Abstract
Chronic hepatitis C virus (HCV) infection is the most common blood-borne infection in the USA. Estimating prevalence is critical for monitoring diagnosis, treatment, and cure and for allocating resources. Surveillance data reported to the New York City (NYC) Health Department, 2000–2015, were used to estimate HCV prevalence in NYC in 2015. The numbers who died, out-migrated or whose last RNA test was negative were removed from the count of people reported with HCV. A simulation model was used to remove estimates of people whose infection spontaneously cleared or was cured and to add an estimate of people unaware of infection. The surveillance-based HCV prevalence in NYC in 2015 overall was 1.4% (95% certainty level (CL) 1.2–1.6%; n ≈ 116 000, 95% CL ≈99 000–135 000) and among adults aged ⩾20 years was 1.8% (95% CL 1.5–2.0%, n ≈ 115 000, 95% CL ≈99 000–134 000), lower than the 2010 estimate among adults aged ⩾20 years of 2.4% (n ≈ 147 000). Contributors to the decrease in HCV prevalence from 2010 to 2015 include both the availability of highly effective treatment and also deaths among an ageing population. The 2015 estimate can be used to set NYC-specific HCV screening and treatment targets and monitor progress towards HCV elimination.
Key words: Estimating, hepatitis C, prevalence of disease, surveillance
Introduction
Chronic hepatitis C virus (HCV) infection has been considered the most common blood-borne infection in the USA [1]. Without linkage to care and treatment, HCV can lead to cirrhosis, liver failure, hepatocellular carcinoma and death [2, 3]. In the USA, deaths attributable to HCV now exceed deaths attributable to all other nationally notifiable conditions combined, including HIV [4]. Despite highly effective treatment with high cure rates, many people infected with HCV remain unaware of their status and are not receiving appropriate care and treatment.
Estimating the prevalence of HCV infection is critical for monitoring diagnosis, treatment and cure rates, as well as for determining resource allocation and capacity for treatment. The National Health and Nutrition Examination Survey (NHANES), a population-based health survey, has been used to estimate HCV prevalence nationally and at the state level. NHANES only samples the non-institutionalised, civilian population, which excludes or underrepresents many groups at higher risk of being infected with HCV (e.g. people who are homeless or incarcerated or who inject drugs), and therefore should not be the only data source used to avoid underestimating HCV prevalence. Consequently, others have refined NHANES estimates using mortality data from the National Vital Statistics System [5] or peer-reviewed and grey literature to estimate prevalence for populations under- or not represented in NHANES [6, 7]. Passive surveillance data for a given jurisdiction will include these individuals if they are tested because positive HCV results from all facilities (e.g. jails and drug treatment programmes) are reported to health departments and are nationally notifiable. However, surveillance data alone might underestimate the true prevalence of HCV because it can take years for symptoms of infection to develop and is therefore often undiagnosed.
Given these considerations, this analysis uses surveillance data to estimate the prevalence of HCV among all ages and among adults aged ⩾20 years in 2015 residing in New York City (NYC), avoiding several limitations of using population health surveys. This analysis updates the NYC 2010 surveillance-based prevalence estimate generated by Balter et al. [8] with an additional 5 years of data, incorporates negative RNA tests reportable in NYC as of July 2014 and accounts for uncertainty around estimates of the percentages of people who spontaneously cleared their infection, were cured pre-2014 and were unaware of their HCV infection status. Revised estimates for HCV prevalence will allow us to assess the burden of HCV in NYC in the era of highly effective direct-acting antivirals, as well as identify gaps in care, treatment and cure to better inform resource allocation and intervention efforts, including setting HCV elimination targets.
Methods
Steps used to estimate the number of people with HCV in NYC
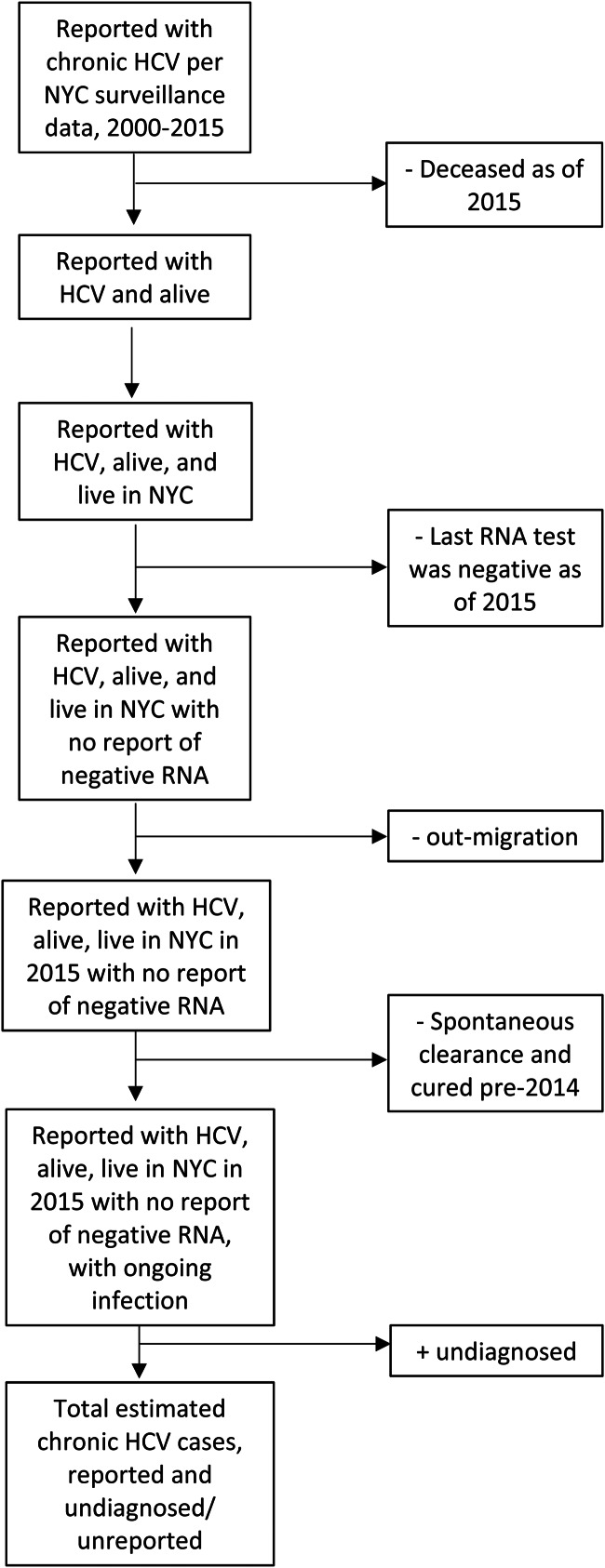
First, the total number of unique people with an HCV test reported to the Health Department between 2000 and 2015 was generated from the NYC HCV surveillance registry (Fig. 1). Using vital statistics and surveillance data, the following people were removed from the total: decedents, people with an unknown borough of residence in the registry at the time of first report, and people whose last RNA test was negative as of 31 December 2015, which accounts for people who were treated with direct-acting antiretroviral (DAA) medications or naturally cleared their infection during 2014–2015. Next, the probability that a person was still living in NYC in 2015 was estimated and applied to the remaining people alive and with no negative RNA reported. Next, estimates for the percentage of people who naturally cleared their infection or were cured prior to 2014, before negative RNA results were reportable to the NYC Health Department, were removed to estimate the number of people living in NYC in 2015 with reported and uncured chronic HCV. Finally, this estimate was adjusted for under-diagnosis. This process was then repeated, restricting to adults aged ⩾20 years in 2015 to make the estimate more comparable to previous local estimates [8, 9]. The all-ages HCV prevalence estimate will be used to guide overall NYC resources and includes two important risk groups who need appropriate testing, monitoring and treatment: perinatally exposed children and young people <20 years-old, who likely acquired HCV through drug use. Each of the steps used to estimate HCV prevalence is described in detail below.
Fig. 1.
Steps used to estimate the number of people with chronic hepatitis C virus (HCV) in New York City (NYC), 2015.
Inputs considered to be known without error
Surveillance data
In NYC, laboratories have been required to electronically report positive HCV tests (EIA, RIBA, RNA and genotype) since 2006, as well as negative RNA test results since July 2014. Electronic laboratory reporting is more complete and timelier than provider reporting [10]. Prior to 2006, health care providers and laboratories reported HCV cases and their corresponding positive test results by fax and mail. Cases and their corresponding test history are stored in an electronic surveillance registry.
In 2013, the Department of Veterans Affairs (VA) required mandatory reporting of infectious diseases designated as reportable to local and state public health authorities [11]. Veterans tested only through the VA health system were unavailable for analysis because the NYC VA health system did not begin reporting hepatitis C laboratory reports until the end of 2016. Also not included in the estimate of people reported with hepatitis C in NYC were cases of acute hepatitis C. Although acute hepatitis C is increasing in other jurisdictions [12], an increase in acute hepatitis C among young people has not been observed in NYC. Most acute cases turn into chronic cases, and if someone tests positive during the acute phase of infection, that test result is reported to the Health Department and appears as a chronic infection, in the absence of provider-reported clinical criteria. Acute hepatitis C is underreported in our surveillance registry because a case is defined using both laboratory and clinical criteria, but most patients are asymptomatic. Even when patients are symptomatic and seek healthcare, most health care providers do not report clinical criteria despite health code reporting requirements. All tests are automatically deduplicated using a matching algorithm, and near-matches are manually reviewed daily. NYC residents reported with any positive HCV test during 1 January 2000–31 December 2015 were included in the analysis. An individual's most recent laboratory result was used for the year at last report.
Match to death certificates
Deduplicated cases were first matched to the analytic dataset used for the NYC 2010 HCV prevalence estimate [8] to remove individuals already known to be deceased. The methods for deduplication for that estimate are reported elsewhere [8]. The remaining cases were matched to death certificates from 2011 to 2015 reported to the NYC Health Department's Bureau of Vital Statistics. At the time of analysis in late 2017, the most recent year of death data available was for 2015. An automated probabilistic de-deduplication algorithm based on key patient identifiers was used to match the remaining HCV cases to NYC Vital Statistics death data. Near matches were manually reviewed.
Tax returns to estimate out-migration
Tax return data from the US Internal Revenue Service (IRS), 2000–2015, were used to estimate the annual proportion of people of all ages who moved outside of NYC during this time period [13]. The IRS estimates migration by matching tax returns filed by households year-to-year and comparing the addresses to identify changes in residence at the time of filing. These data were used to estimate the probability that a person moved from NYC between the year of the last report to the NYC HCV surveillance registry and 2015. The probability that a person in the surveillance registry remained in NYC in 2015 was calculated by multiplying the annual probability that the person has not moved since the year of last laboratory or provider report. Multiplying the probability by the number of people with last HCV report in each year results in the estimated number remaining in NYC by year of the last report. Summing the year-specific estimates results in the total number of people with HCV remaining in NYC in 2015 (Table 1). These methods were used previously for NYC prevalence estimates for hepatitis B [14] and C [8].
Table 1.
Probabilities of out-migration of New York City (NYC) residents applied to individuals with hepatitis C virus (HCV) reported between 1 January 2000 and 31 December 2015 by most recent year of hepatitis C report
| Year | Number of unique persons with last HCV report in a year | Probability remained in NYC in 2015a | Estimated number remaining in NYC in 2015 |
|---|---|---|---|
| 2000 | 2479 | 0.5390 | 1336 |
| 2001 | 1401 | 0.5619 | 787 |
| 2002 | 2157 | 0.5870 | 1266 |
| 2003 | 2817 | 0.6135 | 1728 |
| 2004 | 3140 | 0.6422 | 2016 |
| 2005 | 3110 | 0.6733 | 2094 |
| 2006 | 4504 | 0.7054 | 3177 |
| 2007 | 5245 | 0.7367 | 3864 |
| 2008 | 5658 | 0.7674 | 4342 |
| 2009 | 5801 | 0.7969 | 4623 |
| 2010 | 5812 | 0.8258 | 4800 |
| 2011 | 6189 | 0.8558 | 5297 |
| 2012 | 6781 | 0.8916 | 6046 |
| 2013 | 7420 | 0.9298 | 6899 |
| 2014 | 10 625 | 0.9678 | 10 283 |
| 2015 | 30 236 | 1.0000 | 30 236 |
| Total | 103 375 | 88 794 |
The probability that a person in the surveillance registry remained in NYC in 2015 was based on multiplying the annual probabilities that the person has not moved since the year of the last report.
Inputs considered to be uncertain
Ranges for inputs with uncertainty were used to determine likeliest, minimum and maximum estimates (Table 2).
Table 2.
Inputs considered to be uncertain for estimating hepatitis C virus prevalence in New York City
| Parameter | Likeliest estimate (%) | Minimum, maximum estimates (%) | Data sources |
|---|---|---|---|
| Spontaneous clearance | 20 | 15, 25 | CDC [15] |
| Cured, pre-direct acting antiviral treatment era | 9.3 | 8.9, 9.8 | Yehia et al. [16] |
| Unaware of hepatitis C antibody positivity status | 39.8 | 19.0, 52.0 | Bornschlegel et al. [9], Torian et al. [17], Hagan et al. [22], Denniston et al. [18], NYC DOHMH [21], NYC HANES 2013/2014 (unpublished data) |
Spontaneous clearance
The likeliest estimate for spontaneous clearance ever among people with antibody positive-only test results was considered to be 20%, the mid-point of the low and high values of 15% and 25%, respectively, as reported by CDC [15]. Spontaneous clearance typically happens soon after diagnosis, within the first 6 months of infection. For our estimate, it does not matter when the person cleared the infection; the spontaneous clearance estimate is applied to people with antibody positive-only results by the end of 2015.
Cured pre-2014
The likeliest (9.3%) and minimum and maximum (8.9%, 9.8%) estimates for cure using interferon-based treatments (prior to the introduction of all-oral DAA medications in 2014) were based on a systematic review and meta-analysis by Yehia et al. [16] of the overall pooled percentage of the non-Veteran population estimated to be aware of their diagnosis, have access to healthcare, be prescribed treatment, and have achieved sustained virologic response. Only non-VA studies were used for this estimate. Yehia et al. primarily focused their meta-analysis on non-VA studies because of the differences in the prevalence of comorbid diseases, as well as the care and treatment of Veterans and non-Veterans. Additionally, in NYC the VA rarely or inconsistently reported HCV test results during the analysis period.
Unaware of HCV infection status
The likeliest estimate was based on a 2015 serosurvey in an NYC emergency room, which found that 39.8% of people testing antibody positive were undiagnosed [17]. This estimate might not be representative of all NYC residents undiagnosed with HCV but it is recent, specific to NYC and based on a relatively larger sample (N = 372) than other sources. Although a higher percentage of 50% unaware of HCV infection status is often cited as the likeliest estimate [18], this percentage is based on national data from NHANES surveys conducted from 2001 through 2008. This percentage was not updated in more recent NHANES survey years after 2008. We believe the per cent of people with HCV who were unaware of their infection status likely decreased between the last relevant NHANES survey (2008) and our year of interest for estimating prevalence (2015) because of national and NYS recommendations to screen people born during 1945–1965 for HCV [19, 20].
Multiple sources were used to establish the minimum and maximum estimates. After reviewing the literature (Table 2), the minimum estimate was 19.0% [21] from the NYC National HIV Behavioral Surveillance Survey of people who inject drugs. The maximum estimate was 52.0% from a five-city study, which included young people who inject drugs in NYC [22]. NYC HANES, the NYC version of NHANES, data from the 2004 and 2013–2014 surveys were also included as sources for determining the range of estimates. NYC Health Department staff contacted NYC HANES participants who tested positive for HCV and asked whether they were aware of their diagnosis, and those unweighted percentages were within the range between the 19% minimum and 52% maximum values [9, 21].
To account for spontaneous clearance among the population unaware of their HCV infection status, the likeliest, minimum and maximum estimates for the per cent unaware of their HCV infection were multiplied by the likeliest, minimum and maximum estimates for the per cent who would not have spontaneously cleared. This resulted in the likeliest estimate for unaware of infection status being 31.8% (39.8% unaware of HCV infection × (1–20% spontaneous clearance)), a minimum estimate of 14.3% (19% unaware of HCV infection × (1–25% spontaneous clearance)) and a maximum estimate of 44.2% (52.0% unaware of HCV infection × (1–15% spontaneous clearance)).
Combining inputs using simulations
All inputs were entered into a formula in Microsoft Excel (Microsoft Corp., USA): total estimated number of people living in NYC in 2015 with reported or undiagnosed/unreported chronic HCV = ((number reported with HCV, alive and living in NYC with no report of negative RNA) − (number spontaneously cleared) − (number cured pre-2014))/(1-per cent unaware of HCV infection status). For the three inputs considered to be uncertain, betaPERT distributions based on the minimum, likeliest and maximum estimates were used rather than a single value. The betaPERT distribution is derived from the beta distribution and is used as a ‘smoother’ alternative to the triangular distribution when there are minimum, likeliest and maximum values [23]. To randomly draw from these betaPERT distributions over 50 000 simulations, we used Latin Hypercube sampling in Crystal Ball, an Excel add-in by Oracle. Latin Hypercube sampling generates values more evenly and consistently across the distribution than Monte Carlo sampling.
This modelling approach allowed us to combine certain with uncertain inputs, using repeated sampling from specified input probability distributions, to identify the full range and 95% certainty levels (CLs) of final estimates consistent with the input data. Sensitivity charts were generated to assess which inputs contributed the most variance to the final estimate.
Prevalence estimate
The final estimate of the number and 95% certainty levels (CLs) of people living in NYC in 2015 with chronic HCV was divided by the interpolated intercensal population estimate of the NYC population in 2015 [24]. After calculating prevalence, the number of people reported and undiagnosed/unreported with chronic HCV (all ages and aged ⩾20 years) was rounded to the nearest thousand.
Results
During 1 January 2000–31 December 2015, the NYC Health Department received 161 023 reports of unique people reported with evidence of past or present HCV infection with an NYC borough of residence. Of these, 24 644 (15.3%) matched to death certificates, leaving 136 379 people alive as of 2015 and living in NYC. There were 33 004 (24.2%) people whose last RNA test was negative as of 31 December 2015, leaving 103 375 people with no reported negative RNA test result. Using IRS migration data, an estimated 14 581 people out-migrated, leaving 88 794 people alive as of 2015 with no reported negative RNA test result and still living in NYC in 2015. Using Latin Hypercube sampling to draw from distributions of the percentage of people who spontaneously cleared (among 25 818 with antibody positive-only results), the percentage cured pre-2014, and the percentage unaware of their infection, the number of people estimated to be reported with chronic HCV, alive, living in NYC in 2015 with no report of negative RNA, and with ongoing infection was increased for the percentage of people unaware of their HCV infection status adjusted for spontaneous clearance resulting in a total of 115 516 (≈116 000) (95% CL 99 369–134 564, or ≈99 000–135 000) people reported and undiagnosed/unreported with chronic HCV. With a total NYC 2015 population of 8 550 405, the prevalence of HCV in NYC in 2015 was estimated to be 1.4% (95% CL 1.2–1.6%).
Restricting to adults aged ⩾20 years in 2015, there were 160 359 people with HCV results during this same period. Of these, 24 629 (15.4%) matched to death certificates, leaving 135 730 people alive as of 2015 and living in NYC. A total of 32 925 (24.3%) people whose last RNA test was negative as of 31 December 2015 were removed, leaving 102 805 people with no reported negative RNA test result. An estimated 14 475 people out-migrated, leaving 88 330 people alive as of 2015 with no reported negative RNA test result and still living in NYC in 2015. Increasing the number of people estimated to be reported with chronic HCV, alive, living in NYC in 2015 with no report of negative RNA, and with ongoing infection to account for the percentage unaware of infection status adjusted for spontaneous clearance resulted in a total of 114 954 (≈115 000) (95% CL 98 775–134 089, or ≈99 000–134 000) adults aged ⩾20 years reported and undiagnosed/unreported with chronic HCV. Among 6 558 061 adults aged ⩾20 years in NYC in 2015, HCV prevalence was estimated to be 1.8% (95% CL 1.5–2.0%).
For the 95% CLs for the final prevalence estimates for all ages and also for adults aged ⩾20 years, almost all (99.7%) of the variance was attributable to the uncertainty in the percentage unaware of their infection status.
Discussion
The estimated surveillance-based HCV prevalence in NYC in 2015 was 1.4% overall (n ≈ 116 000) and 1.8% (n ≈ 115 000) among adults aged ⩾20 years, both lower than the previous NYC surveillance-based estimate among adults aged ⩾20 years of 2.4% (n ≈ 147 000) in 2010. With the advancement between 2010 and 2015 of better and shorter treatment, increased resources for HCV care coordinators and peer navigators, deaths among an older population (‘baby boomers’) of people infected with HCV, harm reduction services and a relatively stable number of new reports of confirmed chronic HCV during our analysis period, including among young adults (<30 years of age) who most likely represent acute infection [21], our finding is consistent with our expectation that the prevalence in 2015 would have decreased. A formal statistical comparison is not practical given the different methods used to generate the 2010 and 2015 estimates; however, the 2010 estimate does not fall within the 95% CLs of the 2015 estimate. Using the 2013–2014 NYC HANES, HCV prevalence in NYC was 1.4% (95% confidence interval (CI) 0.8–2.4%) among adults aged ⩾20 years [25]. As mentioned previously, some populations are underrepresented in NYC HANES, but it does not appear that the estimate is markedly different from the 2015 surveillance-based estimate for the same age group (1.8%, 95% CL 1.5–2.0%). Other state and national prevalence estimates are for HCV antibody positivity and use NHANES, so comparing them to NYC is challenging. The most recent national estimate for HCV antibody positivity (past or current infection) using NHANES, adjusting for death, was 1.7% (95% CI 1.5–1.9%) in 2010 and varies vastly by state [5]. We would expect HCV antibody seropositivity to be higher than confirmed chronic infection since it includes people who cleared their infection or were cured and false-positive antibody tests [26].
The input with the largest amount of uncertainty used to generate the prevalence estimates was the percentage of people unaware of their infection status. There is a wide range of estimates from different sources (i.e. NHANES [9, 18], research studies [21, 22] and serosurvey [17]), years and populations (e.g. young people currently injecting drugs [22]). We used a recent, NYC-specific estimate for our likeliest value given efforts to increase screening among people born during 1945–1965. In 2012, CDC recommended that people born during 1945–1965 receive one-time testing because of the disproportionately high prevalence of HCV and HCV-related morbidity and mortality among this cohort [19]. Additionally, in 2014, New York State enacted an HCV testing law that requires healthcare providers to offer HCV screening to all people born during that same time period [20], and evaluation findings show an increase in specimens submitted for testing as well as testing rates among Medicaid recipients [27]. As HCV screening continues to improve after 2015, the percentage of people assumed to be unaware of their infection status in future years can be decreased, with the prevalence estimate decreased accordingly. An additional limitation of the inputs considered to be uncertain is that the proportion of patients with HCV who spontaneously cleared the virus was assumed to be the same between those who were reported and those who were unaware of their infection.
There are several additional limitations to this analysis. While three inputs were formally considered to be uncertain, the uncertainty for the inputs treated as known without error could not be measured. Surveillance and vital statistics data presumably capture all people tested for HCV and people who died in NYC. Reporting or deduplication errors might be present but cannot be ascertained. IRS data capture the universe of NYC residents who file yearly tax returns, but they might be poorly representative of NYC residents with HCV. People who inject drugs, are homeless or incarcerated, or with very low income might be less likely or not required to file tax returns, and are underrepresented in the IRS data for out-migration to an unknown degree. Another limitation is that we did not calculate prevalence estimates by sex or age group. IRS out-migration data could not be stratified by age and sex. Patterns of out-migration might vary by age (e.g. young people might move around more) and sex, but the year-specific probabilities of out-migration were uniformly applied to people based on their year of last HCV report. The US Census Bureau's American Community Survey is another source for out-migration and is available by age and sex; however, those estimates might underrepresent people infected with HCV more than IRS data because of biases related to sample selection and non-response. Furthermore, age-specific estimates for the other inputs were not available. Estimates for spontaneous clearance, cured pre-all-oral DAA medications, and unaware of infection are not available by sex or age group. While we did not include the VA population in the NYC HCV prevalence estimate, nationally in 2015, 79% (174 027/220 605) of those estimated with HCV were diagnosed, and 38% (65 742/174 027) of those diagnosed initiated treatment [28]. We do not have an estimate of the number of those people receiving care through the VA system in NYC and not reported to the NYC surveillance registry to adjust our prevalence estimate; however, the VA system is aggressively diagnosing and treating patients, and their inclusion in target setting and resource allocation should be considered separately from the non-VA population [28]. Finally, we attempted to account for out-migration, but we did not account for in-migration of individuals who are infected with HCV but not tested in NYC, as this is not possible to estimate, given the variability in state surveillance systems, lack of a mechanism for cross-jurisdiction data sharing, and HCV reporting laws. However, people infected with HCV who migrate to NYC and are not tested for HCV are included in the undiagnosed estimate. The NYC registry does not contain HCV test history outside of NYC, so we do not know how many people with HCV are migrating to NYC, and though chronic HCV is a nationally notifiable condition reported to the CDC, data are reported without identifiers and cannot be used to identify a person's last known jurisdiction, or if a person is reported to multiple jurisdictions.
Despite these limitations, we generated a surveillance-based prevalence estimate, accounting for sources of uncertainty. Other jurisdictions with similar surveillance systems can apply these methods to generate a prevalence estimate to guide HCV elimination efforts. Jurisdictions that do not have a robust surveillance system can use these results to advocate for increased funding to establish a surveillance system for reporting and monitoring HCV. As new and better data for the key inputs become available, and matches to death certificates are routinely performed with our surveillance registry, the HCV prevalence estimate can be updated accordingly. Policy and HCV programme initiatives focused on HCV screening and linkage to care navigation services [29] likely contributed to lowering HCV prevalence, and similar efforts should be considered for other jurisdictions. The decrease in the surveillance-based HCV prevalence estimate from 2010 to 2015 is in part attributable to increased treatment and cure in the era of highly effective treatment as shown in the sharp increase in DAA medications uptake in 2014 and 2015 among NYC Medicaid recipients [21]. As more HCV medications became available in 2016, we would expect HCV prevalence to further decrease. Unlike other jurisdictions, we have not seen a large increase in hepatitis C cases related to the opioid epidemic and will need to be vigilant for opioid-related increases that will affect HCV prevalence.
Next steps include generating neighbourhood-level HCV prevalence estimates, which can help better target prevention and programme resources. The updated HCV prevalence estimate can be used to support requests for funding for HCV screening and linkage to care programmes. Additionally, the HCV prevalence estimate will help us estimate the number of people infected but undiagnosed/unreported who need to be screened and the number of people needed to be treated in order to eliminate HCV, as well as allow us to track progress towards HCV elimination as more people are diagnosed and cured.
Acknowledgements
We would like to thank staff from the Bureau of Communicable Disease's Viral Hepatitis Program and Reportable Disease Data, Informatics and Analysis Unit for their data support. In addition, we thank Joseph Kennedy and Hiu Tai Chan for matching the HCV data to death certificates.
References
- 1.Alter MJ et al. (1999) The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. New England Journal of Medicine 341, 556–562. [DOI] [PubMed] [Google Scholar]
- 2.Chen SL and Morgan TR (2006) The natural history of hepatitis C virus (HCV) infection. International Journal of Medical Sciences 3, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis GL et al. (2003) Projecting future complications of chronic hepatitis C in the United States. Liver Transplantation 9, 331–338. [DOI] [PubMed] [Google Scholar]
- 4.Ly KN et al. (2016) Rising mortality associated with hepatitis C virus in the United States, 2003–2013. Clinical Infectious Diseases 62, 1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg ES et al. (2017) Estimation of state-level prevalence of hepatitis C virus infection, US states and District of Columbia, 2010. Clinical Infectious Diseases 64, 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chak E et al. (2011) Hepatitis C virus infection in USA: an estimate of true prevalence. Liver International 31, 1090–1101. [DOI] [PubMed] [Google Scholar]
- 7.Kinnard EN et al. (2014). Estimating the true prevalence of hepatitis C in Rhode Island. Rhode Island Medical Journal July, 19–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Balter S et al. (2014) Estimating the prevalence of hepatitis C infection in New York City using surveillance data. Epidemiology and Infection 142, 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornschlegel K et al. (2009) Prevalence of hepatitis C infection in New York City, 2004. Journal of Urban Health 86, 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stachel AG et al. (2014) Reassessing provider reporting in the age of electronic surveillance. Journal of Public Health Management and Practice 20, 240–245. [DOI] [PubMed] [Google Scholar]
- 11.Department of Veterans Affairs (2013) VHA Direction 2013-008 Infectious Disease Reporting. Washington, DC. Available at https://dhhr.wv.gov/oeps/disease/Reporting/documents/veteran-affairs-infectious-disease-reporting.pdf (Accessed 11 April 2018). [Google Scholar]
- 12.Centers for Diseases Control and Prevention. Surveillance for Viral Hepatitis–United States, 2016. Available at https://www.cdc.gov/hepatitis/statistics/2016surveillance/index.htm (Accessed 04 May 2018).
- 13.Internal Revenue Service (IRS). SOI U.S. Population Migration Data, 2000–2015. Available at https://www.irs.gov/statistics/soi-tax-stats-migration-data (Accessed 10 March 2016 and 24 January 2017 for 2014 to 2015 migration data).
- 14.France AM et al. (2012) Estimating the prevalence of chronic hepatitis B virus infection – New York City, 2008. Journal of Urban Health 89, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Hepatitis C FAQs for Health Professionals. Available at https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm (Accessed 20 September 2017).
- 16.Yehia BR et al. (2014). Treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS ONE 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torian LV et al. (2017) Undiagnosed HIV and HCV in a New York City emergency room, 2015. Presented at Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 13–16 February 2017. Available at http://www.croiconference.org/sessions/undiagnosed-hiv-and-hcv-new-york-city-emergency-room-2015.
- 18.Denniston MM et al. (2012) Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology 55, 1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith BD et al. (2012) Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recommendations and Reports 61, 1–18. [PubMed] [Google Scholar]
- 20.Required Offering of Hepatitis C Screening Testing (2014) NY Pub Health L § 2171 (1 January 2014). Available at http://law.justia.com/codes/new-york/2014/pbh/article-21/title-7/2171.
- 21.NYC Department of Health and Mental Hygiene. Hepatitis B and C in New York City 2016 Annual Report. Published Fall 2017. Available at http://www1.nyc.gov/site/doh/data/data-sets/hepatitis-abc-surveillance-data.page.
- 22.Hagan H et al. (2006) Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Reports 121, 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oracle Crystal Ball (2012) Oracle Crystal Ball User's Guide. Available at https://docs.oracle.com/cd/E17236_01/epm.1112/cb_predictor_user.pdf.
- 24.NYC Department of Health and Mental Hygiene (2017) New York City population estimates, modified from U.S. Census Bureau interpolated intercensal population estimates, 2015, Updated August 2017.
- 25.New York City Department of Health and Mental Hygiene. EpiQuery: NYC Interactive Health Data System – NYC Health and Nutrition Examination Survey 2013–2014. Available at http://nyc/health/epiquery (Accessed 25 October 2017).
- 26.Moorman AC, Drobenuic J and Kamili S (2017) Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012. Journal of Clinical Virology 89, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flanigan CA et al. (2017) Evaluation of the impact of mandating health care providers to offer hepatitis C virus screening to all persons born during 1945–1965 – New York, 2014. MMWR Morbidity Mortality Weekly Report 66, 1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belperio PS et al. (2017) Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Annals of Internal Medicine 167, 499–504. [DOI] [PubMed] [Google Scholar]
- 29.Ford MM et al. (2018) Check Hep C: a community-based approach to hepatitis C diagnosis and linkage to care in high-risk populations. Journal of Public Health Management and Practice 24, 41–48. [DOI] [PubMed] [Google Scholar]