Abstract
Improving access to tuberculosis (TB) care and ensuring early diagnosis are two major aims of the WHO End TB strategy and the Collaborative TB Strategy for England. This study describes risk factors associated with diagnostic delay among TB cases in England. We conducted a retrospective cohort study of TB cases notified to the Enhanced TB Surveillance System in England between 2012 and 2015. Diagnostic delay was defined as more than 4 months between symptom onset and treatment start date. Multivariable logistic regression was used to identify demographic and clinical factors associated with diagnostic delay. Between 2012 and 2015, 22 422 TB cases were notified in England and included in the study. A third (7612) of TB cases had a diagnostic delay of more than 4 months. Being female, aged 45 years and older, residing outside of London and having extra-pulmonary TB disease were significantly associated with a diagnostic delay in the multivariable model (aOR = 1.2, 1.2, 1.2, 1.3, 1.8, respectively). This study identifies demographic and clinical factors associated with diagnostic delay, which will inform targeted interventions to improve access to care and early diagnosis among these groups, with the ultimate aim of helping reduce transmission and improve treatment outcomes for TB cases in England.
Key words: Diagnostic delay, treatment delay, tuberculosis
Introduction
Improving access to tuberculosis (TB) care and ensuring early diagnosis of cases has the potential to reduce transmission and improve treatment outcomes for patients with TB. One of the main pillars of the WHO ‘End TB’ strategy is to promote early TB detection through access to early and accurate diagnostics, information and engagement of patients seeking care and healthcare providers, and the abolition of barriers that people encounter when seeking care [1].
Between 2010 and 2015, there was a small increase in the recorded time between symptom onset and treatment start for pulmonary TB cases in England [2]. In 2015, Public Health England and National Health Service England launched the Collaborative TB Strategy for England (2015–2020) [3]. One of the ten key evidence-based areas for action in the strategy is improving access to care and ensuring early diagnosis. The main actions recommended are raising awareness of signs and symptoms of TB and where to seek care among high-risk communities, and raising awareness and knowledge of TB among the healthcare community.
This study aims to describe the TB patients who experienced a diagnostic delay, and identify demographic and clinical factors associated with diagnostic delay among TB patients in England to inform the development of more targeted interventions to reduce diagnostic delay.
Methods
Study population and data collection
We conducted a retrospective cohort study of TB cases notified to the Enhanced TB Surveillance System (ETS) in England between 2012 and 2015. All TB cases with a symptom onset date and start treatment date were included in the study. Those diagnosed post-mortem were excluded from the analysis.
The following demographic and clinical information were extracted from ETS: age, sex, country of birth, time since entry to the UK for non-UK-born cases, site of disease, social risk factors, area in England where case resided during treatment and previous TB diagnosis. The site of disease was classified into extra-pulmonary TB only, and pulmonary TB with or without extra-pulmonary TB. Data were available on the following social risk factors: current alcohol misuse that would impact on the patient's ability to take treatment without DOT, current or history of drug misuse, homelessness and/or imprisonment. The following clinical dates were extracted from ETS: symptom onset date as reported by the patient, date the patient first presented to health services, date of TB diagnosis, date the first specimen was taken and date the patient started TB treatment.
Descriptive and statistical analysis
Time between symptom onset and treatment start date
The median time (and interquartile range (IQR)) between symptom onset date and treatment start date was described in days. Diagnostic delay was defined as having more than 4 months between symptom onset date and treatment start date. This categorisation was defined based on the distribution of the data, and was consistent with the TB Strategy Monitoring Indicators for England [3]. The trend in diagnostic delay over time was assessed using Poisson regression and statistical significance was tested.
Using knowledge of TB epidemiology in England, as well as the literature, we identified and categorised all variables for which we collected data in our surveillance system into potential confounders or variables potentially on the causal pathway for the diagnostic delay. We compared demographic and clinical factors between TB cases who had a diagnostic delay and those who did not using univariable logistic regression to calculate odd ratios (ORs). We grouped variables according to findings from the univariable logistic regression in order to make logical comparisons. Non-UK-born cases were categorised by the time since entry to the UK and compared to UK-born cases. Having any social risk factor included alcohol misuse, drug misuse, homelessness or imprisonment. The Public Health England Centre a TB case was resident in was categorised into within London or outside of London. A forward stepwise multivariable logistic regression was created including factors with a P-value of <0.5 from the univariable analysis and we assessed the corresponding likelihood ratio tests. All factors identified as significant from the univariable analyses were included as covariates and adjusted for in the final multivariable model, and adjusted odd ratios (aORs) were calculated. Due to an interaction between site of disease (pulmonary/extra-pulmonary) and country of birth (UK-born vs. non-UK born and time since entry to the UK) on the association with diagnostic delay, the multivariable analysis was stratified by site of disease.
Patient and healthcare delay
To take into account missing dates a derived variable, ‘earliest date’, was created to represent the first date a case was reported to have accessed TB health services using the earliest of either: date first presented to health services, date of TB diagnosis, (collection) date of the first specimen or notification date.
Patient delay was defined as the time between symptom onset and the earliest date, and the healthcare delay was defined as the time between earliest date and treatment start date.
All analyses were conducted using Stata V.13.1 (Statacorp, Texas). Public Health England has authority under the Health and Social Care Act 2012 to hold and analyse national surveillance data for public health and research purposes.
Results
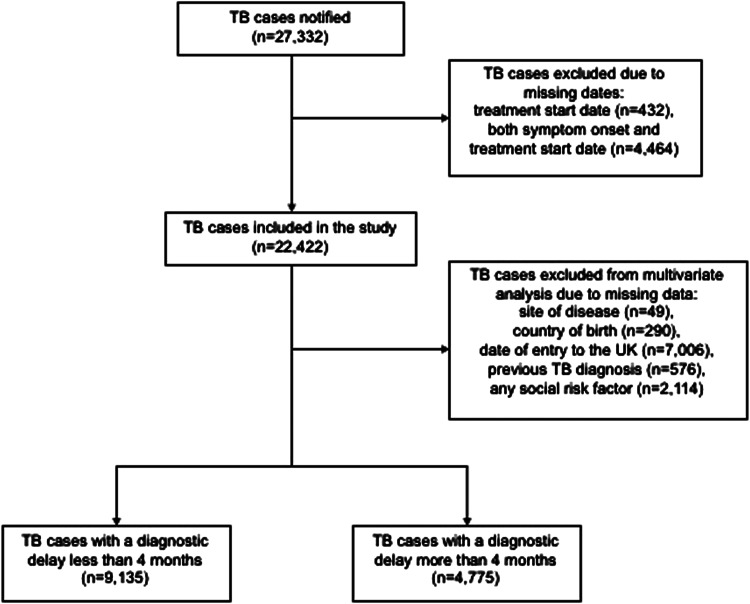
There were 27 332 TB cases notified in England between 2012 and 2015. Of these, 4910 (18.0%) cases were excluded because symptom onset date and/or treatment start date were missing, so 22 422 cases were included in the study (Fig. 1). The proportion of cases with any missing date data decreased over the time period from 27.0% in 2012 to 8.2% in 2015. There were no major differences in the demographic characteristics between cases that were missing dates and those that were not (Table 1).
Fig. 1.
Flow diagram of TB cases included in analysis of diagnostic delay, England, 2012–2015.
Table 1.
Comparison of demographic factors between TB cases missing symptom onset date and/or treatment start date vs. TB cases with dates that were included in the study
| Variable | TB cases missing dates and not included in the study | TB cases with dates and included in the study |
|---|---|---|
| Age (years) | Median 36 (IQR 26–54) | Median 37 (27–52) |
| Sex (%) | 56.7 male | 58.3 male |
| UK-born (%) | 26.2 | 26.3 |
| Pulmonary disease (%) | 49.1 | 52.7 |
| Previous diagnosis (%) | 8.1 | 6.4 |
Time between symptom onset and starting treatment (diagnostic delay)
The median time between symptom onset and starting treatment was 2.8 months (IQR 1.4–5.2 months). 66.1% (14 810) of TB cases started treatment < 4 months after symptom onset, with 16.9% (3801) starting within 1 month, 19.8% (4449) between 1 and 2 months, 16.8% (3772) between 2 and 3 months and 12.4% (2788) between 3 and 4 months.
A total of 7612 (34.0%) TB cases were defined as having a diagnostic delay of more than 4 months and, of these, 1635 had more than 1 year between symptom onset and starting treatment. There was a small but significant increase in the proportion of TB cases with a diagnostic delay of more than 4 months between 2012 and 2015 (32.1% (1879) to 34.1% (1786)) (P-value 0.003).
A higher proportion of female TB cases had a diagnostic delay of more than 4 months compared with male TB cases (36.8%, (3440/9357) vs. 31.9%, (4172/13 065)) (Table 2). Diagnostic delay increased with age from 15.1% (118/780) among TB cases aged < 14 years to 38.4% (1135/2954) among TB cases aged over 65 years. A similar proportion of UK-born and non-UK-born TB cases experienced a diagnostic delay (34.6% (2014/5824) vs. 33.8% (5514/16 308)). A high proportion of TB cases from the Philippines (40.1%, 159/397), Pakistan (37.8%, 1108/2930), Bangladesh (37.0%, 280/757) and Kenya (37.0%, 95/257) experienced diagnostic delay compared with a smaller proportion among TB cases from Nepal (29.8%, 172/577) and India (31.5%, 1453/4612). A higher proportion of TB cases with only extra-pulmonary disease experienced diagnostic delay compared with TB cases with pulmonary disease (40.4%, (4277/10 594) vs. 28.2% (3318/11 779)).
Table 2.
Baseline characteristics of study population
| Characteristic | >4 months DD | Total | |
|---|---|---|---|
| n | % | n | |
| Sex | |||
| Female | 3440 | 36.8 | 9357 |
| Male | 4172 | 31.9 | 13 065 |
| Age (year) | |||
| 0–14 | 118 | 15.1 | 780 |
| 15–44 | 4432 | 32.6 | 13 614 |
| 45–64 | 1927 | 38.0 | 5074 |
| 65+ | 1135 | 38.4 | 2954 |
| Country of birth | |||
| UK | 2014 | 34.6 | 5824 |
| India | 1453 | 31.5 | 4612 |
| Pakistan | 1108 | 37.8 | 2930 |
| Somalia | 283 | 32.0 | 885 |
| Bangladesh | 280 | 37.0 | 757 |
| Nepal | 172 | 29.8 | 577 |
| Nigeria | 164 | 34.9 | 470 |
| Zimbabwe | 126 | 32.4 | 389 |
| Philippines | 159 | 40.1 | 397 |
| Kenya | 95 | 37.0 | 257 |
| Other | 1597 | 33.2 | 4807 |
| Time since entry for foreign-born cases (year) | |||
| 0–1 | 607 | 25.3 | 2403 |
| 2–4 | 1051 | 31.9 | 3294 |
| 5–9 | 1167 | 34.4 | 3390 |
| 10+ | 2441 | 38.6 | 6329 |
| Site of disease | |||
| Extra-pulmonary only | 4277 | 40.4 | 10 594 |
| Pulmonary (with or without extra-pulmonary) | 3318 | 28.2 | 11 779 |
| Previous diagnosis | |||
| No | 7004 | 34.3 | 24 809 |
| Yes | 458 | 32.7 | 1660 |
| Social risk factors | |||
| No risk factors | 6347 | 34.5 | 18 388 |
| At least 1 risk factor | 589 | 30.7 | 1920 |
| Drug misuse | |||
| No | 7058 | 34.2 | 20 653 |
| Yes | 230 | 32.0 | 718 |
| Alcohol misuse | |||
| No | 7056 | 34.4 | 20 542 |
| Yes | 192 | 27.7 | 694 |
| Homeless | |||
| No | 7079 | 34.2 | 20 711 |
| Yes | 196 | 28.2 | 695 |
| Prison | |||
| No | 6916 | 34.1 | 20 293 |
| yes | 242 | 35.2 | 688 |
| PHEC | |||
| East Midlands | 510 | 37.0 | 1380 |
| East of England | 541 | 38.7 | 1397 |
| London | 2585 | 30.0 | 8623 |
| North East | 158 | 30.2 | 523 |
| North West | 763 | 35.0 | 2182 |
| South East | 994 | 40.2 | 2475 |
| South West | 359 | 34.7 | 1034 |
| West Midlands | 1089 | 35.9 | 3038 |
| Yorkshire and Humber | 613 | 34.6 | 1770 |
For cases of pulmonary TB, a higher proportion who were born in the UK (32.1% (1282/3991)) experienced a diagnostic delay compared with those who were non-UK born (26.1% (1985/7611)). Among pulmonary TB cases where sputum smear results were known, 39.9% (954/2389) were sputum smear positive.
The areas of England with the highest proportion of TB cases that experienced diagnostic delay were the South East (40.2%, 994/2475) and East of England (38.7%, 541/1397), whereas a lower proportion of TB cases residing in London or North East had a diagnostic delay (30.0%, 2585/8623 and 30.2%, 158/523, respectively).
Factors associated with diagnostic delay
Cases with missing data on site of disease, country of birth, date of entry to the UK, previous TB diagnosis and any social risk factor were excluded from the multivariable analysis (Fig. 1). In the multivariable analysis adjusted for age, sex and all significant variables from the univariable analysis, the following factors were significantly associated with diagnostic delay: female (aOR = 1.2, 95% CI 1.1–1.3), age 45 years and older (aOR = 1.2, 95% CI 1.1–1.3 and aOR = 1.2, 95% CI 1.1–1.4), residence outside London (aOR = 1.3, 95% CI 1.3–1.4) and having extra-pulmonary disease only (aOR = 1.8, 95% CI 1.6–1.9) (Table 3). Foreign-born TB cases who had entered the UK <10 years ago were significantly less likely to experience diagnostic delay compared with TB cases born in the UK (aOR = 0.6, 95% CI 0.5–0.7; aOR = 0.8, 95% CI 0.7–0.9 and aOR = 0.9, 95% CI 0.8–1.0), whereas foreign-born TB cases who had entered the UK more than 10 years ago had no difference in diagnostic delay compared with the UK-born TB cases. There was no significant difference in diagnostic delay between TB cases with a social risk factor and those without.
Table 3.
Univariable and multivariable logistic regression models comparing TB cases with less than and greater than 4 months between symptom onset and starting treatment
| Characteristics | <4 months | >4 months | col chi | Univariable analysis | Multivariable analysisa | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | N | % | P value | OR | 95% CI | aOR | 95% CI | |
| Sex | |||||||||
| Female | 5917 | 40.0 | 3440 | 45.2 | <0.001 | 1.2 | 1.2–1.3 | 1.2 | 1.1–1.3 |
| Male | 8893 | 60.1 | 4172 | 54.8 | 1.0 | – | 1.0 | – | |
| Age group | |||||||||
| 0–14 | 662 | 4.5 | 118 | 1.6 | <0.001 | 0.4 | 0.3–0.5 | 0.3 | 0.3–0.4 |
| 15–44 | 9182 | 62.0 | 4432 | 58.2 | 1.0 | – | 1.0 | – | |
| 45–64 | 3147 | 21.3 | 1927 | 25.3 | 1.3 | 1.2–1.4 | 1.2 | 1.1–1.3 | |
| 65+ | 1819 | 12.3 | 1135 | 14.9 | 1.3 | 1.2–1.4 | 1.2 | 1.1–1.4 | |
| London | |||||||||
| No | 8772 | 59.2 | 5027 | 66.0 | <0.001 | 1.3 | 1.3–1.4 | 1.3 | 1.3–1.4 |
| Yes | 6038 | 40.8 | 2585 | 34.0 | 1.0 | – | 1.0 | – | |
| UK-born/time since entry | |||||||||
| UK-born | 3810 | 27.3 | 2014 | 27.7 | <0.001 | 1.0 | – | 1.0 | – |
| Non UK-born 0–1 years | 1796 | 12.9 | 607 | 8.3 | 0.6 | 0.6–0.7 | 0.6 | 0.5–0.7 | |
| Non UK-born 2–4 years | 2243 | 16.1 | 1051 | 14.4 | 0.9 | 0.8–0.9 | 0.8 | 0.7–0.9 | |
| Non UK-born 5–9 years | 2223 | 15.9 | 1167 | 16.0 | 1 | 0.9–1.1 | 0.9 | 0.8–1.0 | |
| Non UK-born 10+ years | 3888 | 27.9 | 2441 | 33.5 | 1.2 | 1.1–1.3 | 1.0 | 0.9–1.1 | |
| Extra-pulmonary | |||||||||
| Extra-pulmonary only | 6317 | 42.8 | 4277 | 56.3 | <0.001 | 1.7 | 1.6–1.8 | 1.8 | 1.6–1.9 |
| Pulmonary ± extra-pulmonary | 8461 | 57.3 | 3318 | 43.7 | 1.0 | – | 1.0 | – | |
| Previous diagnosis | |||||||||
| No | 13 443 | 93.5 | 7004 | 93.9 | 0.247 | 1.1 | 0.9–1.2 | 1.1 | 1.0–1.3 |
| Yes | 941 | 6.5 | 458 | 6.1 | 1.0 | – | 1.0 | – | |
| Any risk factor | |||||||||
| No | 12 041 | 90.1 | 6347 | 91.5 | 0.001 | 1.0 | – | 1.0 | – |
| Yes | 1331 | 10.0 | 589 | 8.5 | 0.8 | 0.8–0.9 | 1.0 | 0.9–1.1 | |
Multivariable analysis was adjusted for age, sex, area of residence, country of birth and time since entry to the UK, site of disease and social risk factor.
When analysing the subgroup of cases with extra pulmonary disease only (Table 4), those that were foreign born and had been in the UK for <1 year were significantly less likely to have a diagnostic delay (aOR = 0.7, 95% CI 0.6–0.8), foreign born cases who had been in the UK for 2–10 years had no difference in diagnostic delay and foreign-born cases who had been in the UK for more than 10 years were significantly more likely to have a diagnostic delay (aOR = 1.3, 95% CI 1.1–1.4) than TB cases born in the UK. This trend was different among TB cases with pulmonary disease (Table 5) where all foreign-born cases regardless of the time living in the UK were significantly less likely to have a diagnostic delay compared with TB cases born in the UK (aOR = 0.6, 95% CI 0.5–0.7; aOR = 0.7, 95% CI 0.6–0.8; aOR = 0.8, 95% CI 0.7–0.9; aOR = 0.8, 95% CI 0.7–0.9).
Table 4.
Univariable and multivariable logistic regression models comparing extra-pulmonary TB cases with less than and greater than 4 months between symptom onset and starting treatment
| Characteristics | <4 months | >4 months | col chi | Univariable analysis | Multivariable analysisa | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | P value | OR | 95% CI | aOR | 95% CI | |
| Sex | |||||||||
| Female | 2682 | 42.5 | 2047 | 47.9 | <0.001 | 1.2 | 1.2–1.3 | 1.2 | 1.1–1.3 |
| Male | 3635 | 57.5 | 2230 | 52.1 | 1.0 | – | 1.0 | – | |
| Age group | |||||||||
| 0–14 | 203 | 3.2 | 67 | 1.6 | <0.001 | 0.5 | 0.4–0.7 | 0.5 | 0.4–0.7 |
| 15–44 | 4136 | 65.5 | 2668 | 62.4 | 1.0 | – | 1.0 | – | |
| 45–64 | 1325 | 21.0 | 1054 | 24.6 | 1.2 | 1.1–1.4 | 1.1 | 1.0–1.2 | |
| 65+ | 653 | 10.3 | 488 | 11.4 | 1.2 | 1.0–1.3 | 1.0 | 0.9–1.2 | |
| London | |||||||||
| No | 3515 | 55.6 | 2723 | 63.7 | <0.001 | 1.4 | 1.3–1.5 | 1.4 | 1.3–1.6 |
| Yes | 2802 | 44.4 | 1554 | 36.3 | 1.0 | – | 1.0 | – | |
| UK-born/time since entry | |||||||||
| UK-born | 1089 | 18.3 | 723 | 17.6 | <0.001 | 1.0 | – | 1.0 | – |
| Non UK-born 0–1 years | 760 | 12.8 | 339 | 8.3 | 0.7 | 0.6–0.8 | 0.7 | 0.6–0.8 | |
| Non UK-born 2–4 years | 1138 | 19.2 | 698 | 17.0 | 0.9 | 0.8–1.1 | 1.0 | 0.8–1.1 | |
| Non UK-born 5–9 years | 1116 | 18.8 | 770 | 18.8 | 1 | 0.9–1.2 | 1.1 | 0.9–1.2 | |
| Non UK-born 10+ years | 1838 | 30.9 | 1573 | 38.3 | 1.3 | 1.1–1.4 | 1.3 | 1.1–1.4 | |
| Previous diagnosis | |||||||||
| No | 5813 | 94.4 | 3976 | 94.5 | 0.9 | 1.0 | 0.9–1.2 | 1.1 | 0.9–1.3 |
| Yes | 345 | 5.6 | 233 | 5.5 | 1.0 | – | 1.0 | – | |
| Any social risk factor | |||||||||
| No | 5459 | 95.1 | 3730 | 95.9 | 0.1 | 1.0 | – | 1.0 | – |
| Yes | 280 | 4.9 | 160 | 4.1 | 0.8 | 0.7–1.0 | 0.9 | 0.8–1.1 | |
Multivariable analysis was adjusted for age, sex, area of residence, country of birth and time since entry to the UK, site of disease and social risk factor.
Table 5.
Univariable and multivariable logistic regression models comparing pulmonary TB cases with less than and greater than 4 months between symptom onset and starting treatment
| Characteristics | <4 months | >4 months | col chi | Univariable analysis | Multivariable analysisa | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | P value | OR | 95% CI | aOR | 95% CI | |
| Sex | |||||||||
| Female | 3216 | 38.0 | 1386 | 41.8 | <0.001 | 1.2 | 1.1–1.3 | 1.2 | 1.1–1.3 |
| Male | 5245 | 62.0 | 1932 | 58.2 | 1.0 | – | 1.0 | – | |
| Age group | |||||||||
| 0–14 | 454 | 5.4 | 51 | 1.5 | <0.001 | 0.3 | 0.2–0.4 | 0.2 | 0.2–0.3 |
| 15–44 | 5035 | 59.5 | 1753 | 52.8 | 1.0 | – | 1.0 | – | |
| 45–64 | 1817 | 21.5 | 869 | 26.2 | 1.4 | 1.2–1.5 | 1.3 | 1.1–1.4 | |
| 65+ | 1155 | 13.7 | 645 | 19.4 | 1.6 | 1.4–1.8 | 1.4 | 1.3–1.6 | |
| London | |||||||||
| No | 5226 | 61.8 | 2287 | 68.9 | <0.001 | 1.4 | 1.3–1.5 | 1.2 | 1.1–1.3 |
| Yes | 3235 | 38.2 | 1031 | 31.1 | 1.0 | – | 1.0 | – | |
| UK-born/time since entry | |||||||||
| UK-born | 2709 | 33.9 | 1282 | 40.6 | <0.001 | 1.0 | – | 1.0 | – |
| Non-UK-born 0–1 years | 1036 | 13.0 | 266 | 8.4 | 0.5 | 0.5–0.6 | 0.6 | 0.5–0.7 | |
| Non-UK-born 2–4 years | 1104 | 13.8 | 353 | 11.2 | 0.7 | 0.6–0.8 | 0.7 | 0.6–0.8 | |
| Non-UK-born 5–9 years | 1100 | 13.8 | 395 | 12.5 | 0.8 | 0.7–0.9 | 0.8 | 0.7–0.9 | |
| Non-UK-born 10+ years | 2043 | 25.6 | 865 | 27.4 | 0.9 | 0.8–1.0 | 0.8 | 0.7–0.9 | |
| Previous diagnosis | |||||||||
| No | 7606 | 92.8 | 3013.0 | 93.1 | 0.6 | 1.0 | 0.9–1.2 | 1.2 | 1.0–1.4 |
| Yes | 592 | 7.2 | 224.0 | 6.9 | 1.0 | – | 1.0 | – | |
| Any social risk factor | |||||||||
| No | 6557 | 86.2 | 2601 | 85.8 | 0.6 | 1.0 | – | 1.0 | – |
| Yes | 1051 | 13.8 | 429.0 | 14.2 | 1 | 0.9–1.2 | 1.0 | 0.9–1.1 | |
Multivariable analysis was adjusted for age, sex, area of residence, country of birth and time since entry to the UK, site of disease and social risk factor.
Patient and healthcare delays
The median patient delay (time between symptom onset and earliest date) was 1.3 months (IQR 13 days–3.2 months). The patient delay was longer among TB cases with the extra-pulmonary disease compared with those with pulmonary disease (1.5 months (IQR 14 days–3.7 months) vs. 1.2 months (IQR 13 days–3.0 months), P-value <0.001). The median healthcare delay (time between earliest date and the start date of treatment) was 14 days (IQR 2 days–1.7 months). The healthcare delay was also longer among TB cases with the extra-pulmonary disease compared with those with pulmonary disease (21 days (IQR 1 day–2.2 months) vs. 10 days (IQR 2 days–1.4 months), P-value <0.001).
Discussion
Overall a third of TB cases notified in England between 2012 and 2015 had more than a 4-month delay between the onset of symptoms and starting treatment, and there was a small but significant increase in the proportion of TB cases with a diagnostic delay over the time period.
Our study showed that TB cases who were female, aged 45 years and older, UK-born, resided outside London or had extra-pulmonary TB were significantly more likely to have a delay in diagnosis of more than 4 months between onset of symptoms and starting treatment. Studies in similar countries with low TB incidence have described similar factors associated with diagnostic delay; however, none have used national-level data [4–15]. There has been one previous study in the UK using national level-data to assess diagnostic delay among index cases and their contacts within households, which found that there was a median delay of 65 days for index cases and 61 days for subsequent symptomatic cases [16].
Some previous studies have shown that women with TB have more barriers to accessing care than men, specifically women experience longer healthcare-related delays than men, but similar patient delays to men [15, 17, 18]. This would support our finding that women are more likely to have a diagnostic delay of more than 4 months. Conversely, a recent systematic review and meta-analysis have begun to challenge the idea that men with TB do not experience barriers to accessing care. This study compared TB notification data to data from TB prevalence studies and found there is a higher prevalence among men than women, which suggests men are disadvantaged in seeking and/or accessing TB services [19].
Many studies have, like ours, shown that diagnostic delay increases with increasing age [5, 9, 14, 15]. This is likely to reflect increasing co-morbidities with age, which may make it more difficult to recognise TB symptoms. In addition, studies in Japan, which has a large proportion of TB in the elderly population, show that elderly patients often do not present with typical TB symptoms, which has led to large TB clusters among these populations [20, 21]. The authors conclude that increased knowledge of TB in people who work in care homes could reduce transmission. One study in New York showed the consequences of a long delay in TB diagnosis among elderly populations who often have co-morbidities, which increased the likelihood of transmission and also increased the severity of both the TB disease and the co-morbidity [7].
We found that UK-born TB cases were more likely to have a delay of more than 4 months between symptom onset and starting treatment compared with non-UK-born TB cases who had been in the UK for <10 years. The fact that UK-born cases, or cases born in a low incidence country, have longer diagnostic delay has been shown in several other studies in low TB-incidence countries [5, 6, 12, 15]. The shorter delay for foreign-born TB cases may be influenced by the active screening strategies targeted at these groups. In addition, both patients and healthcare workers may have a higher index of suspicion of TB among these populations, where reactivation of latent TB infection or primary active disease acquired in their home country is more common [5, 12]. Novel to our study, we found interesting patterns in diagnostic delay between short-term and long-term migrants. For instance, non-UK-born cases who had been in the UK for more than 10 years had no difference in diagnostic delay compared with UK-born cases. A possible explanation is that both patients and healthcare workers may believe that those that have lived in the UK for many years no longer have an increased risk of TB. Although it has previously been documented that knowledge of TB impacts diagnostic delay [9, 11], there are no published studies looking at the knowledge of the risk of TB in relation to time spent outside high-TB burden countries, so this would be an interesting hypothesis to explore in a qualitative study.
In our study, extra-pulmonary TB disease was significantly associated with longer diagnostic delays. Approximately half of all TB cases notified in England have extra-pulmonary disease only, so diagnostic delay among these cases has a particularly large impact [2]. A currently unpublished audit of TB cases (pulmonary and extra-pulmonary) in the South East of England found that a poor understanding of referral pathways between primary and secondary care, along with referral to multiple specialties not familiar with TB (particularly oncology and ENT) may contribute to delays. Although extra-pulmonary TB cases do not contribute to transmission, a long delay between symptom onset and starting treatment contributes to increased morbidity at an individual level [22].
TB cases that resided outside London during their treatment were more likely to experience diagnostic delays. Diagnostic delay was highest in the South East, East of England and East Midlands regions of England, which are the areas in the country with the lowest TB incidence [2]. This may reflect less awareness of TB among the populations and healthcare workers where TB is less common. In addition, more specialist services, such as the London mobile chest X-ray unit, which has been shown to reduce diagnostic delay among under-served populations [23–25], are located in London where TB incidence is highest. A previous study in London showed that within the city, differences in TB service capacity and organisational factors appeared to have an impact on diagnostic delay [5]. Although a different context, a study in Colombia found a decreased risk of diagnostic delay in neighbourhoods with stronger healthcare facilities, showing, unsurprisingly, that allocation of resources has an impact on diagnostic delay [10].
We found, somewhat surprisingly, that there was no difference in diagnostic delay among TB cases with a social risk factor and those without a social risk factor, despite the fact that those with social risk factors typically have more difficulty accessing services. This may reflect the existence of services such as the London mobile chest X-ray unit, which specifically target these groups. A study in the USA found diagnostic delay was higher among TB cases without social risk factors (alcohol abuse and homelessness), and suggested that this may be because of the high suspicion of TB among groups with social risk factors [12], which could also be a factor in our context.
Our study has several limitations. While notification of TB cases is mandatory, the reporting of the dates used in this study is not mandatory, and data completion was poor until recently. Therefore, the study was limited to the most recent 4 years of data, when the symptom onset date and treatment initiation date were completed for more than two-thirds of cases. Additionally, as symptom onset is often insidious, the recall of this date may be inaccurate at the individual level. However, this is only likely to have introduced bias into our study if there was differential recall between groups.
We were unable to do detailed analyses on the differences in patient delays and healthcare delays because the date which the patient first presented to a healthcare facility is often missing from the notification, and when it is reported it is unclear whether this is the date the patient first presented to any healthcare facility or to the TB service. Better understanding and completion of this field in the future should enable further exploration of the extent to which delays are due to patient or healthcare issues.
In conclusion, our study supports the need for targeted interventions to increase awareness of TB in both the public and the healthcare community, in order to decrease time to diagnosis as set out in the national TB strategy [3]. To further understand the barriers to diagnosis there is a need for studies to look at the pathway patients take to get diagnosed, and qualitative studies to understand knowledge and perceptions of TB among different populations. Our finding that diagnostic delay is longer outside typical risk groups for TB, and in those with the extra-pulmonary disease, demonstrates the need to increase awareness of TB beyond a focus on high-risk groups and the typical presentation of pulmonary TB disease. We recommend that strategies to reduce diagnostic delay widen their focus to include the specific needs of women, UK-born individuals, long-term migrants and TB cases with the extra-pulmonary disease. Given the epidemiology of TB in England, with the majority of cases occurring outside classic risk groups, among long-term residents in the UK or the UK-born, and with around half of cases having extra-pulmonary TB only, reducing diagnostic delay in these groups has the potential to substantially reduce morbidity from TB in this country.
Acknowledgements
We acknowledge all those who contributed information on TB cases in England, including physicians, nurses, microbiologists, scientists and administrative staff. We also acknowledge all those involved in data management from sub-national and national levels of TB surveillance.
Financial support
None.
Ethics approval
Public Health England has authority under the Health and Social Care Act 2012 to hold and analyse national screening data from the LTBI testing and treatment programme for public health and research purposes.
Competing interests
None.
References
- 1.World Health Organisation (2015) The End TB Strategy. [cited 2017 Dec 2]. http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1.
- 2.Public Health England (2016) Tuberculosis in England: 2016 report (presenting data to the end of 2015). Public Health England.
- 3.Public Health England, NHS-England (2015) Collaborative TB Strategy for England 2015–2020. [cited 2017 Dec 2]. https://www.gov.uk/government/publications/collaborative-tuberculosis-strategy-for-england.
- 4.Golub JE et al. (2006) Delayed tuberculosis diagnosis and tuberculosis transmission. International Journal of Tuberculosis and Lung Disease 10, 24–30. [PubMed] [Google Scholar]
- 5.Paynter S et al. (2004) Patient and health service delays in initiating treatment for patients with pulmonary tuberculosis: retrospective cohort study. International Journal of Tuberculosis and Lung Disease 8, 180–185. [PubMed] [Google Scholar]
- 6.Rodger A et al. (2003) Delay in the diagnosis of pulmonary tuberculosis, London, 1998–2000: analysis of surveillance data. BMJ 326, 909–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris TG, Sullivan Meissner J and Proops D (2013) Delay in diagnosis leading to nosocomial transmission of tuberculosis at a New York city health care facility. American Journal of Infection Control 41, 155–160. [DOI] [PubMed] [Google Scholar]
- 8.Otwombe KN et al. (2013) Predictors of delay in the diagnosis and treatment of suspected tuberculosis in HIV co-infected patients in South Africa. International Journal of Tuberculosis and Lung Disease 17, 1199–1205. [DOI] [PubMed] [Google Scholar]
- 9.Gebreegziabher SB, Bjune GA and Yimer SA (2016) Patients’ and health system's delays in the diagnosis and treatment of new pulmonary tuberculosis patients in West Gojjam Zone, Northwest Ethiopia: a cross-sectional study. BMC Infectious Diseases 16, 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez DA et al. (2016) Monitoring delays in diagnosis of pulmonary tuberculosis in eight cities in Colombia. Pan American Journal of Public Health 39, 12–18. [PubMed] [Google Scholar]
- 11.Dewi C et al. (2016) Improving knowledge and behaviours related to the cause, transmission and prevention of Tuberculosis and early case detection: a descriptive study of community led Tuberculosis program in Flores, Indonesia. BMC Public Health 16, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace RM et al. (2009) Increasing proportions of advanced pulmonary tuberculosis reported in the United States. American Journal of Respiratory and Critical Care Medicine 180, 1016–1022. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim WH et al. (2016) Diagnostic delay among adults with pulmonary tuberculosis in a high gross domestic product Per capita country: reasons and magnitude of the problem. International Journal of Preventive Medicine 7, Published online: 26 Oct 2016. doi: 10.4103/2008-7802.193091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki NDS et al. (2015) Delays in tuberculosis suspicion and diagnosis and related factors. Revista Brasileira de Epidemiologia 18, 809–823. [DOI] [PubMed] [Google Scholar]
- 15.Saldana L et al. (2013) Factors affecting delay in initiation of treatment of tuberculosis in the Thames Valley, UK. Public Health 127, 171–177. [DOI] [PubMed] [Google Scholar]
- 16.Lalor MK et al. (2017). Recent household transmission of tuberculosis in England, 2010–2012: retrospective national cohort study combining epidemiological and molecular strain typing data. BMC Medicine 15, 1–10. Published online: 13 June 2017. doi: 10.1186/s12916-017-0864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long NH et al. (1999) Longer delays in tuberculosis diagnosis among women in Vietnam. International Journal of Tuberculosis and Lung Disease 3, 388–393. [PubMed] [Google Scholar]
- 18.World Health Organization. TDR|Gender and tuberculosis: cross-site analysis and implications of a multi-country study in Bangladesh, India, Malawi, and Colombia [Internet]. WHO. [cited 2017 Mar 27]. Available at http://www.who.int/tdr/publications/tdr-research-publications/gender-tb-multicountry-study/en/.
- 19.Horton KC et al. (2016) Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Medicine 13, e1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seto J et al. (2017) Mycobacterium tuberculosis transmission among elderly persons, Yamagata prefecture, Japan, 2009–2015. Emerging Infectious Diseases 23, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyota E et al. (2010) Clinical investigation among elderly patients with tuberculosis. Kekkaku 85, 655–660. [PubMed] [Google Scholar]
- 22.Virenfeldt J et al. (2014). Treatment delay affects clinical severity of tuberculosis: a longitudinal cohort study. BMJ Open 4, 1–8. Published online: 10 June 2014. doi: 10.1136/bmjopen-2014-004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Story A et al. (2012) Active case finding for pulmonary tuberculosis using mobile digital chest radiography: an observational study. International Journal of Tuberculosis and Lung Disease 16, 1461–1467. [DOI] [PubMed] [Google Scholar]
- 24.Aldridge RW et al. (2015) Effectiveness of peer educators on the uptake of mobile X-ray tuberculosis screening at homeless hostels: a cluster randomised controlled trial. BMJ Open 5, e008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lönnroth K et al. (2015) Towards tuberculosis elimination: an action framework for low-incidence countries. European Respiratory Journal 45, 928–952. [DOI] [PMC free article] [PubMed] [Google Scholar]