SUMMARY
Many infectious diseases in humans may manifest with no or mild symptoms. While numerous studies have estimated the proportion of infectious individuals in whom symptoms are absent during the entire course of infection, the contribution of asymptomatic cases to the overall cumulative incidence is difficult to untangle. Here, with a mathematical model, we provide a simple analytical formula to quantify this contribution and highlight the potential for large errors that can arise when naively estimating it.
Key words: Asymptomatic infection, epidemiology, mathematical modelling, public health
Many infectious diseases in humans may occur without presenting the case-defining symptoms of illness. Numerous studies have estimated the proportion of infectious individuals in whom symptoms are absent during the entire course of infection (herein referred to as the ‘asymptomatic fraction’) for a number of infectious diseases [1–4]. Although such estimates are critically important for devising and implementing effective public health interventions, they may incorrectly quantify the disease burden attributable to asymptomatic transmission during an epidemic. For example, an asymptomatic fraction at 60% is not necessarily responsible for 30% of all transmissions when the infectiousness of asymptomatic cases is on average half that of symptomatic ones. Here, while we do not address the riddle of the disease burden caused by asymptomatic infection, we highlight important factors that should be considered in understanding and quantifying its contribution to the cumulative incidence.
To this end, we define the proportion P as the ratio of the cumulative incidence caused by transmission from asymptomatic cases only, divided by the total cumulative incidence. Hence, P evaluates the fraction of the cumulative incidence attributable to asymptomatic transmission. This proportion is primarily determined by the asymptomatic fraction and the relative infectivity of asymptomatic infection compared with symptomatic infection. We define the relative infectivity as the product r = rcrprd, where:
 |
The ratio rc is largely affected by the nature of infection and its severity, which influences the number of contacts during infection that may be different in the absence of symptoms compared with when symptoms are present. The relative transmissibility, rp, is primarily determined by the amount of pathogen shedding, which depends on the level of infectiousness during illness. The relative duration of infectiousness, rd, may be affected by several factors, including the nature of disease and pathogen–host interactions. While rd ⩽ 1 may characterise several infectious diseases (e.g. Ebola [2], influenza [5] and pertussis [6]) the duration of asymptomatic infection may be significantly longer than that of symptomatic infection for some diseases (e.g. Haemophilus influenzae [7]), suggesting rd > 1. Hence, depending on the pathogen and the social, environmental and individual characteristics, the relative infectivity r can have a broad range of values, smaller or larger than one. The special case r = 1 may arise when the values of its three components compensate for one another. For example, when the average duration of infectiousness is the same for both asymptomatic and symptomatic infections (rd = 1), a twice-larger contact ratio (rc = 2) compensates for a twice-smaller relative transmissibility (rp = 0.5).
We used a standard mathematical model (Supplementary Material) to calculate the proportion P at the end of a simulated epidemic and found that:
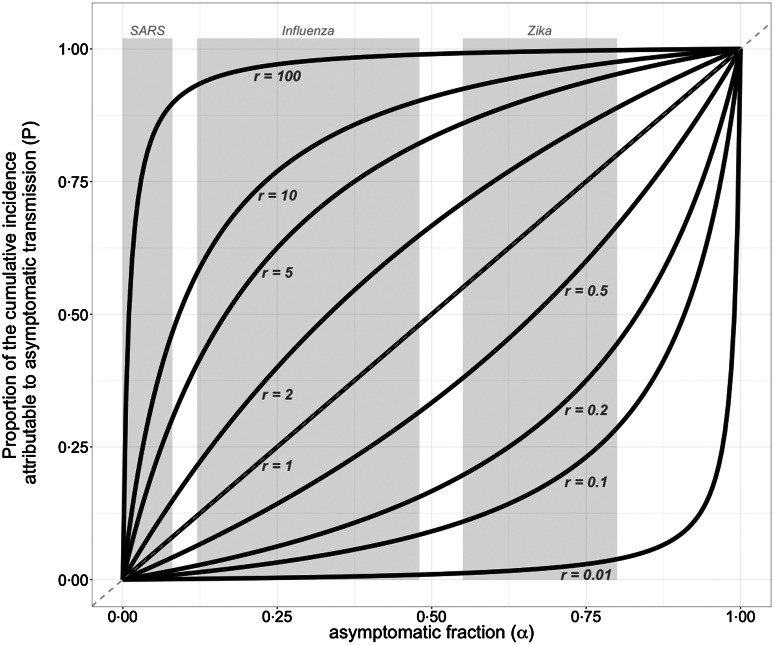
Figure 1 illustrates this proportion as a function of the asymptomatic fraction (α) for different values of the relative infectivity (r). When r = 1, the asymptomatic fraction translates to the same proportion of cumulative incidence caused by asymptomatic infection, i.e. P = α. However, a nonlinear relationship between P and α arises for r ≠ 1, and demonstrates that a simple estimate of P using only α can easily be flawed. For example, Figure 1 shows that for an 80% asymptomatic fraction (α = 0·8), asymptomatic infections with 10% relative infectivity (r = 0·1) are responsible for 28·6% of the total cumulative incidence. This is notably different from a simple estimation of 8% for P that is obtained by calculating the product of α and r. Indeed, the denominator 1 + α(r − 1) in the expression for P can be viewed as an adjustment factor from the trivial estimator αr. The product αr is an acceptable approximation only when the asymptomatic fraction α is very small or when r is close to one.
Fig. 1.
The proportion (P) of cumulative incidence attributable to asymptomatic transmission as a function of the asymptomatic fraction (α), for a given r. Each curve represents this proportion P for a given relative infectivity of asymptomatic infection (r), according to the mathematical model detailed in the Supplementary Material. The shaded rectangles represent the plausible range of the asymptomatic fraction for selected infectious diseases (see Supplementary Material for the sources used to estimate these ranges). The dashed line represents the special case r = 1 where we have P = α. A value of, say, r = 0.1 means that overall–taking into account the relative contact rate, transmissibility and duration of infection–asymptomatic cases are 10 times less infectious than symptomatic ones.
Our simple analytical expression for P provides a straightforward estimate for the proportion of the cumulative incidence attributable to asymptomatic cases. This expression is derived from a relatively generic modelling framework, making it applicable to a broad range of infectious diseases (Supplementary Material). It also underscores the importance of correctly estimating both α and r, even approximately, given their central role in determining the proportion P.
In summary, this simple modelling exercise highlights the potential for large errors that can arise when estimating the contribution of asymptomatic infection to the overall cumulative incidence from either the asymptomatic fraction (α) alone or simply using the product of α and r. We therefore draw attention to the fact that informing optimal health policies and interventions based on the importance of asymptomatic infection in the overall disease burden requires models and methods to tease out critical information and provide more accurate estimates of key epidemiological parameters.
ACKNOWLEDGEMENTS
This study was in part supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Mathematics of Information Technology and Complex Systems (Mitacs), Accelerate Internship Program. The authors would like to thank the anonymous reviewer and Delphine Grynszpan for insightful comments that have greatly improved the paper and its presentation.
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/S0950268817000115.
click here to view supplementary material
DECLARTION OF INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Leung NHL, et al. The fraction of influenza virus infections that are asymptomatic. A systematic review and meta-analysis. Epidemiology 2015; 26: 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leroy EM, et al. Human asymptomatic Ebola infection and strong inflammatory response. Lancet 2000; 355: 2210–2215. [DOI] [PubMed] [Google Scholar]
- 3.Wilder-Smith A, et al. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerging Infectious Diseases 2005; 11: 1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coura JR, Suárez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection – a review. Memórias do Instituto Oswaldo Cruz 2006; 101: 229–237. [DOI] [PubMed] [Google Scholar]
- 5.Loeb M, et al. Longitudinal study of influenza molecular viral shedding in Hutterite Communities. Journal of Infectious Diseases 2012; 206: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 6.Althouse BM, Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Medicine 2015; 13: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaels RH, Norden CW. Pharyngeal colonization with Haemophilus influenzae type b: a longitudinal study of families with a child with meningitis or epiglottitis due to H. influenzae type b. Journal of Infectious Diseases 1977; 136: 222–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://doi.org/10.1017/S0950268817000115.
click here to view supplementary material