Abstract
Purpose
We aimed to determine whether the pan-immune-inflammation value (PIV) of patients with Merkel cell carcinoma (MCC) at primary diagnosis differs from controls and whether it is associated with disease stage and outcome.
Methods
In this retrospective study, we recruited MCC patients with stage I–III. PIV was calculated from absolute complete blood cell counts obtained within one week at MCC diagnosis as follows: [neutrophils (103/mm3) × platelets (103/mm3) × monocytes (103/mm3)]/lymphocytes (103/mm3). As controls, we studied age–gender-matched cutaneous melanoma (CM, stage I–III) patients and healthy controls (HC). Univariate and multivariate statistics were used.
Results
The median PIV in MCC patients was significantly increased compared to both CM patients as well as healthy controls. PIV of MCC patients in stage II and III was significantly higher compared to stage I patients. ROC analysis revealed that MCC recurrence was significantly associated with a PIV greater than 372 [p < 0.0001, Youden index 0.58; hazard ratio: 4 (95% confidence interval: 1.7 to 9.2)]. In multivariate analysis, only a PIV greater than 372 and higher MCC stage were determined as independent predictors for disease recurrence.
Conclusion
We determined, for the first time, the prognostic ability of the promising blood-based biomarker PIV in MCC patients and observed that PIV is increased in MCC patients in dependence on disease stage and independently predicts MCC recurrence.
Keywords: Pan-immune-inflammation value, Neutrophil-to-lymphocyte ratio, Platelet-to-lymphocyte ratio, Merkel cell carcinoma, Biomarker
Introduction
Merkel cell carcinoma (MCC) is a highly aggressive neoplasm of the skin. Major risk factors for MCC development are sun exposure, old age, and immunosuppression. The Merkel cell polyomavirus (MCPyV) is clonally integrated into the majority of MCCs in the Northern hemisphere. By contrast, MCPyV-negative MCC is associated with chronic ultraviolet (UV) exposure and harbors multiple UV-associated DNA mutations (Becker et al. 2017, 2019). In many malignancies, systemic inflammatory responses can be significant determinants of disease progression and survival. As a consequence, several immune-based prognostic scores have been investigated as predictors of prognosis and treatment response in several malignancies including skin cancers such as cutaneous melanoma (CM). These scores include neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) (Zaragoza et al. 2016a; Zaragoza et al. 2016b; Robinson et al. 2020; Hernando-Calvo et al. 2021; Ludwig et al. 2021; Sahin et al. 2021). Recently, a more complex complete blood count (CBC)-based biomarker of systemic inflammation, the pan-immune-inflammation value (PIV), has been developed, but a systematic assessment of its usefulness as a biomarker of prognosis or treatment response in skin cancers has not yet been (Fucà et al. 2020; Hernando-Calvo et al. 2021; Susok et al. 2022). The PIV includes four CBC-based parameters: neutrophil, platelet, monocyte, and lymphocyte counts. In several studies including malignancies, such as metastatic CM, colorectal carcinoma and non-small cell lung carcinoma, PIV was independently associated with overall survival and progression-free survival (Fucà et al. 2020; Corti et al. 2021; Fuca et al. 2021; Zeng et al. 2021). Although biomarkers of systemic inflammation including NLR and PLR, have been investigated in patients with MCC, PIV has not yet been studied in this rare malignancy (Zaragoza et al. 2016b; Garnier et al. 2018; Naseri et al. 2020; Nghiem et al. 2021). Here, we aimed to determine whether PIV of patients with MCC at primary diagnosis differs from controls and whether it is associated with disease stage and outcome.
Materials and methods
MCC patients
In this retrospective study, we recruited MCC patients with stage I–III who were managed at the Skin Cancer Center of the Ruhr-University Bochum also including a CBC count performed at primary diagnosis. This patient group was compared to both an age- and gender-matched healthy control group as well as an age- and gender-matched group of patients with cutaneous melanoma (stage I–III). MCC restaging was performed in accordance with the eighth edition of the AJCC guidelines (Becker et al. 2019). This study was approved by the local ethics review board of the Medical Faculty of the Ruhr-University Bochum (#16-5985). Patients were treated in accordance with the German guidelines for MCC [9]. Accordingly, following primary excision with a 1–2 cm safety margin and sentinel lymph node biopsy, patients received adjuvant local radiotherapy for the tumor bed and draining lymph node basin. Metastatic lymph node disease was treated with complete lymphadenectomy. Patients with un-resectable stage III MCC received radiotherapy, electro-chemotherapy, and/or systemic chemotherapy or immune checkpoint inhibitors (Becker et al. 2019).
Laboratory parameters
PIV was calculated from absolute CBC counts obtained within one week at MCC diagnosis as follows: [neutrophils (103/mm3) × platelets (103/mm3) × monocytes (103/mm3)]/lymphocytes (103/mm3). At the same time, lactate dehydrogenase (LDH) and C-reactive protein (CRP) were also determined (Fucà et al. 2020; Fuca et al. 2021).
Statistical analyses
Data analysis was performed using the statistical package MedCalc Software version 19.1.7 (MedCalc Software, Ostend, Belgium). Distribution of data was assessed by the D ‘Agostino–Pearson test. Receiver operating characteristics (ROC) analyses, including the area under curve (AUC) and the Youden index, were performed to determine optimal cut-off values using continuous PIV values and the dichotomous classification variables “MCC recurrence” and “MCC-specific death” (Schistermann et al. 2008; Polley and Dignam 2021). Univariate analysis was performed using the Mann–Whitney test, Kruskal–Wallis ANOVA, Spearman’s correlation procedure, and Chi2 test. Survival analysis was performed using the Kaplan–Meier method and a multivariate approach by means of Cox proportional hazards regression, in which we included all parameters with p values of 0.1 and smaller on the basis of univariate analysis. p values < 0.05 were considered significant.
Results
Forty-nine patients [median age: 77 years (51–95); 25 males, 24 females)] were studied, including 40 (81.6%) patients with Merkel cell polyomavirus (MCPyV)-positive MCC. Available clinical data for these patients are shown in Table. 1. This patient group was compared to a healthy control group [n = 42; median age: 74 years (37–96); 22 males, 20 females] as well as a group of patients with cutaneous melanoma [stage I–III at first diagnosis; median age: 74 (50–93); 37 males, 34 females]. Importantly, median age and gender of MCC patients did not significantly differ from melanoma and healthy controls (p = 0.13 and p = 0.18 for age, p = 0.72 and p = 0.54 for gender, respectively). Moreover, the distribution of disease stages did not differ between MCC patients and melanoma controls (p = 0.69). Strikingly, the median PIV in MCC patients was significantly increased compared to both CM patients as well as healthy controls [p = 0.0002, MCC PIV = 507 (31–2648), CM PIV = 333 (33–2473), healthy PIV = 206 (52–1638), Fig. 1]. Additionally, as shown in Fig. 2, the PIV of MCC patients in stage II and III was significantly higher compared to stage I patients (p = 0.026). As expected for a marker of inflammation, the PIV of MCC patients significantly correlated with CRP levels (r = 0.44; p = 0.0017), but surprisingly not with the age of MCC patients (p = 0.15).
Table 1.
Clinical characteristics of patients with Merkel cell carcinoma (MCC, n = 49), including data of the pan-immune-inflammation value (PIV) for MCC patients and controls
| Parameters | Data |
|---|---|
| Age at diagnosis | 77 years (51–95) |
| Sex | |
| M/f | 25 (51%)/24 (49%) |
| Primary MCC | |
| High-risk localization: head/neck (no/yes) | 30/19 (61.2/38.8%) |
| MCPyV (negative/positive) | 9/40 (18.4/81.6%) |
| Immunosuppression | |
| No/yes | 39 (79.6%)/10 (20.4%) |
| Tumor stage at diagnosis and median (range) PIV | |
| (AJCC 2018) | I 21 (42.9%)–314 (31–1551) |
| II 17 (34.7%)–507 (226–2648) | |
| III 11 (12.4%)–787 (150–1680) | |
| p value | = 0.027 (stage II and III vs. stage I) |
| Outcome | |
| 5 year MCC recurrence (no/yes) | 26 (53.1%)/23 (46.9%) |
| Median time to recurrence (months) | 14 (IQR: 6–59) |
| 5-year MCC (survived/deceased) | 35 (63.6)/20 (36.4%) |
| Median time to death (months) | 28 (IQR: 11.8–60) |
| Median (range) PIV | |
| MCC patients | 507 (31–2648) |
| Melanoma controls | 333 (38–2473) |
| Healthy controls | 206 (52–1638) |
| p value | = 0.0002 |
| ROC analyses | |
| PIV | |
| MCC recurrence* | AUC: 0.79 (95% CI 0.65–0.89); p < 0.0001; J = 0.58; criterion: > 372 |
| MCC death* | AUC: 0.67 (95% CI 0.52–0.8; p = 0.024; J = 0.44; criterion: > 412 |
Data of ROC curve analyses are also provided
MCPyV Merkel cell polyomavirus; J Youden index
*Classification variables
Fig. 1.
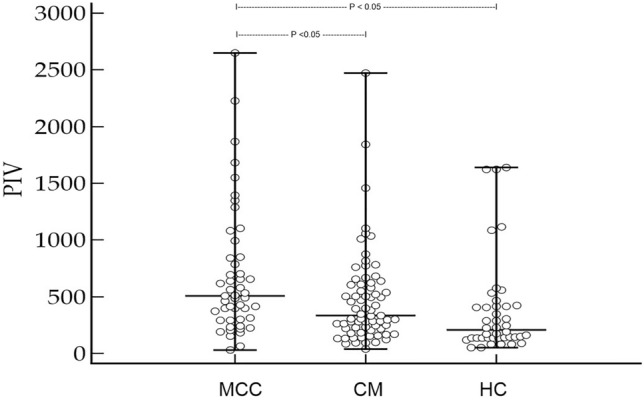
Merkel cell carcinoma (MCC) patients in stage I–III have an significantly increased pan-immune-inflammation value (PIV) when compared to patients with cutaneous melanoma (CM) in stage I–III and healthy controls (HC, Kruskal–Wallis ANOVA p = 0.0002)
Fig. 2.
Showing the pan-immune-inflammation value (PIV) of patients (n = 49) with Merkel cell carcinoma (MCC) in stage I [median (range) PIV: 314 (31–1551)], II [median (range) PIV: 507 (226–2648)], and III median (range) PIV: 787 (150–1680)] at first diagnosis (a). Kruskal–Wallis ANOVA revealed that patients in stage II and III had a significantly higher PIV than patients in stage I (p = 0.026)
ROC analysis revealed that MCC recurrence was significantly associated with a PIV greater than 372 (p < 0.0001, Youden index 0.58). Indeed, logistic regression curves showed that patients with PIV greater than 372 had significantly higher recurrence rates than patients with a PIV below 372 [Fig. 3, p = 0.0015, hazard ratio: 4 (95% confidence interval: 1.7–9.2)]. As expected, univariate analysis confirmed that higher MCC stage was significantly associated with disease recurrence (p = 0.0004). Taking into account different clinical parameters (age, sex, immunosuppression, MCPyV status, LDH, CRP, PIV class, disease stage), age above 75 years, PIV greater than 372 and higher stage of disease were all significantly associated with death (p = 0.011, p = 0.041 and p = 0.049, respectively). However, in multivariate analysis (including PIV class, increased CRP, and MCC stage), only a PIV greater than 372 and higher MCC stage were determined as independent predictors for disease recurrence (p = 0.014, p = 0.028, respectively). With respect to MCC-specific death, multivariate analysis (including age over 75 years, PIV class, disease stage, and elevated CRP) revealed that age over 75 years was the only significant independent predictor for worse survival (p = 0.0068).
Fig. 3.

Kaplan–Meier curve clearly demonstrates that patients with a pan-immune-inflammation value (PIV) > 372 had significantly worse outcome with respect to MCC recurrence [p = 0.0015; hazard ratio: 4 (95% confidence interval: 1.7–9.2)]
Discussion
The investigation of potential biomarkers in patients with MCC is crucial for patient stratification in the era of increasingly for personalized treatment strategies- For example, with respect to adjuvant treatment choices and the frequency of follow-up, biomarkers that accurately predict recurrence, are highly desirable. A wealth of research indicates that systemic inflammation plays a crucial role in tumor development, progression, and metastasis (Liu et al., 2015). On the one hand, pro-tumorigenic cytokines secreted by neutrophil granulocytes and thrombocytes, including vascular endothelial growth factor, tumor necrosis factor-α, and interleukin-10, have been suggested to contribute to tumor progression. On the other hand, monocytes as well as lymphocytes can exert antitumoral effects by increasing immune responses against cancer cells (Mirili et al. 2019). Systemic immune-inflammation prognosis scores, including NLR and PLR, have previously been reported to be of prognostic value in many different types of malignancy (Templeton et al. 2014; Liu et al. 2015; Nishijima et al. 2015; Zaragoza et al. 2016; Zhong et al. 2017; Mirili et al. 2019; Robinson et al. 2020; Hernando-Calvo et al. 2021; Ludwig et al. 2021). Thus, NLR and PLR belong to the most intensely investigated immune-inflammatory blood-based markers in malignancies including, in particular, melanoma, and are also associated with the clinical outcomes of patients treated with immunotherapy and targeted therapy (Zaragoza et al. 2016; Robinson et al. 2020; Hernando-Calvo et al. 2021; Ludwig et al. 2021; Sahin et al. 2021). However, given the complexity of the inflammation network, other cellular components not assessed by NLR and PLR may also have an influence on cancer progression and response to therapy. By capturing changes in four different cell types, the PIV provides a broad assessment of inflammation that might be particularly relevant with respect to potential pro-tumorigenic effects. In support of this consideration, a recent retrospective analysis of metastatic melanoma patients treated first-line with immune checkpoint inhibitors (ICI) or targeted therapy, demonstrated that a high baseline PIV was independently associated with poor progression-free and overall survival (Fucà et al. 2021). Moreover, a high PIV was also associated with primary resistance to both ICI and targeted therapy (Fucà et al. 2021). Another novel CBC-based biomarker represents the systemic immune-inflammation index (SII = platelets × neutrophils/lymphocytes) being a promising prognostic tool in cancers of the liver, pancreas, lungs, and gut (Zhong et al. 2017). Furthermore, Ludwig et al. (2021) assessed the SII in uveal melanoma and observed that low baseline SII was an independent predictor for prolonged overall survival (Ludwig et al. 2021).
Apart from CBC-based markers, many other biomarkers have previously been investigated. Despite routine use by many clinicians, neuron-specific enolase and chromogranin A blood levels do not correlate with MCC recurrence and MCC-specific survival (Gaiser et al. 2015). Similar to the present data, Donizy et al. (2021) showed on multivariate analysis that only ulceration and age were independent predictors of worse survival. However, they also demonstrated that tumoral PD-L1 expression and increased density of intra-tumoral CD8 + cells and FOXP3 + lymphocytes seem to be potential prognostic predictors in a subset of MCC patients (Donizy et al. 2021). Moreover, Harms et al. (2021) recently found that MCPyV-positive MCC patients also showing decreased tumoral expression of granzyme B and IDO-1 have shorter survival when compared to MCPyV-negative MCC patients. Other reports suggested that PD-1 promoter methylation (Ricci et al. 2019), hydroxymethylation (Gambichler et al. 2019), or circulating tumor cells (Riethdorf et al. 2019) are of prognostic relevance in MCC patients. Nevertheless, the data of most studies in this field need to be substantiated by larger prospective investigations.
In the present study, we carefully matched control groups with regard to age and gender. This is of particular importance, since it has been shown that markers of inflammation in peripheral blood increase with age (Fest et al. 2018). However, in our cohort of MCC patients, PIV did not correlate with age (p = 0.15), which could be explained by the fact that almost all patients were of similar, old age. Similar to age, gender-dependent differences of CBC-based inflammatory markers have been reported in patients suffering from different cancers (Fest et al. 2018; Li et al. 2018). Consequently, to avoid these confounders, we matched MCC and control groups investigated with respect to both age and gender. In the present study, we could clearly show that the PIV of MCC patients is significantly increased compared to healthy controls further increases depending on stage of the diseases. These data indicate that systemic immune-inflammation responses might not only be useful as a descriptive biomarker when quantified by the PIV, but also hint toward a causal role in tumor development and progression in MCC patients. Said differently, patients with higher MCC stage were also shown to have a higher PIV indicating that systemic immune-inflammation responses increase with tumor size and progression. In line with these observations, a PIV greater than 372 at time of first diagnosis was significantly and independently associated with MCC recurrence, as also supported by a Youden index of 0.58, which is considered a measure of a diagnostic test's ability to balance sensitivity and specificity. Importantly, a Youden index over 0.50 meets empirical benchmarks for being administered as a diagnostic test (Schistermann et al. 2008; Polley and Dignam 2021). However, such an association could not be demonstrated for MCC-specific death. This finding could be explained by the relatively small sample size leading to a low number of events. This limitation may also be the reason for the observation that other expected survival predictors, including immunosuppression and disease stage, were not significantly associated with MCC-specific survival in the present MCC cohort (Becker et al. 2017, 2019).
In conclusion, we determined, for the first time, the prognostic ability of the promising CBC-based biomarker PIV in MCC patients and observed that PIV is increased in MCC patients in dependence on disease stage and independently predicts MCC recurrence. These data strongly support prospective validation of the PIV in a larger sample size.
Author contributions
TG and JCB conceived and designed the study. Material preparation, data collection, analysis, and interpretation were predominantly performed by SS, RM, CHS, LS, RS, JCB, and TG. The first draft of the manuscript was written by TG and JCB. All authors read the manuscript, revised it critically, and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This was a non-funded study.
Data availability
The data presented in this study are available on reasonable request from the corresponding author.
Declarations
Conflict of interest
Thilo Gambichler has received speakers and/or advisory board honoraria from BMS, Sanofi-Genzyme, MSD, Novartis Pharma, Roche, Abbvie, Almirall, Janssen, Lilly, Pfizer, Pierre Fabre, Merck-Serono, outside the submitted work. Jürgen C. Becker has received speaker honoraria from Amgen, MerckSerono, Pfizer, Recordati, and Sanofi; advisory board honoraria from 4SC, Amgen, eTheRNA, MerckSerono, Novartis and ReProTher, as well as research funding from Alcedis, Boehringer Ingelheim, Bristol-Myers Squibb, IQVIA, and MerckSerono; he also received travel support from 4SC and Incyte. The other authors declare no conflicts of interest.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local Ethics Committee of the Ruhr-University Bochum (IRB Study ID #16-5985).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbé C, Veness M, Nghiem P (2017) Merkel cell carcinoma. Nat Rev Dis Primers 26(3):17077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JC, Eigentler T, Frerich B, Gambichler T, Grabbe S, Höller U, Klumpp B, Loquai C, Krause-Bergmann A, Müller-Richter U, Pföhler C, Schneider-Burrus S, Stang A, Terheyden P, Ugurel S, Veith J, Mauch C (2019) S2k guidelines for Merkel cell carcinoma (MCC, neuroendocrine carcinoma of the skin)—update 2018. J Dtsch Dermatol Ges 17(5):562–577 [DOI] [PubMed] [Google Scholar]
- Corti F, Lonardi S, Intini R, Salati M, Fenocchio E, Belli C, Borelli B, Brambilla M, Prete AA, Quarà V, Antista M, Fassan M, Morano F, Spallanzani A, Ambrosini M, Curigliano G, de Braud F, Zagonel V, Fucà G, Pietrantonio F (2021) The pan-immune-inflammation value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur J Cancer 150:155–167 [DOI] [PubMed] [Google Scholar]
- Donizy P, Wu CL, Kopczynski J, Pieniazek M, Biecek P, Ryś J, Hoang MP (2021) Prognostic role of tumoral PD-L1 and IDO1 expression, and intratumoral CD8+ and FoxP3+ lymphocyte infiltrates in 132 primary cutaneous merkel cell carcinomas. Int J Mol Sci 22(11):5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH (2018) Reference values for white blood-cell-based inflammatory markers in the Rotterdam study: a population-based prospective cohort study. Sci Rep 8(1):10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucà G, Guarini V, Antoniotti C et al (2020) The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the valentino and TRIBE first-line trials. Br J Cancer 123:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucà G, Beninato T, Bini M et al (2021) The pan-immune-inflammation value in patients with metastatic melanoma receiving first-line therapy. Target Oncol 16(4):529–536 [DOI] [PubMed] [Google Scholar]
- Gaiser MR, Daily K, Hoffmann J, Brune M, Enk A, Brownell I (2015) Evaluating blood levels of neuron specific enolase, chromogranin A, and circulating tumor cells as Merkel cell carcinoma biomarkers. Oncotarget 6(28):26472–26482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambichler T, Schmitt K, Rüddel I, Dreißigacker M, Stockfleth E, Becker JC (2019) Decreased 5-hydroxymethylcytosine immunoreactivity in primary Merkel cell carcinoma is a strong predictor for disease-specific death. Br J Dermatol 181(2):389–390 [DOI] [PubMed] [Google Scholar]
- Garnier M, Zaragoza J, Bénéton N, Bens G, Meurisse V, Samimi M, Maillard H, Machet L (2018) High neutrophil-to-lymphocyte ratio before starting anti-programmed cell death 1 immunotherapy predicts poor outcome in patients with metastatic melanoma. J Am Acad Dermatol 79(1):165-167.e2 [DOI] [PubMed] [Google Scholar]
- Harms KL, Zhao L, Johnson B, Wang X, Carskadon S, Palanisamy N, Rhodes DR, Mannan R, Vo JN, Choi JE, Chan MP, Fullen DR, Patel RM, Siddiqui J, Ma VT, Hrycaj S, McLean SA, Hughes TM, Bichakjian CK, Tomlins SA, Harms PW (2021) Virus-positive Merkel cell carcinoma is an independent prognostic group with distinct predictive biomarkers. Clin Cancer Res 27(9):2494–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando-Calvo A, García-Alvarez A, Villacampa G et al (2021) Dynamics of clinical biomarkers as predictors of immunotherapy benefit in metastatic melanoma patients. Clin Transl Oncol 23(2):311–317 [DOI] [PubMed] [Google Scholar]
- Li C, Tian W, Zhao F et al (2018) Systemic immune-inflammation index, SII, for prognosis of elderly patients with newly diagnosed tumors. Oncotarget 9(82):35293–35299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Charles PL, Zhou PB (2015) Inflammation fuels tumor progress and metastasis. Curr Pharm Des 21(21):3032–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig JM, Haubold J, Bauer S et al (2021) Predictive impact of the inflammation-based indices in uveal melanoma liver metastases treated with transarterial hepatic chemoperfusion. Radiol Oncol 55(3):347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirili C, Yılmaz A, Demirkan S, Bilici M, Basol TS (2019) Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol 24(10):1301–1310 [DOI] [PubMed] [Google Scholar]
- Naseri S, Steiniche T, Georgsen JB, Thomsen R, Ladekarl M, Heje M, Damsgaard TE, Bønnelykke-Behrndtz ML (2020) Tumor ulceration, reduced infiltration of CD8-lymphocytes, high neutrophil-to-CD8-lymphocyte ratio and absence of MC virus are negative prognostic markers for patients with Merkel cell carcinoma. Cancers (basel) 12(4):888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, Friedlander PA, Daud A, Kluger HM, Reddy SA, Boulmay BC, Riker A, Burgess MA, Hanks BA, Olencki T, Kendra K, Church C, Akaike T, Ramchurren N, Shinohara MM, Salim B, Taube JM, Jensen E, Kalabis M, Fling SP, Homet Moreno B, Sharon E, Cheever MA, Topalian SL (2021) Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J Immunother Cancer 9(4):002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima TF, Muss HB, Shachar SS et al (2015) Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev 41(10):971–978 [DOI] [PubMed] [Google Scholar]
- Polley MC, Dignam JJ (2021) Statistical considerations in the evaluation of continuous biomarkers. J Nucl Med 62(5):605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci C, Morandi L, Righi A, Gibertoni D, Maletta F, Ambrosi F, Agostinelli C, Uccella S, Asioli S, Sessa F, Pellilli M, Maragliano R, La Rosa S, Papotti MG, Asioli S (2019) PD-1 (PDCD1) promoter methylation in Merkel cell carcinoma: prognostic relevance and relationship with clinico-pathological parameters. Mod Pathol 32(9):1359–1372 [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Hildebrandt L, Heinzerling L, Heitzer E, Fischer N, Bergmann S, Mauermann O, Waldispühl-Geigl J, Coith C, Schön G, Peine S, Schuler G, Speicher MR, Moll I, Pantel K (2019) Detection and characterization of circulating tumor cells in patients with Merkel cell carcinoma. Clin Chem 65(3):462–472 [DOI] [PubMed] [Google Scholar]
- Robinson AV, Keeble C, Lo MCI et al (2020) The neutrophil-lymphocyte ratio and locoregional melanoma: a multicentre cohort study. Cancer Immunol Immunother 69(4):559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şahin AB, Cubukcu E, Ocak B, Deligonul A, Oyucu Orhan S, Tolunay S, Gokgoz MS, Cetintas S, Yarbas G, Senol K, Goktug MR, Yanasma ZB, Hasanzade U, Evrensel T (2021) Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep 11(1):14662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Faraggi D, Reiser B, Hu J (2008) Youden Index and the optimal threshold for markers with mass at zero. Stat Med 27(2):297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susok L, Said S, Reinert D, Mansour R, Scheel CH, Becker JC, Gambichler T. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J Cancer Res Clin Oncol (in press) [DOI] [PMC free article] [PubMed]
- Templeton AJ, Ace O, McNamara MG et al (2014) Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 23(7):1204–1212 [DOI] [PubMed] [Google Scholar]
- Zaragoza J, Caille A, Beneton N, Bens G et al (2016a) High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol 174(1):146–151 [DOI] [PubMed] [Google Scholar]
- Zaragoza J, Kervarrec T, Touzé A, Avenel-Audran M, Beneton N, Esteve E, Wierzbicka Hainaut E, Aubin F, Machet L, Samimi M (2016b) A high neutrophil-to-lymphocyte ratio as a potential marker of mortality in patients with Merkel cell carcinoma: a retrospective study. J Am Acad Dermatol 75(4):712-721.e1 [DOI] [PubMed] [Google Scholar]
- Zeng R, Liu F, Fang C, Yang J, Luo L, Yue P, Gao B, Dong Y, Xiang Y (2021) PIV and PILE score at baseline predict clinical outcome of anti-PD-1/PD-L1 inhibitor combined with chemotherapy in extensive-stage small cell lung cancer patients. Front Immunol 12:724443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JH, Huang DH, Chen ZY (2017) Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 8(43):75381–75388 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.



