Abstract
Derivatives of the widely used laboratory strain Staphylococcus aureus NCTC8325, which are natural rsbU mutants, were shown to be unable to produce RsbU, a positive regulator of the alternative sigma factor ςB. The lack of RsbU prevented the heat-dependent production of ςB-controlled transcripts and resulted in reduced H2O2 and UV tolerance, enhanced alpha-hemolysin activity, and the inability to produce the alkaline shock protein Asp23. After 48 h of growth, rsbU mutant strains failed to accumulate staphyloxanthin, the major stationary-phase carotenoid. Transcription of Asp23 was found to be exclusively controlled by ςB, making it an excellent target for the study of ςB activity in S. aureus. Reporter gene experiments, using the firefly luciferase gene (luc+) fused to the ςB-dependent promoter(s) of asp23, revealed that ςB is almost inactive in 8325 derivatives. cis complementation of the 8325 derivative BB255 with the wild-type rsbU gene from strain COL produced the rsbU+ derivative GP268, a strain possessing a ςB activity profile comparable to that of the rsbU+ wild-type strain Newman. In GP268, the heat inducibility of ςB-dependent genes, Asp23 production, alpha-hemolysin activity, pigmentation, and susceptibility to H2O2 were restored to the levels observed in strain Newman, clearly demonstrating that RsbU is needed for activation of ςB in S. aureus.
Staphylococcus aureus is a major human pathogen causing a wide spectrum of diseases and able to survive under a variety of extreme conditions. In many bacteria, alternative sigma factors have been shown to be important for survival under extreme conditions by regulating the coordinate expression of stress response genes triggered by environmental as well as growth-dependent stimuli. As part of the RNA polymerase holoenzyme, the sigma subunits are responsible for the binding of the catalytic core to specific promoter regions and the initiation of transcription of downstream genes. Thus, sigma factors provide an elegant mechanism in eubacteria to ensure simultaneous transcription of a variety of genetically unlinked genes, provided all these genes share the critical promoter elements. The alternative sigma factor ςB of Bacillus subtilis has been shown to control the transcription of more than 100 genes in response to different stimuli such as heat, ethanol, or salt stress; acid shock; or glucose, oxygen, or phosphate starvation (for reviews see references 23 and 46). In B. subtilis, ςB activity itself is controlled posttranslationally by a multicomponent signal transduction pathway comprising eight regulatory proteins which—with the exception of Obg and RsbP—are coexpressed with the sigma factor as part of the same operon (3, 7, 24, 40, 44, 48, 50). One of these proteins, RsbU, a positive regulator of ςB, is essential for the activation of ςB during exponential growth after environmental stress (45, 48, 50). RsbU activity itself is controlled by the action of further Rsb proteins encoded by the operon (1, 19, 50).
An operon encoding four proteins, sharing strong primary amino acid similarity with RsbU, RsbV, RsbW, and ςB of B. subtilis, has been identified in S. aureus (27, 49). The putative S. aureus ςB was shown to act as a sigma factor initiating the transcription of sarC from the sar P3 promoter (17, 32). RsbW, on the other hand, was shown to be an anti-sigma factor, regulating ςB activity posttranslationally (32). ςB is activated upon heat shock in S. aureus strain MA13 (20) and controls the transcription of at least 30 genes encoding cytoplasmic proteins (21). Although ςB was shown to be involved in the heat and acid shock response of strain MA13, it had no apparent function in strain 8325-4, either in the heat shock response, starvation survival, or pathogenicity, in a mouse abscess model (10, 20).
A phenotypic comparison of genetically distinct wild-type S. aureus strains and their ΔrsbUVWsigB mutants revealed the mutants to be almost unpigmented and to be unable to produce the alkaline shock protein Asp23. Furthermore, the mutants showed increased alpha-hemolysin activity and were more susceptible to hydrogen peroxide (28, 33). Remarkably, the 8325 derivative BB255 showed essentially the same phenotype as ΔrsbUVWsigB mutants. This phenomenon was traced back to an 11-bp deletion in the 5′ part of the rsbU gene of strain BB255, generating a stop codon within a short distance downstream. This 11-bp deletion was also found in the 8325 derivatives 8325-4 and RN4220 (20, 28).
In this study, we demonstrate that 8325 derivatives are unable to produce the positive regulator RsbU. The lack of this protein results in dramatic changes in ςB activity compared to that in rsbU+ strains. cis complementation of the 8325 derivative BB255 with the rsbU+ allele from COL restored the ςB activity profile as well as the ςB-dependent phenotypic properties to the levels seen in the Newman strain.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. S. aureus was routinely grown in Luria-Bertani (LB) medium at 37°C and 200 rpm. Antibiotics were used at the following concentrations: chloramphenicol, 30 μg ml−1; erythromycin and tetracycline, 10 μg ml−1; ampicillin and kanamycin, 50 μg ml−1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotypea | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH10B | F− φ80dlacZΔM15 recA1 | Gibco, Gaithersburg, Md. |
| BL21(DE3) | F−ompT gal [dcm] [lon] hsdSB (rB− mB−), with DE3 | Novagen, Madison, Wis. |
| S. aureus | ||
| RN4220 | NCTC8325-4 r− m+ (restriction minus, modification plus) | 26 |
| BB255 | Essentially the same as NCTC8325 | 5 |
| 8325-4 | NCTC8325, cured of known prophages | 34 |
| RN6390 | Derivative of NCTC8325 that maintains its hemolytic pattern when propagated on sheep erythrocytes | 35 |
| COL | mec, high-Mcr clinical isolate, Tcr | 25 |
| Newman | Clinical isolate, high level of clumping factor (ATCC 25904) | 18 |
| IK181 | BB255 ΔrsbUVWsigB Emr | 28 |
| IK183 | COL ΔrsbUVWsigB Emr | 28 |
| IK184 | Newman ΔrsbUVWsigB Emr | 28 |
| GP268 | BB255 (rsbUVWsigB)+-tetL Tcr | This study |
| GP269 | 8325-4 (rsbUVWsigB)+-tetL Tcr | This study |
| MB25 | RN4220 asp23+ (asp23P::luc+)-pEC-ermB Emr | This study |
| MB32 | Newman asp23+ (asp23P::luc+)-pEC-ermB Emr | This study |
| MB33 | BB255 asp23+ (asp23P::luc+)-pEC-ermB Emr | This study |
| MB49 | BB255 (rsbUVWsigB)+-tetL asp23+ (asp23P::luc+)-pEC-ermB Tcr Emr | This study |
| MB61 | RN4220 asp23+ (asp23P::luc+)-pBT-tetL Tcr | This study |
| MB69 | Newman ΔrsbUVWsigB asp23+ (asp23P::luc+)-pBT-tetL Tcr Emr | This study |
| MB90 | BB255 ΔrsbUVWsigB asp23+ (asp23P::luc+)-pBT-tetL Tcr Emr | This study |
| Plasmids | ||
| pET-24b(+) | Kmr; expression vector | Novagen |
| pSP-luc+ | Apr; firefly luciferase casette vector | Promega |
| pBC SK+ | Cmr; cloning vector | Stratagene |
| pAW8 | Tcr; pAMα1 origin and tetL gene of pHY300PLK, ColE1 origin | A. Wada, unpublished data |
| pBT | Tcr; 1.6-kb PCR fragment of tetL gene of pHY300PLK into Alw26I-digested pBC SK(+) | This study |
| pEC1 | Apr; Emr; 1.45-kb ClaI ermB fragment of Tn551 in pUC18 | 9 |
| pIK6 | Apr; 6.6-kb PstI-EcoRI sigB fragment from strain 8325 in pUC18 | 27 |
| pPG11 | Apr; Tcr; 252-bp MluI-BstXI fragment of rsbU from strain COL replacing the corresponding region of the 6.6-kb PstI-EcoRI sigB fragment from strain 8325 in pUC19 | This study |
| pETasp23 | 510-bp PCR fragment of asp23 from strain 8325 in pET24b | This study |
| pETrsbUCOL | 1-kb PCR fragment of rsbU from strain COL in pET24b | This study |
| pETsigB | 770-bp PCR fragment of sigB from strain 8325 in pET24b | This study |
| pSPasp23P | 1.1-kb PCR fragment of asp23 promoter from strain COL in pSP-luc+ | This study |
| pECasp23P::luc+ | 2.7-kb KpnI-EcoRI asp23P::luc+ fragment of pSPasp23P in pEC1 | This study |
| pBTasp23P::luc+ | 2.7-kb KpnI-EcoRI asp23P::luc+ fragment of pSPasp23P in pBT | This study |
Abbreviations are as follows: Apr, ampicillin resistant; Cmr, chloramphenicol resistant; Emr, erythromycin resistant; Kmr, kanamycin resistant; Mcr, methicillin resistant; Tcr, tetracyclin resistant.
General methods.
All DNA manipulations, basic molecular methods, and handling of Escherichia coli were performed in accordance with standard protocols (39). Genetic manipulation of S. aureus was done as described earlier (27). S. aureus carotenoids were extracted and analyzed according to the methods of Marshall and Wilmoth (31) or Raisig and Sandmann (37). The general transducing phage 80α was used for transductions. Preliminary sequence data were obtained from The Institute for Genomic Research (TIGR) through the website (http://www.tigr.org).
Northern blot analyses.
For the heat shock experiments, isolation of total RNA and analysis of transcription were performed as described by Gertz et al. (20). The specific RNA probes for sigB and crtM were prepared by in vitro translation with T7 polymerase and the appropriate PCR fragments as the template. The PCR fragments were generated by using chromosomal DNA of S. aureus strain COL which was purified with the chromosomal DNA isolation kit (Promega) according to the protocol of the manufacturers and oligonucleotides SasigB+ (5′-AAATAATGGCGAAAGAGTCG-3′) and SasigB(T7)− (5′-CTAATACGACTCACTATAGGGAGACATAATGGTCATCTTGTTGC-3′) (corresponding to nucleotides 2669 to 2688 and 3228 to 3248, respectively, of GenBank accession no. Y07645) and SacrtM+ (5′-CAGAAGATCAAAGAAAGCG-3′) and SacrtM(T7)− (5′-CTAATACGACTCACTATAGGGAGCCTGTCTCAACTTCGTCC-3′) (nucleotides 317 to 335 and 985 to 1002, respectively, of accession no. X73889). Nucleotides corresponding to the T7 promoter consensus are underlined. The hybridizations specific for asp23 were conducted with digoxigenin-labeled RNA as described previously (20).
For all other Northern blot analyses, total RNA was isolated as described by Cheung et al. (13). Eight micrograms of total RNA of each sample was electrophoresed through a 1.5% agarose–0.66 M formaldehyde gel in morpholinepropanesulfonic acid (MOPS) running buffer (20 mM MOPS, 10 mM sodium acetate, 2 mM EDTA [pH 7]). RNA was blotted onto a positively charged nylon membrane (Roche, Basel, Switzerland) with a vacuum blotter (Pharmacia, Uppsala, Sweden). The intensities of the 23S and 16S rRNA bands stained with ethidium bromide were verified to be equivalent in all the samples before transfer. Labeling and hybridization were done by use of the digoxigenin labeling and detection kits according to the manufacturer's instructions (Roche). The following specific primers were used to generate the digoxigenin-labeled DNA probes by PCR labeling: Saasp23A+ (5′-ATGACTGTAGATAACAATAAAGC-3′) and Saasp23A− (5′-TTGTAAACCTTGTCTTTCTTGG-3′) (nucleotides 343 to 365 and 828 to 849, respectively, of accession no. S76213) and luc int+ (5′-GGAGAGCAACTGCATAAGGC-3′) and luc int− (5′-GGCGAAGAAGGAGAATAGG-3′) (nucleotides 111 to 130 and 914 to 932, respectively, of accession no. U47122).
Construction of plasmid pPG11.
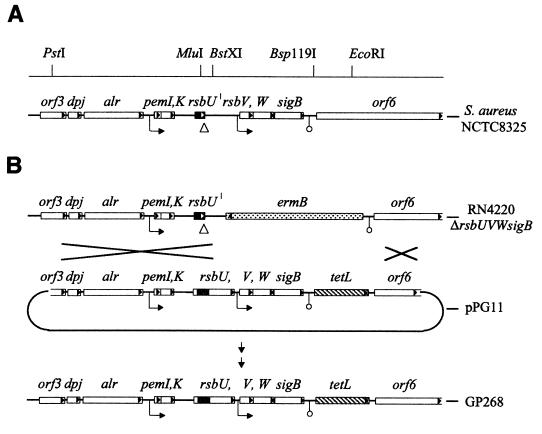
A 6.6-kb PstI-EcoRI fragment of strain BB255, including the whole sigB operon (27), was subcloned into the MCS of pUC19. The plasmid obtained was digested with MluI and BstXI, excising a 252-bp fragment from the rsbU gene including the 11-bp deletion. The excised fragment was replaced by the corresponding fragment of the rsbU+ allele from COL. In a next step, a 1.6-kb PCR fragment of the tetL gene of pAW8 was cloned into a blunted Bsp119I site downstream of the sigB operon (corresponding to positions 3545 to 3550 of the sigB operon of strain 8325, accession no. Y07645). The resulting plasmid was electroporated into S. aureus RN4220 ΔrsbUVWsigB to promote a crossover upstream of rsbU, and screening for double-crossover transformants sensitive to erythromycin and resistant to tetracycline was carried out (Fig. 1). In a last step, the engineered chromosomal region of a positive transformant was transduced into different 8325 derivatives.
FIG. 1.
Genetic organization of the sigB operon. (A) Schematic representation of the sigB operon of S. aureus strain NCTC8325. Open reading frames, putative promoters (→), termination signals (○), and restriction sites used for construction of pPG11 are indicated. The 11-bp deletion within the rsbU gene of strain 8325, resulting in a truncated open reading frame for RsbU (solid area), is indicated by a triangle (▵). (B) Schematic representation of the rsbU+ construct pPG11 and of the strategy for the integration of this construct into the chromosome of S. aureus BB255. In plasmid pPG11, a 252-bp MluI-BstXI restriction fragment of the rsbU gene of strain COL including the 11 bp (shaded area) replaces the corresponding fragment of the rsbU allele from strain BB255 harboring the 11-bp deletion, leading to an open reading frame that encodes a functional RsbU protein. A tetL resistance gene was introduced as a selective marker downstream of the proposed termination signal of the sigB operon, in order not to disrupt the transcriptional control of this locus. Strain RN4220 ΔrsbUVWsigB, in which the major part of the sigB operon is replaced by an ermB resistance cassette (28), was used for electroporation to promote a double crossover of the modified sigB operon of the introduced pPG11 suicide plasmid upstream of the rsbU gene and downstream of the tetR gene. The chromosomal region of a positive transformant was phage transduced into strain BB255 to obtain strain GP268.
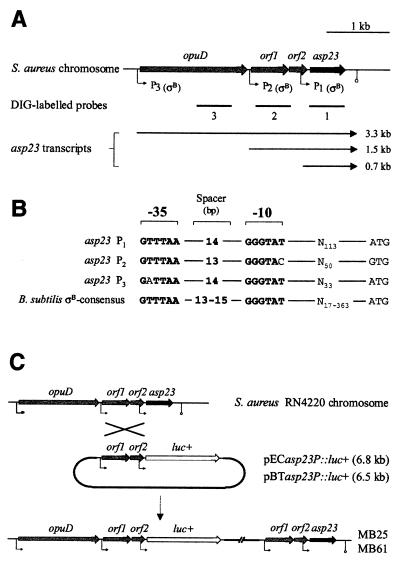
Construction of plasmids pECasp23P::luc+ and pBTasp23P::luc+.
A DNA fragment carrying 1.1 kb of the asp23 gene, including its ςB-dependent promoters, was generated by PCR with primers Saasp23P+ (5′-GGGATCCTTTGAGTGAGGAGAAACC-3′) including a KpnI linker (underlined), and Saasp23P− (5′-CTACAGCCATGGTAGATTCTCCTTTTAC-3′) including an NcoI linker (underlined). The PCR product was digested with KpnI and NcoI and cloned in front of the luciferase gene of plasmid pSP-luc+. The identity of the construct was confirmed by sequence analysis and comparison to the respective COL sequence of the TIGR database. The 2.7-kb KpnI-EcoRI fragment, including the asp23 promoter region fused to the luciferase coding region, was then cloned into plasmids pEC1 and pBT, respectively. The plasmids obtained were electroporated into RN4220 and subsequently transduced into strains BB255, Newman, and GP268 (pECasp23P::luc+) and their respective ΔrsbUVWsigB mutants (pBTasp23P::luc+) (Fig. 2C).
FIG. 2.
Genetic organization of the asp23 operon of S. aureus. (A) Schematic representation of the asp23 operon of S. aureus based on a comparison of the respective sequence region of strain COL, obtained from the unfinished TIGR microbial database. The probes used for Northern blot analyses, open reading frames, putative promoters, and the transcripts detected are indicated. (B) Putative promoter sequences of the asp23 locus. Nucleotides of the −35 and −10 regions of the putative promoters of the asp23 locus which are identical to the ςB-dependent promoter consensus of B. subtilis are boldfaced. Spacer regions between the −35 and −10 hexameric nucleotide sequences, and between the promoter sequence and the proposed start codons of the closest open reading frames, are indicated. (C) Schematic representation of the integration of asp23P::luc+ fusion constructs into the S. aureus chromosome by single crossover. For construction of plasmids pECasp23P::luc+ and pBTasp23P::luc+, and integration of the constructs into the S. aureus chromosome, see Materials and Methods.
Construction of E. coli vectors for overexpression of His-tagged RsbU, ςB, and Asp23.
A DNA fragment carrying 999 bp of the rsbUCOL gene was amplified by PCR using primers SarsbU1+ including an NdeI linker (underlined) (5′-GGAGATATACATATGGAAGAATTTAAGCAAC-3′ [the start methionine shown in boldface type]) and SarsbU1− including an XhoI linker (underlined) (5′-GGTGGTGCTCATTTACTCTTTTTATAATC-3′) (italics correspond to positions 785 to 772 and 1764 to 1782, respectively, in accession no. Y09929). The PCR product was cloned into pET24b to obtain pETrsbUCOL. Similarly, the sigB gene and the asp23 gene were amplified by PCR using, respectively, primer SasigB1+ including an NdeI linker (underlined) (5′-GGAGATATACATATGGCGAAAGAGTCGAAATCAGC-3′) combined with primer SasigB1− including an XhoI linker (underlined) (5′-GTGGTGCTCGAGTTGATGTGCTGCTTCTTG-3′) (italics correspond to positions 2674 to 2696 and 3424 to 3441, respectively, in accession no. Y07645) and primer Saasp2323+ (5′-GGAGATATACATATGACTGTAGATAACAATAAAGC-3′) combined with primer Saasp23− (5′-GGTGGTGCTCGAGTTGTAAACCTTGTCTTTCTTGG-3′) (italics correspond to positions 343 to 365 and 828 to 849, respectively in accession no. S76213). The PCR products were cloned into pET24b to obtain pETsigB or pETasp23, respectively. The junction regions and the introduced PCR products were sequenced to ensure proper ligation and fidelity of the PCR. E. coli strain BL21(DE3) was transformed with the plasmids obtained. Overexpression and purification of the His-tagged proteins were performed using Ni-nitriloacetic acid (NTA) columns according to the recommendations of the manufacturer (Qiagen, Basel, Switzerland). The purified proteins were separated using sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (SDS–12% PAGE), and bands containing the protein were cut out of the gels. N-terminal sequencing confirmed the identities of the desired proteins. The gel slices containing the respective proteins were injected into rabbits to raise anti-RsbU, anti-SigB, and anti-Asp23 polyclonal antibodies (BioScience, Göttingen, Germany). The resulting antisera were purified against the immobilized antigens.
Hydrogen peroxide experiments.
The MICs and minimal bactericidal concentrations (MBCs) of hydrogen peroxide were determined by broth microdilution using the National Committee for Clinical Laboratory Standards protocol with serial dilutions of hydrogen peroxide (2.2 M to 0.125 mM). Microtiter plates were incubated for 24 and 48 h at 37°C.
Luciferase assay.
Bacterial cells were harvested by centrifugation (at 11,000 × g for 1 min. at room temperature), and the cell pellet was resuspended in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4 [pH 7.3]) to an optical density at 600 nm (OD600) of 10 and snap-frozen in liquid nitrogen. Luciferase activity was determined by rapidly mixing PBS-resuspended cells (10 μl) with an equal volume of luciferase assay reagent (Promega, Madison, Wis.). Luminescence was measured on a Turner Designs TD-20/20 Luminometer (Promega) for a period of 10 s with a delay of 2 s.
UV-stress experiments.
Bacterial cells were diluted to McFarland 0.5 and streaked out on LB agar plates. After plating, cells were immediately exposed to far-UV light (254 nm) or near-UV light (312 nm) for different time periods, using a Stratalinker (Stratagene, La Jolla, Calif.) as the light source. The bacteria were then incubated for 24 h at 37°C.
RESULTS
Occurrence of RsbU and ςB in different S. aureus strains.
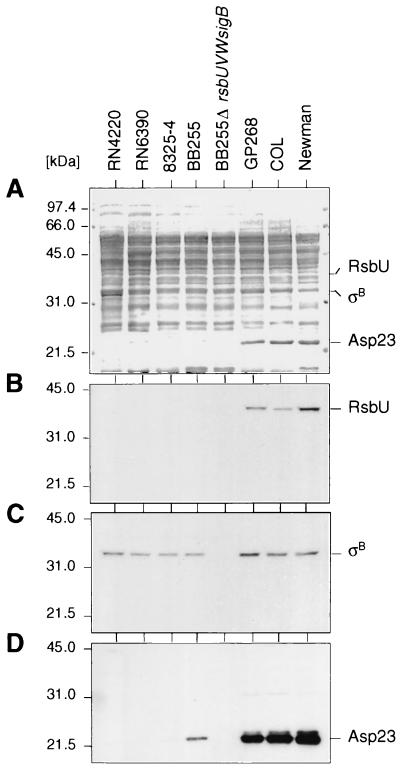
The rsbU gene in S. aureus strain COL encodes a 323-amino-acid open reading frame, while a deletion in the 5′ region of rsbU in strain 8325 generates a premature stop codon, giving rise to an open reading frame of only 74 amino acids. The same deletion was found in all 8325 derivatives tested (BB255, 8325-4, RN4220, RN6390, and BB270) (20, 28). Western blot analyses using antigen-purified polyclonal antibodies revealed the presence of RsbU in the clinical isolates COL and Newman but not in the 8325 derivatives (Fig. 3B), while ςB was detectable in all strains analyzed except BB255 ΔrsbUVWsigB (Fig. 3C).
FIG. 3.
Western blot analyses of different S. aureus strains. Cytoplasmic protein fractions (10 μg/lane) of different S. aureus overnight cultures, grown in LB medium at 37°C and 200 rpm, were separated using SDS–10% PAGE and blotted onto nitrocellulose. The blotted proteins were either stained with amido black (A) or subjected to Western blot analyses using antigen-purified anti-RsbU antibodies (B), anti-SigB antibodies (C), or anti-Asp23 antibodies (D). The broad-range molecular size marker (Gibco-BRL) was used. Relevant protein signals are indicated.
Fifteen independent clinical isolates of S. aureus, selected at the University Hospital of Zürich in 1999, were tested for the presence of the deletion in rsbU by PCR using oligonucleotides flanking the deletion site (28). None of the clinical isolates tested had such a deletion. The presence of RsbU in all clinical isolates was demonstrated by Western blot analysis (data not shown). These results indicated (i) the general presence of RsbU in clinical isolates and (ii) that 8325 derivatives are unable to produce the potential ςB activator RsbU. Furthermore, they excluded the possibility that an RsbU protein, truncated at its N terminus, is translated by use of a cryptic start codon downstream of the deletion. Strain 8325 derivatives are therefore natural rsbU mutants.
Complementation of strain BB255 with rsbUCOL.
The finding that 8325 derivatives are devoid of RsbU and the fact that most studies on ςB have been conducted with these strains prompted us to investigate the effects of RsbU on the phenotype of S. aureus in the 8325 isogenic background by replacing the truncated rsbU gene of BB255 with the intact rsbU+ allele from strain COL. For this purpose, we constructed the suicide plasmid pPG11, harboring a 6.6-kb chromosomal region including the sigB operon of strain BB255 and the rsbU gene of strain COL. In order not to disrupt the transcriptional integrity of the sigB operon, the tetL gene was inserted as a selective marker downstream of the operon (Fig. 1). To promote crossover events upstream of the rsbU region, we used RN4220 ΔrsbUVWsigB mutants for electroporation and selected for transformants that were resistant to tetracycline but sensitive to erythromycin, the selective marker that replaced the sigB operon in the RN4220 derivative (28). Transformants possessing these resistance characteristics should have undergone a double crossover, thereby replacing the ΔrsbUVWsigB deletion region through the sigB operon including the rsbU gene from COL (Fig. 1B). The corresponding chromosomal region of such a transformant was then transduced into 8325 derivatives to obtain the respective tetracycline-resistant rsbU+ derivatives. Transductants were analyzed by Southern hybridization for correct integration and loss of the suicide vector (data not shown). Strain GP268 was thus generated and characterized (see below). As a final proof for correct construction, the chromosomal region of the sigB operon was further phage transduced from GP268 into the natural rsbU+ strain Newman. The phenotypes of the resulting transductants, harboring the chromosomal region of the sigB operon of GP268, and that of the Newman strain were found to be identical (data not shown), confirming that all manipulations had occurred as intended.
Growth of S. aureus strains.
Different S. aureus strains and their respective ΔrsbUVWsigB mutants were analyzed for their stationary-phase cell densities, measured as the OD600. The wild-type strains COL and Newman were found to reach significantly higher OD600 values than their respective ΔrsbUVWsigB mutants, while strain BB255 reached a cell density that was indistinguishable from that of its ΔrsbUVWsigB mutant (Table 2). In contrast, the rsbU+ derivative GP268 reached a cell density that was clearly higher than that of the corresponding strain BB255 or the respective ΔrsbUVWsigB mutant. The ratio between the cell densities of GP268 and BB255 was comparable to those found for the other two rsbU+ strains and their ΔrsbUVWsigB mutants.
TABLE 2.
Cell densities of different S. aureus strainsa
| Strain | OD600b |
|---|---|
| GP268 | 7.97 ± 0.235 |
| BB255 | 6.33 ± 0.225 |
| BB255 ΔrsbUVWsigB | 6.18 ± 0.19 |
| Newman | 8.89 ± 0.16 |
| Newman ΔrsbUVWsigB | 6.52 ± 0.135 |
| COL | 8.65 ± 0.25 |
| COL ΔrsbUVWsigB | 6.99 ± 0.235 |
Strains were grown for 48 h at 37°C and 200 rpm. Cell densities were measured photometrically as OD600.
The OD600 values of rsbU+ strains are boldfaced. Values shown are results of four independent assays.
Increased H2O2 tolerance conferred by RsbU.
Kullik et al. (28) reported the MBC of H2O2 to be four times higher than the MIC in strains COL and Newman, whereas for their ΔrsbUVWsigB mutants as well as for BB255, the MICs and MBCs were found to be identical. Consistent with the data for the rsbU+ strain Newman, we demonstrate here that the MBC of H2O2 for GP268 is four times higher than the MIC (Table 3).
TABLE 3.
Susceptibilities of different S. aureus strains to hydrogen peroxide
| Strain | MIC (mM) | MBC (mM) |
|---|---|---|
| GP268 | 0.5 | 2 |
| BB255 | 0.5 | 0.5 |
| BB255 ΔrsbUVWsigB | 0.5 | 0.5 |
| Newman | 0.5 | 2 |
| Newman ΔrsbUVWsigB | 0.5 | 0.5 |
Alpha-hemolysin activity is negatively correlated to ςB activity.
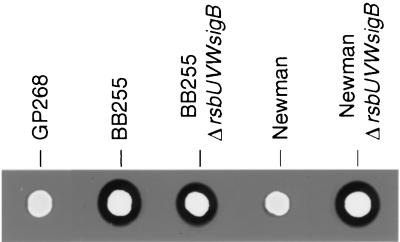
It has been shown previously that ΔrsbUVWsigB mutants possess higher alpha-hemolysin activities than their respective wild-type mutants (15, 33). Alpha-hemolysin activities were analyzed here by examining the lysed zones around spotted colonies grown on horse blood agar (Fig. 4). The ΔrsbUVWsigB mutants as well as strain BB255 produced clearly visible zones of hemolysis. In contrast, the rsbU+ strains Newman and GP268 showed almost no lytic zones. The lytic zones of BB255 and its respective ΔrsbUVWsigB mutant were indistinguishable.
FIG. 4.
Alpha-hemolysin activities of different S. aureus strains. Cells of different S. aureus strains (3 μl of McFarland 0.5 dilutions) were spotted on horse blood agar plates and incubated for 24 h at 37°C. The resulting colonies were scanned and analyzed for their surrounding lytic zones.
Production of Asp23.
The alkaline shock protein Asp23, a 169-amino-acid polypeptide of still unknown function, is known to be highly inducible in S. aureus strains 912 and MA13 by a pH upshift to pH 10 (20, 29). It was, however, neither detectable nor inducible in strain 8325-4 (20). Asp23 was found to be highly abundant in the cytoplasmic fraction of stationary-phase protein extracts of strains COL and Newman, while it was missing in their respective ΔrsbUVWsigB mutants as well as in the 8325 derivatives (20, 28). A ςB-dependent promoter motif has been proposed (20, 28) and recently confirmed (32) upstream of the asp23 open reading frame. Northern blot analysis suggested asp23 expression to be highly dependent on the alternative stress sigma factor ςB (20). Here we present further evidence for asp23 being under the sole control of ςB in S. aureus.
Kuroda et al. (29) reported 0.7- and 1.5-kb asp23 transcripts. Our Northern blot experiments demonstrated that sequences hybridizing to the asp23 probes can be detected on three different RNAs, including a 3.3-kb transcript that was not previously detected. This longer RNA includes an open reading frame with strong homology to OpuD of B. subtilis. Transcription of the asp23 locus (Fig. 5) was analyzed by use of three different DNA probes (as indicated in Fig. 2A; data for probe 2 and 3 not shown). The 0.7- and 1.5-kb transcripts were found to be highly abundant, and all three transcripts were heat inducible in strains Newman and GP268, while they were only weakly expressed and not heat inducible in strain BB255 and were not detectable at all in the ΔrsbUVWsigB mutants of BB255 and Newman (Fig. 5 and 8A). Western blot analysis with anti-Asp23 antibodies confirmed that Asp23 was highly abundant in strains COL, Newman, and GP268, less abundant in strain BB255, and undetectable in the ΔrsbUVWsigB mutant (Fig. 3D).
FIG. 5.
Northern blot analyses of the asp23 operon. (A) Growth curves of the S. aureus strains investigated. Solid squares, BB255; solid circles, IK181 (BB255 ΔrsbUVWsigB); solid triangles, GP268 (BB255 rsbU+); open squares, MB33 (BB255 asp23P::luc+); open circles, MB90 (BB255 ΔrsbUVWsigB asp23P::luc+); open triangles, MB49 (BB255 rsbU+ asp23P::luc+). Time points of sampling are indicated. (B) Total RNAs (8 μg/lane) of S. aureus strains BB255 (lanes 1 to 3), IK181 (BB255 ΔrsbUVWsigB) (lanes 7 to 9), GP268 (BB255 rsbU+) (lanes 13 to 15), MB33 (BB255 asp23P::luc+) (lanes 4 to 6), MB90 (BB255 ΔrsbUVWsigB asp23P::luc+) (lanes 10 to 12), and MB49 (BB255 rsbU+ asp23P::luc+) (lanes 16 to 18), obtained from cells grown in LB medium at 37°C and harvested 1 h (lanes 1, 4, 7, 10, 13, and 16), 3 h (lanes 2, 5, 8, 11, 14, and 17), and 5 h (lanes 3, 6, 9, 12, 15, and 18) after inoculation of the medium with log-phase cells, were blotted onto a positively charged nylon membrane and subjected to Northern blot analysis. The blotted membranes were hybridized using a digoxigenin-labeled DNA probe specific for asp23 (for construction, see Materials and Methods). The RNA molecular size marker I (Roche) was used. Positions of the 16S and 23S rRNAs are indicated by diamonds (⧫) on the left, and relevant transcript signals are indicated on the right.
FIG. 8.
Heat shock induction of ςB-dependent transcripts in S. aureus. Total RNA was isolated from S. aureus GP268 (BB255 rsbU+) (lanes 1 to 7) and S. aureus BB255 (lanes 9 to 15) grown at 37°C (lanes 1, 2, 9, and 10) and 1 min (lanes 3 and 11), 3 min (lanes 4 and 12), 6 min (lanes 5 and 13), 9 min (lanes 6 and 14), and 12 min (lanes 7 and 15) after shifting the cultures to 48°C. The RNAs (15 μg/lane) were blotted onto positively charged nylon membranes and subjected to Northern blot analyses. The blotted membranes were hybridized using digoxigenin-labeled RNA probes specific for asp23 (A), crtM (B), and sigB (C) (for construction see Materials and Methods). A digoxigenin-labeled RNA size marker (lane 8) (Roche) was used as a standard. Relevant transcript signals are given on the left.
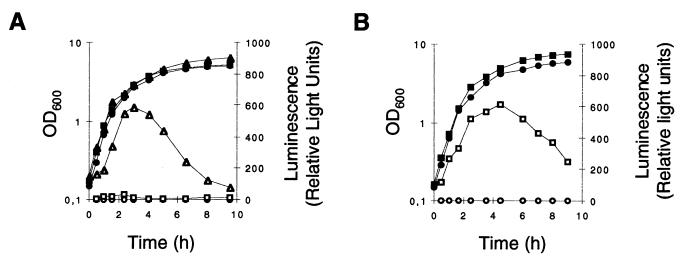
The abundance of asp23 transcripts and their sole dependence on ςB makes the asp23 promoter(s) an ideal candidate for studying ςB activity in S. aureus. We therefore fused the promoter region of asp23 to the firefly luciferase gene (luc+) and integrated the construct into the chromosome (Fig. 2C). Except for the missing 3.3-kb transcript due to the chromosomal integration of asp23P::luc+, transcription was found to be similar to that of the original chromosomal region as demonstrated by Northern blotting (Fig. 5). The ςB activity determined indirectly by the use of the luciferase reporter system confirmed that ςB was almost inactive in strain BB255, while in strain GP268 the ςB activity profile was comparable to that found in strain Newman (Fig. 6). The ςB activity profiles of the above five strains were confirmed by the use of further luciferase fusions to the promoter of csb7, another ςB-controlled gene (21). While luciferase activities derived from csb7P::luc+ strains were found to be 10-fold lower compared to the asp23P::luc+ data, relative intensities in the different strains were essentially identical (data not shown).
FIG. 6.
ςB activity during growth of S. aureus. The expression of asp23P::luc+ during growth of S. aureus strain BB255 (A) and strain Newman (B), grown in LB medium at 37°C, is shown. Bacterial growth was measured as the OD600 (solid symbols). ςB transcriptional activity was determined by measuring the luciferase activity of Luc+ (open symbols), the product of the luc+ reporter gene fused to the ςB-dependent promoters of asp23 (asp23p). (A) Squares, S. aureus strain MB33 (BB255 asp23P::luc+); circles, strain MB90 (BB255 ΔrsbUVWsigB asp23P::luc+); triangles, strain MB49 (BB255 rsbU+ asp23P::luc+). (B) Squares, S. aureus strain MB32 (Newman asp23P::luc+); circles, strain MB69 (Newman ΔrsbUVWsigB asp23P::luc+).
Pigmentation.
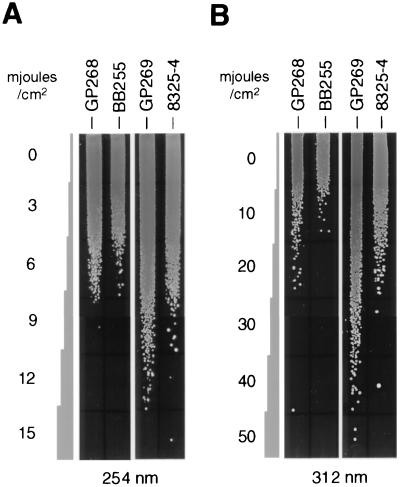
A characteristic feature of many S. aureus strains is the increase in pigmentation, with cells turning bright orange from pale yellow when incubated for 48 h at 37°C. This phenomenon has also been observed in COL, Newman, and MA13 but did not occur in 8325 derivatives, which kept their pale-yellow pigmentation even after 96 h of incubation at 37°C. Although pigment production by S. aureus has been described as a rather unstable characteristic (47), it has clearly been demonstrated by Kullik et al. (28) that the orange pigmentation of S. aureus is influenced by ςB. They showed that sigB deletion mutants of strains COL and Newman were unable to produce the orange pigment, while a sigB-complemented strain of the 8325 derivative BB255 did. Corroborating these findings, we observed that GP268, the BB255 derivative complemented with rsbU+, accumulated staphyloxanthin, the orange end product of S. aureus carotenoid biosynthesis (31), as its major stationary-phase pigment after 48 h of growth (data not shown). In contrast, BB255 produced only trace amounts of the staphyloxanthin precursors 4,4′-diapophytoene (colorless) and 4,4′-diaponeurosporene (yellow), the products of the diapophytoene synthase (CrtM) and diapophytoene desaturase (CrtN), respectively (37, 47). Consistent with its increased pigmentation, GP268 was found to be more tolerant to UV radiation, especially to near-UV light (312 nm), than its unpigmented donor, BB255 (Fig. 7). In the 8325-4 background, the tolerance to UV light was even more pronounced. GP269, the rsbU-complemented 8325-4 derivative, was significantly more tolerant to UV light than its donor (Fig. 7). The differences in UV tolerance observed between the BB255 and the 8325-4 strains are probably due to the fact that the BB255 strains, in contrast to 8325-4 strains (34), still harbor temperate bacteriophages which are known to be excised by UV radiation (41). The marked differences in near-UV-light tolerance between the rsbU+ strains and their unpigmented relatives (Fig. 7B), as opposed to the marginal differences in far-UV-light tolerance (Fig. 7A), reflect the findings of Tuveson et al. (43). These authors, using different light qualities, investigated the UV-protective capacity of pigmentation in an E. coli strain that was transformed with the carotenoid biosynthesis cluster of Erwinia herbicola. They showed that carotenoids protected the transformed E. coli strain against high fluences of near-UV light (320 to 400 nm) but not against far-UV light (200 to 300 nm).
FIG. 7.
UV tolerances of different S. aureus strains. McFarland 0.5 dilutions of different S. aureus strains were streaked out onto LB agar plates and exposed to far-UV light (254 nm) (A) or near-UV light (312 nm) (B) for different time periods. After light exposure, plates were incubated for 24 h at 37°C.
The observation that strain BB255 did not efficiently accumulate the staphyloxanthin precursors 4,4′-diapophytoene and 4,4′-diaponeurosporene argues for an influence of ςB on carotenoid biosynthesis, either on gene products governing the formation of 4,4′-diaponeurosporene or 4,4′-diapophytoene or on a prior synthetic step. Consistent with this assumption that formation of 4,4′-diapophytoene may be affected, we could detect an influence of ςB on the transcription of crtMN by Northern blot analysis (Fig. 8B). Both the transcript levels of GP268 compared with those of BB255 and the heat inducibility of the detected transcripts argue for a ςB dependence of crtMN. However, ςB dependence of crtMN alone is not sufficient to explain the inability of BB255 to produce staphyloxanthin, as overproduction of crtMN under the control of a xylose-inducible promoter resulted, after 24 h of growth, in a strong accumulation of 4,4′-diaponeurosporene, which was not further converted to staphyloxanthin even after 96 h of growth. Overproduction of crtMN in the rsbU+ strain Newman resulted in an equal accumulation of 4,4′-diaponeurosporene after 24 h of growth, but in contrast to the situation in BB255, almost all the 4,4′-diaponeurosporene was converted to staphyloxanthin after 96 h of growth (data not shown). Thus, ςB is likely to control more than one of the intermediate steps of carotenoid biosynthesis in S. aureus.
Induction of ςB-dependent transcripts after heat shock.
In B. subtilis, ςB directs transcription of its own gene when activated through a variety of stress stimuli (4, 7, 8, 22, 45, 50). Transcription starts within the sigB operon upstream of the rsbV gene. A similar situation has been proposed for S. aureus, as a promoter sequence highly similar to the ςB consensus of B. subtilis is found upstream of the rsbV gene (27, 49). Transcription from this promoter would lead to an mRNA of approximately 1.6 kb. In agreement with this prediction, Gertz et al. (20) detected a 1.6-kb transcript that was heat inducible in strain MA13 but was not detectable in strain 8325-4. Here we demonstrate that BB255 cells expressed the 1.6-kb transcript when complemented with rsbU (Fig. 8) and that the transcript was heat inducible as in MA13 (20). In addition to sigB and asp23, we found transcription of crtM to be heat inducible and dependent on ςB (Fig. 8). The time course of transcript induction after heat stress resembled that of the 1.6-kb sigB transcripts in strain MA13 (20), with a maximum induction within the first 3 to 6 min and a decrease thereafter to or below the uninduced level after 12 min.
DISCUSSION
In the gram-positive bacterium B. subtilis, RsbU has been shown to be essential for activation of ςB in response to different environmental stress stimuli such as heat shock, salt stress, or ethanol stress (45, 48, 50). A similar function has been proposed for the RsbU homologue of S. aureus (27, 49). In this study, we clearly demonstrate that RsbU of S. aureus is indeed an essential factor for ςB activity, as strains lacking this protein were unable to render activity from this sigma factor (Fig. 6). Furthermore, the lack of RsbU in 8325 derivatives resulted in phenotypes comparable to those of ΔrsbUVWsigB mutants of rsbU+ strains such as COL or Newman (28). Complementation of strain BB255 with the rsbU+ allele from COL resulted in the rsbU+ derivative GP268. This strain exhibited a ςB activity profile comparable to that of the rsbU+ wild-type strain Newman (Fig. 6) and restored the ςB-dependent phenotypic traits to the levels seen in Newman. Overexpression of RsbU in BB255 altered the phenotype to that found for GP268, while overexpression in the corresponding ΔrsbUVWsigB mutant had no apparent influence, suggesting that RsbU acts primarily through ςB (unpublished data). The observations that ςB is produced by 8325 derivatives (Fig. 3C) and that BB255 was phenotypically indistinguishable from its sigB derivative under the conditions that we tested indicate that although ςB is detectable in 8325 derivatives, it cannot be activated to relevant levels due to the absence of RsbU in this genetic background. However, the presence of detectable amounts of Asp23 and ςB activity at a low level in BB255 suggests that RsbU is not the sole determinant of ςB activity in S. aureus. Significant amounts of Asp23 were detectable in the 8325 isogenic background only in BB255, which harbors at least four prophages, and not in any of the 8325-4 derivatives (i.e., 8325-4, RN4220, and RN6390), which have been cured from the respective prophages, implying that the loss of the prophages from 8325 may influence such residual RsbU-independent ςB activity.
The finding that 8325 derivatives are almost unable to activate ςB is of particular interest, as 8325 derivatives are the laboratory strains most frequently used in S. aureus research. Most studies on starvation survival, pathogenicity, and the regulation of the two global regulators agr (accessory gene regulator) and sar (staphylococcal accessory gene regulator) and, in particular, the influence of ςB in these processes, have been carried out in this genetic background (2, 6, 10, 11, 12, 14, 15, 16, 17, 30, 42). The observed lack of ςB activity in the 8325 isogenic background revives the question if, and to what extent, ςB is involved in these processes. The findings in rsbU+ strains such as COL, Newman, and GP268 of the inducibility of transcription of ςB-dependent genes, of staphyloxanthin accumulation, of reduced susceptibility to hydrogen peroxide (Table 2), and of higher cell densities in overnight cultures compared to those for their respective ΔrsbUVWsigB mutants (Table 3) argue for an influence of ςB on the survival capacity of S. aureus.
ςB has been shown to be a major player in the general stress response of B. subtilis, by controlling the transcription of more than 100 genes under a variety of stress conditions (23, 46). So far, more than 30 genes in S. aureus have been determined to be controlled by ςB (21). These proteins are likely to be involved in the general stress response of S. aureus. In addition, pigmentation of S. aureus by the carotenoid staphyloxanthin, the biosynthesis of which is clearly influenced by ςB, is also likely to be a protective measure against various environmental stress factors, such as UV radiation (Fig. 7) or free radicals. As biological antioxidants, carotenoid pigments have been shown to protect many bacteria against the harmful effects of light, in particular against high fluences of near-UV light (320 to 400 nm). They act as scavengers of reactive molecules that are generated within cells and that can induce oxidative damage, e.g., singlet molecular oxygen (1O2) (36, 43). Thus, pigmented S. aureus cells are very likely to survive longer periods of daylight exposure than their unpigmented relatives.
The lower susceptibility of rsbU+ strains to hydrogen peroxide may also be due to the pigmentation, as carotenoids have been shown to protect efficiently against oxygen radicals (36). Alternatively, the increased resistance of rsbU+ strains to hydrogen peroxide may be due to a ςB-dependent transcriptional control of enzymes directly involved in the degradation of reactive oxygen species, such as catalase or superoxide dismutase. Transcriptional control of the katA gene, coding for the sole catalase thus far identified in S. aureus, however, was found to be independent of ςB in Northern blot analysis (data not shown). Notwithstanding, the higher tolerance of rsbU+ strains to hydrogen peroxide is likely to provide considerable benefit for S. aureus strains invading a host, enabling them to tolerate higher concentrations of oxygen radicals that are produced by the host defense system (38).
We note that rsbU mutants were unable to accumulate the pigment staphyloxanthin, even after 72 h of growth. This finding indicates that—unlike the situation in B. subtilis—ςB is inactive even during the stationary-growth phase, provided that RsbU is absent. Since all analyzed clinical isolates of S. aureus were found to be rsbU+, we consider it important to reinvestigate these processes. Most importantly, the regulation of the two global regulators agr and sar will have to be studied in a genetic background representative for the majority of clinical isolates. Preliminary data suggest a strong impact of ςB on sar expression in rsbU+ strains (M. Bischoff, unpublished data). Strain GP268, which has ςB activity, provides the possibility of studying these processes in the well-characterized 8325 isogenic background.
ACKNOWLEDGMENTS
We thank B. Berger-Bächi, A. Schaller, and M. Hecker for critical reading of, and comments on, the manuscript. We are very grateful to A. Wada for providing plasmid pAW8 and to A. Raisig for HPLC analysis of carotenoids. Preliminary sequence data were obtained from The Institute for Genomic Research (TIGR) through the website at http://www.tigr.org. Sequencing of S. aureus COL was accomplished with support from National Institute of Allergy and Infectious Diseases (NIAID) and the Merck Genome Research Institute (MGRI).
This work was supported by Swiss National Science Foundation grant NF 31-46762.96 to F. H. Kayser.
REFERENCES
- 1.Akbar S, Kang C M, Gaidenko T A, Price C W. Modulator protein RsbR regulates environmental signaling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24:567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- 2.Bayer M G, Heinrichs J H, Cheung A L. The molecular architecture of the sar locus in Staphylococcus aureus. J Bacteriol. 1996;178:4563–4570. doi: 10.1128/jb.178.15.4563-4570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson A K, Haldenwang W G. Characterization of a regulatory network that controls ςB expression in Bacillus subtilis. J Bacteriol. 1992;174:749–757. doi: 10.1128/jb.174.3.749-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson A K, Haldenwang W G. The ςB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J Bacteriol. 1993;175:1929–1935. doi: 10.1128/jb.175.7.1929-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger-Bächi B. Increase in transduction efficiency of Tn551 mediated by the methicillin resistance marker. J Bacteriol. 1983;154:533–535. doi: 10.1128/jb.154.1.533-535.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blevins J S, Gillaspy A F, Rechtin T M, Hurlburt B K, Smeltzer M S. The staphylococcal accessory regulator (sar) represses transcription of the Staphylococcus aureus collagen adhesin gene (cna) in an agr-independent manner. Mol Microbiol. 1999;33:317–326. doi: 10.1046/j.1365-2958.1999.01475.x. [DOI] [PubMed] [Google Scholar]
- 7.Boylan S A, Rutherford A, Thomas S M, Price C W. Activation of Bacillus subtilis transcription factor ςB by a regulatory pathway responsive to stationary-phase signals. J Bacteriol. 1992;174:3695–3706. doi: 10.1128/jb.174.11.3695-3706.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan S A, Redfield A R, Brody M S, Price C W. Stress-induced activation of the ςB transcription factor of Bacillus subtilis. J Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brückner R. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett. 1997;151:1–8. doi: 10.1111/j.1574-6968.1997.tb10387.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan P F, Foster S J, Ingham E, Clements M O. The Staphylococcus aureus alternative sigma factor ςB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan P F, Foster S J. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J Bacteriol. 1998;180:6232–6241. doi: 10.1128/jb.180.23.6232-6241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan P F, Foster S J. The role of environmental factors in the regulation of virulence-determinant expression in Staphylococcus aureus 8325-4. Microbiology. 1998;144:2469–2479. doi: 10.1099/00221287-144-9-2469. [DOI] [PubMed] [Google Scholar]
- 13.Cheung A L, Eberhardt K J, Fischetti V A. A method to isolate RNA from gram-positive bacteria and mycobacteria. Anal Biochem. 1994;222:511–514. doi: 10.1006/abio.1994.1528. [DOI] [PubMed] [Google Scholar]
- 14.Cheung A L, Nast C C, Bayer A S. Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infect Immun. 1998;66:5988–5993. doi: 10.1128/iai.66.12.5988-5993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung A L, Chien Y T, Bayer A S. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect Immun. 1999;67:1331–1337. doi: 10.1128/iai.67.3.1331-1337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chien Y T, Manna A C, Cheung A L. SarA level is a determinant of agr activation in Staphylococcus aureus. Mol Microbiol. 1998;30:991–1001. doi: 10.1046/j.1365-2958.1998.01126.x. [DOI] [PubMed] [Google Scholar]
- 17.Deora R, Tseng T, Misra T K. Alternative transcription factor ςSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J Bacteriol. 1997;179:6355–6359. doi: 10.1128/jb.179.20.6355-6359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duthie E S, Lorenz L L. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 19.Gaidenko T A, Yang X, Lee Y M, Price C W. Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the environmental stress signaling pathway of Bacillus subtilis. J Mol Biol. 1999;288:29–39. doi: 10.1006/jmbi.1999.2665. [DOI] [PubMed] [Google Scholar]
- 20.Gertz S, Engelmann S, Schmid R, Ohlson K, Hacker J, Hecker M. Regulation of ςB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol Gen Genet. 1999;261:558–566. doi: 10.1007/s004380051001. [DOI] [PubMed] [Google Scholar]
- 21.Gertz S, Engelmann S, Schmid R, Ziebandt A K, Tischer K, Scharf C, Hacker J, Hecker M. Characterization of the ςB regulon in Staphylococcus aureus. J Bacteriol. 2000;182:6983–6991. doi: 10.1128/jb.182.24.6983-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecker M, Schuman W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 23.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth restricted Bacillus subtilis cells by the expression of the ςB regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 24.Kalman S, Duncan M L, Thomas S M, Price C W. Similar organization of the sigB and spoIIA operons encoding alternative sigma factors of Bacillus subtilis RNA polymerase. J Bacteriol. 1990;172:5575–5585. doi: 10.1128/jb.172.10.5575-5585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kornblum J, Hartmann B J, Novick R P, Tomasz A. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur J Clin Microbiol. 1986;5:714–718. doi: 10.1007/BF02013311. [DOI] [PubMed] [Google Scholar]
- 26.Kreiswirth B N, Löfdahl S, Betley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 27.Kullik I, Giachino P. The alternative sigma factor ςB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 28.Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor ςB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuroda M, Ohta T, Hayashi H. Isolation and the gene cloning of an alkaline shock protein in methicillin resistant Staphylococcus aureus. Biochem Biophys Res Commun. 1995;207:978–984. doi: 10.1006/bbrc.1995.1281. [DOI] [PubMed] [Google Scholar]
- 30.Manna A C, Bayer M G, Cheung A L. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol. 1998;180:3828–3836. doi: 10.1128/jb.180.15.3828-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall J H, Wilmoth G J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol. 1981;147:900–913. doi: 10.1128/jb.147.3.900-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazaki E, Chen J M, Ko C, Bishai W R. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J Bacteriol. 1999;181:2846–2851. doi: 10.1128/jb.181.9.2846-2851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholas R O, Li T, McDevitt D, Marra A, Sucoloski S, Demarsh P L, Gentry D R. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect Immun. 1999;67:3667–3669. doi: 10.1128/iai.67.7.3667-3669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 35.Novick R P. The Staphylococcus as a molecular genetic system. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 1–40. [Google Scholar]
- 36.Palozza P, Krinsky N I. Antioxidant effects of carotenoids in vivo and in vitro: an overview. Methods Enzymol. 1992;213:403–420. doi: 10.1016/0076-6879(92)13142-k. [DOI] [PubMed] [Google Scholar]
- 37.Raisig A, Sandmann G. 4,4′-Diapophytoene desaturase: catalytic properties of an enzyme from the C30 carotenoid pathway of Staphylococcus aureus. J Bacteriol. 1999;181:6184–6187. doi: 10.1128/jb.181.19.6184-6187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen G M, Pou S, Ramos C L, Cohen M S, Britigan B E. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Scott J M, Haldenwang W G. Obg, an essential GTP binding protein of Bacillus subtilis, is necessary for stress activation of transcription factor ςB. J Bacteriol. 1999;181:4653–4660. doi: 10.1128/jb.181.15.4653-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sompolinsky D, Yiflah Y, Aboud M. The mechanism of ultraviolet induction in lysogenic Staphylococcus aureus. J Gen Virol. 1968;2:347–356. doi: 10.1099/0022-1317-2-3-347. [DOI] [PubMed] [Google Scholar]
- 42.Tegmark K, Morfeldt E, Arvidson S. Regulation of agr-dependent virulence genes in Staphylococcus aureus by RNAIII from coagulase-negative staphylococci. J Bacteriol. 1998;180:3181–3186. doi: 10.1128/jb.180.12.3181-3186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuveson R W, Larson R A, Kagan J. Role of cloned carotenoid genes expressed in Escherichia coli in protecting against inactivation by near-UV light and specific phototoxic molecules. J Bacteriol. 1998;170:4675–4680. doi: 10.1128/jb.170.10.4675-4680.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijay K, Brody M S, Fredlund E, Price C W. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the ςB transcription factor of Bacillus subtilis. Mol Microbiol. 2000;35:180–188. doi: 10.1046/j.1365-2958.2000.01697.x. [DOI] [PubMed] [Google Scholar]
- 45.Völker U, Völker A, Maul B, Hecker M, Dufour A, Haldenwang W G. Separate mechanisms activate ςB of Bacillus subtilis in response to environmental and metabolic stresses. J Bacteriol. 1995;177:3771–3780. doi: 10.1128/jb.177.13.3771-3780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Völker U, Maul B, Hecker M. Expression of the ςB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J Bacteriol. 1999;181:3942–3948. doi: 10.1128/jb.181.13.3942-3948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieland B, Feil C, Gloria-Maercker E, Thumm C, Lechner M, Bravo J-M, Poralla K, Götz F. Genetic and biochemical analysis of the biosynthesis of the yellow carotenoid 4,4′-diaponeurosporene of Staphylococcus aureus. J Bacteriol. 1994;176:7719–7726. doi: 10.1128/jb.176.24.7719-7726.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wise A A, Price C W. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor ςB in response to environmental signals. J Bacteriol. 1995;177:123–133. doi: 10.1128/jb.177.1.123-133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu S, de Lencastre H, Tomasz A. ςB, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X, Kang C M, Brody M S, Price C W. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 1996;10:2265–2275. doi: 10.1101/gad.10.18.2265. [DOI] [PubMed] [Google Scholar]