SUMMARY
Following an unusually heavy rainfall in June 2009, a community-wide outbreak of Campylobacter gastroenteritis occurred in a small Danish town. The outbreak investigation consisted of (1) a cohort study using an e-questionnaire of disease determinants, (2) microbiological study of stool samples, (3) serological study of blood samples from cases and asymptomatic members of case households, and (4) environmental analyses of the water distribution system. The questionnaire study identified 163 cases (respondent attack rate 16%). Results showed a significant dose-response relationship between consumption of tap water and risk of gastroenteritis. Campylobacter jejuni belonging to two related flaA types were isolated from stool samples. Serum antibody levels against Campylobacter were significantly higher in cases than in asymptomatic persons. Water samples were positive for coliform bacteria, and the likely mode of contamination was found to be surface water leaking into the drinking-water system. This geographically constrained outbreak presented an ideal opportunity to study the serological response in persons involved in a Campylobacter outbreak. The serology indicated that asymptomatic persons from the same household may have been exposed, during the outbreak period, to Campylobacter at doses that did not elicit symptoms or alternatively had been exposed to Campylobacter at a time prior to the outbreak, resulting in residual immunity and thus absence of clinical signs.
Key words: Campylobacter, cohort study, outbreak, serology, waterborne
INTRODUCTION
Campylobacter spp. are a common cause of acute human bacterial enteritis. Although generally perceived as foodborne infections, primarily from poultry meat and raw milk, other routes of transmission, including environmental exposures and animal contact, are recognized [1]. Outbreaks of Campylobacter gastroenteritis due to contamination of municipal drinking-water supply systems, in some cases affecting thousands of people, have been reported from several countries, including Denmark, Finland, Norway, and Sweden [2–7].
As for other intestinal infections, the true incidence of Campylobacter infections is believed to be several times higher than the reported incidence, because most cases do not seek medical attention due to short illness duration or mild or absent symptoms [8]. It has been proposed that the number of such cases can be estimated using serological tests, thus obtaining a better measure of the force of transmission in a population (i.e. ‘seroincidence’). In clinical medicine, serology is used for confirming recent Campylobacter infections in the differential diagnosis of patients with suspected Guillain–Barré syndrome or reactive arthritis [9–12]. The serum antibody response to acute Campylobacter infection consists of a sharp rise in immunoglobulin G (IgG), IgA and IgM as measured in an enzyme-linked immunosorbent assay (ELISA), followed by a rapid decrease of IgM and IgA over a period of ~6 weeks [13, 14]. An elevation of at least two of these antibody classes is often considered highly suggestive of recent infection.
In an outbreak situation, serology may be useful to more accurately determine who is infected and who is not, thus providing a better estimate of the true number of affected persons. By avoiding misclassification of asymptomatically infected persons (or persons not seeking medical consultation) as non-cases, serology may also improve the power of analytical epidemiological studies to identify the source of an outbreak.
In the week beginning 15 June 2009, general practitioners (GPs) in the town of Tune, ~30 km south of Copenhagen, noticed an unusual increase in patients with acute gastroenteritis. Campylobacter spp. were detected in stool cultures from some patients. Preliminary inquiries showed that affected persons apparently lived dispersed over the whole town and that they had not attended a common event. No increase of gastroenteritis cases was observed in neighbouring towns. On 11–12 June the area had been affected by an exceptionally heavy rainfall, causing a backflow of water from the common drainage system for rainwater and sewage into the streets. The suspicion of a waterborne gastroenteritis outbreak was raised, an outbreak investigation began and a tap-water boiling advice was issued on 20 June. In the following, we present the outbreak analysis, including a serological follow-up study of persons affected by this waterborne Campylobacter outbreak.
METHODS
Questionnaire survey
A cohort study was initiated using an online questionnaire with questions about signs and symptoms, onset date, duration of illness and exposure to potential sources of Campylobacter infection (e.g. consumption of unboiled tap water, poultry or raw milk, eating ready-to-eat meals from local shops, etc.). All residents of the town were invited to participate in the survey via announcements in the local newspaper, via a link on the municipality website and through informal messages from local key persons. Respondents were asked to provide their complete address (street name and house/apartment number) in order to perform the serological investigation at household level (see below).
Case definitions
A clinical case was defined as a person with diarrhoea (>3 loose stools in 24 h) or abdominal pain with either fever or vomiting, with symptom onset during the period 13–26 June 2009. A confirmed case was defined as a clinical case with a stool culture positive for Campylobacter spp. Asymptomatic persons were defined as persons without any gastrointestinal symptoms. Individuals with ambiguous symptoms (i.e. symptoms indicative of gastrointestinal illness but not fulfilling the case definition) and individuals reporting international travel between 8 and 19 June were excluded from the statistical analysis.
Gender and age distribution (in 10-year age groups 0–9, 10–19, 20–29 years, etc.) of clinical cases and asymptomatic persons were compared using the χ2 test.
Serological study
Among respondents to the questionnaire survey, we selected a sample of individuals who reported drinking tap water between 12 and 20 June and who were either clinical cases or asymptomatic persons living in the same household as a clinical case. These individuals were selected at household level, i.e. asymptomatic persons were only included if they shared a household with a clinical case. To include individuals from as wide a geographical area as possible, households from different areas of the town were selected. The selection at household level was made in order to account for the possibility that the water contamination was not homogenous across the town and between households. Within a single household we assumed that both cases and asymptomatic individuals had been drinking water with identical Campylobacter concentrations and were thus equally at risk of exposure. After informed consent, blood samples were collected from participants during home visits or at appointments with local GPs. Blood samples were drawn about 2, 4, 7, and 13 weeks after the presumed first exposure on 12 June.
Blood was centrifuged within 24 h and serum samples were stored at 4 °C until analysis. IgA, IgG and IgM against Campylobacter spp. was measured by an in-house-developed ELISA at Statens Serum Institut (SSI) as described previously [13].
Ethical permission for the serological study was given by the Committee for Ethics in Science of Copenhagen and Frederiksberg Municipality (reference no. 11-097/02).
In order to assess changes in log-transformed IgG, IgM and IgA optical density (OD) values over time, separate random-effects linear regression models were fitted for both asymptomatic and symptomatic individuals within each antibody class. The outcome variable of these models was log-transformed antibody titre and the explanatory variable was time since assumed exposure (on 12 June 2009) in weeks. The intercept and slope of the models for ill and asymptomatic individuals for each antibody class was compared using t tests (using the asymptomatic model as the reference). Statistical analyses were performed using Stata 14 software (StataCorp., USA) and SAS v. 9.4 (SAS Institute, USA).
Microbiological investigations
Patients consulting their GP with diarrhoea and all participants in the serological study were asked to submit two stool samples, unless they had already tested positive for Campylobacter. Stool samples were cultured for enteric pathogens including Campylobacter by standard methods at the Danish national reference laboratory at SSI. A subset of samples from patients consulting their GP was additionally tested for norovirus, sapovirus and rotavirus.
Isolates of Campylobacter were characterized by flaA typing according to Meinersmann et al. [15].
Environmental investigations
Water samples were repeatedly taken at the waterworks and at multiple points of the water pipe network following the suspected contamination event. Analysis of water samples as well as assessment of the technical condition of the water supply system and scenarios of a possible contamination of the drinking-water supply with sewage water were undertaken by a private civil engineering company commissioned by the municipality. The presence of bacteria, bacterial markers and faecal markers was analysed using quantitative polymerase chain reaction (qPCR) and plate counts.
RESULTS
Questionnaire survey
In 2009, the town of Tune had a population of 5052 persons (50·5% female) with an average age of 40 years. The questionnaire survey was completed by a total of 1039 individuals, response rate 21%, (61% females, 39% males) ranging from 1 to 86 years (mean age 42 years).
Of the 1039 respondees, 69 (7%) were excluded as they did not provide information on the presence or absence of gastrointestinal symptoms, 17 (2%) were excluded due to ambiguous symptoms as described above and 20 persons (2%) were excluded as they reported international travel between 8 and 19 June. Of the remaining 933 persons, 159 (15·3%) were classified as cases and 774 as non-cases, based on their reported symptoms and onset dates. The 159 cases were distributed in 119 households with one case, nine households with two cases, six households with three cases and one household with four cases. Dates for onset of gastroenteritis ranged from 11 to 30 June with a peak on 18–20 June (Fig. 1).
Fig. 1.
Persons with acute gastroenteritis by date of symptom onset, waterborne Campylobacter outbreak, Denmark, June 2009
The only exposure variable showing a statistically significant association with increased risk of illness was consumption of tap water. A significant dose-response relationship was observed with increasing attack rates in persons reporting higher amounts of tap-water consumption (Table 1).
Table 1.
Attack rate by reported amount of tap-water consumption, waterborne Campylobacter outbreak, Denmark, June 2009
| Daily tap-water consumption (in glasses of ~200 ml) | Cases (n) | Non-cases (n) | Attack rate (%) | Risk ratio |
|---|---|---|---|---|
| 0–1 | 5 | 68 | 6·9 | 1 (reference) |
| 2 | 9 | 113 | 7·4 | 1·08 |
| 3 | 25 | 131 | 16·0 | 2·34 |
| 4 | 34 | 145 | 19·0 | 2·77 |
| ⩾5 | 86 | 317 | 21·3 | 3·11 |
Mantel–Haenszel χ2 for linear trend = 18·80, P < 0·001
Serological study
A total of 67 individuals provided blood samples for IgG, IgM and IgA measurement of which 35 were clinical cases and 32 were asymptomatic. Twelve (34%) out of 35 clinical cases and 16 (50%) of asymptomatic persons were males which was not significantly different (P = 0·19). The age distribution of ill and asymptomatic persons also did not differ significantly.
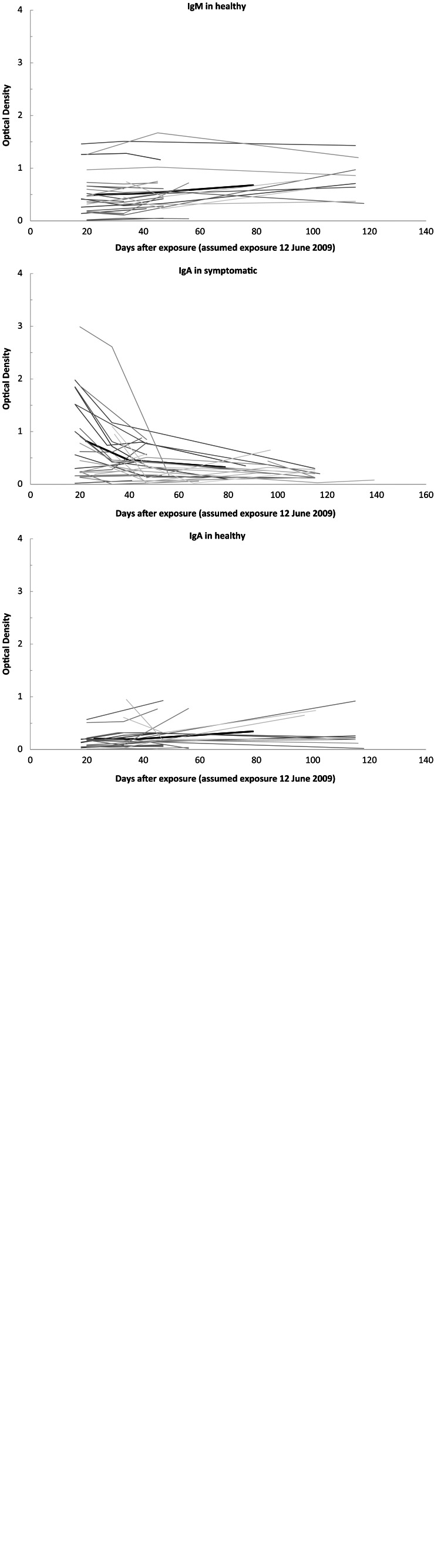
Campylobacter antibody levels following exposure to contaminated water showed IgG and IgA OD values which overall were 3–4 times higher for cases compared to asymptomatic persons, while IgM OD values did not differ significantly (Fig. 2, Table 2). Regarding antibody decay profiles, test for similar slopes showed a significantly faster IgG and IgA antibody decay profile in ill compared to asymptomatic individuals while the IgM antibody decay profile did not differ between the two groups (Fig. 2, Table 2).
Fig. 2.
Serum antibody levels against Campylobacter in persons exposed to tap water presumably contaminated with Campylobacter, Denmark, June 2009. Optical density (OD) (in arbitrary units). Bold lines indicate the mean OD value.
Table 2.
Modelling Campylobacter antibody decay profiles in persons exposed to tap water presumably contaminated with Campylobacter, Denmark, June 2009
| Antibody class | Variable | Clinical form | Coefficient | 95% CI | Regression P value | Comparison between ill and asymptomatic (P value)‡ |
|---|---|---|---|---|---|---|
| IgG | Intercept | Ill | 0·73 | 0·52–1·04 | 0·08 | <0·0001 |
| Asymptomatic | 0·20 | 0·13–0·32 | <0·0001 | |||
| Week | Ill | 1·01 | 0·92–1·11 | 0·76 | 0·03 | |
| Asymptomatic | 1·24 | 1·06–1·45 | 0·008 | |||
| IgA | Intercept | Ill | 0·38 | 0·24–0·57 | <0·0001 | <0·0001 |
| Asymptomatic | 0·08 | 0·05–1·30 | <0·0001 | |||
| Week | Ill | 0·80 | 0·69–0·92 | 0·003 | 0·001 | |
| Asymptomatic | 1·30 | 1·02–1·65 | 0·04 | |||
| IgM | Intercept | Ill | 0·51 | 0·37–0·71 | 0·0002 | 0·08 |
| Asymptomatic | 0·32 | 0·21–0·48 | <0·0001 | |||
| Week | Ill | 0·94 | 0·84–1·05 | 0·25 | 0·09 | |
| Asymptomatic | 1·13 | 0·94–1·37 | 0·19 |
CI, Confidence interval.
Comparing the values for intercept and slope between ill and asymptomatic persons within each antibody class (with asymptomatic as the reference).
Microbiological investigations
Stool samples were received from 100 patients consulting their GP with diarrhoea. Of these, 41 were culture-positive for Campylobacter jejuni. Cultures for other enteric bacteria were negative. Virological investigations detected sapovirus in two (10%) out of 20 samples tested, and one patient tested positive for both sapovirus and C. jejuni. Stool samples were available for 26 cases enrolled in the serological study and 10 (38%) of these were culture positive for Campylobacter. Of the 32 asymptomatic subjects enrolled in the serology study, 12 provided stool samples, of which only one was positive for Campylobacter.
Campylobacter isolates from 27 cases were characterized by flaA sequence analysis. Most isolates belonged to two distinct flaA types (13 and nine isolates, respectively) and the remaining five isolates showed <5% sequence difference from these two clusters, showing that the outbreak was caused by two Campylobacter strains with different but strongly related flaA sequences.
Environmental and technical investigations
The town's drinking water consisted of non-chlorinated groundwater drawn from several boreholes around the town and supplied by the local water plant. Testing of water samples collected on 21 and 26 June and 6, 9 and 10 July from more than 25 points of the water distribution system, including one of the groundwater boreholes, indicated contamination with coliform bacteria (measured by total coliform counts). In addition, following the first positive culture from human stool samples, ~300 water samples were tested specifically for Campylobacter by qPCR. These tests were negative.
During 20 June to 30 July, a boiling order was in place for the whole town and the inhabitants were supplied with drinking water from tank trucks. The technical assessment revealed a faulty installation adjacent to one of the boreholes which may have allowed a backflow of sewage water into the gravel surrounding the borehole, when the sewage system was congested due to the heavy rainfall on 12 June.
DISCUSSION
Here we present results from an investigation of a C. jejuni outbreak in a confined setting, with a particular focus on antibody development in cases and asymptomatic members of case households. The outbreak was most likely caused by drinking water as shown by several lines of evidence: First, the only exposure found to be associated with gastroenteritis in the cohort study was drinking tap water, with a marked dose-response relationship between amount of tap water consumed and the risk of gastroenteritis. Second, an exceptionally heavy rainfall occurred a few days before the outbreak, leading to a drinking-water contamination caused by congestion of the combined rainwater drainage and sewage system. A technical investigation of the water system established a likely scenario as to how sewage-contaminated rainwater could have seeped into one of the groundwater boreholes. Third, detection of coliform bacteria in drinking-water samples confirmed a contamination, even though Campylobacter could not be detected, possibly because the necessary large volume water samples were taken too late after the contamination.
Waterborne outbreaks in Denmark are rare [16], as indeed are Campylobacter outbreaks in general [17]. The infrequent occurrence of waterborne outbreaks in Denmark compared to neighbouring countries has been explained by the fact that drinking water in Denmark is almost exclusively provided as groundwater [16]. The only two other known major waterborne Campylobacter outbreaks in the country occurred in 1995–1996 [2] and in 2010 [7]. Both were traced back to local water distribution systems following point-source contamination events with single clones of C. jejuni leading to widespread illnesses in the local settings. The outbreak described here is in several ways similar, although it differs by being caused by two different clones of C. jejuni. Given that the drinking-water system was likely contaminated by surface water leaking in, it is not surprising to find several related clones of C. jejuni in this outbreak.
Adding serological analysis to outbreak investigations provides the opportunity to study exposure and immune response over time – although recent evidence suggests that, for Campylobacter, seroepidemiological results may sometimes be difficult to interpret. Since the outbreak occurred, we and others have conducted multi-country European seroepidemiological studies for Salmonella and Campylobacter. This has allowed us to measure the exposure rate in the population and thus for instance to compare infection levels between countries [18, 19]; something not feasible to do by comparing numbers of registered cases because of the large differences in surveillance systems both within and between European countries. For Salmonella, such results have been in line with what might be suspected [20, 21]. For Campylobacter, however, a different picture has emerged: the seroincidence is generally very high with much less pronounced variation between countries [22]. The longitudinal serological analysis performed in Denmark does not mirror the quadrupling of registered cases seen throughout the 1990s [23]. This has led to the hypothesis being put forward that Europeans (as indeed probably most populations in the developed world) are frequently (possibly on an almost annual basis) exposed to Campylobacter via a number of routes, including environmental. This will lead to some symptomatic, but also many asymptomatic infections, possibly causing a build-up of immunity to infections throughout life. High dose exposure (and likely also exposure to previously unencountered serovariants) will, however, still lead to symptomatic infection [22]. In other words, a simple correlation (a conversion factor) between seroincidence and registered cases of clinical illness does not exist.
In this situation, interpreting the results of our outbreak serostudy is not straightforward. In the outbreak, illness was shown to be associated with water consumption. In the survey, drinking tap water was very commonly reported for both cases and asymptomatic respondents. Thus, a priori, we might expect almost all participants in the study to seroconvert – irrespective of case status. We might even expect most participants to have been seropositive even before water exposure, since the level of Campylobacter seropositivity in the population is, as mentioned, generally high (which indeed may explain the findings of measurable Campylobacter antibody levels in almost all individuals sampled). However, it seems likely that the bacteria would not have been uniformly suspended in the water. Even in the questionnaire respondents who reported drinking ⩾1 litre tap water daily, the attack rate was a moderate 22%. A possible explanation for the results of our serological study is that only individuals unfortunate enough to have drunk the (most heavily) contaminated water, receiving a large dose of Campylobacter, developed symptoms and were most likely to seroconvert.
It is often hypothesized that, following massive exposure, a large number of individuals become infected (as documented by high antibody levels) but do not develop symptoms. We could not provide support for this hypothesis. In contrast, our findings indicate that asymptomatic persons have low, but measurable, antibody levels and were most likely asymptomatic either due to low levels of exposure or a combination of the low exposure and build-up of immunity from repeated previous exposure episodes. Further, our findings demonstrate that the use of seroepidemiology in an outbreak situation can provide additional understanding of the dynamics of the outbreak. We suggest that other researchers use outbreaks as ‘natural experiments’ to study antibody response in asymptomatic but exposed individuals, thereby gradually increasing the insight into advantages and limitations of seroepidemiology as a method to monitor bacterial gastrointestinal infections in the community.
In conclusion, we investigated one of the very few known waterborne Campylobacter outbreaks in Denmark, establishing source, agent and mode of contamination. Further, we explored the use of serology as a tool for investigating outbreaks or – in another perspective – using outbreaks to study the seroepidemiology of Campylobacter, highlighting serology as a potentially valuable method to gain a better understanding of the dynamics of the most frequently occurring bacterial enteric infection in Europe.
ACKNOWLEDGEMENTS
We are grateful for the enthusiasm and patience of all study participants, who provided blood and stool samples. We thank our colleagues Mette Malling, Mads Riise, Sabrina Bacci, and Katarina Widgren who helped with blood sample collection. We are also grateful to Katharina P. Olsen and her technical staff for culturing of stool samples.
This study was financially supported by European Centres for Disease Control and Prevention (ECDC), framework contract ECDC/09/032.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Evers EG et al. Campylobacter source attribution by exposure assessment. International Journal of Risk Assessment Management 2008; 8: 174–190. [Google Scholar]
- 2.Engberg J et al. Water-borne Campylobacter jejuni infection in a Danish town – a 6-week continuous source outbreak. Clinical Microbiology and Infection 1998; 4: 648–656. [DOI] [PubMed] [Google Scholar]
- 3.Kuusi M, et al. An outbreak of gastroenteritis from a non-chlorinated community water supply. Journal of Epidemiology and Community Health 2004; 58: 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuusi M, et al. A large outbreak of campylobacteriosis associated with a municipal water supply in Finland. Epidemiology and Infection 2005; 133: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin S, et al. A case-cohort study to investigate concomitant waterborne outbreaks of Campylobacter and gastroenteritis in Söderhamn, Sweden, 2002–3. Journal of Water Health 2006; 4: 417–24. [PubMed] [Google Scholar]
- 6.Jakopanec I, et al. A large waterborne outbreak of campylobacteriosis in Norway: the need to focus on distribution system safety. BMC Infectious Diseases 2008; 8: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubbels SM, et al. A waterborne outbreak with a single clone of Campylobacter jejuni in the Danish town of Køge in May 2010. Scandinavian Journal of Infectious Diseases 2012; 44: 586–594. [DOI] [PubMed] [Google Scholar]
- 8.Hardnett FP, et al. Epidemiological issues in study design and data analysis related to FoodNet activities. Clinical Infectious Diseases 2004; 38: S121–126. [DOI] [PubMed] [Google Scholar]
- 9.Ang CW, et al. Validation of an ELISA for the diagnosis of recent Campylobacter infections in Guillain-Barré and reactive arthritis patients. Clinical Microbiology and Infection 2007; 13: 915–922. [DOI] [PubMed] [Google Scholar]
- 10.Tam CC, et al. Guillain-Barré syndrome and preceding infection with Campylobacter, influenza and Epstein–Barr virus in the general practice research database. PLoS ONE 2007; 2: e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn KG, et al. Detection of antibodies to Campylobacter in humans using enzyme-linked immunosorbent assays: a review of the literature. Diagnostic Microbiology and Infectious Diseases 2012; 74: 113–118. [DOI] [PubMed] [Google Scholar]
- 12.Mortensen NP, et al. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes and Infection 2009; 11: 988–994. [DOI] [PubMed] [Google Scholar]
- 13.Strid MA, et al. Antibody responses to Campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clinical and Diagnostic Laboratory Immunology 2001; 8: 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor BV, et al. Sensitivity and specificity of serology in determining recent acute Campylobacter infection. Internal Medicine Journal 2004; 34: 250–258. [DOI] [PubMed] [Google Scholar]
- 15.Meinersmann RJ, et al. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. Journal of Clinical Microbiology 1997; 35: 2810–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzman-Herrador B, et al. Waterborne outbreaks in the Nordic countries, 1998 to 2012. Eurosurveillance 2015; 20: 1–10. [DOI] [PubMed] [Google Scholar]
- 17.Statens Serum Institut. EPI-NEWS No. 11, 2016 (http://www.ssi.dk/English/News/EPI-NEWS/2016/No11-2016.aspx).
- 18.Simonsen J, et al. Estimation of incidences of infectious diseases based on antibody measurements. Statistics in Medicine 2009; 28: 1882–1895. [DOI] [PubMed] [Google Scholar]
- 19.Mølbak K, et al. Seroincidence of human infections with nontyphoid Salmonella compared with data from public health surveillance and food animals in 13 European countries. Clinical Infectious Diseases 2014; 59: 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falkenhorst G et al. Serological cross-sectional studies on Salmonella incidence in eight European countries: no correlation with incidence of reported cases. BMC Public Health 2012; 16: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonsen J, et al. Sero-epidemiology as a tool to study the incidence of Salmonella infections in humans. Epidemiology and Infection 2008; 136: 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teunis PFM, et al. Campylobacter seroconversion rates in selected countries in the European Union. Epidemiology and Infection 2013; 141: 2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emborg HD, et al. Was the increase in culture-confirmed Campylobacter infections in Denmark during the 1990s a surveillance artefact? Eurosurveillance 2015; 20: pii = 30041. [DOI] [PubMed] [Google Scholar]