Abstract
Hepatitis delta virus (HDV) is a defective RNA virus that depends on the presence of hepatitis B virus (HBV) for the creation of new virions and propagation of the infection to hepatocytes. Chronic infection with HDV is usually associated with a worsening of HBV infection, leading more frequently to cirrhosis, increased risk of liver decompensation and hepatocellular carcinoma (HCC) occurrence. In spite of a progressive declining prevalence of both acute and chronic HDV infection observed over several years, mainly due to increased global health policies and mass vaccination against HBV, several European countries have more recently observed stable HDV prevalence mainly due to migrants from non-European countries. Persistent HDV replication has been widely demonstrated as associated with cirrhosis development and, as a consequence, development of liver decompensation and occurrence of HCC. Several treatment options have been attempted with poor results in terms of HDV eradication and improvement of long-term prognosis. A global effort is deemed urgent to enhance the models already existing as well as to learn more about HDV infection and correlated tumourigenesis mechanisms.
Key words: Cirrhosis, epidemiology, hepatitis delta virus, hepatocellular carcinoma, natural history, treatment
Introduction
Hepatitis delta virus (HDV) is a defective RNA virus that requires the presence of hepatitis B virus (HBV) for virion assembly and propagation [1–5]. It is a small RNA virus with a circular RNA genome of approximately 1.7 Kb in length (Fig. 1) [6]. The virus does not code any enzyme to replicate its genome using instead RNA polymerase II from hepatocytes for the synthesis of its RNAs, both with positive and negative polarity. HDV encodes a single ORF responsible for the expression of the delta antigen (HDAg), in two isoforms, the small (S-HDAg) and large HDAg (L-HDAg) [7]. HDAg and HDV RNA are subsequently encased in an envelope embedded with HBV surface antigen (HBsAg) to form infectious virions (Fig. 1) [7]. As a consequence, the early step of HDV entry into hepatocytes follows the same mechanism for both viruses. Once in the hepatocytes, a nuclear localisation signal on L-HDAg triggers the translocation of HDV nucleocapsid to the nucleus where viral genome is replicated.
Fig. 1.
Hepatitis delta virus virion.
HDV infection can be acquired either as coinfection of the two viruses or as superinfection of an already chronic carrier of HBV. Normally, in coinfection, HDV activation depends on HBV activation. As a consequence, HDV expression in coinfection can vary widely, ranging from an abortive infection to an extremely virulent form of disease [8–10]. In the vast majority of coinfections, however, HBV infection is self-limiting and HDV infection recovers, with a progression to a chronic disease in about 2% of cases, only [11].
Superinfection with HDV in chronic hepatitis B causes a more threatening form of liver disease that increases the liver damage leading to more rapid progression to cirrhosis in up to 90% of cases [12]. Early studies in the general population demonstrated a benign, non-progressive disease in about 15% of cases, while the vast majority of cases experience a rapid progression to cirrhosis within few years [13]. Subsequent studies demonstrated that HDV-positive cirrhotic patients had significantly higher risk of progression towards hepatocellular carcinoma (HCC) development and liver decompensation.
This article provides an update on the relationship between HDV infection and development of HCC, highlighting the possible mechanisms of carcinogenesis. We also review the most recent epidemiological data as well as advances in HDV treatment.
Epidemiology of HDV-related HCC
Liver cancer is an important health problem worldwide, being the second cause of cancer-related death. More than 90% of the primary liver cancers are HCC having a particularly poor prognosis, with 700 000 new cases and 600 000 deaths occurring every year [14]. Infections with hepatitis B, C and delta viruses, all having a great propensity to progress towards chronicity, are the aetiological agent of more than 80% of all HCCs. It has been calculated that approximately 170 million chronic HCV carriers, 350 million chronic HBV carriers and 15–20 million HDV carriers represent the burden of chronic hepatitis worldwide [14, 15]. HBV/HDV coinfected patients experience rapid progression to more advanced stages of liver disease and also have the highest mortality rate (20%) of any of the viral hepatitis [16–18].
Several mechanisms for HCC development and progression in the presence of viral hepatitis have been proposed. These include antiviral inflammatory response, immune clearance of infected cells and subsequent hepatocytes regeneration all leading to genetic and epigenetic changes, predisposing to HCC development [19–22].
The International Agency for Research on Cancer (IARC) has recently categorised several infectious agents into four groups, according to the evidence for carcinogenicity to humans [23]: group 1 (high evidence of carcinogenicity to humans), group 2A (possibility of carcinogenic effects), group 2B (low carcinogenic effects) and group 3 (not sufficient evidences of carcinogenicity). While HBV and HCV are both allocated in IARC group 1, HDV was allocated several years ago in group 3 [24], as the demonstration of HDV contribution to HCC induction on HBV was not adequate. However, more recently, it has been suggested that the risk of HCC is higher when HBV is superinfected by HDV [25].
The analysis of several epidemiological studies published lately has shown the existence of some controversies about the increasing risk of HCC development in HDV chronically infected patients. An important study by the European Concerted Action on Viral Hepatitis (Eurohep) demonstrated that HBV/HDV-positive cirrhotics followed for a median 6.6 years had a twofold mortality risk than HBV cirrhosis. Moreover, the estimated risk for HCC was 13% for HBV/HDV cirrhotics as compared with 2–4% for HBV cirrhotics, thus increasing the risk to threefold [26]. A retrospective study on data collected in South London showed that despite the increasing prevalence of HDV infection, the risk of HCC was similar in HDV-positive and negative patients [27]. Very high prevalence (18.8% and 23%) of HDV markers in HCC patients was shown in two Turkish studies [28, 29], as well as in a small study published in Jordan [30].
We published the results of 299 patients with chronic HDV infection followed for a mean interval of 28 years. The study demonstrated that 30% of patients developed cirrhosis and <15% of all patients died of liver-related causes [31]. These results are in contrast to the data published previously but more in line with subsequently published results, suggesting that our cohort probably reflected a change in the epidemiology of the infection, but also one could suggest that our patients were rather the result of a long-lasting asymptomatic disease that eventually lead to cirrhosis than a recent rapidly progressive infection. This same conclusion had already been suggested by others indicating a biphasic course of HDV-related disease, characterised by an initially florid infection and a rapid progression towards liver failure with high mortality rates [32]. Patients surviving the initial phase experienced a slowly progressing disease leading to liver decompensation later [33]. Our results also demonstrated that persistent HBV/HDV infection was associated with cirrhosis development and HCC occurrence at 4% and 2.8%, respectively. Moreover, persistent HDV replication was the only predictor of liver-related mortality [31].
Similar results as ours were obtained by Buti et al. [19] in a cohort of 158 chronic HDV patients, followed for a median of 158 months. In this study, 18% experienced liver decompensation, while 3% developed HCC. A recently published study calculated the standardised risk (standardised incidence ratios (SIRs)) for HDV patients of developing HCC, demonstrating an increased risk for HDV as compared with HBV (SIR 6.11, 95% CI 2.77–11.65) [33].
It has been recently demonstrated that high levels of HDV replication in non-cirrhotic patients are associated with an increasing progression to cirrhosis and to HCC development (multivariate analysis OR 1.42, 95% CI 1.04–1.95; P = 0.03), while the role of HDV viraemia as a predictor of negative outcome in cirrhotic patients lessens [34].
In a study from Japan, HDV superinfection increases the risk of cirrhosis and HCC. The proportion of HCC per 1000 person years was 7.84 among cases with anti-HDV and 2.73 among those without anti-HDV. The overall relative risk of HCC was 2.87, 95% CI 1.03–6.23 [35]. A study from Taiwan failed to show any acceleration in the development of HCC in patients with HDV superinfection. Nevertheless, the numbers of patients in HDV group were small compared with HBV monoinfection group (42 vs. 255) [36].
A recently published case–control study aimed at the determination of the role of HBV, HCV and HDV in HCC development in Cameroon, Central Africa [36]. The sera of 88 consecutive HCC patients and 85 controls without known liver disease were analysed for the presence of markers of viral hepatitis. The analysis of risk factors associated with the development of HCC demonstrated a strong association with the presence of HBV, HCV and HDV markers, being the OD (95% CI) of 16.3 (7.2–37.1), 9.6 (2.8–33.6) and 29.3 (4.1–1231) for the three viruses, respectively.
Finally, a very recent publication on the epidemiological characteristics of HDV infection on a nationwide Swiss HIV Cohort Study, aiming at the evaluation of the impact and clinical outcome of the infection, indicates a prevalence of HDV infection of 15.4% (119/771, 95% CI 12.9–18.0) in HIV-infected patients, with HDV replication in 62.9% [37]. Moreover, HDV infection appeared as strongly associated with overall death (adjusted hazard ratio 2.33, 95% CI 1.41–3.84), liver-related death (7.71, 3.13–18.97) and HCC occurrence (9.30, 3.03–28.61). Noteworthy, results were similar when HCV-coinfected or people injecting drugs were excluded from the analysis.
Taken together, the results of the above-mentioned studies, summarised in Table 1, emphasise the need for developing new treatments for chronic HDV infection as the presently available drugs are largely ineffective for viral eradication and control of disease progression.
Table 1.
The epidemiological studies on the role of hepatitis D virus infection in increasing the risk of hepatocellular carcinoma
| Fattovich et al. [26] | Estimated risk for HCC 13% for HBV/HDV cirrhotics as compared with 2–4% for HBV cirrhotics |
| Cross et al. [27] | Risk of HCC similar in HDV-positive and negative patients |
| Uzunalimoğlu et al. [28] | High prevalence (18.8%) of HDV markers in HCC patients |
| Değertekin et al. [29] | High prevalence (23.3%) of HDV markers in HCC patients |
| Toukan et al. [30] | The highest prevalence of HCC was found in patients co-infected with HDV |
| Romeo et al. [31] | 299 HDV-infected patients investigated over 28 years. Persistent HDV leads to cirrhosis and HCC at annual rates of 4% and 2.8% |
| Buti et al. [19] | 158 chronic HDV patients, followed for a median of 158 months, 18% experienced liver decompensation while 3% developed HCC |
| Ji et al. [33] | 650 out of 9160 HBV patients had HDV. The median follow-up was 11 years. The risk of HCC was increased. HDV was a strong risk factor |
| Romeo et al. [34] | 193 patients with HDV co-infection were investigated for a median of 9.5 years. HDV RNA levels appeared significantly associated with HCC |
| Tamura et al. [35] | 1127 patients were followed for at least 3 years. The prevalence was 4.05 per 1000 person years in HDV co-infection patients compared with 2.73 in patients with HBV alone |
| Huo et al. [36] | 42 HDV co-infected patients were compared with 255 HBV patients, all with HCC, over a period of 8 years. HDV co-infection does not accelerate HCC development, and the outcomes are the same as HBV mono-infection |
| Amougou et al. [37] | 88 consecutive HCC patients and 85 controls without known liver disease were analysed. The analysis of risk factors associated with the development of HCC demonstrated a strong association with the presence of HBV, HCV and HDV markers (OD = 16.3, 9.6 and 29.3) |
| Béguelin et al. [38] | HDV infection appeared strongly associated with overall death (adjusted hazard ratio 2.33), liver-related death (7.71) and HCC occurrence (9.30) |
Treatment of HDV infection
Ideally, a successful treatment for chronic HDV infection would eradicate HDV as well as its helper virus. Clearance of HDV is considered as obtained when both HDV RNA and liver HDAg become persistently undetectable. A complete resolution is achieved when HBsAg clearance is obtained [38]. The persistence of HBsAg corresponds to an HDV-persistent infectivity, even with low viral titres. Unfortunately, despite inhibition of HBV replication and, in few cases, HBsAg clearance, no clearance of HDV has been observed when oral antivirals against HBV were used alone. Lamivudine, ribavirin, famciclovir, adefovir and entecavir have shown little or no effect when used alone in HDV treatment, regardless of the length of drug administration [39–43].
Interferon (IFN) is still the only recognised treatment for HDV chronic infection, even though results published over the years have demonstrated that this drug is far from being the optimal treatment [44]. Response to treatment is extremely variable and it may occur even spontaneously, at the end of treatment, in analogy with what already known for HBV infection. The response is normally proportionate to drug dosage, as demonstrated by an early publication, demonstrating higher efficacy of 9 MU of INF administered subcutaneously thrice a week, as compared with 3 MU thrice a week [45]. The results of this study demonstrated that by the end of treatment, negative HDV RNA and normal ALT were found in 71% and 71%, respectively, of patients treated with higher dosage, against 36% and 29%, respectively, obtained in patients treated with lower IFN dose [43].
By 2006, IFN-α was largely replaced by pegylated IFN (PEG-IFN) [44–46] with improved pharmacokinetic. Studies of PEG-IFN in hepatitis delta have shown HDV RNA suppression during treatment in 17–47% of patients, with a post-treatment week 24 virological response, observed in 25–40% of patients [38, 42, 44]. Several studies have emphasised the importance of negative HDV RNA after 6 months of treatment as a predictor of response at 12 months, selecting patients that should be treated for longer periods [42, 43, 46]. The analysis of factors as potential predictors of response has indicated that naïve patients with low levels of serum GGT appeared to have a better response [47]. More recently, several publications have demonstrated that the therapeutic effect of PEG-IFN combined to several analogues does not increase the proportion of patients with complete HDV eradication [41, 44, 48–51]. One possible explanation could be that since these drugs are not effective as inhibitors of the only HBV component necessary for HDV survival, HBsAg, HBV DNA suppression alone does not lead to HDV eradication.
However, recently published findings have pointed out that in spite of the disappointing results so far shown, IFN treatment is independently associated with a more benign clinical long-term outcome [52]. Indeed, the results of this retrospective single-centre study demonstrate that the frequency of hepatic decompensation is reduced in IFN-α treated patients, while there is no difference in terms of HCC development. Finally, loss of HDV RNA by IFN-α did not influence HCC development [53].
Recently, new strategies for HDV treatment are under investigation. Being HDV infection always associated with HBV presence, it must be considered that the two viruses may interfere with each other at several steps of the replication cycles. The optimal treatment should have as a gold standard the loss of HBsAg, resulting in an automatic eradication of HDV replication and propagation. With this in mind, the new therapeutic approaches act at different levels of HDV lifecycle. The sodium taurocholate co-transporting polypeptide (NCTP) receptor and the myristoylated N-terminal pre-S1 domain of the L-HBsAg are essential for viral entry into cells [54, 55]. Myrcludex B is a mirystoylated lipopeptide that has been demonstrated as capable of blocking HBV entry into hepatocytes [56]. In vitro studies have shown that myrcludex B is able to inhibit HDV entry as well [57]. Interim results of a phase II study on the administration of myrcludex B in humans have been recently published [58]. This study indicates that myrcludex B is associated with HDV RNA decline in a good proportion of patients but the result is encumbered HDV RNA reappearance after drug stop, in a good proportion of cases. Once the virus is within the hepatocyte, the replicative cycle happens through the host polymerase, giving rise to L-HDAg that will later interact with HBsAg, forming new viral particles. Prenylation of the newly formed L-HDAg is required for particle formation. The inhibition of such a step prevents the appearance of new viral particles [59, 60].
Finally, once the new viral particles are formed, they are released from the cell. At this stage, very recent studies indicate that the use of nucleic acid polymers (NAPs) can block HBsAg release by binding to class I surface glycoproteins [61]. Few studies have already been published on the administration of NAPs, demonstrating serum HBsAg and HBV DNA reduction with viral rebound occurring in the majority of cases after treatment stop [62].
Overall, the treatment results so far presented indicate that we are still not ready for a curative treatment against HDV infection, leaving the road open to progression towards more severe clinical outcomes, with HCC development as the final step.
Oncogenic mechanisms
It is still not clear whether HDV adds additional oncogenic effects beyond promoting fibrosis deposition and development of cirrhosis. HDAg expression alone does not appear to be cytopathic and does not appear to have oncogenic potential. On the other hand, it has been indicated that since high levels of antigen and viral RNA cause cell cycle arrest in G1 phase, this mechanism could be responsible for cell death in the acute phase of the infection, when viral replication is high [4]. However, the intrahepatic expression of L-HDAg appears to be associated with high levels of liver inflammation through NF-κB activation signalling and the immune response against the virus [63, 64]. These proteins seem to be implicated in cell transformation and tumourigenesis, indeed STAT3 over expression is associated with leukaemia, prostate cancer and melanoma [65, 66]. Niu et al. [67] demonstrated that L-HDAg (p27) significantly increases the NFκB activity also via tumour necrosis factor α (TNFα), TNF receptor-associated factor (TRAF2), IKKβ and p65-mediated induction. Park et al. [68] have shown that HDV p27 activates STAT3 via phosphorylation of tyrosine 705 residue. STAT3, in turn, regulates DNA methyltransferases (DNMT1) and causes the overexpression of DNMT3b. Since DNMT1 is responsible for the maintenance of methylation patterns, whereas DNMT 3a and 3b catalyse new methylation events, their overexpression can be potentially oncogenic.
Interestingly, it has been postulated an epigenetic control of HDV over HBV transcription and regulation, that would explain why HBV replication is inhibited in the presence of HBV, implying an HCC development as depending on HDV alone [69]. Against this hypothesis is the evidence that long-lasting active proliferation of the two viruses leads to more aggressive disease and HCC development as already mentioned.
Recently, it has been suggested that long non-coding RNAs (lncRNAs) may play a role in the pathogenesis of HDV-related HCC, through the dysregulation of lncRNAs unique to HDV [70].
Early studies demonstrated that the lymphocytes-mediated cell lysis has a non-relevant role in determining liver disease, indicating that HDV is not able to raise an autoimmune response [71]. By the same token, since lymphocytes T CD4+ specific for HD antigen have been observed within the liver of chronically HDV-infected patients, it has been postulated that T lymphocytes-specific clones against HD antigen may be involved in B and T cellular response, leading to the immune activation against HDV [72]. Finally, more recent findings again propose that hepatitis D is an immune-mediated disease, showing a CD4+ T cell rise in HDV infection [73]. Since several autoantibodies are commonly detected in HDV-infected patients, it is still not completely understood whether this represents an accompanying pattern rather than a true autoimmune manifestation. As long as we do not have conclusive evidences for or against the true pathogenic mechanism of HDV-related liver disease, we keep exploring several different mechanisms as possibly involved in HCC development.
In conclusion, the mechanism by which HDV causes HCC still remains to be elucidated, even if several events occurring during HDV infection, like oxidative stress as result of the severe necroinflammation HDV-related, the aberrant silencing of tumour suppressor genes by DNA methyltransferases, increased levels of histone H3 acetylation of the clusterin promoter and the targeted inhibition of STAT3 and cyclophilin might have a potential role in explaining the oncogenetic role of HDV [74].
Genetic heterogeneity
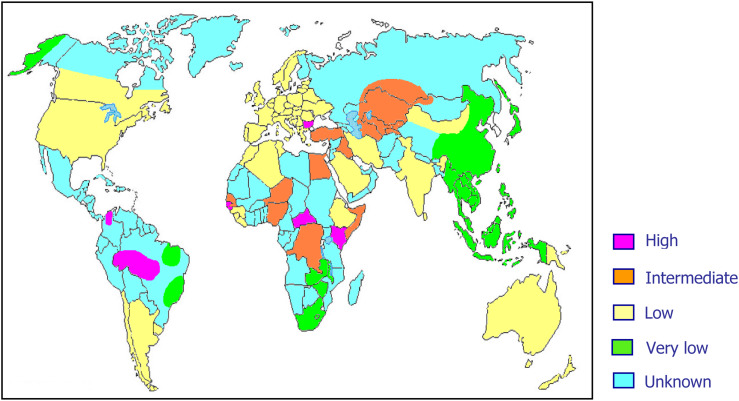
As previously mentioned, about 15–20 million people worldwide are considered to be chronic HDV carriers. However, it is highly probable that these data are largely underestimated mainly because of lack of data on HDV prevalence from several areas of the world (Fig. 2).
Fig. 2.
World prevalence of hepatitis delta virus infection.
HDV RNA, like all RNAs, is characterised by high degree of genetic variability, resulting in at least eight major clades distributed worldwide [75].
The pathogenetic role of viral heterogeneity in HDV-related clinical outcome is still unknown. However, early data have demonstrated that HDV genotype III, endemic in the Amazonian area and northern South America, is associated with very aggressive forms of liver disease [8, 76], while genotype II, prevalent in Far East and Japan, is associated with a milder clinical course [77] and genotype I, present mostly in Europe and North America, is associated with a wide variety of clinical conditions [78].
A recent finding has demonstrated the occurrence of a natural intergenotypic recombination between HDV genotypes I and II [79, 80], suggesting that the occurrence of mixed genotypes as well as recombinant HDV genotypes may be responsible for alterations leading to different clinical outcomes and possibly HCC development.
Conclusions
HDV remains a potential risk factor for serious liver damage, either for HBV chronic carriers or for all those still susceptible to HBV infection, for whom HBV vaccination is strongly recommended. Early long-term treatment of chronic HDV infection with IFN still offers better chances of infection control, lowering the risk of progression to cirrhosis and liver cancer. A better understanding of the mechanisms responsible for HCC development in chronic HDV infection is necessary also for directing research toward the development of efficient antiviral compounds able to eradicate the infection and redesign the natural history of the disease. A global effort is deemed urgent to enhance the models already existing as well as to learn more about viral infection and correlated tumorigenesis mechanisms.
Author ORCIDs
Raffaella Romeo (0000-0002-7858-4670); Arnolfo Petruzziello (0000-0003-0353-9151); Eve Isabel Pecheur (0000-0002-8613-862X); Floriana Facchetti (0000-0001-5252-4744); Riccardo Perbellini (0000-0002-4412-1256); Enrico Galmozzi (0000-0001-8831-2401); Najeeb Ullah Khan (0000-0003-2568-9484); Lucia Di Capua (0000-0002-6113-688X); Rocco Sabatino (0000-0003-1113-8665); Gerardo Botti (0000-0002-6287-733X); Giovanna Loquercio (0000-0002-7539-2075).
Author contributions
Romeo R, Facchetti F, Galmozzi E, Perbellini R, Di Capua L and Sabatino R acquired the data; Romeo R drafted the article and contributed to the conception and design in collaboration with Loquercio G; Botti G, Petruzziello A and Pecheur EI contributed to the critical revision for important intellectual content; all authors approved the final version to be published.
Data sharing statement
Participants gave informed consent for data sharing.
Conflict of interest
None.
References
- 1.Sureau C, Guerra B and Lanford RE (1993) Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. Journal of Virology 67, 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzetto M et al. (1988) Hepatitis delta virus infection: clinical and epidemiological aspects. In: Zuckerman AJ (ed), Viral Hepatitis and Liver Disease, New York: Alan R. Liss, pp. 389–394. [Google Scholar]
- 3.Gerin JL, Casey JL and Purcell RH (2002) Hepatitis delta virus. In: Hollinger FB, Purcell RH, Gerin JL, Ganem DE, Feinstone FM (eds), Viral Hepatitis, United States: Lippincott Williams and Wilkins, pp. 169–192. [Google Scholar]
- 4.Taylor JM (2006) Hepatitis delta virus. Virology 344, 71–76. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JM (1996) Hepatitis delta virus and its replication. In Fields BN, Knipe DM, Howley PM (eds), Fundamental Virology, 3rd Edn. Philadelphia: Lippincott-Raven Publishers, pp. 1235–1244. [Google Scholar]
- 6.Flores R, Ruiz-Ruiz S and Serra P (2012) Viroids and hepatitis delta virus. Seminars in Liver Disease 32, 201–210. [DOI] [PubMed] [Google Scholar]
- 7.Shirvani-Dastgerdi E and Tacke F (2015) Molecular interactions between hepatitis B virus and delta virus. World Journal of Virology 4, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey JL et al. (1996) Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: the roles of HDV genotype III and HBV genotype F. Journal of Infectious Diseases 174, 920–926. [DOI] [PubMed] [Google Scholar]
- 9.Sagnelli E et al. (1991) Interaction between HDV and HBV infection in HBsAg-chronic carriers. Infection 19, 155–158. [DOI] [PubMed] [Google Scholar]
- 10.Stroffolini T et al. (1992) Hepatitis B virus DNA in chronic HBsAg carriers: correlation with HBeAg/anti-HBe status, anti-HD and liver histology. Hepato-gastroenterology 39, 62–65. [PubMed] [Google Scholar]
- 11.Caredda F et al. (1983) Prospective study of epidemic delta infection in drug addicts. Progress in Clinical and Biological Research 143, 245–250. [PubMed] [Google Scholar]
- 12.Smedile A et al. (1982) Influence of delta infection on severity of hepatitis B. The Lancet 2, 945–947. [DOI] [PubMed] [Google Scholar]
- 13.Rizzetto M et al. (1983) Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of the delta antigen. An active and progressive disease unresponsive to immunosuppressive treatment. Annals of Internal Medicine 98, 437–441. [DOI] [PubMed] [Google Scholar]
- 14.Petruzziello A (2018) Epidemiology of hepatitis B virus (HBV) and hepatitis C virus (HCV) related hepatocellular carcinoma. The Open Virology Journal 12(Suppl.-1, M3) 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petruzziello A et al. (2017) Hepatitis C virus (HCV) genotypes distribution among hepatocellular carcinoma patients in Southern Italy: a three year retrospective study. Infectious Agents and Cancer 12, 52. [Google Scholar]
- 16.Bray F et al. (2013) Global estimates of cancer prevalence for 27 sites in the adult population in 2008. International Journal of Cancer 132, 1133–1145. [DOI] [PubMed] [Google Scholar]
- 17.Ferlay J et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 136, E359–E386. [DOI] [PubMed] [Google Scholar]
- 18.Farci P and Niro GA (2012) Clinical features of hepatitis D. Seminars in Liver Disease 32, 228–236. [DOI] [PubMed] [Google Scholar]
- 19.Buti M et al. (2011) Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. Journal of Viral Hepatitis 18, 434–442. [DOI] [PubMed] [Google Scholar]
- 20.Grabowski J and Wedemeyer H (2010) Hepatitis delta: immunopathogenesis and clinical challenges. Digestive Diseases 28, 133–138. [DOI] [PubMed] [Google Scholar]
- 21.Ajiro M and Zheng ZM (2014) Oncogenes and RNA splicing of human tumor viruses. Emerging Microbes and Infection 3, e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesri EA, Feitelson MA and Munger K (2014) Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe 15, 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Agency for Research on Cancer (2012) Vol 100B, 31–33. [Google Scholar]
- 24.International Agency for Research on Cancer (1994) Vol 59, 223–242. [Google Scholar]
- 25.Ghamari S et al. (2013) Prevalence of hepatitis delta virus (HDV) infection in chronic hepatitis B patients with unusual clinical pictures. Hepatitis Monthly 13, e6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattovich G et al. (2000) Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 46, 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross TJ et al. (2008) The increasing prevalence of hepatitis delta virus (HDV) infection in South London. Journal of Medical Virology 80, 277–282. [DOI] [PubMed] [Google Scholar]
- 28.Uzunalimoğlu O et al. (2001) Risk factors for hepatocellular carcinoma in Turkey. Digestive Diseases and Sciences 46, 1022–1028. [DOI] [PubMed] [Google Scholar]
- 29.Değertekin H, Yalçin K and Yakut M (2006) The prevalence of hepatitis delta virus infection in acute and chronic liver diseases in Turkey: an analysis of clinical studies. Turkish Journal of Gastroenterology 17, 25–34. [PubMed] [Google Scholar]
- 30.Toukan AU et al. (1987) The epidemiology and clinical outcome of hepatitis D virus (delta) infection in Jordan. Hepatology 7, 1340–1345. [DOI] [PubMed] [Google Scholar]
- 31.Romeo R et al. (2009) A 28-year study of the course of hepatitis delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 136, 1629–1638. [DOI] [PubMed] [Google Scholar]
- 32.Rosina F et al. (1999) Changing pattern of chronic hepatitis D in Southern Europe. Gastroenterology 117, 161–166. [DOI] [PubMed] [Google Scholar]
- 33.Ji J, Sundquist K and Sundquist J (2012) A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. Journal of the National Cancer Institute 104, 790–792. [DOI] [PubMed] [Google Scholar]
- 34.Romeo R et al. (2014) High serum levels of HDV RNA are predictors of cirrhosis and liver cancer in patients with chronic hepatitis delta. PLoS ONE 9, e92062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura I et al. (1993) Risk of liver cirrhosis and hepatocellular carcinoma in subjects with hepatitis B and delta virus infection: a study from Kure, Japan. Journal of Gastroenterology and Hepatology 8, 433–436. [DOI] [PubMed] [Google Scholar]
- 36.Huo TI et al. (1996) Comparison of clinico-pathological features in hepatitis B virus-associated hepatocellular carcinoma with or without hepatitis D virus superinfection. Journal of Hepatology 25, 439–444. [DOI] [PubMed] [Google Scholar]
- 37.Amougou MA et al. (2016) A prominent role of hepatitis D virus in liver cancers documented in Central Africa. BMC Infectious Diseases 16, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Béguelin C et al. (2017) Swiss HIV Cohort Study. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. Journal of Hepatology 66, 297–303. [DOI] [PubMed] [Google Scholar]
- 39.Alves C, Branco C and Cunha C (2013) Hepatitis delta virus: a peculiar virus. Advances in Virology 2013, 560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niro GA et al. (2005) Lamivudine therapy in chronic delta hepatitis: a multicentre randomized-controlled pilot study. Aliment Pharmacology Therapy 22, 227–232. [DOI] [PubMed] [Google Scholar]
- 41.Garripoli A et al. (1994) Ribavirin treatment for chronic hepatitis D: a pilot study. Liver 14, 154–157. [DOI] [PubMed] [Google Scholar]
- 42.Yurdaydin C et al. (2002) Famciclovir treatment of chronic delta hepatitis. Journal of Hepatology 37, 266–271. [DOI] [PubMed] [Google Scholar]
- 43.Lau DT et al. (1999) Lamivudine for chronic delta hepatitis. Hepatology 30, 546–549. [DOI] [PubMed] [Google Scholar]
- 44.Wedemeyer H et al. (2011) HIDIT study group. Peginterferon plus adefovir versus either drug alone for hepatitis delta. The New England Journal of Medicine 364, 322–331. [DOI] [PubMed] [Google Scholar]
- 45.Niro GA, Rosina F and Rizzetto M (2005) Treatment of hepatitis D. Journal of Viral Hepatitis 12, 2–9. [DOI] [PubMed] [Google Scholar]
- 46.Farci P et al. (1994) Treatment of chronic hepatitis D with interferon alfa-2a. The New England Journal of Medicine 330, 88–94. [DOI] [PubMed] [Google Scholar]
- 47.Niro GA et al. (2006) Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 44, 713–720. [DOI] [PubMed] [Google Scholar]
- 48.Castelnau C et al. (2006) Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology 44, 728–735. [DOI] [PubMed] [Google Scholar]
- 49.Erhardt A et al. (2006) Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver International 26, 805–810. [DOI] [PubMed] [Google Scholar]
- 50.Gheorghe L et al. (2011) Weight-based dosing regimen of peg-interferon α−2b for chronic hepatitis delta: a multicenter Romanian trial. Journal of Gastrointestinal and Liver Diseases 20, 377–382. [PubMed] [Google Scholar]
- 51.Yurdaydin C et al. (2008) Treatment of chronic delta hepatitis with lamivudine vs lamivudine+ interferon vs interferon. Journal of Viral Hepatitis 15, 314–321. [DOI] [PubMed] [Google Scholar]
- 52.Wedemeyer H, Yurdaydin C and Caruntu FA (2014) Pegylated-interferon-alpha2a plus tenofovir or placebo for the treatment of hepatitis delta. 49th Annual Meeting of the European Association for the Study of the Liver (EASL), London, United Kingdom. 9–13 April 2014.
- 53.Wranke A et al. (2017) Antiviral treatment and liver-related complications in hepatitis delta. Hepatology 65, 414–425. [DOI] [PubMed] [Google Scholar]
- 54.Meier A et al. (2013) Myristoylated PreS1-domain of the hepatitis B virus L-protein mediates specific binding to differentiated hepatocytes. Hepatology 58, 31–42. [DOI] [PubMed] [Google Scholar]
- 55.Zhong G et al. (2013) Sodium taurocholate cotransporting polypeptide mediates woolly monkey hepatitis B virus infection of Tupaia hepatocytes. Journal of Virology 87, 7176–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ni Y et al. (2014) Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146, 1070–1083. [DOI] [PubMed] [Google Scholar]
- 57.Li W and Urban S (2016) Entry of hepatitis B and hepatitis D virus into hepatocytes: basic insights and clinical implications. Journal of Hepatology 64, S32–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze A et al. (2010) Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. Journal of Virology 84, 1989–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bogomolov P et al. (2016) Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. Journal of Hepatology 65, 490–498. [DOI] [PubMed] [Google Scholar]
- 60.Bordier BB et al. (2002) A prenylation inhibitor prevents production of infectious hepatitis delta virus particles. Journal of Virology 76, 10465–10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koh C et al. (2015) Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. The Lancet Infectious Diseases 15, 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kocisko DA et al. (2006) Potent antiscrapie activities of degenerate phosphorothioate oligonucleotides. Antimicrobial Agents Chemotherapy 50, 1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Mahtab M, Bazinet M and Vaillant A (2016) Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS ONE 11, e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guilhot S et al. (1994) Expression of the hepatitis delta virus large and small antigens in transgenic mice. Journal of Virology 68, 1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Negro F et al. (1988) Chronic HDV (hepatitis delta virus) hepatitis. Intrahepatic expression of delta antigen, histologic activity and outcome of liver disease. Journal of Hepatology 6, 8–14. [DOI] [PubMed] [Google Scholar]
- 66.Benekli M et al. (2003) Signal transducer and activator of transcription proteins in leukemias. Blood 101, 2940–2954. [DOI] [PubMed] [Google Scholar]
- 67.Niu G et al. (2002) Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 21, 7001–7010. [DOI] [PubMed] [Google Scholar]
- 68.Park CY et al. (2009) Hepatitis delta virus large antigen sensitizes to TNF-alpha-induced NF-kappa B signaling. Molecular Cell 28, 49–55. [DOI] [PubMed] [Google Scholar]
- 69.Benegiamo G et al. (2013) Hepatitis delta virus induces specific DNA methylation processes in Huh-7 liver cancer cells. FEBS Letters 587, 1424–1428. [DOI] [PubMed] [Google Scholar]
- 70.Dastgerdi ES, Herbers U and Tacke F (2012) Molecular and clinical aspects of hepatitis D virus infections. World Journal of Virology 1, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Q et al. (2016) Analysis of long noncoding RNA expression in hepatocellular carcinoma of different viral etiology. Journal of Translational Medicine 14, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McFarlane BM et al. (1995) Autoimmune mechanisms in chronic hepatitis B and delta virus infections. European Journal of Gastroenterology and Hepatology 7, 615–621. [PubMed] [Google Scholar]
- 73.Nisini R et al. (1997) Human CD4+ T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profiles. Journal of Virology 71, 2241–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wedemeyer H and Manns MP (2010) Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nature Reviews Gastroenterology and Hepatology 7, 31–40. [DOI] [PubMed] [Google Scholar]
- 75.Abbas Z et al. (2015) Hepatitis D and hepatocellular carcinoma. World Journal of Hepatology 7, 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radjef N et al. (2004) Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. Journal of Virology 78, 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakano T et al. (2001) Characterization of hepatitis D virus genotype III among Yucpa Indians in Venezuela. Journal of General Virology 82, 2183–2189. [DOI] [PubMed] [Google Scholar]
- 78.Lee CM et al. (1996) Characterization of a new genotype II hepatitis delta virus from Taiwan. Journal of Medical Virology 49, 145–154. [DOI] [PubMed] [Google Scholar]
- 79.Niro GA et al. (1997) The predominance of hepatitis delta virus genotype I among chronically infected Italian patients. Hepatology 25, 728–734. [DOI] [PubMed] [Google Scholar]
- 80.Sy BT et al. (2015) Identification of a natural intergenotypic recombinant hepatitis delta virus genotype 1 and 2 in Vietnamese HBsAg-positive patients. Journal of Viral Hepatitis 22, 55–63. [DOI] [PubMed] [Google Scholar]