Abstract
Pulmonary embolism (PE) is one of the most common etiologies of cardiovascular mortality. It could be linked to several risk factors including advanced age. The pathogenesis of PE is dictated by the Virchow's triad that includes venous stasis, endothelial injury, and a hypercoagulable state. The diagnosis of PE is difficult and is often missed due to the nonspecific symptomatology. Hypoxia is common in the setting of PE, and the degree of respiratory compromise is multifactorial and influenced by underlying cardiac function, clot location, and ability to compensate with respiratory mechanics. Right ventricular dysfunction/failure is the more profound cardiovascular impact of acute PE and occurs due to sudden increase in afterload. This is also the primary cause of death in PE. High clinical suspicion is required in those with risk factors and presenting signs or symptoms of venous thromboembolic disease, with validated clinical risk scores such as the Wells, Geneva, and pulmonary embolism rule out criteria in estimating the likelihood for PE. Advancement in capture time and wider availability of computed tomographic pulmonary angiography and D-dimer testing have further facilitated the rapid evaluation and diagnosis of suspected PE. Treatment is dependent on clinical presentation and initially involves providing adequate oxygenation and stabilizing hemodynamics. Anticoagulant therapy is indicated for the treatment of PE. Treatment is guided by presence or absence of shock and ranges from therapeutic anticoagulation to pharmacologic versus mechanical thrombectomy. The prognosis of patients can vary considerably depending on the cardiac and pulmonary status of patient and the size of the embolus.
Keywords: pulmonary embolism, pulmonary angiography, pulmonary hypertension, hypoxia, Virchow's triad, thromboembolic disease, deep venous thrombosis
Venous thromboembolic (VTE) disease presents as a major burden to health care and affects approximately 10 million cases per year. 1 2 3 4 In clinical practice, it is encountered as either deep vein thrombosis (DVT) and/or pulmonary embolism (PE) and is thought to affect as many as 900,000 individuals in the United States each year and associated with substantial morbidity and mortality.
It is estimated that there are approximately 10 million new cases of VTE in the world on an annual basis. In the United States, it is estimated that the incidence of diagnosed VTE is 117 per 100,000, but this number may be an underestimation as most cases are frequently not diagnosed or only discovered on autopsy. 1
PE is thought to develop in approximately 10% of patients with acute DVT and it can lead to approximately 10% of hospital deaths. It is also a known fact that most patients (up to 75%) with PE are asymptomatic. It is further thought that about one-third of the hospitalized patients in the United States are at high risk of developing VTE and about 100,000 deaths are related to this disease per year. 2 3 4
The annual incidence of venous thrombosis, including DVT and PE, is estimated to occur in 1 out of 1,000 adults. Rates increase significantly after age 45 years and are higher in males than in females. 3
In this article, we discuss the pathophysiology of PE and management of this condition.
Pathophysiology
The pathogenesis of PE is dictated by the Virchow's triad like other intravascular thrombi and is a combination of venous stasis, endothelial injury, and a hypercoagulable state ( Table 1 ). The etiology/risk factors leading to culmination of the triad can be inherited or acquired ( Table 2 ).
Table 1. Virchow's triad.
| Venous stasis |
| Endothelial injury |
| Hypercoagulable state |
Table 2. Risk factors for pulmonary emboli.
| Acquired |
| Immobilization |
| Major trauma or surgery within 4 weeks |
| Active cancer (treatment within 6 months or palliative therapy) |
| Prior history of thromboembolism |
| Reduced cardiac output/Heart Failure |
| Obesity |
| Pregnancy, early puerperium |
| Estrogen/ oral contraceptives |
| Indwelling catheters |
| Antiphospholipid antibodies |
| Thrombocytosis |
| Postsplenectomy |
| Heparin induced thrombocytopenia |
| Primary hypercoagulable states/ Thrombophilia |
| Deficiency of antithrombin III, protein C or S |
| Resistance to activated protein C (factor V Leiden) |
| Elevated plasminogen activator inhibitor |
| Hyperhomocysteinemia |
| High plasma concentration of factor VIII |
| Prothrombin gene mutation (G20210A polymorphism) |
Most PEs originate from the deep veins of lower extremity. The common sites of thrombus formation are in the calf veins followed by femoropopliteal veins and finally the iliac veins. A blood clot dislodges from the vessel wall and travels into the pulmonary system, eventually lodging in the pulmonary arteries. When large pulmonary vessels are involved, it could cause severe hemodynamic instability including right ventricular (RV) pressure overload, RV failure, and eventually death ( Fig. 1 ).
Fig. 1.
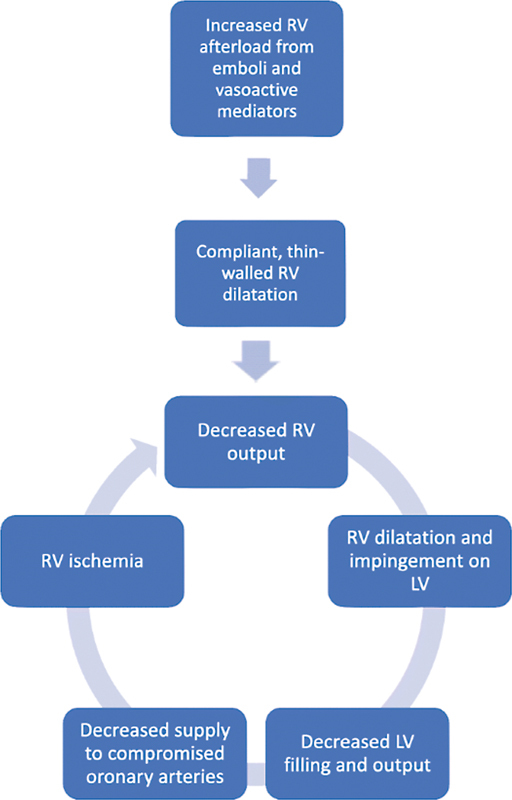
Pathophysiology of right ventricular failure. LV, left ventricle; RV, right ventricle.
Hypoxia is common in the setting of PE, and the degree of respiratory compromise is multifactorial and influenced by underlying cardiac function, clot location, and ability to compensate with respiratory mechanics. 5 6 Embolus size does not correlate with severity of hypoxia. 7 There are multiple mechanisms that eventually lead to hypoxia, including (1) ventilation perfusion mismatch causing redistribution of perfusion to nonoccluded areas, leading to regional lower perfusion and hence skewing the V/Q ratio; (2) regional bronchoconstriction leading to reduced ventilation atelectasis, which further causes intrapulmonary shunting leading to hypoxia; (3) reduced cardiac output leading to reduced central venous oxygen pressures and hence hypoxia; (4) increase in right atrial pressure that can open the patent foramen ovale, thereby leading to intracardiac right-to-left shunting and hence hypoxia. 5 8 9 10 11 12
RV dysfunction/failure is the more profound cardiovascular impact of acute PE and occurs due to sudden increase in afterload. This is also the primary cause of death in PE. At baseline, the RV is a thin-walled chamber that pumps against a low-pressure and low-resistance pulmonary vasculature. Increase in afterload is due to mechanical obstruction of the pulmonary vasculature that impedes blood flow and release of vasoconstrictors like TXA2. (Thromboxane) due to hypoxia. 6 13 14 15 TXA2 is a hormone of the prostacyclin type released from blood platelets. It induces platelet aggregation and produces local vasoconstriction. This increase in afterload and pulmonary vascular resistance stretches the RV, increases wall tension, and causes compensatory increase in chronotropy and inotropy to maintain the Frank-Starling mechanism and adequate cardiac function at the expense of pulmonary hypertension. 16 17 18 The compensation reaches a tipping point, beyond which the increase in PA pressures cannot generate further RV dilation, causing RV failure. Increased myocardial wall tension and transmural pressure cause ischemia by impeding coronary flow. This, in the presence of hypoxia and increased afterload, precipitates a vicious cycle of worsening myocardial ischemia, reduced RV contractility, reduced cardiac output, and eventual RV failure. Progressive RV dilation will also exacerbate previously nascent tricuspid regurgitation that can then trigger arrhythmias and hence worse cardiac performance. 19 20 There is also an inflammatory component, as evidenced by the influx of granulocytes and monocytes that suggests myocarditis. 21 22
Diagnostic Evaluation
Thorough history and identification of risk factors and physical exam are essential in guiding the appropriate diagnostic evaluations. Preliminary evaluation would include 12-lead electrocardiogram, on which the most common findings are sinus tachycardia (44%), RV strain pattern/T-wave inversions (34%), right axis deviation (16%), and right bundle branch block (18%). 23
Chest X-ray features of acute PE include enlarged pulmonary artery (Fleischner sign), regional oligemia (Westermark sign), and Hampton hump (wedge-shaped distal infarct) that have high specificity, but very low sensitivity.
Multiple risk scores have been developed to quantify the pre-test probability of PE and help guide the diagnostic process and triage them accordingly. The Wells score and modified Wells score use seven clinical indicators to stratify in to “PE likely” and “PE unlikely” groups ( Table 3 ). The Geneva score relies only on objective data to stratify in to low-, intermediate-, and high-risk categories ( Table 4 ). The pulmonary e mbolism rule out criteria rule can be used in low-risk patients to avoid unnecessary diagnostics ( Table 5 ).
Table 3. Wells criteria and modified Wells criteria: clinical assessment for pulmonary embolism.
| Clinical symptoms of DVT (leg swelling, pain with palpation) | 3.0 |
| Other diagnosis less likely than PE | 3.0 |
| Heart rate >100 | 1.5 |
| Immobilization (≥3 days) or surgery in the previous 4 weeks | 1.5 |
| Previous DVT/PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy | 1.0 |
| Probability | Score |
| Traditional clinical probability assessment (Wells criteria) | |
| High | >6.0 |
| Moderate | 2.0–6.0 |
| Low | <2.0 |
| Simplified clinical probability assessment (modified Wells criteria) | |
| PE likely | >4.0 |
| PE unlikely | ≤4.0 |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism.
Source: Data from van Belle A, Buller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172.
Table 4. Modified Geneva score.
| Variables | Points | |
|---|---|---|
| Risk factors | Age >65 years | 1 |
| Previous deep venous thrombosis or pulmonary embolism | 3 | |
| Surgery under general anesthesia or fracture of the lower limbs within 1 month | 2 | |
| Active malignancy (solid or hematologic; currently active or cured within the last year) | 2 | |
| Symptoms | Unilateral lower-limb pain | 3 |
| Hemoptysis | 2 | |
| Signs | Heart rate 75 to 94 beats per minute | 3 |
| ≥95 beats per minute | 5 | |
| Pain on lower limb deep venous palpation and unilateral edema | 4 | |
| Total points | ||
| Pre-test probability assessment | Low | 0 to 3 |
| Intermediate | 4 to 10 | |
| High | ≥ 11 | |
Source: From Annals of Internal Medicine, Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144(3);165-71. Copyright © 2006 American College of Physicians. All rights reserved. Reprinted with the permission of American College of Physicians, Inc.
Table 5. Pulmonary embolism rule out criteria (PERC rule) a .
| Age <50 years |
| Heart rate <100 bpm |
| Oxyhemoglobin saturation ≥95% |
| No hemoptysis |
| No estrogen use |
| No prior DVT or PE |
| No unilateral leg swelling |
| No surgery/trauma requiring hospitalization within the prior 4 weeks |
Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolus; bpm: beats per minute.
This rule is only valid in patients with a low clinical probability of PE (gestalt estimate <15%). In patients with a low probability of PE who fulfill all eight criteria, the likelihood of PE is low and no further testing is required. All other patients should be considered for further testing with sensitive D-dimer or imaging.
Source: Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost 2008;6:772 .
In clinically stable patients obtaining a D-dimer, a soluble fibrin degradation product, is typically the next diagnostic step. Due to the high diagnostic sensitivity (>95%) and negative predictive value, it is primarily used to help exclude PE when the levels are normal. D-dimer, however, has poor diagnostic specificity (41%), and when elevated, it can be due to other various conditions such as sepsis, trauma, cancer, surgery, other thrombosis, or disseminated intravascular coagulation, among others. 24
In patients with high clinical probability or abnormal D-dimer, computed tomography (CT) angiography of the pulmonary vasculature is the next diagnostic step ( Fig. 2 ) Advancements in imaging have reduced acquisition times to a few seconds, making it possible to get high-resolution images even in the presence of dyspnea. CT also provides assessment for other conditions in the differential diagnosis of dyspnea and tachycardia like pneumonia, coronavirus disease 2019, and effusions. 25 If computed tomography angiography (CTA) is contraindicated (renal failure, allergy, pregnancy), ventilation perfusion scans can be utilized, which have a sensitivity and specificity of 85 and 93%, respectively. 26
Fig. 2.
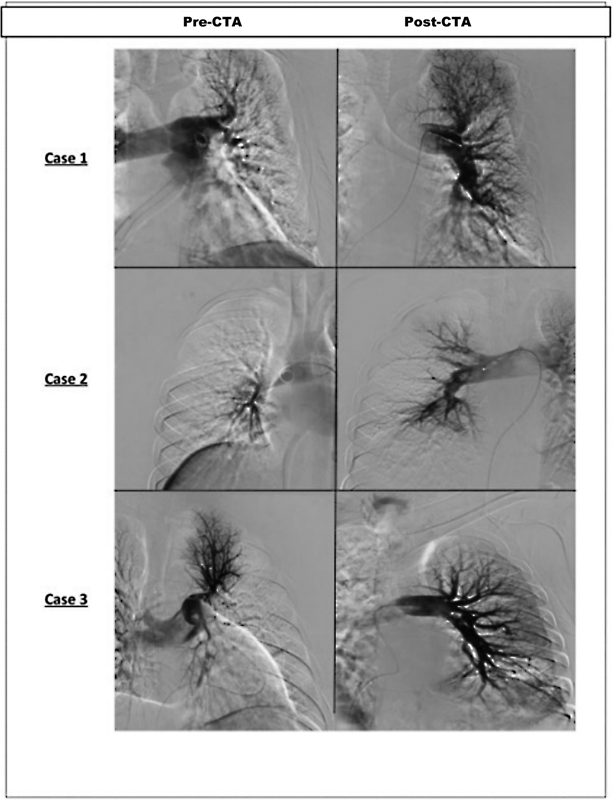
Pre- and post-computed tomography angiography (CTA) images following thrombolysis showing significant improvement in pulmonary.
Echocardiography can be helpful in assessing RV function and subsequent risk stratification since it is typically associated with worse outcomes. PE can also cause RV failure and cardiogenic shock due to obstruction and increased pulmonary vasoconstriction. 27
Treatment
PE can range from acute massive PE causing pulmonary infarction or could be secondary to small emboli that do not cause hemodynamic instability. Patients with PE can present with minor nonspecific symptoms like dyspnea, to symptoms of shock with systolic blood pressures less than 90 mm Hg or a decrease in systolic arterial pressure of at least 40 mm Hg for 15 minutes or more.
Treatment is dependent on clinical presentation and initially involves providing adequate oxygenation and stabilizing hemodynamics. Treatment of the PE per se is guided by presence or absence of shock.
Patients without shock are low risk and can be treated with anticoagulation, which is the mainstay of most PE treatments. Parenteral treatment can be started with heparin or enoxaparin and transitioned to warfarin for a goal international normalized ratio between 2 and 3. Direct-acting oral anticoagulants, on the other hand, have quick onset of action and do not require bridging with parenteral anticoagulants 28 ( Table 6 ). Duration of anticoagulation is 3 to 6 months but is required lifelong if there is a recurrence of PE/DVT. 29
Table 6. Dosing of direct oral anticoagulants.
| Anticoagulant | Nonvalvular AF -stroke prophylaxis | VTE treatment | VTE primary prophylaxis |
|---|---|---|---|
| Dabigatran (Pradaxa) | 150 mg twice daily | Parenteral anticoagulation for 5 to 10 days; then dabigatran 150 mg twice daily | 110 mg for the first day, then 220 mg once daily |
| Apixaban (Eliquis) | 5 mg twice daily | 10 mg twice daily for 1 week, then 5 mg twice daily | 2.5 mg twice daily |
| Edoxaban (Savaysa, Lixiana) | 60 mg once daily | Parenteral anticoagulation for 5 to 10 days; then edoxaban 60 mg once daily | |
| Rivaroxaban (Xarelto) | 20 mg once daily with the evening meal | 15 mg twice daily with food for 3 weeks; then 20 mg once daily with food | 10 mg once daily, with or without food |
Abbreviations: AF, atrial fibrillation; VTE, venous thromboembolic.
Thrombolytic therapy is reserved for patients with cardiorespiratory compromise/shock. It can also be used with severe right heart strain or RV failure. Thrombolytics cause clot-lysis, hence, resuming circulation and reducing RV strain. tPA, or tissue plasminogen activator, is the most commonly use thrombolytic and administered as a 100 mg infusion over 2 hours. For catheter-directed thrombolysis, tPA is infused at 0.5 to 2 mg/h for 2 to 15 hours. Contraindications for tPA are outlined in Table 7 .
Table 7. Contraindications to fibrinolytic therapy.
| Absolute contraindications |
| Prior intracranial hemorrhage |
| Known structural cerebral vascular lesion |
| Known malignant intracranial neoplasm |
| Ischemic stroke within 3 months (excluding stroke within 3 hours a ) |
| Suspected aortic dissection |
| Active bleeding or bleeding diathesis (excluding menses) |
| Significant closed-head trauma or facial trauma within 3 months |
| Relative contraindications |
| History of chronic, severe, poorly controlled hypertension |
| Severe uncontrolled hypertension on presentation (SPB >180 mm Hg or DBP > 110 mm Hg) |
| History of ischemic stroke more than 3 months prior |
| Traumatic or prolonged (>10 minute) CPR or major surgery less than 3 weeks |
| Recent (within 2–4 weeks) internal bleeding |
| Noncompressible vascular punctures |
| Recent invasive procedure |
| For streptokinase/anistreplase—prior exposure (> 5 days ago) or prior allergic reaction to these agents |
| Pregnancy |
| Active peptic ulcer |
| Pericarditis or pericardial fluid |
| Current use of anticoagulant (e.g., warfarin sodium) that has produced an elevated INR >1.7 or PT >15 seconds |
| Age >75 years |
| Diabetic retinopathy |
Abbreviations: CPR, cardiopulmonary resuscitation; DBP, diastolic blood pressure; INR, international normalized ratio; PT, prothrombin time; SBP, systolic blood pressure.
The American College of Cardiology suggests that select patients with stroke may benefit from thrombolytic therapy within 4.5 hours of the onset of symptoms.
Conclusion
PE is a relatively common and serious complication of VTE disease. Its incidence appears to be steadily increasing, possibly due to earlier recognition of symptoms and more accurate diagnosis. The increased accuracy of CTA in detecting PE is an important milestone in this regard. The overall mortality risk is directly related to abnormalities of gas exchange in the pulmonary vasculature and cardiovascular complications resulting from obstruction, which lead to increase in pulmonary vascular resistance and RV pressure overload and RV systolic dysfunction. The underlying cardiopulmonary disease status also contributes significantly to above-mentioned hemodynamic complications. Inherited and acquired risk factors like blood dyscrasias, immobilization post-surgery, and malignancy can increase the likelihood of developing VTE and PE. Anticoagulation treatment is mandatory and needs to be initiated as soon as the diagnosis of PE is established. Newer (novel) oral anticoagulation agents are now available in the market and show promise in being used like warfarin, as parenteral agents, in the treatment of PE.
Footnotes
Conflict of Interest None declared.
References
- 1.ISTH Steering Committee for World Thrombosis Day . Raskob G E, Angchaisuksiri P, Blanco A N. Thrombosis: a major contributor to global disease burden. Semin Thromb Hemost. 2014;40(07):724–735. doi: 10.1055/s-0034-1390325. [DOI] [PubMed] [Google Scholar]
- 2.Anderson F A, Jr, Zayaruzny M, Heit J A, Fidan D, Cohen A T. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82(09):777–782. doi: 10.1002/ajh.20983. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein M D, Heit J A, Mohr D N, Petterson T M, O'Fallon W M, Melton L J., III Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(06):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 4.Dudzinski D M, Giri J, Rosenfield K. Interventional treatment of pulmonary embolism. Circ Cardiovasc Interv. 2017;10(02):e004345. doi: 10.1161/CIRCINTERVENTIONS.116.004345. [DOI] [PubMed] [Google Scholar]
- 5.Huet Y, Lemaire F, Brun-Buisson C. Hypoxemia in acute pulmonary embolism. Chest. 1985;88(06):829–836. doi: 10.1378/chest.88.6.829. [DOI] [PubMed] [Google Scholar]
- 6.Tsang J Y, Hogg J C. Gas exchange and pulmonary hypertension following acute pulmonary thromboembolism: has the emperor got some new clothes yet? Pulm Circ. 2014;4(02):220–236. doi: 10.1086/675985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson J E, III, Pierce A K, Johnson R L., Jr Hypoxemia in pulmonary embolism, a clinical study. J Clin Invest. 1971;50(03):481–491. doi: 10.1172/JCI106516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein P D, Terrin M L, Hales C A. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100(03):598–603. doi: 10.1378/chest.100.3.598. [DOI] [PubMed] [Google Scholar]
- 9.Calkovska A, Mokra D, Calkovsky V. Lung surfactant alterations in pulmonary thromboembolism. Eur J Med Res. 2009;14 04:38–41. doi: 10.1186/2047-783X-14-S4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurewich V, Thomas D, Stein M, Wessler S. Bronchoconstriction in the presence of pulmonary embolism. Circulation. 1963;27:339–345. doi: 10.1161/01.cir.27.3.339. [DOI] [PubMed] [Google Scholar]
- 11.Lele E E, Hantos Z, Bitay M. Bronchoconstriction during alveolar hypocapnia and systemic hypercapnia in dogs with a cardiopulmonary bypass. Respir Physiol Neurobiol. 2011;175(01):140–145. doi: 10.1016/j.resp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Konstantinides S, Geibel A, Kasper W, Olschewski M, Blümel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation. 1998;97(19):1946–1951. doi: 10.1161/01.cir.97.19.1946. [DOI] [PubMed] [Google Scholar]
- 13.Haddad F, Doyle R, Murphy D J, Hunt S A. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117(13):1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 14.Goldhaber S Z, Visani L, De Rosa M.Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet 1999353(9162):1386–1389. [DOI] [PubMed] [Google Scholar]
- 15.Smulders Y M. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000;48(01):23–33. doi: 10.1016/s0008-6363(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 16.Cingolani H E, Pérez N G, Cingolani O H, Ennis I L. The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol. 2013;304(02):H175–H182. doi: 10.1152/ajpheart.00508.2012. [DOI] [PubMed] [Google Scholar]
- 17.Nootens M, Kaufmann E, Rector T. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26(07):1581–1585. doi: 10.1016/0735-1097(95)00399-1. [DOI] [PubMed] [Google Scholar]
- 18.Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013;18(02):129–138. [PMC free article] [PubMed] [Google Scholar]
- 19.Vlahakes G J, Turley K, Hoffman J I. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation. 1981;63(01):87–95. doi: 10.1161/01.cir.63.1.87. [DOI] [PubMed] [Google Scholar]
- 20.van Wolferen S A, Marcus J T, Westerhof N. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29(01):120–127. doi: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]
- 21.Iwadate K, Doi M, Tanno K.Right ventricular damage due to pulmonary embolism: examination of the number of infiltrating macrophages Forensic Sci Int 2003134(2-3):147–153. [DOI] [PubMed] [Google Scholar]
- 22.Watts J A, Zagorski J, Gellar M A, Stevinson B G, Kline J A. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol. 2006;41(02):296–307. doi: 10.1016/j.yjmcc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Ullman E, Brady W J, Perron A D, Chan T, Mattu A. Electrocardiographic manifestations of pulmonary embolism. Am J Emerg Med. 2001;19(06):514–519. doi: 10.1053/ajem.2001.27172. [DOI] [PubMed] [Google Scholar]
- 24.Perrier A, Desmarais S, Goehring C.D-dimer testing for suspected pulmonary embolism in outpatients Am J Respir Crit Care Med 1997156(2 Pt 1):492–496. [DOI] [PubMed] [Google Scholar]
- 25.Schoepf U J, Costello P. CT angiography for diagnosis of pulmonary embolism: state of the art. Radiology. 2004;230(02):329–337. doi: 10.1148/radiol.2302021489. [DOI] [PubMed] [Google Scholar]
- 26.Sostman H D, Miniati M, Gottschalk A, Matta F, Stein P D, Pistolesi M. Sensitivity and specificity of perfusion scintigraphy combined with chest radiography for acute pulmonary embolism in PIOPED II. J Nucl Med. 2008;49(11):1741–1748. doi: 10.2967/jnumed.108.052217. [DOI] [PubMed] [Google Scholar]
- 27.Lucassen W, Geersing G J, Erkens P M. Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med. 2011;155(07):448–460. doi: 10.7326/0003-4819-155-7-201110040-00007. [DOI] [PubMed] [Google Scholar]
- 28.ESC Working Group on Thrombosis Task Force on Anticoagulants in Heart Disease . Husted S, de Caterina R, Andreotti F. Non-vitamin K antagonist oral anticoagulants (NOACs): no longer new or novel. Thromb Haemost. 2014;111(05):781–782. doi: 10.1160/TH14-03-0228. [DOI] [PubMed] [Google Scholar]
- 29.ESC Scientific Document Group . Konstantinides S V, Meyer G, Becattini C. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41(04):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]


