) | 0.891 | ||
| Acute phase | 64 (84.2%) | 101 (83.5%) | |
| Chronic phase | 12 (15.8%) | 20 (16.5%) | |
| Laboratory findings | |||
| ESR (mm/h), median (IQR) | 35.5 (11.5–52.0) | 21.0 (10.0–36.0) | 0.006 |
| CRP (mg/L), median (IQR) | 20.9 (9.3–43.0) | 14 0.0 (5.0–35.5) | 0.151 |
| Leukocyte (×109/L), median (IQR) | 36.8 ± 4.9 | 36.6 ± 4.7 | 0.377 |
| Neutrophil (×109/L), median (IQR) | 3.1 (2.3–4.5) | 2.6 (1.8–4.0) | 0.013 |
| Lymphocyte (×109/L), median (IQR) | 1.9 (1.5–2.8) | 2 (1.4–2.9) | 0.950 |
| Monocyte (×109/L), median (IQR) | 0.45 (0.4–0.6) | 0.36 (0.3–0.5) | 0.011 |
| Hemoglobin (g/L), mean ± SD | 121.9 ± 19.8 | 120.6 ±19.3 | 0.648 |
| Albumin (g/L) | 36.8 ± 4.9 | 36.6 ± 4.7 | 0.805 |
| Platelet count (×109/L), median (IQR) | 231.5 (192.5–324.5) | 195.5 (125.3–245.3) | <0.001 |
| Mean platelet volume (fL) | 9.6 (9.0–10.3) | 10.3 (9.5–10.9) | 0.001 |
| ALT (U/L), median (IQR) | 24.7 (15.5–42.1) | 39.2 (27.5–68.3) | <0.001 |
| AST (U/L), median (IQR) | 25.1 (18.4–40.7) | 40.3 (29.2–70.3) | <0.001 |
| TBIL (μmol/L), median (IQR) | 12.1 (8.9–17.9) | 11.9 (8.3–19.8) | 0.741 |
| Creatinine (μmol/L), median (IQR) | 58.3 (46.0–68.0) | 58.0 (45.5–67.6) | 0.883 |
Note: Bold values denote statistically significant differences (p < 0.05).
Abbreviations: SD, standard deviation; IQR, interquartile range; ESR, erythrocyte sedimentation rate; CRP, C - reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin.
Risk Factors for Focal Brucellosis
Patients with focal brucellosis may suffer from persistent complications and should be treated with different regimes. Thus, we analyzed the risk factors for focal brucellosis. Via univariate analysis, we found the male, arthralgia, the level of ALT, AST, ESD and the number of platelets and monocytes were associated with focal brucellosis (Table 4). However, through multivariate logistic regression analysis, only male (OR 2.76, 95% CI 1.15–6.64, p = 0.023), arthralgia (OR 6.23, 95% CI 2.36–16.43, p < 0.001) and the count of platelet (OR 1.01, 95% CI 1.00–1.01, p < 0.001) were the independent risk factors for focal brucellosis (Table 4). Considering that all patients with epididymoorchitis were male, we next performed adjusted-analysis (excluding epididymoorchitis) and found platelet (OR 11.38, 95% CI 3.06–42.39, p < 0.001) and arthralgia (OR 1.01, 95% CI 1.00–1.01, p < 0.001) were still independent risk factors for focal complications.
Table 4.
Univariate and Multivariate Logistic Regression Analysis of Risk Factors for Focal Brucellosisa
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Male, n (%) | 2.531 | (1.272–5.036) | 0.008 | 2.849 | (1.189–6.829) | 0.019 |
| Arthralgia, n (%) | 0.120 | (0.053–0.270) | <0.001 | 6.786 | (2.586–17.805) | <0.001 |
| ESR (mm/h), median (IQR) | 1.018 | (1.006–1.031) | 0.005 | |||
| Platelet count (×109/L), median (IQR) | 1.005 | (1.002–1.008) | 0.001 | 1.006 | (1.002–1.010) | 0.002 |
| Mean platelet volume (fL), median (IQR) | 0.695 | (0.520–0.927) | 0.013 | |||
| Monocyte (×109/L), median (IQR) | 6.902 | (1.406–33.894) | 0.017 | |||
| ALT (U/L), median (IQR) | 0.993 | (0.986–1.000) | 0.040 | |||
| AST (U/L), median (IQR) | 0.984 | (0.974–0.994) | 0.002 | |||
Note: aOnly the values found to be significant in univariate logistic regression analysis were evaluated in multivariate logistic regression analysis.
Abbreviations: IQR, interquartile range; ESR, erythrocyte sedimentation rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
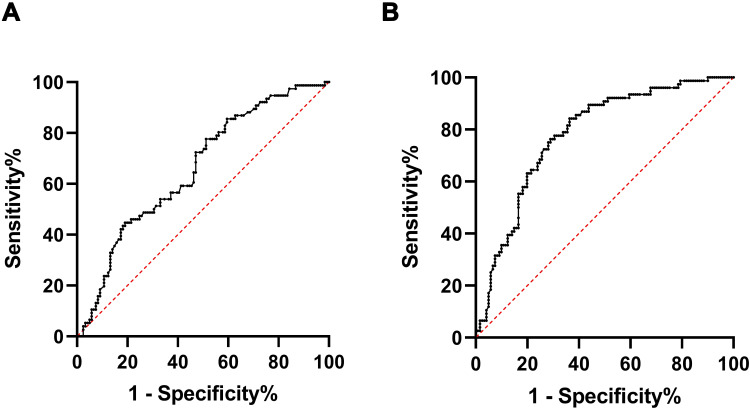
Moreover, we performed the ROC curves to determine the cutoff value of platelet to maximize the sensitivity and specificity as the predictor of focal brucellosis. The cutoff value of platelet is 191.5 (×109/L), the sensitivity and specificity were 77.6% and 51.2%, respectively, with an AUC of 0.66 (95% CI 0.58–0.74, p = 0.039) (Figure 2A). The AUC value of the mode including male, arthralgia and the number of platelets was 0.78 (95% CI 0.72–0.85, p < 0.001) with a sensitivity of 84.2% and a specificity of 63.6% (Figure 2B).
Figure 2.
Receiver operating characteristic curves (ROC) curves for the number of platelets (A) and the mode including gender of men, arthralgia and the number of platelets (B) in predicting focal brucellosis.
Discussion
Brucellosis is an old zoonotic disease causing serious economic losses and public health problems, especially in developing countries. This study retrospectively illustrated the epidemiological and clinical features of brucellosis patients in an infectious disease hospital in Beijing, China, and assessed the risk factors systematically for focal brucellosis.
The retrospective study reported the cases of human brucellosis did reduce before 2019, but reemerged since that according to the data in a tertiary hospital in Beijing from 2010 to 2021, which is not a rare phenomenon, but it has been reported in several countries in Asia and the Middle East.12,13 Undoubtedly, we have achieved some progress according to the National Brucellosis Prevention and Control Plan (2016–2020),2 but it is still important for high-risk populations to improve the awareness of brucellosis and the ability to protect against the severe complications of brucellosis, especially in the context of COVID-19 pandemic.14
Human brucellosis is traditionally described to have protean manifestations. We have found that fever, arthralgia, fatigue, hyperhidrosis and weight loss are the five most common symptoms in both acute and chronic phases, which is similar to the systematic reviews of the clinical manifestations of human brucellosis. Additionally, Shi et al15 reported that patients in acute phase were more likely to present splenomegaly and less likely to have arthralgia than patients in chronic phase in Anhui. Splenomegaly is linked with the increase of lymphohistiocytic cells and macrophages in the spleen, and the decrease of splenic CD4+ and CD8+ T cells.16 The data from Xinjiang17 showed that systematic manifestations, such as fever, anorexia and weight loss, were more frequent in acute cases, while arthralgia, back pain and joint tenderness occurred more frequently in patients with chronic brucellosis. The classic categorization of brucellosis as acute and chronic is somewhat subjective but with great clinical interest. Patients in chronic phase at the time of diagnosis accounted for 16.2% in our study, which is similar to previous studies (10–30%),7,18 indicating that some patients were not diagnosed and treated in time, especially those old farmers and herdsmen who live in the countryside. It is necessary to explore new methods of early diagnosis and hence choose optimum treatment regimens for prolonged periods to prevent the chronicity and relapse of symptoms.
When Brucella enters into phagocytic cells and replicates, it has several mechanisms to evade the attack of host immune response and contributes to chronicity, such as inhibition of apoptosis of infected mononuclear, dendritic cell maturation, presentation of antigen and activation of naive T cells.16 Subsequently, chronic infection eventually results in cardiovascular, hepatic, lymphoreticular and osteoarticular disease. Osteoarticular disease is the most common complication of brucellosis, which is observed in 27–36% patients,4 while respiratory diseases, cardiovascular diseases, central nervous system dysfunction and other complications are less common,10 which consists of the data of patients in this study. Some studies found that patients with brucellosis in chronic phase were more likely to have osteoarticular disease.4,17,19–21 In contrast, patients at acute stage seemed more susceptible to develop epididymoorchitis, gastrointestinal and respiratory involvement.22 The previous studies also indicated that children were more likely to present arthritis, while adults were more likely to present spondylitis. In addition to age, recent data suggested that osteoarticular complications were related to a genetic predisposition and HLA-B39.6 Brucella is characterized by strong tissue tropism for reproductive systems,16 and drives clinical disease manifestations and pathology. The prevalence of epididymoorchitis ranged from 3.4% to 10%,8,23 and most patients with epididymoorchitis were in acute stage. Nevertheless, the difference was not significant statistically between acute brucellosis and chronic brucellosis in this study, possibly due to the small sample size. Liver involvement usually manifests as elevated ALT and AST, while liver abscess and jaundice are rare.24,25 The infection of liver (liver abscess) was not found in this study. Alternatively, elevated transaminase was more common in active brucellosis and those without focal brucellosis. It has been reported that 5% to 52% of patients with acute brucellosis have liver involvement.22 Therefore, patients in acute phase, who are almost in active infection, are more susceptible to liver injury, leading to limited choice of antibiotics, and liver protection drugs should be used when necessary.
As brucellosis patients complicated with focal involvements are more difficult to treat, it is vital to identify risk factors early and hence make an accurate diagnosis in time. Notably, we found platelet count was associated with the focal brucellosis. It was reported that mean platelet volume (MPV), regarded as a marker of platelet function, might help in assessment of brucellosis. However, Cengiz Beyan doubted that MPV might not relate to complications in human brucellosis due to the reliability of the measurement results and age.26 In this study, after adjusted by MPV and age, as well as other possible influencing factors, platelet count was still an independent risk factor. A recent study found that platelets established complexes with infected monocytes/macrophages, further enhanced the secretion of IL-1β, TNF-α, IL-8, and MCP-1, whereas inhibited IL-10 secretion, which promotes proinflammatory.27 Moreover, platelets interact with neutrophils, which may contribute to the control of the infection.23 Actually, the AUC of platelet count to predict focal brucellosis was only 0.66, but it rose to 0.781 when it combined with male and arthralgia, which may contribute to the prediction of focal infection. A recent study also reported anti-Brucella IgG were highly associated with focal complications with an AUC of 0.885 (95% CI, 0.847–0.924), which unfortunately was not provided in this study. In addition to these factors, studies investigated the risk factors for focal brucellosis also reported that older age (OR 1.02, 95%Cl 1.00–1.04), lymphadenomegaly (OR 7.22, 95%Cl 1.07–48.71), AST (OR 1.03, 95%Cl 1.01–1.06), and neutrophil-lymphocyte ratio (OR 1.74, 95%Cl 1.07–2.83) were independent risk factors for predicting focal involvement.11,28 Of note, most of these studies were data from Turkey,11,21,28 and we first investigated the factors associated with focal complications in China. Therefore, it is of great help for Chinese clinicians to focus on the gender, the symptom of arthralgia and the count of platelet in making early diagnosis of focal infection.
Our study has several limitations. First, this is a retrospective study, and more details such as follow-up data and previous treatments before admission are unavailable. Second, this is a single-center study with small sample size. It would be more convincing to carry out a multicenter study with large sample size to further illustrate the epidemiology of brucellosis in Beijing and verify the results.
Conclusion
The cases of human brucellosis re-emerged in Beijing, China, which indicated that the control of human brucellosis still cannot be ignored. Similar to other studies, the most common symptoms of human brucellosis in Beijing were fever, arthralgia, hyperhidrosis, fatigue and weight loss. Patients with acute brucellosis were more likely to present splenomegaly and abnormal liver function. There were 38.6% patients were reported with focal complications, including 8.1% peripheral arthritis, 18.3% spondylitis, 8.6% epididymoorchitis, 4.1% meningitis and 1.3% endocarditis. Patients with focal involvements were older than those without focal complications. And there were more male patients and arthralgia in patients with focal complications. Additionally, male, arthralgia and high level of platelets were the independent risk factors for focal brucellosis, which could be helpful to identify patients with focal complications early.
Funding Statement
This research received no external funding.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Beijing Youan Hospital, Capital Medical University; written informed consent was waived owing to the use of retrospective data from the hospital’s electronic medical record system and the impossibility of obtaining contact with all patients. All data analyzed were anonymised. The privacy rights of all patients were observed.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yagupsky P, Morata P, Colmenero J. Laboratory diagnosis of human brucellosis. Clin Microbiol Rev. 2019;33(1):e00073–00019. doi: 10.1128/CMR.00073-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao Z, Chen Q, Chen Y, et al. Epidemiological characteristics of human brucellosis - China, 2016–2019. China CDC Weekly. 2021;3(6):114–119. doi: 10.46234/ccdcw2021.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Wang Y, Zhang L, et al. An epidemiological study of brucellosis on mainland China during 2004–2018. Transbound Emerg Dis. 2021;68(4):2353–2363. doi: 10.1111/tbed.13896 [DOI] [PubMed] [Google Scholar]
- 4.Adetunji S, Ramirez G, Foster M, Arenas-Gamboa A. A systematic review and meta-analysis of the prevalence of osteoarticular brucellosis. PLoS Negl Trop Dis. 2019;13(1):e0007112. doi: 10.1371/journal.pntd.0007112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell J, Lan N, Phuong P, et al. Human Brucella melitensis infections in southern Vietnam. Clin Microbiol Infect. 2017;23(11):788–790. doi: 10.1016/j.cmi.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Li S, Wang L, et al. Pathogenesis of Brucella epididymoorchitis-game of death. Crit Rev Microbiol. 2021;48:1–25. [DOI] [PubMed] [Google Scholar]
- 7.Budak F, Bal S, Tezcan G, Akalın H, Goral G, Oral H. Altered expressions of miR-1238-3p, miR-494, miR-6069, and miR-139-3p in the formation of chronic brucellosis. J Immunol Res. 2016;2016:4591468. doi: 10.1155/2016/4591468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Feng L, Lu J. Updated guidelines for the diagnosis of human brucellosis - China, 2019. China CDC Weekly. 2020;2(26):487–489. doi: 10.46234/ccdcw2020.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Editorial board of Chinese Journal of infectious diseases. Consensus of experts in diagnosis and treatment of brucellosis. Chin J Infect Dis. 2017;35(12):705–710. [Google Scholar]
- 10.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352(22):2325–2336. doi: 10.1056/NEJMra050570 [DOI] [PubMed] [Google Scholar]
- 11.Demirdal T, Sen P. Risk factors for focal involvement in brucellosis. Diagn Microbiol Infect Dis. 2020;97(1):115003. doi: 10.1016/j.diagmicrobio.2020.115003 [DOI] [PubMed] [Google Scholar]
- 12.Bagheri Nejad R, Krecek RC, Khalaf OH, Hailat N, Arenas-Gamboa AM. Brucellosis in the Middle East: current situation and a pathway forward. PLoS Negl Trop Dis. 2020;14(5):e0008071. doi: 10.1371/journal.pntd.0008071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amro A, Mansoor B, Hamarsheh O, Hjaija D. Recent trends in human brucellosis in the West Bank, Palestine. Int J Infect Dis. 2021;106:308–313. doi: 10.1016/j.ijid.2021.04.037 [DOI] [PubMed] [Google Scholar]
- 14.Lin S, Wang Z, Liu X, et al. Serological prevalence survey among the high-risk populations of brucellosis-endemic areas - China, 2019–2020. China CDC Weekly. 2021;3(6):101–105. doi: 10.46234/ccdcw2021.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi C, Wang L, Lv D, et al. Epidemiological, clinical and laboratory characteristics of patients with brucella infection in Anhui Province, China. Infect Drug Resist. 2021;14:2741–2752. doi: 10.2147/IDR.S319595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella–host interactions. Am J Pathol. 2015;185(6):1505–1517. doi: 10.1016/j.ajpath.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia B, Zhang F, Lu Y, et al. The clinical features of 590 patients with brucellosis in Xinjiang, China with the emphasis on the treatment of complications. PLoS Negl Trop Dis. 2017;11(5):e0005577. doi: 10.1371/journal.pntd.0005577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen S, Palmer M. Advancement of knowledge of Brucella over the past 50 years. Vet Pathol. 2014;51(6):1076–1089. doi: 10.1177/0300985814540545 [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Chen J, Li Q, et al. Epidemiological characteristics, clinical manifestations and laboratory findings in 850 patients with brucellosis in Heilongjiang Province, China. BMC Infect Dis. 2019;19(1):439. doi: 10.1186/s12879-019-4081-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Z, Lin G, He W, et al. IL-6 and INF-γ levels in patients with brucellosis in severe epidemic region, Xinjiang, China. Infect Dis Poverty. 2020;9(1):47. doi: 10.1186/s40249-020-00666-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu N, Dong X, Yao Y, et al. Improved early detection of focal brucellosis complications with anti-Brucella IgG. J Clin Microbiol. 2020;58(10):e00903–00920. doi: 10.1128/JCM.00903-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Gao H, Pappas G, et al. Clinical features of 2041 human brucellosis cases in China. PLoS One. 2018;13(11):1–15. doi: 10.1371/journal.pone.0205500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trotta A, Milillo MA, Serafino A, et al. Brucella abortus-infected platelets modulate the activation of neutrophils. Immunol Cell Biol. 2020;98(9):743–756. doi: 10.1111/imcb.12373 [DOI] [PubMed] [Google Scholar]
- 24.Amsilli M, Epaulard O, Brion J, et al. Hepatic brucelloma diagnosis and long-term treatment, France. Emerg Infect Dis. 2019;25(5):1021–1023. doi: 10.3201/eid2505.180613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giambartolomei G, Delpino M. Immunopathogenesis of Hepatic Brucellosis. Front Cell Infect Microbiol. 2019;9:423. doi: 10.3389/fcimb.2019.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyan C, Beyan E. Mean platelet volume may not be a risk factor for focal involvement in patients with brucellosis. Diagn Microbiol Infect Dis. 2020;97(2):115036. doi: 10.1016/j.diagmicrobio.2020.115036 [DOI] [PubMed] [Google Scholar]
- 27.Trotta A, Velásquez LN, Milillo MA, et al. Platelets promote Brucella abortus monocyte invasion by establishing complexes with monocytes. Front Immunol. 2018;9:1000. doi: 10.3389/fimmu.2018.01000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copur B, Sayili U. Laboratory and clinical predictors of focal involvement and bacteremia in brucellosis. Eur J Clin Microbiol Infect Dis. 2022;41(5):793–801. doi: 10.1007/s10096-022-04436-1 [DOI] [PubMed] [Google Scholar]