PhoP-PhoQ is a two-component system that governs virulence, mediates the adaptation to Mg2+-limiting environments, and regulates numerous cellular activities in several gram-negative species. It consists of the inner membrane sensor PhoQ and the cytoplasmic regulator PhoP. The PhoP-PhoQ system is encoded by the phoP locus, which was first identified in Salmonella enterica serovar Typhimurium as controlling the expression of a nonspecific acid phosphatase (51). This is the reason for the pho in phoP, a designation typically denoting loci involved in phosphate metabolism. However, the PhoP-PhoQ system responds to the levels of Mg2+ and Ca2+ (29) and should not be confused with PhoB-PhoR or PhoP-PhoR, two-component systems governing the adaptation to phosphate-limiting conditions in Escherichia coli (83) and Bacillus subtilis (49), respectively. The realization that PhoP-PhoQ controls virulence in Salmonella (31, 58) promoted new interest in the system in the late 1980s and has rendered the PhoP regulon one of the best characterized regulons in enteric bacteria.
Here, I first discuss how the PhoP-PhoQ two-component system responds to environmental cues and interacts with other regulatory systems to integrate multiple signals into a coordinated cellular response and then I describe the PhoP-regulated genes mediating the various PhoP-controlled functions, including virulence. The Salmonella-centric tone of this review reflects the fact that most of the work on PhoP-PhoQ has been carried out with this enteric pathogen. However, many of the findings discussed about Salmonella PhoP-PhoQ apply to PhoP-PhoQ homologues in other gram-negative species. A model of the PhoP-PhoQ system is presented in Fig. 1, and the genes and cellular activities regulated by Phop-PhoQ in Salmonella are listed in Table 1.
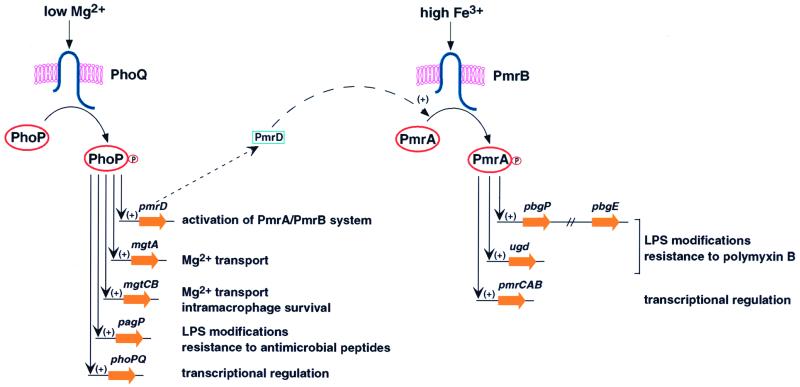
FIG. 1.
Model describing the signals controlling expression of PhoP-PhoQ-regulated determinants and the interaction between the PhoP-PhoQ and PmrA-PmrB two-component systems, as well as some of the genes and phenotypes governed by the PhoP-PhoQ system.
TABLE 1.
Genes and cellular activities regulated by PhoP-PhoQ in Salmonella
| Gene | Function and /or properties of gene product | Present in E. coli K-12? |
|---|---|---|
| hilA | Transcriptional regulator of invasion genes | No |
| mgtA | P-type ATPase Mg2+ transporter | Yes |
| mgtB | P-type ATPase Mg2+ transporter | No |
| mgtC | Mg2+ acquisition; intramacrophage survival | No |
| pagC | Outer membrane protein with sequence similarity to Enterobacter OmpX, Yersinia Ail, and phage lambda Lom | No |
| pagP | Outer membrane enzyme mediating transfer of palmitate to lipid A; resistance to peptide C18G | Yes |
| pbgPE operon | Synthesis and/or incorporation of 4-aminoarabinose into lipid A; resistance to polymyxin | Yes |
| pcgL | Periplasmic d-Ala-d-Ala dipeptidase | Noa |
| pgtE | Outer membrane protease; resistance to peptide C18G | Noa |
| phoN | Periplasmic nonspecific acid phosphatase | No |
| phoPQ | Mg2+ -responding two-component system | Yes |
| pmrAB | Fe3+ -responding two-component system | Yes |
| pmrD | Mediator of transcriptional activation of pmrA-regulated genes during growth in low Mg2+ | Yes |
| prgHIJK | Components of Inv-Spa type III secretion system | No |
| spvB | Mono(ADP-ribosyl)transferase encoded in Salmonella virulence plasmid | No |
| ugd | UDP-d-glucose dehydrogenase | Yes |
| ugtL | Putative membrane protein | No |
E. coli K-12 harbors two genes, ecovanX and ompT, encoding products that are only 40 and 46% identical to the Salmonella pcgL and pgtE gene products, respectively. However, these genes are not true homologs and, like Salmonella pcgL and pgtE, the E. coli genes appear to have been acquired horizontally.
PHOP-PHOQ RESPONDS TO EXTRACYTOPLASMIC LEVELS OF MG2+ AND CA2+
PhoP-PhoQ constitutes the first example of a regulatory system that uses extracellular Mg2+ as a primary signal. Growth in micromolar concentrations of Mg2+ promotes transcription of PhoP-activated genes in a PhoP-and PhoQ-dependent manner, whereas growth in millimolar concentrations of Mg2+ represses expression of PhoP-activated genes to the levels displayed by phoP or phoQ null mutants (29, 50, 78). In addition to Mg2+, Ca2+ and Mn2+ can repress transcription of PhoP-activated genes, whereas Ni2+, Cu2+, Co2+, and Ba2+ have no effect (29).
Consistent with Mg2+ and Ca2+ being the physiological signals controlling the PhoP-PhoQ system, several PhoP-dependent phenotypes are regulated by these divalent cations in wild-type microorganisms. For example, wild-type Salmonella grown in Luria-Bertani broth is >1,000-fold more resistant to the antimicrobial peptide magainin 2 than organisms grown in Luria-Bertani broth supplemented with 25 mM Mg2+ (29). Likewise, the ability to modify the lipid A moiety of the lipopolysaccharide (LPS) and to exhibit resistance to the antibiotic polymyxin B is regulated by Mg2+ in both Salmonella (33, 41) and Pseudomonas aeruginosa (18, 55). Moreover, the PhoP-PhoQ homologue PehR-PehS governs transcription of the Ca2+-regulated virulence protein PehA in the plant pathogen Erwinia carotovora subsp. carotovora (23, 24). On the other hand, the PhoQ protein of Providencia stuartii does not appear to respond to Mg2+ but to a yet undefined signal (68).
HOW DOES MG2+ CONTROL THE PHOP-PHOQ SYSTEM?
The Salmonella PhoQ protein features two transmembrane regions that define a long cytoplasmic C-terminal tail harboring the histidine residue predicted to be the site of autophosphorylation and a periplasmic domain harboring several acidic residues that could be involved in sensing divalent cations. In vivo experiments indicate that the PhoQ protein has distinct noninteracting binding sites for Mg2+ and Ca2+ (28) and that the PhoP-PhoQ system responds to the periplasmic (as opposed to cytoplasmic) levels of these divalent cations (29). Moreover, in vitro experiments demonstrated that the purified 146-amino-acid periplasmic domain of PhoQ binds Mg2+ but not Ba2+, a divalent cation unable to repress transcription of PhoP-regulated genes (28, 82). A cluster of acidic residues in the periplasmic region of the PhoQ protein has been implicated in Mg2+ sensing by E. coli (82); however, its role is presently unclear because the acidic cluster is not conserved in the PhoQ protein of some gram-negative species that respond to Mg2+.
What are the biochemical consequences resulting from Mg2+ binding to the periplasmic domain of the PhoQ protein? High Mg2+ promotes the dephosphorylation of phospho-PhoP by the PhoQ protein (as opposed to regulating the autophosphorylation of PhoQ or the phosphorylation of the PhoP protein from phospho-PhoQ.) When grown in the presence of Mg2+, PhoQ abolishes transcription of PhoP-activated genes in a strain harboring a PhoP variant that autophosphorylates from acetyl phosphate (15). And in vitro, Mg2+ stimulates dephosphorylation of phospho-PhoP by membranes enriched for the PhoQ protein (14). It has been proposed that dephosphorylation of phospho-PhoP involves the reversal of phosphate transfer from aspartate 57 in PhoP back to histidine 243 in PhoQ because a mutant PhoQ protein harboring a valine residue at position 243 exhibits no phosphatase activity (14). However, additional PhoQ mutants will need to be tested to validate this model because when similar experiments were carried out with the related sensor EnvZ of E. coli, only certain amino acid substitutions in the histidine site of phosphorylation resulted in EnvZ proteins lacking phosphatase activity towards phospho-OmpR (48).
PH REGULATES TRANSCRIPTION OF A SUBSET OF PHOP-ACTIVATED GENES
Because mild acid pH promotes transcription of certain PhoP-activated genes, it has been proposed that the Salmonella PhoQ protein senses acid pH (4) or responds to both pH and Mg2+ (7). However, PhoQ is unlikely to be a pH sensor because growth in mild acid pH promotes transcription only in a subset of PhoP-activated genes and this activation still takes place in a phoQ null mutant (7, 29, 79). Likewise, the mild-acid induction of these genes is not affected by mutation of the phoP gene, arguing against the possibility of the PhoP protein itself sensing pH or being activated by a pH sensor. Therefore, although transcription of PhoP-regulated genes can be regulated by signals other than Mg2+ and Ca2+, these signals are sensed typically by sensors other than PhoQ, activate regulators other than PhoP, and affect only a subset of the genes belonging to the PhoP-PhoQ regulon.
AUTOGENOUS REGULATION OF THE PHOP-PHOQ SYSTEM
The phoPQ operon is autogenously controlled in a positive fashion by the PhoP and PhoQ proteins both in Salmonella (77) and E. coli K-12 (50). The phoPQ operon is transcribed from two promoters, one active only during growth in low Mg2+ and dependent on the PhoP and PhoQ proteins and another that is constitutive (i.e., active regardless of the Mg2+ concentration or presence of PhoP and PhoQ proteins) (29). However, the regulation of the phoPQ operon differs in Salmonella and E. coli K-12 in that PhoP-PhoQ regulates the promoter distal to the phoP initiating codon in Salmonella (77) but controls the promoter that is proximal to phoP in E. coli (50). Thus, while the relative position of the constitutive and regulated promoters is different in Salmonella and E. coli, both species rely on a positive regulatory loop to control phoPQ gene expression, perhaps as a means to amplify the signals sensed by the PhoQ protein.
In P. aeruginosa, the phoPQ genes are preceded by oprH (a gene encoding an outer membrane protein of unknown function) and transcribed from two promoters. There is an Mg2+- and PhoP-regulated polycistronic transcript that expresses the oprH, phoP, and phoQ genes, whereas a second promoter located within or downstream of oprH allows low levels of phoPQ transcription (54). This organization is reminiscent of that present in the Salmonella locus encoding the two-component system PmrA-PmrB: the pmrAB genes are preceded by the pmrC gene (which also encodes a putative outer membrane protein) and transcribed by two promoters, one upstream of pmrC and one within pmrC (39).
THE PHOP-PHOQ TWO-COMPONENT SYSTEM ACTIVATES THE PMRA-PMRB TWO-COMPONENT SYSTEM DURING GROWTH IN LOW MG2+ CONCENTRATIONS
A subset of PhoP-activated genes is regulated via another two-component system (39, 43, 79), PmrA-PmrB (70), where PmrA is the response regulator and PmrB is a sensor kinase that responds to extracytoplasmic ferric iron (86). Thus, either low Mg2+ or high Fe3+ concentrations promote transcription of PhoP-activated PmrA-dependent genes. In contrast, transcription of those PhoP-activated genes which are PmrA independent is promoted in low Mg2+ concentrations. but does not respond to Fe3+.
We are beginning to understand how the Mg2+ and Fe3+ signals are sensed and transduced into an appropriate cellular response that activates only the correct set of genes. The low Mg2+ activation of PmrA-regulated genes, which requires the phoQ, phoP, pmrD, pmrA, and pmrB genes, occurs by the PhoQ protein serving as an Mg2+ sensor that modulates the ability of the PhoP protein to promote transcription of the pmrD gene. The pmrD gene product then activates PmrA-PmrB at a posttranscriptional level by a yet undefined mechanism (52). On the other hand, Fe3+-promoted activation of PmrA-regulated genes occurs by Fe3+ binding to the periplasmic domain of the PmrB protein (86), which presumably promotes PmrB autophosphorylation and the ensuing phosphorylation of the PmrA protein. This would result in transcription of PmrA-activated genes because the PmrA protein binds to the promoters it regulates with higher affinity in its phosphorylated form (85). The Fe3+-promoted pathway is independent of the phoP, phoQ (86), and pmrD (52) genes, allowing transcription of PmrA-activated genes to take place independently of the PhoP-PhoQ system. In addition, transcription of PmrA-dependent genes can be induced by mild acid pH in strains lacking functional phoQ, phoP (79), or pmrB (F. C. Soncini and E. A. Groisman, unpublished results) genes by an unknown mechanism.
Why does the PmrA regulon respond to multiple signals? We hypothesize that this is because the PmrA-regulated gene products are useful under the various growth conditions that promote their expression. For example, when bacteria experience low Mg2+ environments the PhoP-PhoQ system is activated, resulting in expression of several proteins required for growth in low Mg2+ concentrations such as the Mg2+ transporters MgtA and MgtB (78). By also promoting pmrD transcription, PhoP-PhoQ activates the PmrA-PmrB system, which controls transcription of genes necessary for growth in low Mg2+ solid media (78). Perhaps transcription of PhoP-regulated genes is induced at dissimilar Mg2+ thresholds depending on whether they are regulated directly or indirectly (e.g., via pmrD) by the PhoP protein. On the other hand, the possibility to activate the PmrA-PmrB system independently of PhoP-PhoQ allows the synthesis of proteins conferring protection from the toxic effects of Fe3+ in environments with repressing levels of Mg2+ (86).
The PhoP-PhoQ and PmrA-PmrB systems are widely distributed among enteric bacteria and have been identified also in gram-negative organisms such as P. aeruginosa (31, 55; F. Solomon and E. A. Groisman, unpublished results). In contrast, pmrD homologues have not been detected in the genomes of species harboring phoP-phoQ and pmrA-pmrB genes (M. Winfield and E. A. Groisman, unpublished results). This indicates that PhoP-PhoQ and PmrA-PmrB can exist as “free-standing” two-component systems, each responding to their specific signals. Moreover, it suggests that during evolution the PhoP-PhoQ and PmrA-PmrB systems became connected via the “pmrD shunt” in species such as Salmonella. This enabled expression of PmrA-activated genes not only when Fe3+ concentrations are high but also when Mg2+ concentrations are low, thereby providing pmrD-containing organisms with the opportunity to expand their niches.
PHOP-REGULATED PROMOTERS
A direct repeat, (T/G)GTTTA, has been identified 25 bp upstream of the transcription start site of three Mg2+-regulated PhoP-activated genes in E. coli K-12: phoPQ, mgtA, and mgrB (50). This repeat is also present in the promoter region of the Salmonella phoPQ (77) and phoN (35) genes and has been detected in the putative regulatory region of four additional open reading frames in E. coli and three in Salmonella (50). It is tempting to speculate that the identified motif represents a bona fide PhoP-binding site. However, this awaits DNAse footprinting analysis of PhoP-regulated promoters because the identified motif is also present in the promoter region of the PhoP-activated PmrA-dependent ugd gene (38, 79), which has been shown to bind the PmrA protein (1).
ECLECTIC GENES AND PHENOTYPES CONTROLLED BY PHOP-PHOQ
The PhoP-PhoQ system governs expression of at least 40 proteins in Salmonella (59), which constitutes approximately 1% of the open reading frames encoded in the Salmonella genome. Some two dozen PhoP-regulated determinants have been identified by classical genetic methods (9, 66, 78) and MALDI-TOF analysis (36). And while additional PhoP-regulated genes are likely to be uncovered with the use of high-density DNA arrays, some general principles have emerged from the analysis of the PhoP-regulated genes already identified. First, the PhoP-PhoQ regulon mediates the adaptation to Mg2+-limiting environments. Second, PhoP-PhoQ governs virulence in several gram-negative species. Third, many of the genes identified as PhoP regulated are species specific and confer unique properties upon the microorganism. And fourth, PhoP-PhoQ governs the modification of many components in the bacterial cell envelope.
MG2+ TRANSPORTERS, LPS MODIFICATIONS, AND ADAPTATION TO MG2+-LIMITING ENVIRONMENTS
The PhoP-PhoQ system controls the expression of several genes that are necessary for growth in low Mg2+ concentrations (78). These include the mgtA and mgtB genes, which encode two of the three Mg2+ transporters of Salmonella (75). Like a phoP null mutant, mgtA and mgtCB null mutants exhibit wild-type logarithmic growth but reach a plateau earlier than wild-type Salmonella in low Mg2+ liquid media, forming elongated cells and losing the ability to form bacterial colonies (78). That lack of either MgtA or MgtB results in a growth defect suggests that both transporters are necessary to maintain physiological levels of Mg2+ in the Salmonella cytoplasm. Alternatively, the growth defect may be due to the inability of these transporters to take up or export a ligand(s) that is cotransported along with Mg2+. The MgtA and MgtB proteins are P-type ATPases that transport Mg2+ down an electrochemical gradient, supporting the notion that a ligand is likely cotransported along with Mg2+ (75). While the mgtA and mgtB genes encode proteins that are 50% identical, they differ in phylogenetic distribution and expression profile: mgtB has been detected in only 3 of 10 gram-negative species harboring mgtA-hybridizing sequences (11), and acid pH abolishes mgtA transcription but has minimal effect on mgtB expression (74).
A different set of PhoP-regulated genes is required for growth in low Mg2+ solid media (but dispensable for growth in low Mg2+ liquid media): the ugd gene and the seven-gene operon pbgPE (78) (the pbgPE operon is designated pmrF in reference 38), which are regulated via the PmrA-PmrB system. The ugd-and pbgPE-encoded proteins mediate the synthesis and/or incorporation of 4-aminoarabinose in the lipid A portion of the LPS (38). This reduces the negative charge of the LPS and renders Salmonella resistant to the cationic peptide antibiotic polymyxin B (33, 38), presumably by decreasing binding of the positively charged polymyxin to the surface of the bacterial cell (20, 73).
What is the physiological role of the low Mg2+-promoted PmrA-regulated LPS modifications? We have hypothesized that the LPS is an Mg2+ reservoir and constitutes the initial source of Mg2+ for the MgtA and MgtB transporters when bacteria face Mg2+-limiting environments (33). According to this hypothesis, the PmrA-controlled modifications of the LPS serve to neutralize negative charges normally neutralized by Mg2+ and Ca2+ ions. Thus, the failure to modify the surface charge in a pmrA mutant would result in electrostatic repulsion between negatively charged LPS molecules, thereby preventing the intimate side-by-side alignments required for the formation of bacterial colonies, which could be important in biofilm formation.
PHOP-REGULATED RESISTANCE TO ANTIMICROBIAL PEPTIDES
Salmonella phoP mutants are highly susceptible to a variety of antimicrobial peptides, including the mammalian defensins, the frog-derived magainin 2, the insect-derived melittin and mastoparan, and the peptide antibiotic polymyxin B, produced by the soil bacterium Paenibacillus polymyxa (21, 33, 34, 39, 60). Despite similarities among many of these antimicrobial peptides—such as being small, cationic, and amphipathic (5, 64)—different PhoP-regulated determinants often mediate resistance to different antimicrobial peptides. Some of these determinants modify the LPS, whereas others encode extracytoplasmic proteases with the capacity to cleave antimicrobial peptides.
PagP is a PhoP-activated outer membrane protein responsible for the incorporation of palmitate into the lipid A moiety of the LPS (10), but dispensable for other PhoP-regulated modifications, such as addition of 2-hydroxymyristate or 4-aminoarabinose into lipid A (42). A Salmonella pagP mutant exhibits hypersensitivity to the synthetic α-helical peptide C18G but displays wild-type resistance towards polymyxin and protegrin (42). In addition to PagP, the outer membrane protease PgtE contributes to resistance against C18G, at least when the pgtE gene is overexpressed to high levels, since a pgtE mutant exhibits wild-type resistance to C18G (36). Resistance is associated with C18G cleavage by strains expressing PgtE, whose expression is reportedly controlled by PhoP-PhoQ at a posttranscriptional level (36).
Polymyxin resistance requires both PmrA-dependent and -independent PhoP-activated loci. The PmrA-dependent loci include ugd and the seven-gene pbgPE operon (also designated pmrF) (33, 38), both of which mediate the synthesis and/or incorporation of 4-aminoarabinose into the lipid A moiety of the LPS (38, 40). A PhoP-activated PmrA-independent gene(s) also appears to participate in polymyxin resistance because when Salmonella is grown in low Mg2+ concentrations (to promote transcription of the whole PhoP regulon) a phoP mutant is >20 times more susceptible to polymyxin than a pmrA mutant (86). Moreover, when bacteria are grown in high Fe3+ concentrations (to promote expression of PmrA-activated genes independently of PhoP) the phoP mutant remains >20 times more susceptible to polymyxin than wild-type Salmonella (86).
It was originally suggested that increased susceptibility to antimicrobial peptides is in part responsible for virulence attenuation in phoP and phoQ mutants (21). However, it is presently unclear what contribution PhoP-regulated peptide resistance makes to Salmonella virulence because pagP mutants retain wild-type virulence (9) and pmrA mutants are only slightly attenuated (40).
PhoP and Mg2+ also control resistance to antimicrobial peptides in other gram-negative species. For example, a phoP E. coli strain is hypersensitive to killing by magainin 2 and mastoparan (32), and Mg2+ modulates resistance of Shigella flexneri to magainin 2 in a PhoP-dependent manner (62). In P. aeruginosa, wild-type organisms grown in low Mg2+ concentrations are more resistant to killing by C18G (18) or polymyxin B (18, 55) than organisms grown in high Mg2+ concentrations. On the other hand, the role of PhoP in polymyxin resistance remains controversial in Pseudomonas. One group found the phoP null mutant more susceptible to polymyxin (18), whereas another group reported the phoP mutant to be as resistant as wild-type Pseudomonas (55), although phoP expression from a heterologous promoter conferred polymyxin resistance even in cells grown in high Mg2+ (55).
ROLE OF PHOP-PHOQ IN SALMONELLA PATHOGENESIS
Salmonellae are facultative intracellular pathogens that infect a wide variety of animals causing different disease conditions (see reference 72 for a review on Salmonella pathogenesis). Salmonella strains harboring null alleles of the phoP or phoQ gene are highly attenuated for virulence: their median lethal doses in intraperitoneally innoculated BALB/c mice are 5 orders of magnitude higher than that of wild-type Salmonella (21, 26, 58). This attenuation could be due to one or more of the various in vitro virulence defects displayed by phoP and phoQ null mutants. These include the inability to survive within macrophages (22, 58) and an increased susceptibility to killing by antimicrobial peptides (21, 34, 36, 42, 60), bile salts (81), and acid pH (7, 25). While many of these PhoP-regulated functions are mediated by genes present also in E. coli, PhoP-PhoQ appears to control virulence primarily by regulating transcription of Salmonella-specific virulence genes.
The PhoP-activated mgtC gene is required for intramacrophage survival in vitro and mouse virulence in vivo (11). Encoded within a Salmonella-specific gene cluster designated SPI-3 pathogenicity island, MgtC appears to function in Mg2+ acquisition, because an mgtC mutant is defective for growth in a low Mg2+ medium (11) and the MgtC protein is predicted to localize to the inner membrane (76). MgtC may mediate Mg2+ uptake by working in conjunction with another protein(s) because, by itself, it could not mediate transport of radioactive Ni2+ or Co2+ when used as a surrogate for Mg2+ (61). The MgtC protein appears to confer intramacrophage survival by insuring that proper levels of Mg2+ are available to the microorganism proliferating within the phagosome because intramacrophage survival was partially restored to both mgtC and phoP mutants upon addition of Mg2+ to the tissue culture media (11).
It is interesting that the phylogenetically distant pathogen Mycobacterium tuberculosis harbors an MgtC homolog that is also required for growth in low Mg2+ concentrations, proliferation within macrophages, and virulence in mice (13). In contrast, the closely related E. coli is missing the mgtC gene and cannot grow in Mg2+ concentrations as low as Salmonella (11). This suggests that acquisition of the mgtC gene, and the ensuing ability to grow in low Mg2+ concentrations, were important steps in the development of Salmonella as an intracellular pathogen.
Because mgtC mutants are not as attenuated as phoP null mutants, additional PhoP-regulated loci likely mediate Salmonella virulence in mice. Two such candidate loci, which have been implicated in intramacrophage survival, are the spv genes in the Salmonella virulence plasmid (53) and the ssa and sse genes in the SPI-2 pathogenicity island (16, 45, 63). However, the role of PhoP in transcription of the spv (19, 43, 44, 57) and SPI-2 (17, 80) genes remains controversial, suggesting the existence of other, yet unidentified, PhoP-activated virulence genes.
The attenuated phenotype and immunogenic potential of phoP mutants of serovar Typhimurium in mice (26, 58) have prompted the development of a vaccine strain of serovar Typhi that appears to be safe and immunogenic in humans (47). Moreover, the PhoP-PhoQ system influences the processing and presentation of antigens by Salmonella because activated macrophages process a phoP null mutant more efficiently than wild-type Salmonella, which in turn, is processed more efficiently than a pho-24 mutant [84]; see below).
POTENTIAL ROLE FOR PHOP-PHOQ IN PEPTIDOGLYCAN REMODELING
PhoP governs transcription of two adjacent Salmonella-specific genes that could be involved in peptidoglycan remodeling: pcgL, encoding a d-Ala-d-Ala dipeptidase, and ugtL, encoding a protein with sequence similarity to a chitin synthetase from Schizosaccharomyces pombe (46). While d-Ala-d-Ala dipeptidases have been traditionally associated with vancomycin-resistant enterococci (69), the PcgL protein is unlikely to be involved in resistance to this glycopeptide antibiotic because gram-negative bacteria are naturally resistant to vancomycin. The Salmonella and enterococcal d-Ala-d-Ala dipeptidases exhibit virtually identical substrate specificity in spite of the fact that they are only 40% identical at the amino acid level. On the other hand, these enzymes differ in their subcellular location: periplasmic for PcgL and cytoplasmic for the enterococcal enzyme. While the physiological role of the PcgL and UgtL proteins remains undetermined, these proteins could function in some aspect of peptidoglycan metabolism because d-Ala-d-Ala is produced only for its incorporation into the peptidoglycan and chitin is the yeast equivalent of bacterial peptidoglycan. For example, these proteins could participate in the peptidoglycan remodeling experienced by Salmonella during growth in host epithelial cells (67), an environment that promotes expression of PhoP-activated genes (4, 27, 44, 80).
VIRULENCE ATTENUATION ASSOCIATED WITH HYPERACTIVATION OF THE PHOP-PHOQ SYSTEM
One of the paradoxical virulence phenotypes associated with PhoP-PhoQ is that displayed by a strain harboring the pho-24 allele of phoQ, which overexpresses PhoP-activated proteins and further represses PhoP-repressed proteins and is as attenuated for mouse virulence as a phoP null mutant (59). Often referred to as PhoPc (for PhoP constitutive [59]), the pho-24 mutation is neither in the phoP gene nor constitutive. The pho-24 mutant harbors a single amino acid substitution in the periplasmic domain of PhoQ (28) and responds to Mg2+ virtually like wild-type Salmonella. On the other hand, its PhoQ protein has an altered set point for Ca2+, requiring eight times higher concentrations of this divalent cation to repress transcription to the levels achieved by wild-type PhoQ (28).
The pho-24 mutant is unable to invade nonphagocytic cells due to repression of transcription of the invasion gene regulator hilA (6), which controls expression of several genes within the SPI-1 pathogenicity island (8, 66). However, the pho-24 mutant is highly attenuated even when mice are inoculated intraperitoneally (i.e., when the invasion genes are not required), unable to replicate within macrophages in vitro, and defective in inducing spacious phagosome formation in macrophages (3).
The virulence attenuation exhibited by the pho-24 mutant has been attributed to disregulation of PhoP-controlled targets (59), which is consistent with increased phosphorylation of the PhoP protein promoted by membranes from a pho-24 mutant relative to that promoted by membranes prepared from wild-type Salmonella (37). While the mechanism by which hyperactivation of the PhoP-PhoQ system reduces virulence remains unknown, it appears to require the spv plasmid virulence genes because constitutive expression of the phoP gene from a heterologous promoter results in virulence attenuation only in strains harboring the spv genes (57).
EXPRESSION OF PHOP-REGULATED GENES DURING INFECTION: MG2+ LEVELS AS A MEANS OF TELLING INTRACELLULAR FROM EXTRACELLULAR ENVIRONMENTS
The majority of Salmonella promoters preferentially induced during infection of host cells are under transcriptional control of the PhoP-PhoQ system: 8 of 14 promoters recovered from macrophages by differential fluorescence induction [80] and 7 of 8 promoters isolated from the spleen and/or cultured macrophages by in vivo expression technology (44) were dependent on phoP for intracellular expression. The seven PhoP-dependent promoters recovered by in vivo expression technology are induced in vitro both by low pH and by low Mg2+ concentrations and are transcriptionally active in macrophages and epithelial cells (44). This indicates that the signals promoting expression of PhoP-activated genes are common to both cell types and argues against early proposals that PhoP-activated genes are expressed in macrophages but not in epithelial cells (4).
All PhoP-regulated genes mediating Salmonella virulence appear to have been acquired by horizontal gene transfer. We hypothesize that these genes have been put under PhoP-PhoQ control to insure that Salmonella expresses its virulence genes at the right time and in the right places (e.g., mgtC inside the macrophage phagosome). According to this hypothesis, Salmonella determines its subcellular location (at least in part) by examining the Mg2+ levels in its surroundings via the PhoQ protein: a low Mg2+ concentration is an indication of an intracellular environment (i.e., the phagosome), whereas a high Mg2+ concentration denotes an extracellular environment (30). (Ca2+ and Mn2+ also regulate the PhoP-PhoQ system in vitro, but the concentrations of these divalent cations are too low in host tissues to control PhoP-PhoQ in vivo.) This hypothesis is consistent with the expression pattern of PhoP-activated genes, which are transcriptionally induced when the Mg2+ concentration is low in vitro (28, 78) and inside host cells in vivo (4, 27, 43, 44, 80). According to this model, PhoP-repressed genes, such as the invasion determinant prgH, should be transcribed outside host cells, an environment where the concentrations of Mg2+ and Ca2+ are high, thereby eliciting entry into host cells.
VIRULENCE ROLE OF PHOP-PHOQ IN OTHER GRAM-NEGATIVE SPECIES
The PhoP-PhoQ system is required for virulence in several species that have very different lifestyles from that of Salmonella. For example, S. flexneri—the agent of bacillary dysentery—requires a functional phoP gene for full virulence (62) despite causing a different disease and normally residing in a different subcellular compartment than Salmonella (see reference 71 for a review on Shigella pathogenesis). A Shigella phoP mutant is hypersensitive to killing by neutrophils (62), the only cell type in which Shigella, like Salmonella, remains within a membrane bound vacuole (56) and does not escape to the cytosol by lysing the phagosomal membrane.
PhoP also controls virulence in Yersinia pestis, the etiologic agent of bubonic plague (see reference 12) for a review on Yersinia pathogenesis): a Yersinia phoP mutant has a mean lethal dose that is 75-fold higher than that of the isogenic wild-type strain (65). Even though Yersinia is normally present extracellularly, initially in the infection it undergoes an intracellular phase within phagocytic cells, where PhoP may be important because a Yersinia phoP mutant is defective for survival within macrophages and exhibits increased sensitivity to low pH, oxidative killing, and high osmolarity (65).
Thus, while Salmonella, Shigella, and Yersinia cause different disease conditions, inactivation of the phoP gene prevents proliferation within phagocytic cells of all three species, suggesting a role for these PhoP-PhoQ systems in survival within the phagosome. Surprisingly, PhoP-PhoQ also controls virulence functions in Erwinia carotovora supsb. carotovora, a plant pathogen that resides in the intercellular fluid and is not known to enter plant cells (see reference 2 for a review on Erwinia pathogenesis). Erwinia secretes a large number of cell wall-degrading enzymes that damage different plant tissues, and one of them is an endopolygalacturonase (designated PehA) that is transcriptionally regulated by the PhoP-PhoQ homologue PehR-PehS (23). Ca2+ controls the levels of the PehA protein in Erwinia and transcription of the pehA gene in E. coli, in both cases requiring a functional phoP gene. In addition, mutants of Erwinia defective in either pehR or pehS are attenuated for virulence on tobacco seedlings. This indicates that PhoP-PhoQ can control virulence even in pathogens that do not have an intracellular lifestyle.
CONCLUSIONS
The PhoP-PhoQ two-component system governs the adaptation to low Mg2+ environments and the response to other stress conditions by regulating expression of as much as 1% of the genes in certain gram-negative species. PhoP-PhoQ mediates its effects often indirectly: via activation of other regulatory systems such as the PmrA-PmrB two-component system. By controlling expression of many horizontally acquired virulence determinants, the PhoP-PhoQ system has become a major regulator of virulence in Salmonella.
ACKNOWLEDGMENTS
I thank S. Garlich for preparation of Fig. 1, M. Winfield for help with Table 1, and S. Chamnongpol and two anonymous reviewers for comments on the manuscript. I also thank present and past members of my laboratory who contributed to our present understanding of PhoP-PhoQ.
Research on the PhoP-PhoQ and PmrA-PmrB systems is supported by grants GM54900 and AI42236 from the NIH to E.A.G, who is an Associate Investigator of the Howard Hughes Medical Institute.
Footnotes
Dedicated to Philip Matsumura and Robert S. Munson, Jr., in appreciation of their mentorship.
REFERENCES
- 1.Aguirre A, Lejona S, Véscovi E G, Soncini F C. Phosphorylated PmrA interacts with the promoter region of ugd in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182:3874–3876. doi: 10.1128/jb.182.13.3874-3876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfano J R, Collmer A. Mechanisms of bacterial pathogenesis in plants: familiar foes in a foreign kingdom. In: Groisman E A, editor. Principles of bacterial pathogensis. New York, N.Y: Academic Press; 2001. pp. 180–211. [Google Scholar]
- 3.Alpuche-Aranda C M, Racoosin E L, Swanson J A, Miller S I. Salmonella stimulates macrophage macropinocytosis and persists within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpuche-Aranda C M, Swanson J A, Loomis W P, Miller S I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreu D, Rivas L. Animal antimicrobial peptides: an overview. Biopolymers. 1998;47:415–433. doi: 10.1002/(SICI)1097-0282(1998)47:6<415::AID-BIP2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 7.Bearson B L, Wilson L, Foster J W. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J Bacteriol. 1998;180:2409–2417. doi: 10.1128/jb.180.9.2409-2417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belden W J, Miller S I. Further characterization of the PhoP regulon: identification of new PhoP-activated virulence loci. Infect Immun. 1994;62:5095–5101. doi: 10.1128/iai.62.11.5095-5101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop R E, Gibbons H S, Guina T, Trent M S, Miller S I, Raetz C R. Transfer of palmitate from phospholipids to lipid A in outer membranes of Gram-negative bacteria. EMBO J. 2000;19:5071–5080. doi: 10.1093/emboj/cdd507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanc-Potard A-B, Groisman E A. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd A P, Cornelis G R. Yersinia. In: Groisman E A, editor. Principles of bacterial pathogenesis. New York, N.Y: Academic Press; 2001. pp. 228–253. [Google Scholar]
- 13.Buchmeier N, Blanc-Potard A, Ehrt S, Piddington D, Riley L, Groisman E A. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol Microbiol. 2000;35:1375–1382. doi: 10.1046/j.1365-2958.2000.01797.x. [DOI] [PubMed] [Google Scholar]
- 14.Castelli M E, García Véscovi E, Soncini F C. The phosphatases activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J Biol Chem. 2000;275:22948–22954. doi: 10.1074/jbc.M909335199. [DOI] [PubMed] [Google Scholar]
- 15.Chamnongpol S, Groisman E A. Acetyl-phosphate-dependent activation of a mutant PhoP response regulator that functions independently of its cognate sensor kinase. J Mol Biol. 2000;300:291–305. doi: 10.1006/jmbi.2000.3848. [DOI] [PubMed] [Google Scholar]
- 16.Cirillo D M, Valdivia R H, Monack D M, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 17.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 18.Ernst R K, Yi E C, Guo L, Lim K B, Burns J L, Hackett M, Miller S I. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 19.Fang F C, Krause M, Roudier C, Fierer J, Guiney D G. Growth regulation of a Salmonella plasmid gene essential for virulence. J Bacteriol. 1991;173:6783–6789. doi: 10.1128/jb.173.21.6783-6789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farley M M, Shafer W M, Spitznagel J K. Lipopolysaccharide structure determines ionic and hydrophobic binding of a cationic antimicrobial neutrophil granule protein. Infect Immun. 1988;56:1589–1592. doi: 10.1128/iai.56.6.1589-1592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 22.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flego D, Marits R, Eriksson A R, Koiv V, Karlsson M B, Heikinheimo R, Palva E T. A two-component regulatory system, pehR-pehS, controls endopolygalacturonase production and virulence in the plant pathogen Erwinia carotovora subsp. carotovora. Mol Plant Microbe Interact. 2000;13:430–438. doi: 10.1094/MPMI.2000.13.4.447. [DOI] [PubMed] [Google Scholar]
- 24.Flego D, Pirhonen M, Saarilahti H, Palva T K, Palva E T. Control of virulence gene expression by plant calcium in the phytopathogen Erwinia carotovora. Mol Microbiol. 1997;25:831–838. doi: 10.1111/j.1365-2958.1997.mmi501.x. [DOI] [PubMed] [Google Scholar]
- 25.Foster J W, Hall H K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990;172:771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galán J E, Curtiss R., 3d Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989;6:433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- 27.García-del Portillo F, Foster J W, Maguire M E, Finlay B B. Characterization of the micro-environment of Salmonella typhimurium-containing vacuoles within MDCK epithelial cells. Mol Microbiol. 1992;6:3289–3297. doi: 10.1111/j.1365-2958.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 28.García Véscovi E, Ayala M, Di Cera E, Groisman E A. Characterization of the bacterial sensor protein PhoQ. J Biol Chem. 1997;272:1440–1443. doi: 10.1074/jbc.272.3.1440. [DOI] [PubMed] [Google Scholar]
- 29.García Véscovi E, Soncini F C, Groisman E A. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 30.Groisman E A. The ins and outs of virulence gene expression: Mg2+ as a regulatory signal. Bioessays. 1998;20:96–101. doi: 10.1002/(SICI)1521-1878(199801)20:1<96::AID-BIES13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Groisman E A, Chiao E, Lipps C J, Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci USA. 1989;86:7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groisman E A, Heffron F, Solomon F. Molecular genetic analysis of the Escherichia coli phoP locus. J Bacteriol. 1992;174:486–491. doi: 10.1128/jb.174.2.486-491.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groisman E A, Kayser J, Soncini F C. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol. 1997;179:7040–7045. doi: 10.1128/jb.179.22.7040-7045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groisman E A, Parra C A, Salcedo M, Lipps C J, Heffron F. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11939–11943. doi: 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groisman E A, Saier M H, Jr, Ochman H. Horizontal transfer of a phosphatase gene as evidence for mosaic structure of the Salmonella genome. EMBO J. 1992;11:1309–1316. doi: 10.1002/j.1460-2075.1992.tb05175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guina T, Yi E C, Wang H, Hackett M, Miller S I. A PhoP-regulated outer membrane protease of Salmonella enterica serovar Typhimurium promotes resistance to alpha-helical antimicrobial peptides. J Bacteriol. 2000;182:4077–4086. doi: 10.1128/jb.182.14.4077-4086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunn J S, Hohmann E L, Miller S I. Transcriptional regulation of Salmonella virulence: a PhoQ periplasmic domain mutation results in increased net phosphotransfer to PhoP. J Bacteriol. 1996;178:6369–6373. doi: 10.1128/jb.178.21.6369-6373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunn J S, Lim K B, Krueger J, Kim K, Guo L, Hackett M, Miller S I. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol. 1998;27:1171–1182. doi: 10.1046/j.1365-2958.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 39.Gunn J S, Miller S I. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178:6857–6864. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunn J S, Ryan S S, Van Velkingurgh J C, Ernst R K, Miller S I. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2000;69:6139–6146. doi: 10.1128/iai.68.11.6139-6146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo L, Lim K B, Gunn J S, Bainbridge B, Darveau R P, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 42.Guo L, Lim K B, Poduje C M, Daniel M, Gunn J S, Hackett M, Miller S I. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95:189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 43.Heithoff D M, Conner C P, Hanna P C, Julio S M, Hentschel U, Mahan M J. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci USA. 1997;94:934–939. doi: 10.1073/pnas.94.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heithoff D M, Conner C P, Hentschel U, Govantes F, Hanna P C, Mahan M J. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol. 1999;181:799–807. doi: 10.1128/jb.181.3.799-807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hensel M, Shea J E, Waterman S R, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang F C, Holden D W. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 46.Hilbert F, García del Portillo F, Groisman E A. A periplasmic d-alanyl-d-alanine dipeptidase in the gram-negative bacterium Salmonella enterica. J Bacteriol. 1999;181:2158–2165. doi: 10.1128/jb.181.7.2158-2165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hohmann E L, Oletta C A, Killeen K P, Miller S I. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. I Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 48.Hsing W, Silhavy T J. Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor protein for porin osmoregulation in Escherichia coli. J Bacteriol. 1997;179:3729–3735. doi: 10.1128/jb.179.11.3729-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hulett F M. The signal-transduction network for Pho regulation in Bacillus subtilis. Mol Microbiol. 1996;19:933–939. doi: 10.1046/j.1365-2958.1996.421953.x. [DOI] [PubMed] [Google Scholar]
- 50.Kato A, Tanabe H, Utsumi R. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J Bacteriol. 1999;181:5516–5520. doi: 10.1128/jb.181.17.5516-5520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kier L D, Weppelman R M, Ames B N. Regulation of nonspecific acid phosphatase in Salmonella: phoN and phoP genes. J Bacteriol. 1979;138:155–161. doi: 10.1128/jb.138.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kox L F F, Wösten M M S M, Groisman E A. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 2000;19:1861–1872. doi: 10.1093/emboj/19.8.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libby S J, Adams L G, Ficht T A, Allen C, Whitford H A, Buchmeier N A, Bossie S, Guiney D G. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect Immun. 1997;65:1786–1792. doi: 10.1128/iai.65.5.1786-1792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacFarlane E L A, Kwasnicka A, Hancock R E W. Role of Pseudomonas aeruginosa PhoP-PhoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology. 2000;146:2543–2554. doi: 10.1099/00221287-146-10-2543. [DOI] [PubMed] [Google Scholar]
- 55.Macfarlane E L A, Kwasnicka A, Ochs M M, Hancock R E W. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol Microbiol. 1999;34:305–316. doi: 10.1046/j.1365-2958.1999.01600.x. [DOI] [PubMed] [Google Scholar]
- 56.Mandic-Mulec I, Weiss J, Zychlinsky A. Shigella flexneri is trapped in polymorphonuclear leukocyte vacuoles and efficiently killed. Infect Immun. 1997;65:110–115. doi: 10.1128/iai.65.1.110-115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsui H, Kawakami T, Ishikawa S, Danbara H, Gulig P A. Constitutively expressed phoP inhibits mouse virulence of Salmonella typhimurium in an Spv-dependent manner. Microbiol Immunol. 2000;44:447–454. doi: 10.1111/j.1348-0421.2000.tb02519.x. [DOI] [PubMed] [Google Scholar]
- 58.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller S I, Mekalanos J J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller S I, Pulkkinen W S, Selsted M E, Mekalanos J J. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect Immun. 1990;58:3706–3710. doi: 10.1128/iai.58.11.3706-3710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moncrief M B C, Maguire M E. Magnesium and the role of mgtC in growth of Salmonella typhimurium. Infect Immun. 1998;66:3802–3809. doi: 10.1128/iai.66.8.3802-3809.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moss J E, Fisher P E, Vick B, Groisman E A, Zychlinsky A. The regulatory protein PhoP controls the resolution of Shigella flexneri infections. Cell Microbiol. 2001;2:443–452. doi: 10.1046/j.1462-5822.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 63.Ochman H, Soncini F C, Solomon F, Groisman E A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oren A, Shai Y. Mode of action of linear amphipathic α-helical peptides. Biopolymers. 1998;47:451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 65.Oyston P C F, Dorrell N, Williams D, Li S-R, Green M, Titball R W, Wren B W. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect Immun. 2000;68:3419–3425. doi: 10.1128/iai.68.6.3419-3425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 67.Quintela J C, de Pedro M A, Zöllner P, Allmaier G, Garcia-del Portillo F. Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol Microbiol. 1997;23:693–704. doi: 10.1046/j.1365-2958.1997.2561621.x. [DOI] [PubMed] [Google Scholar]
- 68.Rather P N, Paradise M R, Parojcic M M, Patel S. A regulatory cascade involving AarG, a putative sensor kinase, controls the expression of the 2′-N-acetyltransferase and an intrinsic multiple antibiotic resistance (Mar) response in Providencia stuartii. Mol Microbiol. 1998;28:1345–1353. doi: 10.1046/j.1365-2958.1998.00900.x. [DOI] [PubMed] [Google Scholar]
- 69.Reynolds P E. Control of peptidoglycan synthesis in vancomycin-resistant enterococci: D,D-peptidases and D,D-carboxypeptidases. Cell Mol Life Sci. 1998;54:325–331. doi: 10.1007/s000180050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roland K L, Martin L E, Esther C R, Spitznagel J K. Spontaneous pmrA mutants of Salmonella typhimurium LT2 define a new two-component regulatory system with possible role in virulence. J Bacteriol. 1993;175:4154–4164. doi: 10.1128/jb.175.13.4154-4164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sansonetti P J, Egile C, Wennerås C. Shigellosis: from disease symptoms to molecular and cellular pathogenesis. In: Groisman E A, editor. Principles of bacterial pathogenesis. New York, N.Y: Academic Press; 2001. pp. 336–373. [Google Scholar]
- 72.Scherer C A, Miller S I. Molecular pathogenesis of salmonellae. In: Groisman E A, editor. Principles of bacterrial pathogenesis. New York, N.Y: Academic Press; 2001. pp. 266–316. [Google Scholar]
- 73.Shafer W M, Casey S G, Spitznagel J K. Lipid A and resistance of Salmonella typhimurium to antimicrobial granule proteins of human neutrophils. Infect Immun. 1984;43:834–838. doi: 10.1128/iai.43.3.834-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith R L, Kaczmarek M T, Kucharski L M, Maguire M E. Magnesium transport in Salmonella typhimurium: regulation of mgtA and mgtCB during invasion of epithelial and macrophage cells. Microbiology. 1998;144:1835–1843. doi: 10.1099/00221287-144-7-1835. [DOI] [PubMed] [Google Scholar]
- 75.Smith R L, Maguire M E. Microbial magnesium transport: unusual transporters searching for identity. Mol Microbiol. 1998;28:217–226. doi: 10.1046/j.1365-2958.1998.00810.x. [DOI] [PubMed] [Google Scholar]
- 76.Snavely M D, Miller C G, Maguire M E. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991;266:815–823. [PubMed] [Google Scholar]
- 77.Soncini F C, García Véscovi E, Groisman E A. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol. 1995;177:4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soncini F C, García Véscovi E, Solomon F, Groisman E A. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J Bacteriol. 1996;178:5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soncini F C, Groisman E A. Two-component regulatory systems can interact to process multiple environmental signals. J Bacteriol. 1996;178:6796–6801. doi: 10.1128/jb.178.23.6796-6801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valdivia R H, Falkow S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science. 1997;277:2007–2010. doi: 10.1126/science.277.5334.2007. [DOI] [PubMed] [Google Scholar]
- 81.van Velkinburgh J C, Gunn J S. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect Immun. 1999;67:1614–1622. doi: 10.1128/iai.67.4.1614-1622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waldburger C D, Sauer R T. Signal detection by PhoQ: characterization of the sensor domain and a response-impaired mutant that identifies ligand-binding determinants. J Biol Chem. 1996;271:26630–26636. doi: 10.1074/jbc.271.43.26630. [DOI] [PubMed] [Google Scholar]
- 83.Wanner B L. Signal transduction and cross regulation in the Escherichia coli phosphate regulon by PhoR, CreC, and acetyl phosphate. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 203–221. [Google Scholar]
- 84.Wick M J, Harding C V, Twesten N J, Normark S J, Pfeifer J D. The phoP locus influences processing and presentation of Salmonella typhimurium antigens by activated macrophages. Mol Microbiol. 1995;16:465–476. doi: 10.1111/j.1365-2958.1995.tb02411.x. [DOI] [PubMed] [Google Scholar]
- 85.Wösten M M S M, Groisman E A. Molecular characterization of the PmrA regulon. J Biol Chem. 1999;274:27185–27190. doi: 10.1074/jbc.274.38.27185. [DOI] [PubMed] [Google Scholar]
- 86.Wösten M M S M, Kox L F F, Chamnongpol S, Soncini F C, Groisman E A. A signal transduction system that responds to extracellular iron. Cell. 2000;103:113–125. doi: 10.1016/s0092-8674(00)00092-1. [DOI] [PubMed] [Google Scholar]