SUMMARY
Positive Deviance (PD) is a process to achieve a social and cultural change. This strategy has been used for the control of methicillin-resistant Staphylococcus aureus (MRSA) infection in some health institutions in the United States, but has rarely been adopted in institutions from developing countries where resources are limited. We describe our experience of PD in the control of healthcare-associated infections (HAIs) due to MRSA in a Colombian hospital with the aim of reducing HAI rates through a cultural change in processes. A time-series study was conducted based on the MRSA-HAI rate and the number of months with zero MRSA infections before and after application of PD (2001–2012). On comparing the pre-intervention and intervention periods, the mean overall rates of MRSA-HAI was 0·62 and 0·36, respectively (P = 0·0005); the number of months with zero MRSA-HAIs were 3/70 and 12/74 (odds ratio 0·264, 95% confidence interval 0·078–0·897); the percentage of MRSA-HAIs was 53·2% and 41·0%. These results are consistent with other published data. Implementation of PD was associated with a significant reduction of MRSA-HAIs, it did not involve high costs and the changes have been lasting.
Key words: Colombia, healthcare-associated infections, MRSA, Positive Deviance
INTRODUCTION
The emergence and dissemination of microorganisms that are resistant to antibiotics is a growing global phenomenon with substantial repercussions for public health due to increase in medical costs and the risk of death. Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most common microorganisms responsible for healthcare-associated infections (HAIs) and is a frequent cause of invasive and surgical wound infections [1]. In the United States, it is classified by the Centers for Disease Control and Prevention (CDC) as a serious public health threat and in 2014 it was reported that 47·9% of S. aureus HAIs across all clinical settings were MRSA [2]. Unpublished data from the National Institute of Health, Colombia suggest MRSA rates over 36% in high-complexity hospitals, and as widely found elsewhere in the world, MRSA-HAIs are most often associated with an increase in hospital length of stay and higher mortality rates [1, 3].
The Positive Deviance (PD) approach is based on the observation that in every community, there are certain individuals or groups whose uncommon behaviour and strategies enable them to find better solutions to problems than their peers while still having access to the same resources and facing similar or worse challenges [4]. This strategy has been used for MRSA infection control in several health institutions in the United States [5–7]. However, it has been poorly applied in institutions in developing countries, which usually have limited healthcare resources. We report here the results obtained for the prevention and control of MRSA infections through the implementation of the PD process in a hospital in Colombia.
METHODS
The study was performed in El Tunal Hospital which is a high-complexity general hospital with 222 beds that has critical-care services for adults (18 beds), infants (eight beds) and newborns (28 beds). The hospital serves a catchment area of 1 500 000 inhabitants of low socioeconomic status and is a reference centre for patients from remote regions of the country.
Implementation of PD (intervention)
Since 2000, improved hand hygiene, stricter patient isolation and education programmes were established as infection prevention measures in all units of the study hospital. However, a review 6 years later failed to show a significant reduction of HAIs and MRSA infections and on the contrary, revealed an increasing trend. Following early meeting reports in 2006, subsequently published, of the experience of several hospitals in the United States using PD methodology for MRSA control [5–7], it was decided to implement this approach in the study hospital in October 2006 following training of personnel by the Plexus Institute (http://www.plexusinstitute.org/).
The PD method is summarized by the following stages: define, determine, discover, design and monitor. This entails defining the problem or opportunity, and the outcome sought; determination of individuals in the organization who are already exhibiting the desired behaviour and/or producing the desired results; discovery of uncommon practices and behaviours that could enable sets of healthcare workers to outperform others in their organization; design of interventions that allow others to access the new behaviours, and monitoring of results.
A preliminary meeting was held of health workers, administrative staff and community representatives and participants (medical, nursing, administrative staff) were invited voluntarily to attend work sessions where opinions were shared on the topics under review. Groups were asked to address the issue of MRSA-HAI and share their findings with other groups. There was general consensus of the need to improve adherence to existing infection prevention and control measures such as hand hygiene and patient isolation, and that PD should be implemented in the institution, taking into account that it did not require additional financial resources. A PD implemention group comprising 30 individuals was therefore established with the goal of reducing MRSA infections in the hospital to ‘zero’; this initiative received approval by the chief executive officer of the hospital. The implementation staff were then trained in the technique of Discovery and Action Dialogues (DADs), the centrepiece of PD methodology usage (see Supplementary material) which focuses on the solution rather than the problem. The aim of DADs is to ensure that in the presence of a facilitator, a group of around eight people (Positive Deviants) discuss the identified problem and how barriers may be overcome to find a solution. All ideas were considered to be of value. The group made proposals and commitments and identified specific actions to be taken forward as interventions.
From each DAD, the most feasible and low-cost economic proposals were selected for implementation (Table 1) in January 2007 and currently remain in place. In the first year of PD, 200 DAD groups were held in which 1600 people participated. Subsequently, DADs were performed whenever a suspected outbreak or increased infection rates were observed. Early DAD groups (2007) identified that the most important measures to combat MRSA and other HAIs were hand hygiene, isolation precautions, and staff education as well as discipline in compliance and adherence to practice recommendations. Other important contributions were the need for regular feedback and recognition of successes in prevention of infections. As a consequence, three new measures were proposed: (i) identification of asymptomatic MRSA carriers in patients in intensive care units (ICUs) and maintaining isolation precautions until discharge [8], (ii) introduction of bathing of ICU patients with chlorhexidine gluconate-impregnated cloths, and (iii) improvement of oral hygiene through chlorhexidine mouthwash for intubated patients.
Table 1.
Implemented measures in the Positive Deviance (PD) approach
| Measure | Group | Actions | Date |
|---|---|---|---|
| Hand-hygiene (alcohol gel) dispensers | Facility staff | Located at entrance to all hospital areas. | Nov. 2006 |
| Clinical/Surgical | Located in wards and surgical areas | Jan. 2007 | |
| Learning group | Stimulated the use of personal alcohol gel units. | Jan. 2007 | |
| Community leaders | Disseminated information on hand-hygiene | 2007 and 2008 | |
| Isolation precautions | Learning group | Designed posters illustrating PD methodology | June 2007 |
| Nursing assistant | Dsigned educational brochures for the patient families. | Jan. 2008 | |
| Surgeons | Green bed sheets for patients in isolation. | Oct. 2007 | |
| Laundry staff | Green bed sheets better conserved after washing. | ||
| Identification of nasal carriage | Medical, nursing, bacteriology staff | MRSA nasal screening on admission to ICU, contact precautions instituted until discharge from hospital. | Feb. 2007 |
| Decolonization | ICU nursing staff | Implemented bathing of patients with chlorhexidine gluconate-impregnated cloths. | Aug. 2011 |
| Physiotherapists | Implemented oral hygiene with chlorhexidine to intubated patients | ||
| Education | Learning group | Videos shown on hand-hygiene, isolation precautions and surgical site infection prevention | Jan. 2008 |
| Installation of floor graphics with PD illustrations. | Jan. 2008 | ||
| Educational materials made available to all staff through web | Jan. 2008 | ||
| Implemented ‘make visible the invisible’ strategy using glitter to simulate hand transmission of microorganisms and examples of bacteria on hands before and decontamination | June 2007 | ||
| Feedback | DAD participants | Public acknowledgment of hospital management officials, hospital areas and ICU housekeeping staff for good practices in infection prevention. | 2007, 2008 and 2011 |
| ICU staff awarded for goal of zero MRSA infections in 1 month with a chocolate bar and an emblematic diploma. | |||
| Feb. 2008 | |||
| Learning group | Periodic epidemiological bulletin on HAI information sent to personal email of hospital officials. | Jan. 2008 |
DAD, Discovery and Action Dialogue; ICU, intensive care unit; MRSA, methicillin-resistant S. aureus.
Measurements
HAI data and antimicrobial resistance profiles of isolates were collected by the same individual for the duration of the 12-year study beginning in 2000. An HAI incident case was defined as a minimum 48-h hospital stay according to CDC guidelines; infection was classified as a localized or systemic condition resulting from an infectious agent, and no evidence of infection on patient admission to the acure care setting [9]. Clinical samples for microbiology were processed using standard procedures and organisms were identified by the MicroScanWalkAway® plus System (Dade Behring, USA).
In order to evaluate the effects of the application of PD the pre-intervention period ran from January 2001 to October 2006 (70 months) and the post-intervention period was November 2006 to December 2012 (74 months). HAI rates/100 patient-days for the hospital as a whole, and for the adult ICU, the number of months with zero MRSA infections, and the overall percentage of MRSA were recorded.
Statistical analysis
Primary data were entered in Excel (Microsoft Corp., USA). A time-series analysis was performed in a SARIMA model (R Project v. 3.0.2 for Windows) based on an exploratory analysis using a correlogram and a partial correlogram. This analysis showed that the assumptions of constant mean and variance through time were fulfilled for the additive decomposition. Subsequently, a descriptive analysis was performed with frequencies and central tendency measurements. For the inferential analysis, the associations were determined using the χ2 test to calculate the odds ratio (OR); comparison of number of MRSA in the hospital and ICU over each time period, and difference between the latter two was performed using the Mann–Whitney U test (SPSS v. 20; IBM Corp., USA). All tests met a 95% confidence interval (CI) assuming significant values of P < 0·05.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by The Tunal Hospital Institutional Review Board. Informed consent was not obtained as patient-specific data is not reported and data had already been abstracted in our infection control software program.
RESULTS
The statistical variance and proportional homogeneity of the pre- and intervention periods showed the suitability of the data for comparative analysis for all of the hospital excluding the adult ICU, and for the ICU alone. In the 144-month study period for the hospital, 106 121 days of hospitalization were recorded with 1013 HAIs caused by S. aureus; 215 (23·2%) patients died. MRSA infections were identified in 509 (50·2%) patients and the most frequent types were: surgical site infections (250, 49·1%), a large number of cases with superficial incisional, bloodstream infection (122, 24·0%), and pneumonia and other lower respiratory tract infection (79, 15·5%). Of the total patients with MRSA infection, 134 (28·2%) died.
The ICU registered 69 118 days of hospitalization with 202 S. aureus infections (87, 43·1% MRSA). The monthly average of S. aureus infections for the entire institution decreased from 8·16 to 5·97 during the intervention period (P = 0·0001), and from 74 to 1·08 for the ICU (P = 0·001) (Table 2). There was a similar decrease in MRSA-HAIs across the hospital from 4·43 to 2·69 (P = 0·0001), and from 0·77 to 0·45 (P = 0·047) in the ICU. Further, the overall rate for MRSA infections/1000 patient-days at risk ranged from 0 to 1·4, with mean values of 0·48 for the total, and 0·62 for the pre-intervention periods, respectively; a decreasing trend in rates was observed post-intervention of PD (Fig. 1).
Table 2.
Analysis of MRSA-HAI infections pre- and post intervention of PD methodology
| Infections | All hospital | ICU | |||
|---|---|---|---|---|---|
| No. of cases | Mean ± s.d. | No. of cases | Mean ± s.d. | ||
| (median) | (median) | ||||
| MRSA-HAI infections per thousand patient-days at risk rate*† | Pre-intervention | n.a. | 0·62 ± 0·36 | n.a. | 1·79 ± 2·33 |
| (0·565) | (0·0) | ||||
| Post-intervention | n.a. | 0·36 ± 0·30 | n.a. | 0·84 ± 1·40 | |
| (0·27) | (0·0) | ||||
| Number of S. aureus infections† | Pre-intervention | 571 | 8·16 ± 3·26 | 122 | 1·74 ± 1·36 |
| (8·0) | (1·5) | ||||
| Post-intervention | 442 | 5·97 ± 3·24 | 80 | 1·08 ± 1·21 | |
| (5·5) | (1·0) | ||||
| Number of MRSA infections† | Pre-intervention | 310 | 4·43 ± 2·66 | 54 | 0·77 ± 1·04 |
| (4·0) | (0·0) | ||||
| Post-intervention | 199 | 2·69 ± 2·26 | 33 | 0·45 ± 0·76 | |
| (2·0) | (0·0) | ||||
| Percentage of MRSA isolates† | Pre-intervention | n.a. | 53·19 ± 24·39 | n.a. | 33·42 ± 40·14 |
| (56·35) | (0·0) | ||||
| Post-intervention | n.a. | 41·04 ± 26·14 | n.a. | 21·35 ± 34·10 | |
| (40·0) | (0·0) | ||||
ICU, Intensive care unit; MRSA, methicillin-resistant S. aureus; n.a., not applicable.
Risk rate: MRSA-HAI/1000 patient-days.
P < 0·05.
Fig. 1.
MRSA infections/1000 patient-days at risk rate during both study periods. (a) All hospital services, (b) ICU, (c) all hospital services (except ICU). Dashed black lines show data trends pre- and post-intervention.
The MRSA-HAI rate across all the hospital over the study period was 47·0 % with 53·2% and 41% pre- and post-intervention, respectively, giving a reduction of 12%. The corresponding rates for the ICU were 27·2% overall, 33·4% pre-, and 33·4% post-intervention. During the intervention period, the overall MRSA-HAI rate ranged from 0 to 1·29/1000 days, with a mean of 0·36 (P = 0·0005). Two peaks of increase in rates were observed in December 2006 and May 2007. Overall rates ranged from 0 to 8·33 and displayed a decreasing tendency over time; the mean pre-intervention period was 1·79, and 0·74 (range 0–7·27) post-intervention (P = 0·003) (Table 2).
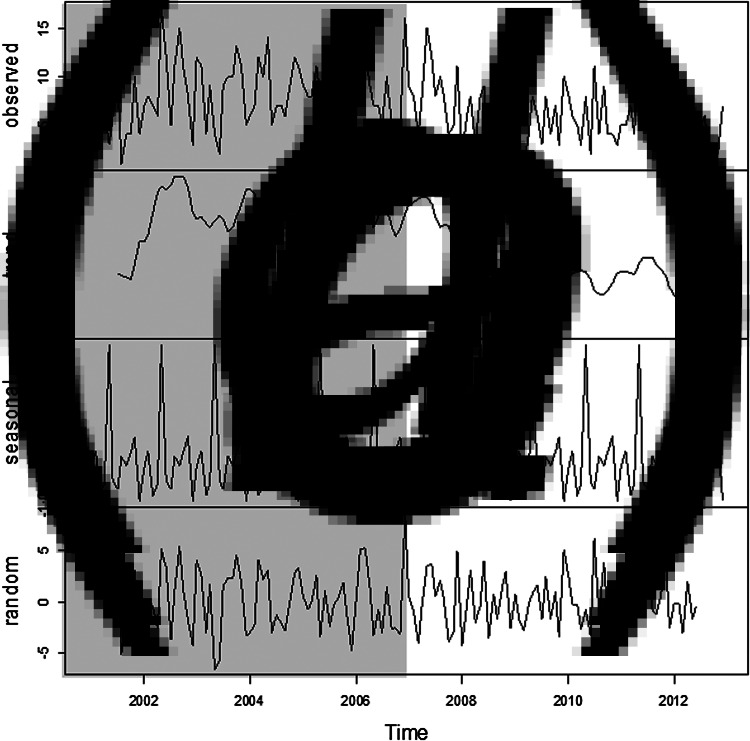
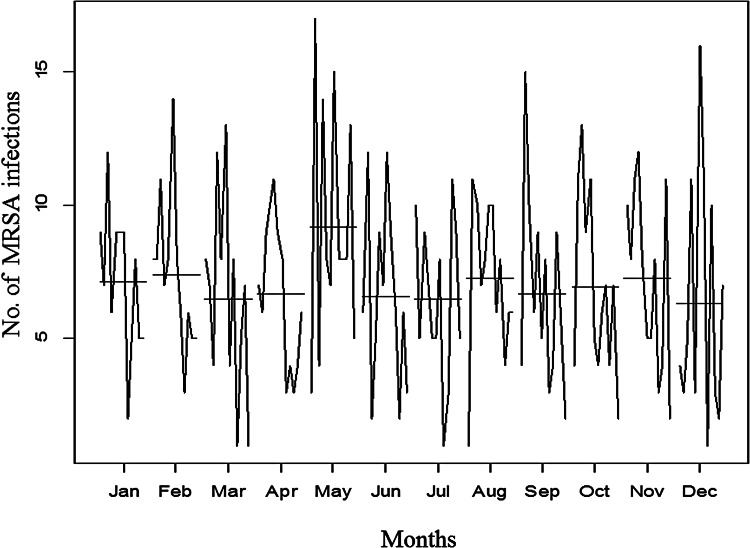
The additive decomposition of the time-series analysis was used to identify whether the emergence of MRSA infections occurred randomly. The trend graphic in Figure 2b shows that the number of infections in the entire hospital remained high and constant from the beginning of the observation period. Nevertheless, a gradual decrease in the frequency of MRSA infections was noted from the end of 2007, which bottomed out in 2009 with a slight resurgence in the following year until declining to non-detection levels. There was evidence of seasonal variation in MRSA infection rates with the highest peaks being recorded in May (9·17 ± 4·53) of each year over the pre- and post-intervention periods (see Fig. 3); this periodicity did not appear to be random.
Fig. 2.
Additive decomposition of the series of patients with MRSA. (a) Original series, (b) trend, (c) seasonality, (d) random residues. Shaded areas indicate pre-intervention period.
Fig. 3.
Distribution of MRSA infections by month during each year of the total study period.
The impact of intervention was assessed by the number of MRSA infection-free months. Over the total study period for all of the hospital, 15/144 (10·4%) months were free of MRSA cases and 12 (80%) of these months were in the post-intervention period (P = 0·017). These data suggest that implementation of PD together with standard infection control measures was effective in significantly reducing the acquisition of MRSA infections (OR 0·264, 95% CI 0·078–0·897). For the adult ICU, the corresponding number of MRSA infection-free months was 86/144 (59·7%), and of these 49 (57·2%) occurred in the post-intervention period; no significant associations were found but a risk reduction trend was evident (OR 0·798, 95% CI 0·607–1·051).
DISCUSSION
In our hospital, poor adherence to conventional infection prevention practice prior to the introduction of PD did not allow adequate control of HAIs and resulted in an endemic MRSA situation in the hospital, as experienced by others [10]. It is widely accepted that effective prevention and control of HAIs necessitates the inclusion of all healthcare practitioners, the generation of appropriate environments for change, a transition from knowledge to sustained action, elimination of barriers to achieve adherence, and a culture change.
Our results show a sustained reduction in MRSA-HAI rates in the period after the application of PD as the number of MRSA-free months increased and the number of cases decreased (see Fig. 4). These results are consistent with similar studies, which showed that the application of a bundle of universal surveillance, contact precautions, hand hygiene, and PD, was associated with a decrease in MRSA-HAI transmissions in an ICU from 1·64 to 0·62/1000 patients-days [6, 11]. Similarly, a collaborative project, employing a panel of infection prevention strategies, and engaging employees through PD, resulted in a significant decline of bloodstream infections in outpatient haemodialysis centres [7].
Fig. 4.
Incidence of MRSA infections in both study periods. (a) Numbers of MRSA infection-free months in all hospitals and ICUs. (b) Percentage of the MRSA isolates over time. Shaded areas indicate pre-intervention period; dashed black lines show data trends pre- and post-intervention.
In our experience, although the reduction of MRSA-HAI rates became evident soon after implemetation of PD at the end of 2007, it did not become sustained until 2009, when most of the people in the hospital were involved in the strategy. The two unexpected increases in cases in 2006 and 2007 coincided with a number of patients with community-acquired MRSA infections attending the hospital, and reflected a similar increase at that time in cases in other hospitals in Bogotá [12].
Most hospital staff bought into the strategy which involved educational talks on local MRSA-HAI epidemiology and clinical consequences which resulted in direct actions to increase patient isolation and hand hygiene adherence across the hospital. Prior to admission to the adult ICU, microbiological screening was undertaken to identify MRSA carriers and such patients were isolated until discharge; ICU patients were also subjected to chlorhexidine baths. Interestingly, although only implemented in the ICU, MRSA carrier screening also resulted in a decrease of MRSA in both the ICU and the hospital as a whole. These results reflect findings of other groups with interventions focused on cultural change and/or the modification of behaviour to improve adherence, including PD [7, 11, 13]. Although not quantified in this study, there appeared to be a general trend towards a reduction in the overall HAI rate during the PD intervention in our hospital.
The acceptance of the PD strategy across the wider hospital staff resulted in increased engagement in the decisions and actions that involved or affected them. Indeed, it was observed that some DAD participants progressed to become leaders in HAI prevention in their work areas even when there was constant staff turnover, and administrative and economic difficulties. In general, all DAD participants showed knowledge of the infection prevention measures and discussions focused mainly on how these measures could be applied by all staff irrespective of their status and function. This approach led to expansion of staff being involved from initially four individuals in infection control, to hundreds. The establishment of self-control measures were evidence of the cultural change through PD, i.e. identify, imitate, disseminate, and improve good practices. Patient safety initiatives usually tend to focus on system failures after an adverse event and involve identifying the problem, its root cause, or identifying the individual responsible (negative deviance), rather than attempting prospectively to cultivate effective practices [14]. The PD leadership model allows everyone to take ownership of both the problem and the solution. In our study, a product of the DAD and learning groups was the slogan ‘Some people find problems in the solutions, some people find solutions in the problems, and some people prevent problems, and it is the last group that comprises our Positive Deviants’. Implementation of PD as a preventive strategy did not involve a significant increase in the HAI budget and is therefore suitable for resource-poor institutions.
The reasons for the observed spike in numbers of MRSA infection in May of each year are unclear. However, seasonal variation of S. aureus and MRSA infections has been previously described, particularly skin and soft tissue infections occurring more frequently in summer and autumn [15, 16]. It is noteworthy that Bogotá, Colombia is 2600 m above sea level, with average temperatures of 15–20 °C without extreme seasonal variation. Rainfall is highest in May and some local studies have shown an increase of lower respiratory tract infections in children in this month. However, surgical site infections were the most predominant in our study in May and so further studies are warranted to seek an explanation for their increased frequency.
Currently, there is insufficient evidence to determine the most effective interventions to achieve optimal adherence to HAI prevention measures [10], but our experience suggests that implementation of PD offers a practical and beneficial addition to infection control practice, and merits further research into its application to wider nosocomial infection issues. Nevertheless the study has some limitations. Although we were able to show a significant reduction in MRSA infection in the post-intervention period, this could have been influenced by other epidemiological factors such as dissemination of community-associated MRSA strains in the hospital and differences in the patient populations at risk over the two study periods. However, over the 12 years of follow-up, there were no major changes in the types of patients seen in the hospital, except for the opening in an oncology service in 2011.
In recent years the rates of MRSA infections have generally decreased around the world, particularly in developed nations [17, 18] but they remain high in some parts of the world, particularly Asia [19]. Information on such rates in Colombia is limited but a reduction of MRSA infections was recorded between 2004 and 2008 from 60% to 35%, respectively [20]; which could be attributed to wider adoption of care bundles, better use of antimicrobials, improved surveillance of resistant bacteria, and decolonization of patients. Other contributory factors such as differences in clonal populations and virulence of MRSA strains, spread from environmental reservoirs and inanimate surfaces, etc., also need to be considered. In our study, the trends of increasing MRSA-HAI observed prior to 2006 was markedly reversed following implemetation of PD in 2007. This also coincided with a fall in the number of infections due to other common nosocomial pathogens (Escherichia coli, Klebsiella pneumoniae and Acinetobacter baumannii, data not shown) which supports the efficacy of PD methodology to improve infection prevention and control for other microorganisms, as reported by Jain et al. [6].
ACKNOWLEDGEMENTS
The authors are grateful to Plexus Institute for providing the Positive Deviance methodology in our hospital, to Dr Elkin Lemos and Dr Ernesto Giraldo for valuable comments and technical review of the manuscript and to Dr Miguel Otero (El Bosque University) for invaluable support.
This work was financially supported by El Tunal Hospital, El Bosque University and partially by the Administrative Department of Science, Technology and Innovation/Colciencias grant 1308-569-33463.
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/S095026881600306X.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.World Health Organization. Antimicrobial resistance: global report on surveillance. World Health Organization, 2014, pp. 257 (http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf). Accessed January 2016.
- 2.Hidron AI, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infection Control and Hospital Epidemiology 2008; 29: 996–1011. [DOI] [PubMed] [Google Scholar]
- 3.Castillo JS, et al. Mortality among critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia: a multicenter cohort study in Colombia. Revista Panamericana de Salud Publica 2012; 32: 343–350. [DOI] [PubMed] [Google Scholar]
- 4.Marsh DR, et al. The power of positive deviance. British Medical Journal 2004; 329: 1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindberg C, et al. Letting go, gaining control: positive deviance and MRSA prevention. Clinical Leader 2009; 2: 60–67. [Google Scholar]
- 6.Jain R, et al. Veterans affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. New England Journal of Medicine 2011; 364: 1419–1430. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg C, et al. Embracing collaboration: a novel strategy for reducing bloodstream infections in outpatient hemodialysis centers. American Journal of Infection Control 2013; 41: 513–519. [DOI] [PubMed] [Google Scholar]
- 8.Olarte NM, et al. Methicillin-resistant Staphylococcus aureus colonization in a Colombian hospital intensive care unit: phenotypic and molecular characterization [in Spanish]. Biomedica 2010; 30: 353–361. [PubMed] [Google Scholar]
- 9.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 2008; 36: 309–332. [DOI] [PubMed] [Google Scholar]
- 10.Flodgren G, et al. Interventions to improve professional adherence to guidelines for prevention of device-related infections. Cochrane Database of Systematic Reviews 2013; 3: CD006559. [DOI] [PubMed] [Google Scholar]
- 11.Muder RR, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infection Control and Hospital Epidemiology 2008; 29: 702–708. [DOI] [PubMed] [Google Scholar]
- 12.Cortes JA, et al. Community-acquired methicillin-resistant Staphylococcus aureus in Bogota, Colombia: Public health implications [in Spanish]. Revista de Salud Publica (Bogota) 2007; 9: 448–454. [DOI] [PubMed] [Google Scholar]
- 13.Ellingson K, et al. Sustained reduction in the clinical incidence of methicillin-resistant Staphylococcus aureus colonization or infection associated with a multifaceted infection control intervention. Infection Control and Hospital Epidemiology 2011; 32: 1–8. [DOI] [PubMed] [Google Scholar]
- 14.Lawton R, et al. Positive deviance: a different approach to achieving patient safety. BMJ Quality & Safety 2014; 23: 880–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leekha S, Diekema D, Perencevich E. Seasonality of staphylococcal infections. Clinical Microbiology and Infection 2012; 18: 927–933. [DOI] [PubMed] [Google Scholar]
- 16.Mermel LA, Machan JT, Parenteau S. Seasonality of MRSA infections. PLoS ONE 2011; 6: e17925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore DM. Fourteen years in resistance. International Journal of Antimicrobial Agents 2012; 39: 283–294. [DOI] [PubMed] [Google Scholar]
- 18.Lagace-Wiens PR, et al. Trends in antibiotic resistance over time among pathogens from Canadian hospitals: results of the CANWARD study 2007–11. Journal of Antimicrobial Chemotherapy 2013; 68: i23–29. [DOI] [PubMed] [Google Scholar]
- 19.Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clinical Microbiology and Infection 2014; 20: 605–623. [DOI] [PubMed] [Google Scholar]
- 20.Espinosa CJ, et al. Systematic review of antimicrobial resistance among Gram positive cocci in hospitals in Colombia [in Spanish]. Biomedica 2011; 31: 27–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://doi.org/10.1017/S095026881600306X.
click here to view supplementary material