Abstract
Background
Schizophrenia is accompanied by widespread alterations in static functional connectivity associated with symptom severity and cognitive deficits. Improvements in aerobic fitness have been demonstrated to ameliorate symptomatology and cognition in people with schizophrenia, but the intermediary role of macroscale connectivity patterns remains unknown.
Objective
Therefore, we aim to explore the relation between aerobic fitness and the functional connectome in individuals with schizophrenia. Further, we investigate clinical and cognitive relevance of the identified fitness-connectivity links.
Methods
Patients diagnosed with schizophrenia were included in this cross-sectional resting-state fMRI analysis. Multilevel Bayesian partial correlations between aerobic fitness and functional connections across the whole brain as well as between static functional connectivity patterns and clinical and cognitive outcome were performed. Preliminary causal inferences were enabled based on mediation analyses.
Results
Static functional connectivity between the subcortical nuclei and the cerebellum as well as between temporal seeds mediated the attenuating relation between aerobic fitness and total symptom severity. Functional connections between cerebellar seeds affected the positive link between aerobic fitness and global cognition, while the functional interplay between central and limbic seeds drove the beneficial association between aerobic fitness and emotion recognition.
Conclusion
The current study provides first insights into the interactions between aerobic fitness, the functional connectome and clinical and cognitive outcome in people with schizophrenia, but causal interpretations are preliminary. Further interventional aerobic exercise studies are needed to replicate the current findings and to enable conclusive causal inferences.
Trial registration
The study which the manuscript is based on is registered in the International Clinical Trials Database (ClinicalTrials.gov identifier [NCT number]: NCT03466112) and in the German Clinical Trials Register (DRKS-ID: DRKS00009804).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00406-022-01411-x.
Keywords: Schizophrenia, Exercise, Fitness, Neuroimaging, fMRI, Functional connectivity
Introduction
Schizophrenia is described as a disorder of dysconnectivity characterized by deficits in synaptic functioning and myelination [1]. Those micro-scale alterations lead to impairments within neural macro-scale circuits which in turn drive psychopathological symptoms and cognitive deficits [2]. In recent years, various large-scale resting-state functional magnetic resonance imaging (fMRI) examinations have confirmed abnormalities of static functional connectivity (FC) patterns in severe mental disorders [3–8]. In resting-state fMRI, FC is defined as the temporal similarity of the blood-oxygen-level-dependent (BOLD) signal of two brain regions during rest and quantifies the degree of their connectedness [9–11]. Compared to healthy controls, hypo- and hyperconnectivities within and between core intrinsic connectivity networks (ICNs) represent typical functional deviations across multiple psychiatric conditions (e.g., schizophrenia, depression, anxiety disorder) [3–7]. Current large-scale evidence identifies schizophrenia-specific functional disconnections of particular seed regions in the salience network (SN), default-mode network (DMN), fronto-parietal network (FPN) and the limbic network [3].
ICNs are generally associated with essential aspects of human behavior like cognition, emotion, perception, interoception and action [12]. Correspondingly, across different psychiatric disorders FC alterations within and between the DMN, SN and FPN are related to deficits in different components of neurocognition such as inhibition control, fluid intelligence, spatial orientation or alertness [5]. In patients with schizophrenia, negative symptom severity is related to FC alterations within the DMN [6], while the cognitive domains of processing speed and working memory performance reveal associations with FC within the SN, the auditory network, the sensorimotor network and the visual network [7]. Both, negative symptoms and cognitive deficits in schizophrenia, remain difficult to treat using antipsychotic medication [13, 14], persist over the long term in most of the patients [15] and contribute to poor social and occupational functioning [16, 17] as well as to low recovery rates [18].
In healthy individuals, compelling evidence demonstrates that moderate exercise ameliorates general health [19] and improves cognitive functioning [20], while aerobic fitness is linked to widespread adaptations of FC across the whole brain [21]. In people with schizophrenia, different kinds of exercise treatments show small to medium beneficial effects on positive [22–26] and negative symptom severity [22–25, 27, 28], depressive symptoms [24, 29], several cognitive domains [25, 26, 29, 30], quality of life [24, 29] and global functioning [24, 26, 31]. In particular, exercise interventions aiming at enhancing aerobic fitness represent promising adjunctive treatment strategies in schizophrenia [22, 26, 30, 32].
Beneficial effects of such interventions are assumed to be mediated by multiple neurophysiological processes such as structural plasticity changes (e.g., increases in grey and white matter volumes) and molecular adaptations (e.g., changes in growth factor and neurotransmitter concentrations) [33, 34]. However, the mechanistic role of changes in macro-scale FC patterns that potentially drive the beneficial effects of aerobic exercise on psychiatric symptoms and cognition in schizophrenia has been neglected yet. Importantly, even the general association between aerobic fitness and global FC patterns in patients with schizophrenia has not been studied to date. Consequently, we do not know to which particular functional connections aerobic fitness is generally linked in people with schizophrenia and thus cannot derive hypotheses on behaviorally relevant, regional FC adaptations induced by aerobic exercise interventions.
The current cross-sectional study addresses this gap to enable hypothesis-driven aerobic exercise intervention approaches that investigate the mediating role of region-specific changes in FC. We aim to provide first insights into potential FC mechanisms that drive the beneficial link between aerobic fitness and psychopathological outcome. Therefore, we examine the relation between aerobic fitness and multiple functional connections across the whole brain (defined as the functional connectome) in patients with schizophrenia using a global, exploratory approach. Further, we investigate if those functional connections associated with aerobic fitness also demonstrate clinical or cognitive relevance and mediate the association between aerobic fitness and psychiatric symptoms and cognition.
Methods
The ESPRIT C3 study is a clinical, randomized-controlled, multicenter trial examining the effects of an aerobic exercise intervention on multiple health outcomes in people with schizophrenia [35]. All patients were diagnosed with schizophrenia in accordance with DSM-IV and the majority received antipsychotic mediation. For inclusion and exclusion criteria as well as other study details see Maurus et al. [35]. The current cross-sectional investigation utilized the baseline data of the ESPRIT C3 study prior to intervention onset.
Study sample
A sample of 101 patients with schizophrenia recruited at study centers LMU Hospital in Munich and Central Institute of Mental Health in Mannheim underwent MRI scans (for sample characteristics see Table 1). In case of four subjects, inclusion and exclusion criteria were not fulfilled although MRI data were available. Nine subjects had no resting-state fMRI sequences, while further nine subjects were excluded due to lacking image quality (suppl. S2). Depending on the corresponding statistical analysis (“Statistical data analysis”), a different number of subjects was included: regarding the correlations between aerobic fitness and the functional connectome, further 21 subjects had to be excluded because no fitness data were available resulting in 58 individuals considered in this analysis. With respect to the correlations between the functional connectome and clinical and cognitive scores, different amounts of participants had to be excluded depending on the number of invalid or missing values in each cognitive test battery resulting in 72 to 79 individuals included in this analysis (suppl. S6). In the context of the mediation analyses, the aforementioned 21 subjects were removed due to lacking fitness data. The number of missing values differed between clinical and cognitive tests leading to 51–58 patients included in this approach (suppl. S6).
Table 1.
Sample characteristics
| Attribute | n | Mean ± SD |
|---|---|---|
| Sample | ||
| Full | 101 | – |
| After exclusion of screening failures | 97 | – |
| After exclusion of subjects with lacking or noisy fMRI sequences | 79 | – |
| After exclusion of subjects with lacking fitness data | 58 | – |
| Age (years) | 97 | 37.35 ± 12.20 |
| Sex | – | |
| Male | 62 (63.9%) | – |
| Female | 35 (36.1%) | – |
| Site | ||
| Munich | 72 (74.2%) | – |
| Mannheim | 25 (25.8%) | – |
| Chlorpromazine equivalents (CPZ) | 97 | 372.11 ± 245.74 |
| Years of education | 97 | 14.48 ± 4.14 |
| Disorder duration (years) | 97 | 9.29 ± 9.24 |
| Body-mass-index (BMI) | 97 | 28.30 ± 5.31 |
Important sample characteristics of the whole sample are listed. All numbers relate to the study sample after exclusion of screening failures. Screening failures reflect cases that were removed due to inclusion and exclusion criteria. Data from other sub-samples are neglected because they are fairly similar
Operationalization of aerobic fitness
Subjects performed a stepwise lactate threshold test on a stationary bicycle ergometer. A function describing the relation between wattage and lactate concentration was estimated. Lactate concentrations at around 2 mmol/l are supposed to represent the aerobic threshold [36]. We identified the individual aerobic threshold at lactate concentrations between 1.8 and 2.5 mmol/l according to the previously defined exercise protocol [35]. Achieved wattage at a subject-specific lactate concentration within this range divided by body weight represents individuals’ performance capability at an aerobic exercise intensity. We refer to this value using the term aerobic fitness.
fMRI data acquisition and pre-processing
MRI data at both study centers were acquired in a whole-body 3.0 Tesla MRI Scanner (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany). Subjects at both study sites underwent at least one echo-planar imaging (EPI) sequence and one T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) sequence (see suppl. S1 for scanning parameters). Raw data files from the scanners were converted from DICOM to NIFTI format using dcm2niix software [37]. NIFTI files were embedded into a BIDS data structure [38].
Quality control was performed utilizing the automated software MRIQC [39]. Pre-processing was done using FMRIPREP [40] (suppl. S3). Within FMRIPREP, automatic removal of motion artifacts based on independent component analysis (ICA-AROMA) was utilized to extract aggressive noise regressors [41]. Framewise displacement [42, 43] and DVARS [43] as well as the temporal signal-to-noise ratio calculated via fslmaths from FSL v 6.0.4 [44] were evaluated again after pre-processing. The slicer attribute from NiBabel v3.2.1 [45] was administered to remove the first ten dummy scans of every pre-processed fMRI file. Images were smoothed (FWHM = 6 mm) with the smooth_img function from Nilearn v0.8.0 which was built on scikit-learn [46] and the temporal signal-to-noise ratio was checked again. Confound regression, detrending, low- and high-pass filtering (0.008–0.1 Hz) and signal standardization were performed within one step utilizing the clean_img function from Nilearn v0.8.0. Global signal, cerebrospinal fluid, white matter and the extracted noise components from ICA-AROMA were regressed from BOLD timeseries according to current findings on different denoising strategies [47].
fMRI data post-processing
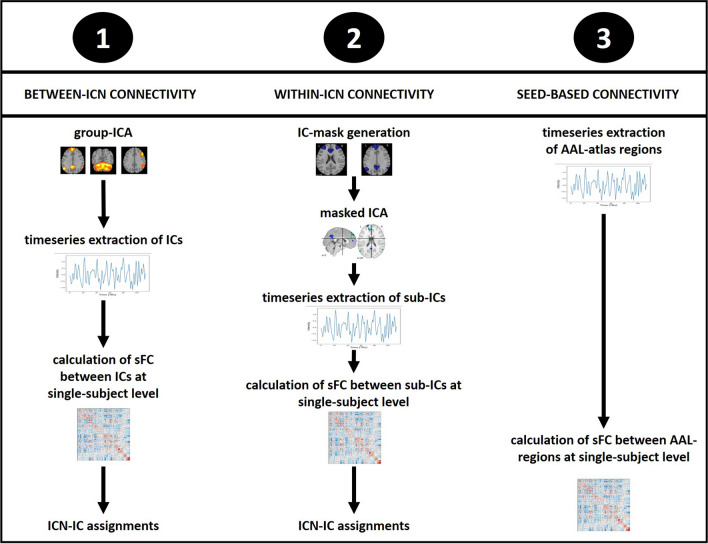
To explore the functional connectome in patients with schizophrenia, three different analyses were applied on denoised fMRI data (Fig. 1): (1) computation of FC between ICNs, (2) assessment of FC within ICNs and (3) a seed-based examination of FC between different brain regions. These approaches were selected to cover different perspectives on the broad concept of the functional connectome. While the first and second analysis provide insights into the functional organization of the human brain based on widespread ICNs, the seed-based approach takes into account the anatomical organization of the brain with a higher spatial resolution. Since we examine associations between aerobic fitness and the functional connectome from a global and exploratory perspective, we aimed to target multiple facets of the functional connectome. All three approaches are common in neuropsychiatric fMRI research.
Fig. 1.
Workflow of the three resting-state fMRI analysis approaches. The analysis steps of the three fMRI approaches that aim to examine the functional connectome from different perspectives are visualized. Details on the workflows are described in the manuscript text
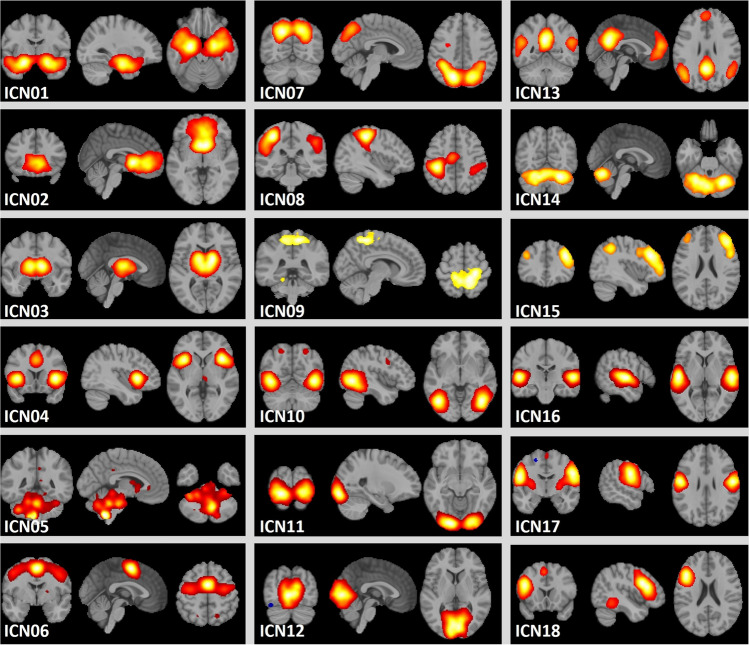
Due to differences in various scanning parameters, all analyses were executed separately for data from Munich and Mannheim. Table 2 and Fig. 2 illustrate the 18 ICNs with their included regions proposed by Laird et al. [12].
Table 2.
ICNs and the corresponding anatomical regions
| Group | Network | Regions |
|---|---|---|
| 1 | ICN01 | Limbic and medial-temporal areas |
| Emotional and autonomic processes | ICN02 | Subgenual anterior cingulate cortex, orbitofrontal cortex |
| ICN03 | Basal ganglia and thalamus | |
| ICN04 | Anterior insula, frontal opercula, anterior cingulate cortex (SN) | |
| ICN05 | Midbrain | |
| 2 | ICN06 | Premotor and supplementary motor area, frontal eye field |
| Motor and visuospatial integration, coordination, execution | ICN07 | Dorsolateral prefrontal cortex, posterior parietal cortex |
| ICN08 | Primary sensorimotor cortices (extremities) | |
| ICN09 | Medial posterior parietal association areas | |
| 3 | ICN10 | Middle temporal visual association areas |
| Visual perception | ICN11 | Primary, secondary and tertiary visual cortices |
| ICN12 | Primary, secondary and tertiary visual cortices | |
| 4 | ICN13 | Medial prefrontal and posterior cingulate cortex (DMN) |
| Divergent networks | ICN14 | Cerebellum |
| ICN15 | Right-lateralized fronto-parietal regions (right FPN) | |
| ICN16 | Transverse temporal gyri, primary auditory cortices | |
| ICN17 | Primary sensorimotor cortices (mouth) | |
| ICN18 | Left-lateralized fronto-parietal regions (left FPN) |
The 18 ICNs based on Laird et al. [12] including the anatomical regions that they cover are listed
Fig. 2.
Visualization of the ICNs. The 18 ICNs based on Laird et al. [12] are illustrated. ICNs are z-standardized, thresholded at z > 4 and mapped onto the MNI152NLin2009cAsym standard space with a resolution of 2 mm in neurological convention. Color mapping is not standardized across ICNs
Between-ICN connectivity
Using melodic from FSL v6.0.4 [44, 48], a group ICA was computed to extract 20 sample-specific independent components (ICs). Dual regression from FSL v6.0.4 [44, 48] was administered to extract the BOLD-timeseries of every IC for each subject. ConnectivityMeasure function from Nilearn v0.8.0 was used to calculate subject-specific FC between ICs quantified as Pearson’s correlation coefficient. The latter were converted to z values using Fisher’s r-to-z transformation. ICs were cross-correlated with the 18 ICNs of the functional atlas provided by Laird et al. [12] utilizing fslcc of FSL v6.0.4 [44]. Each IC was assigned to a corresponding ICN depending on the magnitude of their statistical overlap (r ≥ 0.2). In ambiguous cases, ICs were inspected visually and assignments were adjusted if necessary (see suppl. S4 for cross-correlation results and ICN/IC visualizations). Data from study sites Munich and Mannheim were concatenated after ICN-assignments. In sum, 148 between-ICN FC measures per subject (one for every unique ICN combination) were computed.
Within-ICN connectivity
The melodic_IC output from the abovementioned group ICA was splitted using fslsplit from FSL v6.0.4 [44]. The created single IC files were thresholded (z = 4) and binarized with fslmaths providing masks of every IC. Canonical ICA [49] within those masks was performed with the CanICA function of Nilearn v0.8.0 aiming at extracting two sub-ICs within every main IC. To determine the BOLD-timeseries of each sub-IC at single-subject level, the fit_transform attribute from Nilearn v0.8.0 was applied. FC within every IC was again calculated with the ConnectivityMeasure function from Nilearn v0.8.0 by correlating the timeseries of both corresponding sub-ICs and performing Fisher’s transformation. Data from study sites Munich and Mannheim were merged and site-specific ICs were labelled based on ICN-assignments. In total, 18 within-ICN FC measures per subject (one for each ICN) were extracted.
Seed-based connectivity
NiftiLabelsMasker function from Nilearn v0.8.0 was employed to extract the BOLD-timeseries of 116 brain regions defined by the Automated Anatomical Labelling (AAL) atlas [50]. FC between these regional, subject-specific timeseries was again calculated using ConnectivityMeasure function from Nilearn v0.8.0 and Fisher’s transformation. Data from both study centers were concatenated analogously. In sum, 6670 seed-based FC measures per subject (one for every unique AAL-region combination) were calculated.
Clinical and cognitive data acquisition
The Positive and Negative Syndrome Scale (PANSS) [51] was employed to assess positive (PANSS-positive), negative (PANSS-negative) and general psychopathological symptoms (PANSS-psychopath) as well as summarized symptom severity (PANSS-total). Calgary Depression Scale for Schizophrenia (CDSS) was utilized to measure depressive symptoms [52]. Covering global disorder severity, the Clinical Global Impression (CGI) scale was administered [53]. Global cognition was targeted by Trail Making Tests A and B (TMT) [54], the category naming part of the Brief Cognitive Assessment Tool for Schizophrenia (B-CATS) [55] and the Digit Symbol Substitution Test (DSST) [56]. The forward and backward versions of the Digit Span Test (DST) [56] were used to measure verbal working memory performance, while verbal declarative memory was covered by seven different measures of the Verbal Learning and Memory Test (VLMT) [57]. Emotion recognition capability was examined by an adjusted version of the Emotion Recognition Test (ERT) [58]. For detailed descriptions of the co gnitive tests and the corresponding abbreviations see suppl. S5.
Statistical data analysis
Rstudio v1.4.1717 based on R v4.1.2 was used for statistical data analysis [59, 60]. We detected outliers in the distributions of FC data as well as clinical and cognitive data (for details see suppl. S6). Thereafter, behavioral data were z-standardized and multilevel Bayesian partial correlations between aerobic fitness and all functional connections (between-ICN: 148 connections, within-ICN: 18, seed-based: 6670) were calculated using the correlation package in R v4.1.2 [61]. Age, body-mass-index (BMI), disorder duration, education years and chlorpromazine equivalents were included as covariates, while sex and study site were treated as random factors within a mixed effect model. Chlorpromazine equivalents were computed based on the defined daily dose method [62]. The main output of interest was Jeffrey’s default Bayes factor (BF10) representing a continuous, relative measure of evidence the data is providing for the alternative hypothesis (H1: r ≠ 0) compared to the null hypothesis (H0: r = 0) [63, 64]. For instance, if the BF10 = 3, it is three times more likely to observe the current data under the alternative hypothesis than under the null hypothesis. The BF10 can be separated in different categories of evidence strength facilitating interpretations and conclusions (suppl. tab. S7) [65]. In addition, Pearson’s correlation coefficient with its corresponding highest density interval (HDI), the probability of direction (PD) and the region of practical equivalence (ROPE) were considered to evaluate the existence of an association between the variables of interest [66] (for detailed description of Bayesian parameters and prior selection see suppl. S7). Regarding between- and within-ICN FC, we focused on single associations between aerobic fitness and the corresponding functional connection. We correlated between- and within-ICN connections with clinical and cognitive scores, if they tended to relate to aerobic fitness. Considering the large number of 6670 FC measures in the seed-based approach, we examined if seed connections of specific anatomical clusters defined by the AAL atlas [50] were related to aerobic fitness most robustly (for a detailed description of cluster definition and evaluation of robustness see suppl. S8). The most prominent anatomical clusters were related to clinical and cognitive scores. In all three approaches, the mediation package in R v4.1.2 [67] was used to compute a mediation analysis, if correlations between aerobic fitness and FC as well as FC and clinical/cognitive scores existed. Finally, a Bayes factor design analysis (BFDA) [68, 69] was performed aiming to evaluate the probabilities to obtain a BF10 > 3 under the alternative and null hypothesis within the current study design. BFDA was done utilizing the BFDA package in R v4.1.2 [70] (see suppl. S9).
Results
Aerobic fitness, between-ICN connectivity and clinical/cognitive outcome
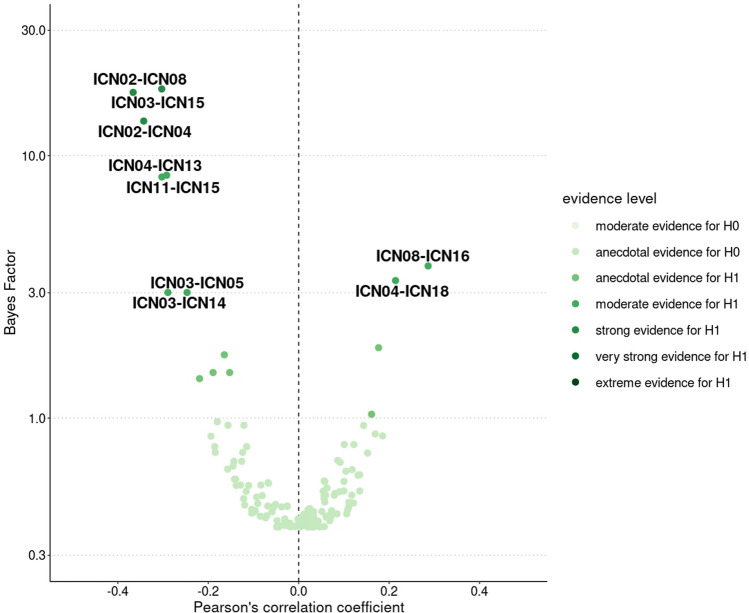
15 of 148 investigated functional connections demonstrated at least anecdotal evidence in favor of a correlation to aerobic fitness (BF10 > 1, Fig. 3). The following eight functional connections revealed the most robust associations ranging from moderate to very strong evidence strengths. ICN05 and ICN14 were covered by the same IC in our sample (suppl. S4) resulting in equal findings:
Fig. 3.
BFs and partial correlations between aerobic fitness and between-ICN connectivity. Correlation tests resulting in a BF10 around three or higher are labelled with the corresponding between-ICN connection. Categorical scheme of the BF10 according to Lee and Wagenmarkers [65]: BF10 > 1: anecdotal evidence for H1, BF10 > 3: moderate evidence for H1, BF10 > 10: strong evidence for H1, BF10 > 30: very strong evidence for H1, BF10 > 100: extreme evidence for H1, N = 58
Aerobic fitness was positively correlated with FC between ICN08 (primary sensorimotor cortices) and ICN16 (primary auditory cortices) (BF10 = 3.80, r = 0.26 [0.06, 0.43], PD = 98.6%, ROPE = 9.4%) as well as ICN04 (SN) and ICN18 (left FPN) (BF10 = 3.34, r = 0.25 [0.05, 0.42], PD = 98.3%, ROPE = 10.4%). Aerobic fitness revealed negative correlations with FC between ICN02 (subgenual anterior cingulate and orbitofrontal cortex) and ICN08 (primary sensorimotor cortices) (BF10 = 17.94, r = − 0.33 [− 0.50, − 0.17], PD = 99.9%, ROPE = 2.3%), ICN03 (basal ganglia and thalamus) and ICN15 (right FPN) (BF10 = 17.41, r = − 0.33 [− 0.50, − 0.17], PD = 99.9%, ROPE = 2.4%), ICN02 (subgenual anterior cingulate and orbitofrontal cortex) and ICN04 (SN) (BF10 = 3.09, r = − 0.25 [− 0.44, − 0.07], PD = 98.0%, ROPE = 11.3%), ICN04 (basal ganglia and thalamus) and ICN13 (DMN) (BF10 = 13.54, r = − 0.32 [− 0.49, − 0.15], PD = 99.8%, ROPE = 2.6%), ICN11 (visual cortices) and ICN15 (right FPN) (BF10 = 8.29, r = − 0.30 [− 0.47, − 0.12], PD = 99.2%, ROPE = 4.2%) and ICN03 (basal ganglia and thalamus) and ICN05/14 (midbrain and cerebellum) (BF10 = 3.01, r = − 0.24 [− 0.43, − 0.07], PD = 97.9%, ROPE = 11.7%).
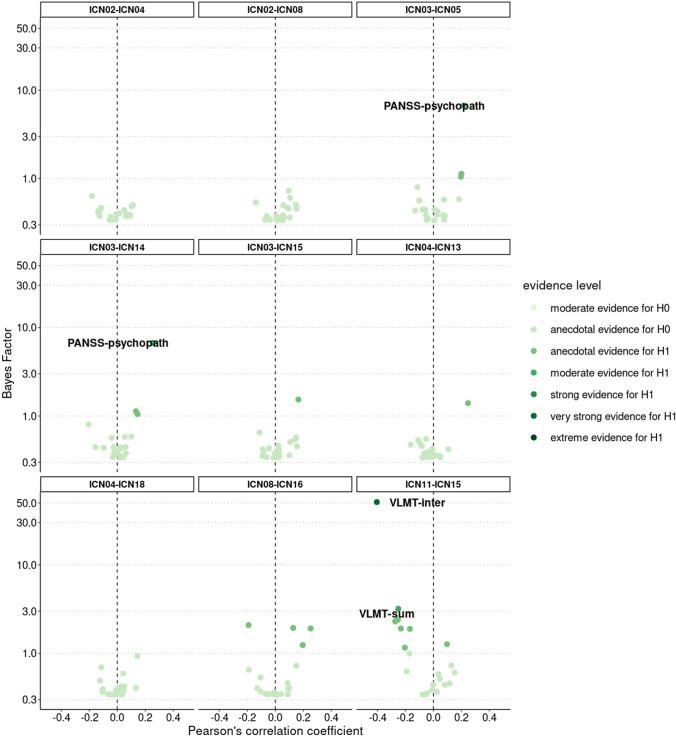
Two of the eight functional connections linked to aerobic fitness were associated with clinical or cognitive scores (Fig. 4) accompanied by two significant mediation effects:
Fig. 4.
BFs and partial correlations between-ICN FC and clinical and cognitive scores. Correlation tests resulting in a BF10 around three or higher are labelled with the corresponding name of the test battery. Categorical scheme of the BF10 according to Lee and Wagenmarkers [65]: BF10 > 1: anecdotal evidence for H1, BF10 > 3: moderate evidence for H1, BF10 > 10: strong evidence for H1, BF10 > 30: very strong evidence for H1, BF10 > 100: extreme evidence for H1, N = 72–79
The functional connection between ICN03 (basal ganglia and thalamus) and ICN05/14 (midbrain and cerebellum) was positively correlated with PANSS-psychopath (BF10 = 6.71, r = 0.25 [0.08, 0.40], PD = 99.1%, ROPE = 7.2%) including a significant negative mediation effect (p = 0.022, β = − 0.15 [− 0.30, − 0.03]). Hence, higher patients’ aerobic fitness levels were accompanied by lower FC between the basal ganglia/thalamus and the midbrain/cerebellum, which in turn led to lower PANSS-psychopath scores. The functional connection between ICN11 (visual cortices) and ICN15 (right FPN) revealed a negative correlation with VLMT-inter (BF10 = 51.15, r = − 0.34 [− 0.49, − 0.19], PD = 100%, ROPE = 1.2%) and VLMT-sum score (BF10 = 3.19, r = − 0.23 [− 0.39, − 0.07], PD = 98.4%, ROPE = 12.1%). Positive mediation effects were significant in case of VLMT-inter (p = 0.044, β = 0.13 [0.18, 0.27]), but not for VLMT-sum (p = 0.25, β = 0.05 [− 0.01, 0.13]). Consequently, the higher patients’ aerobic fitness was, the lower was FC between the visual network and the right FPN leading to better performance in VLMT-inter.
Aerobic fitness, within-ICN connectivity and clinical/cognitive outcome
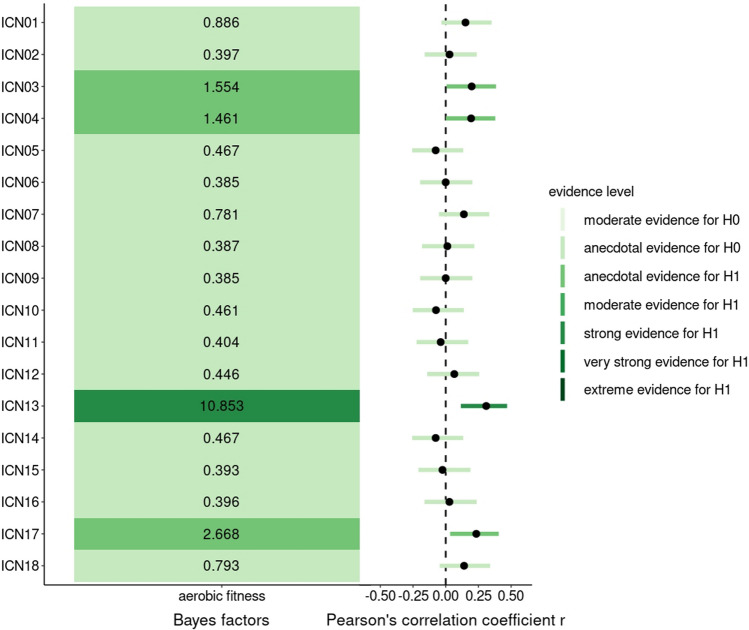
Four of 18 investigated functional connections indicated at least anecdotal evidence in favor of a correlation to aerobic fitness (BF10 > 1, Fig. 5). The most robust finding was the positive correlation between aerobic fitness and FC within ICN13 (DMN) (BF10 = 10.85, r = 0.31 [0.12, 0.47], PD = 99.4%, ROPE = 3.7%) (Fig. 5).
Fig. 5.
BFs and partial correlations between aerobic fitness and within-ICN connectivity. On the left-hand side, BFs of the multilevel partial correlation tests between aerobic fitness and within-ICN FC are displayed and colored according to evidence strength. On the right, the corresponding correlation coefficients and the HDIs are visualized. Categorical scheme of the BF10 according to Lee and Wagenmarkers [65]: BF10 > 1: anecdotal evidence for H1, BF10 > 3: moderate evidence for H1, BF10 > 10: strong evidence for H1, BF10 > 30: very strong evidence for H1, BF10 > 100: extreme evidence for H1, N = 58
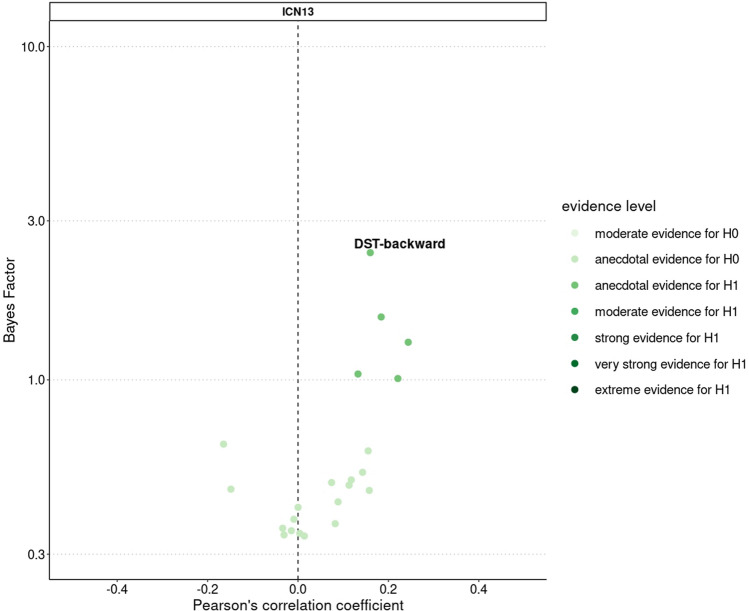
With respect to clinical and cognitive relevance (Fig. 6), anecdotal evidence in favour of a positive correlation between FC within ICN13 (DMN) and DST-backward performance (BF10 = 2.41, r = 0.21 [0.04, 0.38], PD = 97.7%, ROPE = 14.7%) was observed, but a significant mediation effect was lacking (p = 0.21, β = 0.07 [− 0.01, 0.17]).
Fig. 6.
BFs and partial correlations between within-ICN FC and clinical and cognitive scores. Visualization of the BFs and correlation coefficients of the Bayesian multilevel partial correlation tests including within-ICN connectivity and clinical and cognitive scores. Correlation tests resulting in a BF10 of two or higher are labelled with the corresponding name of the test battery. Categorical scheme of the BF10 according to Lee and Wagenmarkers [65]: BF10 > 1: anecdotal evidence for H1, BF10 > 3: moderate evidence for H1, BF10 > 10: strong evidence for H1, BF10 > 30: very strong evidence for H1, BF10 > 100: extreme evidence for H1, N = 72–79
Aerobic fitness, seed-based connectivity and clinical/cognitive outcome
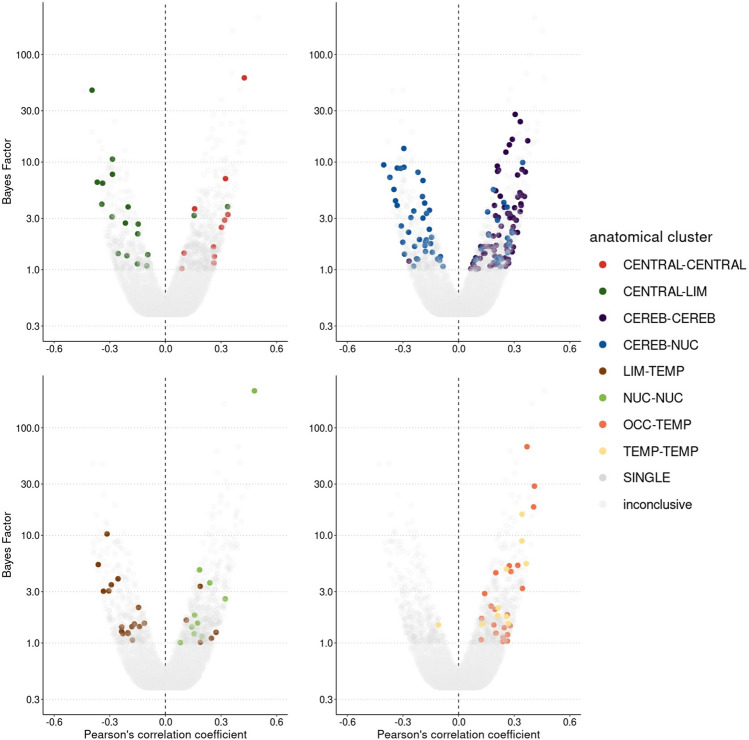
The 6670 functional connections between all AAL-regions were assigned to 45 anatomical clusters based on the anatomical description proposed by Tzourio-Mazoyer et al. [50]. These 45 anatomical clusters were evaluated in terms of the robustness of their relation to aerobic fitness (suppl. S8). Eight anatomical clusters demonstrated the most robust associations with aerobic fitness (Fig. 7):
Fig. 7.
BFs and partial correlations between aerobic fitness and seed-based connectivity of anatomical clusters. Tests resulting in a BF10 < 1 are labelled as inconclusive. Tests resulting in a BF10 > 1 are either assigned to the eight most consistent clusters CENTRAL–CENTRAL, CENTRAL–LIM, CEREB–CEREB, CEREB–NUC, LIM–TEMP, NUC–NUC, OCC–TEMP, TEMP–TEMP or labelled as SINGLE. N = 58, CENTRAL: central cortical structures, LIM: limbic lobe, CEREB: cerebellum, NUC: subcortical nuclei, TEMP: temporal lobe, OCC: occipital lobe, SINGLE = single connections that were linked to aerobic fitness, but were not part of a robust cluster
The first cluster consisted of 15 functional connections between seeds from central cortical regions (CENTRAL–CENTRAL). Eleven of these 15 functional connections (73.3%) were linked to aerobic fitness. All eleven correlations were positive (100%) and evidence strengths ranged from anecdotal to very strong (BF10mean = 7.87, BF10median = 2.48, BF10range = 1.02–60.66, r = 0.16–0.38, PD = 91.5–100%, ROPE = 27.5–1.2%).
The second cluster comprised 84 functional connections between seeds from central cortical regions and seeds from the limbic lobe (CENTRAL–LIM). 18 of these 84 functional connections (21.43%) revealed correlations with aerobic fitness. 16 correlations were negative (88.89%) accompanied by anecdotal to very strong evidence strengths (BF10mean = 6.42, BF10median = 2.91, BF10range = 1.09–46.60, r = − 0.17 to − 0.37, PD = 92.3–100%, ROPE = 25.0–1.0%).
325 functional connections between cerebellar seeds formed the third cluster (CEREB–CEREB). 80 of these 325 functional connections (24.62%) demonstrated correlations with aerobic fitness. 79 correlations were positive (98.75%) ranging from anecdotal to strong evidence levels (BF10mean = 3.94, BF10median = 2.09, BF10range = 1.00–27.76, r = 0.16–0.35, PD = 91.3–99.8%, ROPE = 28.1–1.8%).
The fourth cluster included 260 functional connections between cerebellar seeds and seeds from the subcortical nuclei (CEREB–NUC). 57 of these 260 functional connections (21.92%) revealed correlations with aerobic fitness. 39 correlations were negative (68.42%) including anecdotal to strong evidence strengths (BF10mean = 3.72, BF10median = 2.37, BF10range = 1.07–13.34, r = − 0.17 to − 0.32, PD = 92.2–99.8%, ROPE = 25.4–2.7%).
The fifth cluster consisted of 112 functional connections between seeds from the limbic lobe and seeds from the temporal lobe (LIM-TEMP). 22 of these 112 functional connections (19.64%) correlated with aerobic fitness. 17 correlations were negative (77.27%) and evidence levels ranged from anecdotal to strong (BF10mean = 2.62, BF10median = 1.50, BF10range = 1.07–10.32, r = − 0.17 to − 0.31, PD = 92.2–99.5%, ROPE = 25.6–3.5%).
45 functional connections between seeds from the subcortical nuclei formed the sixth cluster (NUC–NUC). Ten of these 45 functional connections (22.22%) exhibited correlations with aerobic fitness. All ten correlations were positive (100%) ranging from anecdotal to extreme evidence levels (BF10mean = 23.96, BF10median = 1.67, BF10range = 1.01–220.48, r = 0.16–0.42, PD = 91.3–100%, ROPE = 28.0–0.3%).
The seventh cluster comprised 112 functional connections between seeds from the occipital lobe and seeds from the temporal lobe (OCC–TEMP). 22 of these 112 functional connections (19.64%) revealed correlations with aerobic fitness. All 22 correlations were positive (100%) accompanied by anecdotal to very strong evidence strengths (BF10mean = 7.19, BF10median = 1.94, BF10range = 1.04–66.69, r = 0.16 0.38, PD = 91.7–100%, ROPE = 26.9–1.4%).
The eighth cluster included 28 functional connections between seeds from the temporal lobe (TEMP–TEMP). Ten of these 28 functional connections (35.71%) correlated with aerobic fitness. Nine of ten correlations were positive (90%) ranging from anecdotal to strong evidence levels (BF10mean = 4.85, BF10median = 2.11, BF10range = 1.50–15.71, r = 0.20–0.33, PD = 95.3–99.5%, ROPE = 19.4–2.8%).
Of these eight anatomical clusters linked to aerobic fitness, the following five exhibited consistent relations to clinical and cognitive outcomes across multiple functional connections (suppl. figs. S10.2, S10.3, S10.4, S10.7 and S10.8):
Four of 18 functional connections linked to aerobic fitness in the CENTRAL-LIM cluster correlated with performance in the ERT (BF10range = 6.93–41.53, r = − 0.26 to − 0.33, PD = 99.1–100%, ROPE = 6.0–1.4%). Two of four mediation effects were significant suggesting a positive impact of aerobic fitness on ERT performance mediated by two functional connections between seeds from central cortical regions and seeds from limbic lobe (Table 3).
Table 3.
Results of the mediation analyses
| IV | Mediator | DV | Eff | Estimate | CI low | CI high | p | n |
|---|---|---|---|---|---|---|---|---|
| CENTRAL–LIM | ||||||||
| Aerobic fitness | CingulumAntR-PostcentralR | ERT | ACME | 0.03 | − 0.04 | 0.12 | 0.550 | 55 |
| Aerobic fitness | RolandicOperL-CingulumPostR | ERT | ACME | 0.09 | 0.004 | 0.21 | 0.080 | 55 |
| Aerobic fitness | RolandicOperR-CingulumPostL | ERT | ACME | 0.15 | 0.03 | 0.30 | 0.040* | 55 |
| Aerobic fitness | RolandicOperR-CingulumPostR | ERT | ACME | 0.12 | 0.02 | 0.25 | 0.036* | 55 |
| CEREB–CEREB | ||||||||
| Aerobic fitness | Cerebellum3R-Cerebellum45L | TMT-A | ACME | − 0.02 | − 0.10 | 0.06 | 0.740 | 57 |
| Aerobic fitness | Cerebellum9R-Vermis10 | TMT-A | ACME | − 0.10 | − 0.21 | − 0.02 | 0.028* | 57 |
| Aerobic fitness | CerebellumCrus1R-Cerebellum6L | TMT-A | ACME | − 0.05 | − 0.14 | 0.00 | 0.130 | 57 |
| Aerobic fitness | CerebellumCrus2R-Cerebellum10R | TMT-A | ACME | − 0.04 | − 0.11 | 0.01 | 0.31 | 57 |
| Aerobic fitness | CerebellumCrus2R-Cerebellum9L | TMT-A | ACME | − 0.11 | − 0.23 | − 0.03 | 0.024* | 57 |
| Aerobic fitness | CerebellumCrus2R-Cerebellum9L | TMT-B | ACME | − 0.12 | − 0.24 | − 0.02 | 0.044* | 56 |
| Aerobic fitness | CerebellumCrus2R-Vermis9 | TMT-A | ACME | − 0.06 | − 0.15 | 0.00 | 0.098 | 57 |
| Aerobic fitness | Cerebellum3R-Cerebellum45R | B-CATS-fruits | ACME | 0.05 | − 0.02 | 0.14 | 0.320 | 55 |
| Aerobic fitness | Cerebellum7bR-Cerebellum9L | B-CATS-fruits | ACME | 0.08 | 0.00 | 0.21 | 0.11 | 55 |
| Aerobic fitness | Cerebellum7bR-Cerebellum9L | B-CATS-vegetables | ACME | 0.06 | − 0.03 | 0.19 | 0.290 | 51 |
| Aerobic fitness | Cerebellum7bR-Vermis9 | B-CATS-fruits | ACME | 0.05 | − 0.02 | 0.15 | 0.280 | 55 |
| Aerobic fitness | Cerebellum7bR-Vermis9 | B-CATS-vegetables | ACME | 0.03 | − 0.03 | 0.11 | 0.550 | 51 |
| Aerobic fitness | Cerebellum9L-Vermis7 | B-CATS-vegetables | ACME | 0.10 | − 0.01 | 0.23 | 0.146 | 51 |
| Aerobic fitness | Cerebellum9L-Vermis7 | B-CATS-animals | ACME | 0.06 | − 0.01 | 0.16 | 0.244 | 55 |
| Aerobic fitness | Cerebellum9L-Vermis8 | B-CATS-vegetables | ACME | 0.10 | 0.00 | 0.22 | 0.098 | 51 |
| Aerobic fitness | Cerebellum9L-Vermis8 | B-CATS-animals | ACME | 0.08 | − 0.01 | 0.19 | 0.140 | 55 |
| Aerobic fitness | Cerebellum9R-Vermis7 | B-CATS-vegetables | ACME | 0.12 | 0.01 | 0.27 | 0.054 | 51 |
| Aerobic fitness | Cerebellum9R-Vermis8 | B-CATS-vegetables | ACME | 0.13 | 0.02 | 0.28 | 0.050 | 51 |
| Aerobic fitness | Cerebellum9R-Vermis9 | B-CATS-animals | ACME | 0.09 | − 0.01 | 0.21 | 0.166 | 55 |
| Aerobic fitness | CerebellumCrus1R-Vermis6 | B-CATS-animals | ACME | 0.00 | − 0.11 | 0.12 | 0.980 | 55 |
| Aerobic fitness | CerebellumCrus2R-Cerebellum9L | B-CATS-vegetables | ACME | 0.04 | − 0.04 | 0.15 | 0.450 | 51 |
| CEREB–NUC | ||||||||
| Aerobic fitness | AmygdalaR-CerebellumCrus2L | PANSS-negative | ACME | − 0.07 | − 0.18 | 0.00 | 0.140 | 57 |
| Aerobic fitness | CaudateL-CerebellumCrus1R | PANSS-negative | ACME | − 0.09 | − 0.21 | 0.00 | 0.140 | 57 |
| Aerobic fitness | CaudateR-Cerebellum45L | PANSS-positive | ACME | − 0.07 | − 0.19 | 0.00 | 0.120 | 58 |
| Aerobic fitness | CaudateR-Cerebellum45L | PANSS-total | ACME | − 0.06 | − 0.16 | 0.01 | 0.220 | 58 |
| Aerobic fitness | CaudateR-Cerebellum45L | CDSS | ACME | − 0.09 | − 0.20 | − 0.01 | 0.044* | 58 |
| Aerobic fitness | PallidumR-Vermis3 | CDSS | ACME | 0.03 | − 0.3 | 0.11 | 0.420 | 58 |
| Aerobic fitness | PallidumR-Vermis8 | CGI | ACME | − 0.09 | − 0.21 | 0.00 | 0.110 | 58 |
| Aerobic fitness | PallidumR-Vermis8 | PANSS-total | ACME | − 0.08 | − 0.21 | 0.00 | 0.120 | 58 |
| Aerobic fitness | PallidumR-Vermis8 | PANSS-psychopath | ACME | − 0.08 | − 0.18 | 0.00 | 0.140 | 58 |
| Aerobic fitness | PallidumR-Vermis8 | PANSS-positive | ACME | − 0.05 | − 0.15 | 0.01 | 0.250 | 58 |
| Aerobic fitness | PutamenL-Vermis45 | PANSS-psychopath | ACME | 0.05 | − 0.01 | 0.15 | 0.250 | 58 |
| Aerobic fitness | PutamenL-Vermis45 | CDSS | ACME | 0.05 | − 0.01 | 0.13 | 0.230 | 58 |
| Aerobic fitness | ThalamusL-Cerebellum9R | CGI | ACME | − 0.06 | − 0.16 | 0.01 | 0.25 | 58 |
| Aerobic fitness | ThalamusR-Cerebellum9R | CGI | ACME | − 0.15 | − 0.30 | − 0.04 | 0.022* | 58 |
| Aerobic fitness | CaudateL-Cerebellum6L | B-CATS-fruits | ACME | 0.05 | − 0.01 | 0.15 | 0.260 | 55 |
| Aerobic fitness | CaudateL-Cerebellum6L | B-CATS-vegetables | ACME | 0.02 | − 0.05 | 0.11 | 0.574 | 51 |
| Aerobic fitness | CaudateL-Cerebellum6R | B-CATS-vegetables | ACME | 0.05 | − 0.06 | 0.18 | 0.500 | 51 |
| Aerobic fitness | CaudateR-Cerebellum8L | B-CATS-fruits | ACME | 0.04 | − 0.02 | 0.14 | 0.330 | 55 |
| Aerobic fitness | PallidumR-CerebellumCrus1L | B-CATS-fruits | ACME | 0.08 | − 0.02 | 0.20 | 0.220 | 55 |
| Aerobic fitness | PutamenL-CerebellumCrus1L | B-CATS-fruits | ACME | 0.07 | − 0.02 | 0.20 | 0.230 | 55 |
| Aerobic fitness | PutamenL-CerebellumCrus2L | B-CATS-fruits | ACME | 0.03 | − 0.05 | 0.12 | 0.610 | 55 |
| Aerobic fitness | PutamenR-Cerebellum10L | B-CATS-vegetables | ACME | 0.05 | − 0.02 | 0.15 | 0.316 | 51 |
| Aerobic fitness | PutamenR-CerebellumCrus1L | B-CATS-fruits | ACME | 0.07 | − 0.01 | 0.17 | 0.190 | 55 |
| Aerobic fitness | CaudateL-Cerebellum7bL | DSST | ACME | − 0.14 | − 0.27 | − 0.03 | 0.018* | 56 |
| Aerobic fitness | CaudateL-CerebellumCrus1L | DSST | ACME | − 0.09 | − 0.19 | − 0.01 | 0.076 | 56 |
| Aerobic fitness | CaudateL-CerebellumCrus1R | DSST | ACME | − 0.14 | − 0.28 | − 0.04 | 0.022* | 56 |
| Aerobic fitness | CaudateR-CerebellumCrus1L | DSST | ACME | − 0.09 | − 0.20 | − 0.01 | 0.074 | 56 |
| Aerobic fitness | PallidumL-Cerebellum6L | DSST | ACME | 0.03 | − 0.02 | 0.10 | 0.390 | 56 |
| Aerobic fitness | PallidumL-Cerebellum8L | DSST | ACME | 0.08 | 0.00 | 0.18 | 0.090 | 56 |
| Aerobic fitness | PallidumL-Cerebellum8R | DSST | ACME | 0.09 | 0.01 | 0.20 | 0.060 | 56 |
| Aerobic fitness | PallidumL-Vermis6 | DSST | ACME | 0.03 | − 0.02 | 0.10 | 0.440 | 56 |
| Aerobic fitness | PallidumL-Vermis7 | DSST | ACME | 0.09 | 0.00 | 0.20 | 0.100 | 56 |
| Aerobic fitness | ThalamusR-Cerebellum9R | DSST | ACME | 0.14 | 0.04 | 0.27 | 0.008* | 56 |
| OCC–TEMP | ||||||||
| Aerobic fitness | OccipitalInfL-HeschlL | PANSS-negative | ACME | 0.08 | 0.00 | 0.21 | 0.138 | 57 |
| Aerobic fitness | OccipitalInfL-HeschlL | PANSS-total | ACME | 0.11 | 0.01 | 0.25 | 0.054 | 58 |
| Aerobic fitness | OccipitalMidL-HeschlL | PANSS-psychopath | ACME | 0.09 | 0.00 | 0.20 | 0.110 | 58 |
| Aerobic fitness | OccipitalMidL-HeschlR | PANSS-psychopath | ACME | 0.07 | 0.00 | 0.18 | 0.140 | 58 |
| Aerobic fitness | OccipitalMidL-HeschlR | PANSS-total | ACME | 0.06 | − 0.01 | 0.17 | 0.190 | 58 |
| Aerobic fitness | OccipitalMidR-TemporalMidL | PANSS-negative | ACME | 0.10 | 0.00 | 0.22 | 0.088 | 57 |
| Aerobic fitness | OccipitalSupL-HeschlL | PANSS-psychopath | ACME | 0.07 | 0.00 | 0.18 | 0.150 | 58 |
| Aerobic fitness | OccipitalSupL-HeschlR | PANSS-psychopath | ACME | 0.09 | 0.00 | 0.20 | 0.110 | 58 |
| Aerobic fitness | OccipitalSupL-HeschlR | CGI | ACME | 0.09 | 0.00 | 0.20 | 0.100 | 58 |
| Aerobic fitness | OccipitalSupL-HeschlR | PANSS-total | ACME | 0.07 | 0.00 | 0.19 | 0.130 | 58 |
| Aerobic fitness | OccipitalSupR-HeschlR | PANSS-psychopath | ACME | 0.07 | − 0.01 | 0.17 | 0.180 | 58 |
| Aerobic fitness | OccipitalSupR-HeschlR | CGI | ACME | 0.07 | 0.00 | 0.18 | 0.150 | 58 |
| Aerobic fitness | OccipitalSupR-TemporalMidL | PANSS-negative | ACME | 0.09 | 0.00 | 0.21 | 0.122 | 57 |
| TEMP–TEMP | ||||||||
| Aerobic fitness | HeschlR-TemporalMidL | PANSS-total | ACME | − 0.14 | − 0.29 | − 0.02 | 0.024* | 58 |
| Aerobic fitness | HeschlR-TemporalMidL | CGI | ACME | − 0.14 | − 0.29 | − 0.03 | 0.024* | 58 |
| Aerobic fitness | HeschlR-TemporalMidL | PANSS-positive | ACME | − 0.13 | − 0.27 | − 0.03 | 0.022* | 58 |
| Aerobic fitness | TemporalSupR-TemporalMidR | PANSS-negative | ACME | − 0.14 | − 0.28 | − 0.02 | 0.044* | 58 |
Summary of the results of the mediation analyses. IV = independent variable, DV = dependent variable, Eff. = type of the effect, estimate = β-coefficient of the mediation effect, CI = confidence interval, n = sample size, ACME = average causal mediation effect, CENTRAL–LIM = functional connections between seeds from central cortical structures and limbic lobe, CEREB–CEREB = functional connections between cerebellar seeds, CEREB–NUC = functional connections between cerebellar seeds and seeds from the subcortical nuclei, OCC–TEMP = functional connections between seeds from the occipital lobe and temporal lobe, TEMP–TEMP = functional connections between seeds from the temporal lobe
*p < 0.05
Six of 80 functional connections related to aerobic fitness in the CEREB–CEREB cluster correlated with performance in the TMT-A and -B (BF10range = 2.89–82.52, r = − 0.22 to − 0.35, PD = 98.3–100%, ROPE = 12.6–0.9%). Three of seven mediation effects were significant proposing a positive impact of aerobic fitness on TMT speed mediated by three functional connections between cerebellar seeds (Table 3). Further nine functional connections in the CEREB-CEREB cluster correlated with B-CATS-animals, -fruits and -vegetables (BF10range = 2.53–15.26, r = 0.21–0.30, PD = 97.7–99.5%, ROPE = 13.8–3.0%). No significant mediation effects were observed (Table 3).
Five of 57 functional connections related to aerobic fitness in the CEREB–NUC cluster correlated with the four PANSS scores as well as CDSS and CGI score (BF10range = 2.52–16.36, r = − 0.28 to 0.29, PD = 98.1–99.6%, ROPE = 13.9–2.9%). Two of 14 mediation effects were significant underlying an attenuating effect of aerobic fitness on total symptom severity mediated by two functional connections between cerebellar seeds and seeds from subcortical nuclei (Table 3). Further eight functional connections in the CEREB-NUC cluster correlated with B-CATS-fruits and -vegetables (BF10range = 3.60–24.88, r = − 0.23 to − 0.31, PD = 98.3–100%, ROPE = 11.1–2.0%). No significant mediation effects were found (Table 3). Finally, ten functional connections in the CEREB-NUC cluster showed correlations with DSST performance (BF10range = 2.94–35.09, r = 0.22–0.32, PD = 98.1–99.9%, ROPE = 12.7–1.4%). Three of ten mediation effects were significant suggesting both a positive and a negative influence of aerobic fitness on DSST performance mediated by three functional connections between cerebellar seeds and seeds from the subcortical nuclei (Table 3).
Eight of 22 functional connections associated with aerobic fitness in the OCC-TEMP cluster correlated with three PANSS scores as well as CGI score (BF10range = 2.50–9.45, r = 0.21–0.27, PD = 97.7–99.4%, ROPE = 14.6–5.4%). No significant mediation effects existed (Table 3).
Two of ten functional connections linked to aerobic fitness in the TEMP–TEMP cluster were correlated with PANSS-total, -positive and -negative scores as well as CGI score (BF10range = 2.61–4.17, r = − 0.21 to − 0.24, PD = 98.2–98.6%, ROPE = 12.7–9.8%). All mediation effects were significant supporting the attenuating effect of aerobic fitness on total symptom severity mediated by functional connections between seeds from the temporal lobe (Table 3).
Discussion
The present study was the first to explore the association between aerobic fitness and the whole-brain functional connectome in patients with schizophrenia while considering clinical and cognitive relevance of the identified fitness-connectivity relations.
First, we showed that higher patients’ aerobic fitness levels were associated with lower FC between the basal ganglia/thalamus and the midbrain/cerebellum network leading to lower psychopathological symptom severity. Second, FC between parts of the visual network and the right-lateralized FPN mediated the beneficial impact of aerobic fitness on verbal declarative memory. Third, higher patients’ aerobic fitness was accompanied by higher FC within the DMN, but clinical and cognitive relevance was lacking. Fourth, functional connections between cerebellar seeds and seeds from the subcortical nuclei as well as between seeds from the temporal lobe influenced the beneficial effect of aerobic fitness on total symptom severity. Fifth, FC between cerebellar seed connections mediated the positive influence of aerobic fitness on global cognition. Finally, functional connections between seeds from central cortical areas and from the limbic lobe drove the positive impact of aerobic fitness on emotion recognition.
Higher patients’ aerobic fitness levels are linked to lower FC between parts of the subcortical nuclei (basal ganglia, thalamus and amygdala [50]) and the cerebellum leading to attenuated total symptom severity. This is in line with previous studies suggesting a beneficial relation between aerobic fitness and different domains of psychiatric symptoms [22, 71, 72]. Within this association, our results are the first underlining the mediating role of FC between the subcortical nuclei and the cerebellum. Recent evidence proposes a state-independent cerebello-thalamo-cortical hyperconnectivity compared to healthy controls as a heritable neural signature in schizophrenia related to domains of positive symptomatology, especially disorganized thoughts and behavior [73, 74]. Based on the NMDA receptor hypofunction hypothesis of schizophrenia [75, 76], cerebello-thalamo-cortical hyperconnectivity is supposed to result from NMDA receptor deficits impeding the functioning of cortical parvalbumin-containing gamma-aminobutyric acid (GABA) interneurons which fail to inhibit pyramidal glutamatergic neurons eliciting upregulated FC within this circuitry [73, 77]. In line with the cognitive dysmetria theory of schizophrenia [78], the cerebello-thalamo-cortical hyperconnectivity may represent psychosis-related increased efforts in processing motion and cognition errors accurately [73]. Consequently, our findings could indicate that parts of the schizophrenia-specific cerebello-thalamo-cortical hyperconnectivity pattern are attenuated if patients have higher aerobic fitness levels leading to ameliorations in total symptom severity.
The beneficial impact of aerobic fitness on total symptom severity is also mediated by FC between temporal seeds as indicated by our results. An association between aerobic fitness and functional connections within temporal regions has already been proposed in healthy subjects [21]. Our finding supports this outcome and provides novel evidence on the mediating role of FC between temporal seeds. Specifically, the functional connections between the right Heschl’s gyrus and the left middle temporal gyrus and between the right superior temporal and the right middle temporal gyrus mediated the attenuating effect of aerobic fitness on total symptom severity. These regions are part of the auditory system and fulfil a broad range of different auditory and verbal tasks such as sound perception and recognition as well as language comprehension and production [79, 80]. In schizophrenia, functional deteriorations of the activation of the auditory system are consistently related to the severity of auditory hallucinations [81–84] and to disorganized speech [85] both reflecting positive symptoms. Functional overactivation of the middle and superior temporal gyri has been found in patients suffering from auditory hallucinations [83]. Accordingly, functional disconnections of the superior temporal gyrus have been related to the predisposition to develop auditory hallucinations [86]. Hence, it seems conceivable that the beneficial effect of aerobic fitness on total symptom severity is mediated by FC between auditory seed regions.
Furthermore, our findings suggest that patients’ aerobic fitness strengthened FC between cerebellar seeds leading to ameliorations in global cognition. Correspondingly, aerobic fitness and global cognition have been found to be correlated positively in patients with schizophrenia [87]. Simultaneously, recent findings in healthy subjects indicate that higher aerobic fitness levels are related to increased FC within the cerebellum [21]. In schizophrenia, hypoconnectivity patterns within the cerebellum have been reported [88]. Generally, the cerebellum—as part of the cortico-cerebellar-thalamic-cortical circuit—acts as a modulating system detecting patterns, changes and errors in motion and cognitive processes and providing adaptive neural feedback to cortical areas [89]. Multimodal cerebellar disturbances in schizophrenia are linked to deteriorations in multiple higher-order cognitive domains such as memory or attentional processes [78, 89]. Taken together, fitness-induced improvements in global cognition mediated by strengthened functional connections within the cerebellum seem plausible.
Finally, we observe a positive influence of aerobic fitness on emotion recognition capability mediated by FC between central and limbic seeds. A positive link between aerobic fitness and emotion recognition has recently been demonstrated in healthy participants [90]. We could replicate this finding in people with schizophrenia and provide new evidence on the mediating role of FC between the right Rolandic operculum and the bilateral posterior cingulate gyrus. The former integrates different kinds of sensory signals guiding interoceptive awareness and physical self-consciousness and is involved in emotion processing [91]. The posterior cingulate cortex represents the central node of the DMN facilitating internally directed cognition such as the retrieval of autobiographical memories, but is also supposed to regulate the focus of attention [92]. Consequently, it seems reasonable that the functional connections between the right Rolandic operculum and the bilateral posterior cingulate gyrus mediates the beneficial impact of aerobic fitness on emotion recognition.
Our exploratory examination comes along with a few limitations yielding important implications for future research: As indicated by the BFDA, the probability to detect a BF10 > 3 assuming a small population effect is only 30.6% using our study design (for details see suppl. S9). Furthermore, we do not correct for multiple comparisons. Because in Bayesian statistics no classical statistical test is performed resulting in a binary decision (effect vs. no effect), it is not common to correct the BF in case of a multiple test situation. Therefore, it is essential to consider the magnitude of the BF and the corresponding evidence level in favor of the alternative hypothesis instead of defining every BF > 3 as a robust effect. Importantly, in case of the mediation analysis, we do not correct the p values neither because we aim to detect even small effects. Future studies can build upon our preliminary findings using a hypothesis-driven approach based on an a-priori BFDA to ensure sufficient statistical power.
Further, the causal interpretations based on the results from mediation analysis in our cross-sectional study design have to be interpreted carefully. Although we control for age, sex, BMI, education years, disorder duration and chlorpromazine equivalents, we cannot rule out that other influencing variables exist, affecting our mediation analysis and leading to spurious interactions. Therefore, we interpret our preliminary findings in consideration of current literature knowledge on behavioral tasks of the ICNs and anatomical regions as well as on reported connectivity-fitness associations in other populations. Randomized-controlled intervention studies including an aerobic exercise program are needed to provide stable causal inferences concerning the FC-mediated effects of aerobic exercise on clinical and cognitive outcome.
Finally, we have no healthy control group available, but we still can draw first cautious conclusions on fitness-induced changes in FC leading to specific clinical or cognitive outcome. However, future studies should include a healthy control group and compare an aerobic exercise program to other types of physical activity interventions to verify possible compensatory effects and FC-mediated beneficial impacts of aerobic fitness on clinical and cognitive outcomes.
Conclusion
To the best of our knowledge, our findings provide first insights into the role of fitness-induced adaptations of macro-scale FC patterns underlying benefits in symptomatology and cognition in people with schizophrenia. We emphasize that the results of this global exploratory analysis need further replication within a hypothesis-driven, randomized-controlled, interventional aerobic exercise study design.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The study was funded by the German Federal Ministry of Education and Research (BMBF) through the research network on psychiatric diseases ESPRIT (Grant number 01EE1407E) to AML, PF, AH, and AS. Furthermore, the study was supported by the Else Kröner-Fresenius Foundation to IM, PF and AS. The authors thank the Clinical Trials Centre Cologne (CTC Cologne) for developing the database and performing data management and monitoring and the Institute of Medical Statistics and Computational Biology of the University Cologne (IMSB) for statistical support. Finally, the authors like to express their appreciation to the “Studienstiftung des Deutschen Volkes” for providing a PhD-scholarship to LR.
Abbreviations
- AAL
Automated anatomical labelling
- ACME
Average causal mediation effect
- B-CATS
Brief Cognitive Assessment Tool for Schizophrenia
- BF10
Relative evidence for alternative hypothesis compared to null hypothesis
- BFDA
Bayes factor design analysis
- BIDS
Brain imaging data structure
- BMI
Body-mass-index
- BOLD
Blood oxygenation level dependent signal
- CDSS
Calgary Depression Scale for Schizophrenia
- CENTRAL–CENTRAL
Functional connections between seeds from central regions
- CENTRAL–LIM
Functional connections between seeds from central regions and the limbic lobe
- CEREB–CEREB
Functional connections between cerebellar seeds
- CEREB–NUC
Functional connections between cerebellar seeds and seeds from subcortical nuclei
- CGI
Clinical global impression
- CI
Confidence interval
- DICOM
Digital imaging and communications in medicine
- DMN
Default mode network
- DSM
Diagnostic and statistical manual of mental disorders
- DSST
Digit symbol substitution test
- DST
Digit span test
- DV
Dependent variable
- ICN
Intrinsic connectivity network
- ESPRIT
Enhancing schizophrenia prevention and recovery through innovative treatments
- Eff.
Effect (within mediation analysis)
- EPI
Echo-planar imaging
- fMRI
Functional magnetic resonance imaging
- FPN
Fronto-parietal network
- FWHM
Full width at half maximum
- GABA
Gamma-aminobutyric acid
- H0
Null hypothesis assuming that the true correlation equals zero
- H1
Null hypothesis assuming that the true correlation does not equal zero
- HDI
Highest density interval
- ICA-AROMA
Automatic removal of motion artifacts based on independent component analysis
- IV
Independent variable
- LIM-TEMP
Functional connections between seeds from limbic and temporal lobe
- MP-RAGE
T1-weighted magnetization prepared rapid gradient echo
- NIFTI
Neuroimaging Informatics Technology Initiative
- NMDA
N-Methyl-D-aspartate
- OCC-TEMP
Functional connections between seeds from occipital and temporal lobe
- p
Uncorrected p value
- PANSS
Positive and Negative Syndrome Scale for Schizophrenia
- PD
Probability of direction
- ROPE
Region of practical equivalence
- r
Pearson’s correlation coefficient
- FC
Static functional connectivity
- SN
Salience network
- TEMP-TEMP
Functional connections between seeds from temporal lobe
- TMT-A
Trail making test A
- TMT-B
Trail making test B
- VLMT
Verbal learning and memory test
Author contributions
PF, DH, AML, AS, BM, KV and AH were involved in the conception and design of the study. LR and IM were involved in acquisition, analysis and interpretation of data, manuscript writing, revision and final approval of the version to be submitted. BP, DK, TK, SS, ES and BEW have assisted with MRI data acquisition and analysis. SM, VS, MC, EW, LL, AR and IP assessed clinical data. ML, DG and KV conducted fitness assessment. All authors were involved in revising the article, read and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The data evaluated were derived from the ESPRIT C3 study, a subproject of the research network ESPRIT (Enhancing Schizophrenia Prevention and Recovery through Innovative Treatments; coordinator: Andreas Meyer-Lindenberg), which is sponsored by the Federal Ministry of Education and Research (BMBF; funding identifier 01EE1407A).
Availability of data, code and material
Imaging data, results from the quality control and the Jupyter and R-scripts for the whole analysis as well as demographic, physical, clinical and cognitive data files are published on OSF (https://osf.io/tr3nx/?view_only=d2b15fb0503043328a7a27d0ba3a801f). Additional data can be made available upon request.
Declarations
Conflict of interest
AS was an honorary speaker for TAD Pharma and Roche and a member of Roche advisory boards. AH is co-editor of the German (DGPPN) schizophrenia treatment guidelines and first author of the WFSBP schizophrenia treatment guidelines; he has been on the advisory boards and has received speaker fees from Janssen-Cilag, Lundbeck, and Otsuka. PF is a co-editor of the German (DGPPN) schizophrenia treatment guidelines and a co-author of the WFSBP schizophrenia treatment guidelines; he is on the advisory boards and receives speaker fees from Janssen, Lundbeck, Otsuka, Servier, and Richter. AML has received consultant fees from Boehringer Ingelheim, Elsevier, Brainsway, Lundbeck Int. Neuroscience Foundation, Lundbeck A/S, Sumitomo Dainippon Pharma Co., Academic Medical Center of the University of Amsterdam, Synapsis Foundation-Alzheimer Research Switzerland, IBS Center for Synaptic Brain Dysfunction, Blueprint Partnership, University of Cambridge, Dt. Zentrum für Neurodegenerative Erkrankungen, Zürich University, Brain Mind Institute, L.E.K. Consulting, ICARE Schizophrenia, Science Advances, Fondation FondaMental, v Behring Röntgen Stiftung, The Wolfson Foundation, and Sage Therapeutics; Additionally, he has received speaker fees from Lundbeck International Foundation, Paul-Martini-Stiftung, Lilly Deutschland, Atheneum, Fama Public Relations, Institut d'investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Janssen-Cilag, Hertie Stiftung, Bodelschwingh-Klinik, Pfizer, Atheneum, University of Freiburg, Schizophrenia Academy, Hong Kong Society of Biological Psychiatry, Fama Public Relations, Spanish Society of Psychiatry, Italian Society of Biological Psychiatry, Reunions I Ciencia S.L., and Brain Center Rudolf Magnus UMC Utrecht as well as the Prix Roger de Spoelberch grant and the CINP Lilly Neuroscience Clinical Research Award 2016. BEW’s spouse is an employee of Siemens Healthineers. IM, LR, DK, TK, BP, DH, ES, SM, VS, MC, EW, LL, AR, ML, KV, IP, BM, JP and SS report no conflicts of interest.
Informed consent
Before participation subjects provided written informed consent. All study procedures complied with the Declaration of Helsinki and were approved by the local ethic committee (project nr. 706-15, date 18.05.2016). The study was registered on the U.S. Department of Health`s website, a database of clinical trials conducted worldwide (NCT number: NCT03466112), and on the German Clinical Trials Register (DRKS-ID: DRKS00009804).
References
- 1.Schmitt A, Hasan A, Gruber O, Falkai P. Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci. 2011;261:150–154. doi: 10.1007/s00406-011-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016) Schizophr Res. 2016;176:83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandl F, Avram M, Weise B, Shang J, Simões B, Bertram T, Hoffmann Ayala D, Penzel N, Gürsel DA, Bäuml J, Wohlschläger AM, Vukadinovic Z, Koutsouleris N, Leucht S, Sorg C. Specific substantial dysconnectivity in schizophrenia: a transdiagnostic multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. Biol Psychiatry. 2019;85:573–583. doi: 10.1016/j.biopsych.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44:168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sha Z, Wager TD, Mechelli A, He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019;85:379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill A, Mechelli A, Bhattacharyya S. Dysconnectivity of large-scale functional networks in early psychosis: a meta-analysis. Schizophr Bull. 2019;45:579–590. doi: 10.1093/schbul/sby094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adhikari BM, Hong LE, Sampath H, Chiappelli J, Jahanshad N, Thompson PM, Rowland LM, Calhoun VD, Du X, Chen S, Kochunov P. Functional network connectivity impairments and core cognitive deficits in schizophrenia. Hum Brain Mapp. 2019;40:4593–4605. doi: 10.1002/hbm.24723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Hu N, Zhang W, Tao B, Dai J, Gong Y, Tan Y, Cai D, Lui S. Dysconnectivity of multiple brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Front Psychiatry. 2019;10:482. doi: 10.3389/fpsyt.2019.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fmri functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 11.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 12.Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892–899. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen RE, Levander S, Kjaersdam Telléus G, Jensen SO, Østergaard Christensen T, Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia—a meta-analysis of randomized clinical trials. Acta Psychiatr Scand. 2015;131:185–196. doi: 10.1111/acps.12374. [DOI] [PubMed] [Google Scholar]
- 15.Heilbronner U, Samara M, Leucht S, Falkai P, Schulze TG. The longitudinal course of schizophrenia across the lifespan: clinical, cognitive, and neurobiological aspects. Harv Rev Psychiatry. 2016;24:118–128. doi: 10.1097/hrp.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsang HW, Leung AY, Chung RC, Bell M, Cheung WM. Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiatry. 2010;44:495–504. doi: 10.3109/00048671003785716. [DOI] [PubMed] [Google Scholar]
- 17.Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res. 2009;113:189–199. doi: 10.1016/j.schres.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Eck RM, Burger TJ, Vellinga A, Schirmbeck F, de Haan L. The relationship between clinical and personal recovery in patients with schizophrenia spectrum disorders: a systematic review and meta-analysis. Schizophr Bull. 2018;44:631–642. doi: 10.1093/schbul/sbx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posadzki P, Pieper D, Bajpai R, Makaruk H, Könsgen N, Neuhaus AL, Semwal M. Exercise/physical activity and health outcomes: an overview of cochrane systematic reviews. BMC Public Health. 2020;20:1–12. doi: 10.1186/s12889-020-09855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talukdar T, Nikolaidis A, Zwilling CE, Paul EJ, Hillman CH, Cohen NJ, Kramer AF, Barbey AK. Aerobic fitness explains individual differences in the functional brain connectome of healthy young adults. Cereb Cortex. 2018;28:3600–3609. doi: 10.1093/cercor/bhx232. [DOI] [PubMed] [Google Scholar]
- 22.Sabe M, Kaiser S, Sentissi O. Physical exercise for negative symptoms of schizophrenia: systematic review of randomized controlled trials and meta-analysis. Gen Hosp Psychiatry. 2020;62:13–20. doi: 10.1016/j.genhosppsych.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015;45:1343–1361. doi: 10.1017/s0033291714003110. [DOI] [PubMed] [Google Scholar]
- 24.Dauwan M, Begemann MJ, Heringa SM, Sommer IE. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2016;42:588–599. doi: 10.1093/schbul/sbv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashdown-Franks G, Firth J, Carney R, Carvalho AF, Hallgren M, Koyanagi A, Rosenbaum S, Schuch FB, Smith L, Solmi M, Vancampfort D, Stubbs B. Exercise as medicine for mental and substance use disorders: a meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med. 2020;50:151–170. doi: 10.1007/s40279-019-01187-6. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Abascal B, Suárez-Pinilla P, Cobo-Corrales C, Crespo-Facorro B, Suárez-Pinilla M. In- and outpatient lifestyle interventions on diet and exercise and their effect on physical and psychological health: a systematic review and meta-analysis of randomised controlled trials in patients with schizophrenia spectrum disorders and first episode of psychosis. Neurosci Biobehav Rev. 2021;125:535–568. doi: 10.1016/j.neubiorev.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Vogel JS, van der Gaag M, Slofstra C, Knegtering H, Bruins J, Castelein S. The effect of mind-body and aerobic exercise on negative symptoms in schizophrenia: a meta-analysis. Psychiatry Res. 2019;279:295–305. doi: 10.1016/j.psychres.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Lutgens D, Gariepy G, Malla A. Psychological and psychosocial interventions for negative symptoms in psychosis: systematic review and meta-analysis. Br J Psychiatry. 2017;210:324–332. doi: 10.1192/bjp.bp.116.197103. [DOI] [PubMed] [Google Scholar]
- 29.Dauwan M, Begemann MJH, Slot MIE, Lee EHM, Scheltens P, Sommer IEC. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: a transdiagnostic systematic review and meta-analysis of randomized controlled trials. J Neurol. 2021;268:1222–1246. doi: 10.1007/s00415-019-09493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Firth J, Stubbs B, Rosenbaum S, Vancampfort D, Malchow B, Schuch F, Elliott R, Nuechterlein KH, Yung AR. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43:546–556. doi: 10.1093/schbul/sbw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falkai P, Maurus I, Schmitt A, Malchow B, Schneider-Axmann T, Röll L, Papiol S, Wobrock T, Hasan A, Keeser D. Improvement in daily functioning after aerobic exercise training in schizophrenia is sustained after exercise cessation. Eur Arch Psychiatry Clin Neurosci. 2021;271:1201–1203. doi: 10.1007/s00406-021-01282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt A, Reich-Erkelenz D, Hasan A, Falkai P. Aerobic exercise in mental disorders: from basic mechanisms to treatment recommendations. Eur Arch Psychiatry Clin Neurosci. 2019;269:483–484. doi: 10.1007/s00406-019-01037-6. [DOI] [PubMed] [Google Scholar]
- 33.Maurus HA, Röh A, Takahashi S, Rauchmann B, Keeser D, Malchow B, Schmitt A, Falkai P. Neurobiological effects of aerobic exercise, with a focus on patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269:499–515. doi: 10.1007/s00406-019-01025-w. [DOI] [PubMed] [Google Scholar]
- 34.Firth J, Cotter J, Carney R, Yung AR. The pro-cognitive mechanisms of physical exercise in people with schizophrenia. Br J Pharmacol. 2017;174:3161–3172. doi: 10.1111/bph.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurus HA, Schmitt A, Roeh A, Keeser D, Malchow B, Schneider-Axmann T, Hellmich M, Schmied S, Lembeck M, Keller-Varady K, Papazova I, Hirjak D, Topor CE, Walter H, Mohnke S, Vogel BO, Wölwer W, Schneider F, Henkel K, Meyer-Lindenberg A, Falkai P. Aerobic endurance training to improve cognition and enhance recovery in schizophrenia: design and methodology of a multicenter randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2020;271:315–324. doi: 10.1007/s00406-020-01175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faude O, Kindermann W, Meyer T. Lactate threshold concepts: how valid are they? Sports Med (Auckl, NZ) 2009;39:469–490. doi: 10.2165/00007256-200939060-00003. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: dicom to nifti conversion. J Neurosci Methods. 2016;264:47–56. doi: 10.1016/j.jneumeth.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Gorgolewski KJ, Auer T, Calhoun VD, Craddock RC, Das S, Duff EP, Flandin G, Ghosh SS, Glatard T, Halchenko YO, Handwerker DA, Hanke M, Keator D, Li X, Michael Z, Maumet C, Nichols BN, Nichols TE, Pellman J, Poline JB, Rokem A, Schaefer G, Sochat V, Triplett W, Turner JA, Varoquaux G, Poldrack RA. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data. 2016;3:1–9. doi: 10.1038/sdata.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ. Mriqc: advancing the automatic prediction of image quality in mri from unseen sites. PLoS ONE. 2017;12:e0184661. doi: 10.1371/journal.pone.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ. Fmriprep: a robust preprocessing pipeline for functional mri. Nat Methods. 2019;16:111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. Ica-aroma: a robust ica-based strategy for removing motion artifacts from fmri data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 42.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fmri. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity mri networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Brett M, Hanke M, Markiewicz C, Côté M-A, McCarthy P, Ghosh S, Wassermann D. 2020. Nipy/nibabel: 2.3.1. Zenodo. [DOI]
- 46.Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, Gramfort A, Thirion B, Varoquaux G. Machine learning for neuroimaging with scikit-learn. Front Neuroinform. 2014;8:1–10. doi: 10.3389/fninf.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parkes L, Fulcher B, Yücel M, Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional mri. Neuroimage. 2018;171:415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- 48.Beckmann C, Ce M, Filippini N, Sm S. Group comparison of resting-state fmri data using multi-subject ica and dual regression. Neuroimage. 2009;47:148. doi: 10.1016/S1053-8119(09)71511-3. [DOI] [Google Scholar]
- 49.Varoquaux G, Sadaghiani S, Pinel P, Kleinschmidt A, Poline JB, Thirion B. A group model for stable multi-subject ica on fmri datasets. Neuroimage. 2010;51:288–299. doi: 10.1016/j.neuroimage.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the mni mri single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 51.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (panss) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 52.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry. 1993;163:39–44. doi: 10.1192/S0007125000292581. [DOI] [PubMed] [Google Scholar]
- 53.Gu W. Ecdeu assessment manual for psychopharmacology. Rockville: US Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 54.Reitan R, Wolfson D. The halstead-reitan neuropsychological test battery: theory and clinical interpretation. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 55.Hurford IM, Marder SR, Keefe RS, Reise SP, Bilder RM. A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull. 2011;37:538–545. doi: 10.1093/schbul/sbp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tewes U. Hamburg-wechsler intelligenztest für erwachsene—revision 1991 (hawie-r) Bern: Huber; 1994. [Google Scholar]
- 57.Helmstaedter C, Wietzke J, Lutz MT. Unique and shared validity of the "wechsler logical memory test", the "california verbal learning test", and the "verbal learning and memory test" in patients with epilepsy. Epilepsy Res. 2009;87:203–212. doi: 10.1016/j.eplepsyres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Ekman P, Friesen WV. Detecting deception from the body or face. J Pers Soc Psychol. 1974;29:288–298. doi: 10.1037/h0036006. [DOI] [Google Scholar]
- 59.RStudio Team (2020) Rstudio: Integrated development environment for r. In: RStudio, PBC, Boston, MA
- 60.R Core Team (2021) R: A language and environment for statistical computing. In: Computing RFfS (ed) R foundation for statistical computing, Vienna
- 61.Makowski D, Ben-Sachar M, Patil I, Lüdecke D. Methods and algorithms for correlation analysis in r. J Open Source Softw. 2020;5:2306. doi: 10.21105/joss.02306. [DOI] [Google Scholar]
- 62.Leucht S, Samara M, Heres S, Davis JM. Dose equivalents for antipsychotic drugs: the ddd method. Schizophr Bull. 2016;42(1):90–94. doi: 10.1093/schbul/sbv167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeffreys H. The theory of probability. Oxford: Oxford Univerity Press; 1998. [Google Scholar]
- 64.Ly A, Verhagen J, Wagenmakers E-J. Harold Jeffreys’s default bayes factor hypothesis tests: explanation, extension, and application in psychology. J Math Psychol. 2016;72:19–32. doi: 10.1016/j.jmp.2015.06.004. [DOI] [Google Scholar]
- 65.Lee MD, Wagenmakers E-J. Bayesian cognitive modeling: a practical course. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 66.Makowski D, Ben-Shachar MS, Chen SHA, Lüdecke D. Indices of effect existence and significance in the bayesian framework. Front Psychol. 2019;10:2767. doi: 10.3389/fpsyg.2019.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Soft. 2014;59:1–38. doi: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- 68.Schönbrodt FD, Wagenmakers E-J. Bayes factor design analysis: planning for compelling evidence. Psychon Bull Rev. 2018;25:128–142. doi: 10.3758/s13423-017-1230-y. [DOI] [PubMed] [Google Scholar]
- 69.Stefan A, Gronau Q, Schönbrodt F, Wagenmakers E-J. A tutorial on bayes factor design analysis using an informed prior. Behav Res Methods. 2019;51:1042–1058. doi: 10.3758/s13428-018-01189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schönbrodt FD, Stefan AM (2018) Bfda: an r package for bayes factor design analysis (version 0.4.0). Retrieved from https://github.com/nicebread/BFDA
- 71.Hassan S, Ross J, Marston L, Osborn D, Walters K. Factors prospectively associated with physical activity and dietary related outcomes in people with severe mental illness: a systematic review of longitudinal studies. Psychiatry Res. 2019;273:181–191. doi: 10.1016/j.psychres.2018.12.115. [DOI] [PubMed] [Google Scholar]
- 72.Vancampfort D, Rosenbaum S, Probst M, Soundy A, Mitchell AJ, De Hert M, Stubbs B. Promotion of cardiorespiratory fitness in schizophrenia: a clinical overview and meta-analysis. Acta Psychiatr Scand. 2015;132:131–143. doi: 10.1111/acps.12407. [DOI] [PubMed] [Google Scholar]
- 73.Cao H, Chén OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Carrión RE, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Thermenos H, Tsuang MT, van Erp TGM, Walker EF, Hamann S, Anticevic A, Woods SW, Cannon TD. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018;9:1–9. doi: 10.1038/s41467-018-06350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao H, Ingvar M, Hultman CM, Cannon T. Evidence for cerebello-thalamo-cortical hyperconnectivity as a heritable trait for schizophrenia. Transl Psychiatry. 2019;9:1–8. doi: 10.1038/s41398-019-0531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coyle JT. Nmda receptor and schizophrenia: a brief history. Schizophr Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29:97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of nmda receptor hypofunction on gabaergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 2015;167:98–107. doi: 10.1016/j.schres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andreasen NC, Paradiso S, O'Leary DS. "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 79.Alho K, Rinne T, Herron TJ, Woods DL. Stimulus-dependent activations and attention-related modulations in the auditory cortex: a meta-analysis of fmri studies. Hear Res. 2014;307:29–41. doi: 10.1016/j.heares.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Yi HG, Leonard MK, Chang EF. The encoding of speech sounds in the superior temporal gyrus. Neuron. 2019;102:1096–1110. doi: 10.1016/j.neuron.2019.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zmigrod L, Garrison JR, Carr J, Simons JS. The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2016;69:113–123. doi: 10.1016/j.neubiorev.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 82.Kühn S, Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr Bull. 2012;38:779–786. doi: 10.1093/schbul/sbq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- 84.Kompus K, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49:3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Wensing T, Cieslik EC, Müller VI, Hoffstaedter F, Eickhoff SB, Nickl-Jockschat T. Neural correlates of formal thought disorder: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2017;38:4946–4965. doi: 10.1002/hbm.23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diederen KM, Neggers SF, de Weijer AD, van Lutterveld R, Daalman K, Eickhoff SB, Clos M, Kahn RS, Sommer IE. Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol Med. 2013;43:1685–1696. doi: 10.1017/s0033291712002541. [DOI] [PubMed] [Google Scholar]
- 87.Kimhy D, Vakhrusheva J, Bartels MN, Armstrong HF, Ballon JS, Khan S, Chang RW, Hansen MC, Ayanruoh L, Smith EE, Sloan RP. Aerobic fitness and body mass index in individuals with schizophrenia: implications for neurocognition and daily functioning. Psychiatry Res. 2014;220:784–791. doi: 10.1016/j.psychres.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding Y, Ou Y, Pan P, Shan X, Chen J, Liu F, Zhao J, Guo W. Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: a meta-analysis. Psychiatry Res Neuroimaging. 2019;283:24–33. doi: 10.1016/j.pscychresns.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 89.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ludyga S, Schilling R, Colledge F, Brand S, Pühse U, Gerber M. Association between cardiorespiratory fitness and social cognition in healthy adults. Scand J Med Sci Sports. 2020;30:1722–1728. doi: 10.1111/sms.13730. [DOI] [PubMed] [Google Scholar]
- 91.Triarhou LC. Cytoarchitectonics of the rolandic operculum: morphofunctional ponderings. Brain Struct Funct. 2021;226:941–950. doi: 10.1007/s00429-021-02258-z. [DOI] [PubMed] [Google Scholar]
- 92.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Brett M, Hanke M, Markiewicz C, Côté M-A, McCarthy P, Ghosh S, Wassermann D. 2020. Nipy/nibabel: 2.3.1. Zenodo. [DOI]
Supplementary Materials
Data Availability Statement
Imaging data, results from the quality control and the Jupyter and R-scripts for the whole analysis as well as demographic, physical, clinical and cognitive data files are published on OSF (https://osf.io/tr3nx/?view_only=d2b15fb0503043328a7a27d0ba3a801f). Additional data can be made available upon request.