Abstract
Purpose of Review
This scoping review summarises the literature on HIV prevention and management interventions utilizing behavioural economic principles encapsulated in the MINDSPACE framework.
Recent Findings
MINDSPACE is an acronym developed by the UK’s behavioural insights team to summarise nine key influences on human behaviour: Messenger, Incentives, Norms, Default, Salience, Priming, Affect, Commitment, and Ego. These effects have been used in various settings to design interventions that encourage positive behaviours. Currently, over 200 institutionalised behavioural insight teams exist internationally, which may draw upon the MINDSPACE framework to inform policy and improve public services. To date, it is not clear how behavioural insights have been applied to HIV prevention and management interventions.
Summary
After screening 899 studies for eligibility, 124 were included in the final review. We identified examples of interventions that utilised all the MINDSPACE effects in a variety of settings and among various populations. Studies from high-income countries were most common (n = 54) and incentives were the most frequently applied effect (n = 100). The MINDSPACE framework is a useful tool to consider how behavioural science principles can be applied in future HIV prevention and management interventions. Creating nudges to enhance the design of HIV prevention and management interventions can help people make better choices as we strive to end the HIV/AIDS pandemic by 2030.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11904-022-00615-z.
Keywords: HIV, Behavioural economics, Nudge, Behavioural insights
Introduction
HIV continues to be a major public health issue. In 2020, 37.7 million people were living with HIV, with 1.5 million new infections globally [1]. Multiple HIV prevention and management interventions are needed to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS)’s 95–95-95 targets by 2030. Biomedical prevention interventions, including pre-exposure prophylaxis (PrEP), provide an effective HIV prevention strategy, and the use of antiretroviral therapy (ART) for HIV management closes the gap on life expectancy outcomes between affected patients and the general population [2]. However, HIV prevention and management strategies are often underutilised in key populations such as men who have sex with men (MSM), intravenous drug users, and sex workers [3–5].
HIV prevention and management interventions grounded in psychological theories could make them more effective in that these understandings of human behaviours underpin the design and implementation of health interventions. Behavioural economics blends psychological and economic principles to provide unique insights into decision-making and behaviour change. Behavioural economics posits that human decision making is influenced by heuristics and cognitive biases. People adapt decision-making depending on resource constraints, context, and other exogenous factors, including whether decisions are made using automatic (system 1 — inherent biases and heuristics) or deliberative processing (system 2 — rational, reasoned thinking) [6].
Nudging is one popular application of the principles described in behavioural economics. A nudge is defined as any aspect of the choice architecture that predictably alters behaviour without forbidding any options or significantly changing their economic incentives [7]. Nudges tend to be low cost and preserve the freedom to choose, and can be used to overcome suboptimal behaviour [8]. Examples of nudges include automatic enrollment into employee retirement savings [9], attaching a fly-shaped sticker on urinals to reduce spillage [10] and highlighting the positive behaviour of others, for instance ‘9 out of 10 people pay their tax on time’ [11]. Nudges used in public health interventions to encourage behaviour change can be summarised in the behavioural economics framework ‘MINDSPACE’ [12]. MINDSPACE is an acronym that summarises nine effects on people’s behaviour that, once realised, can be harnessed to influence their future decisions and ultimately improve public health (Box 1).
We used a scoping review methodology to assess the extent of HIV prevention and management interventions which can be described by behavioural economic principles, and highlight gaps whereby use of behavioural economic principles may further enhance the design of future interventions.
Box 1 Definitions of the MINDSPACE effects [12]
| Definitions | |
|---|---|
| Messenger | Who communicates the information |
| Incentive | Response to incentives can depend on how they are presented |
| Norms | People are influenced by what others do |
| Default | Preset options will be activated unless an active choice occurs |
| Salience | Attention is drawn to something novel or that seems relevant to the target population |
| Priming | Exposure to subconscious cues may influence people’s performance or choice on a subsequent task |
| Affect | Emotional associations that shape actions |
| Commitment | Consistency with public promises and reciprocate acts |
| Ego | Acting in ways to make one feel better about themselves |
Methods
We used Arksey and O’Malley’s scoping review methodology to examine the literature on HIV prevention and management interventions which can be described by behavioural economics principles [13]. This consisted of five stages: (1) identification of a research question; (2) identification of relevant articles; (3) selection of articles; (4) extraction and charting of data; and (5) synthesizing, summarizing, and reporting the results.
Search Strategy
Our search included studies published between January 2000 and August 2021 on HIV prevention and management interventions using behavioural economic principles. We searched the following databases on 11th August, 2021: Medline, PsycInfo, Scopus, and CINAHL. We used the following search terms: HIV, AIDS, prevention, testing, medication, PrEP, PEP, condoms, incentives, reinforcement, economic, and behavioural economics. Detailed information of search terms used in the search strategy is listed in the Appendix (Supplementary Table 1).
Inclusion and Exclusion Criteria
Studies were included if they contained information to describe HIV prevention interventions (such as HIV testing, PrEP) or HIV management interventions (such as linkage to care, ART medication adherence). We excluded studies not published in English, only described theoretical principles, and systematic or literature reviews.
Screening and Data Extraction
After removing duplicate studies, the titles and abstracts of the remaining articles were independently evaluated for relevance by two reviewers (AA and JT) using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Any discrepancies were assessed by a third reviewer (JO). Similarly, data were independently extracted by two reviewers (AA and JT) with any discrepancies assessed by a third reviewer (JO). The following data were extracted: authors’ name, year of publication, paper title, country and purpose of the intervention, intervention description, study period, recruitment site, and demographic characteristics of targeted populations.
Data Analysis
We report our findings using the PRISMA for scoping review guidelines [14]. We categorised the interventions according to their deployment of the nine MINDSPACE categories. The studies were further grouped based on whether the intervention applied to HIV prevention or management. We also grouped interventions by country income level according to the World Bank classification [15]. We used descriptive statistics to summarise the characteristics of the included studies. While we cannot give an account of all the studies, we describe a few key studies in the text, illustrating the wide range of ways the nudge effects (using the MINDSPACE framework) may describe the interventions deployed in the field. We provide the list of relevant studies for each effect in Table 2.
Table 2.
Summary of the use of MINDSPACE for HIV prevention and management studies
| Examples | References | |
|---|---|---|
| Messenger | ||
| HIV prevention | Peer groups and/or mentoring in HIV prevention methods | [16–28] |
| Clinical staff, case managers, and community HIV programmes to facilitate HIV prevention | [28–40] | |
| Respected medical establishment to facilitate HIV prevention | [41–48] | |
| HIV management | Case managers to facilitate recruitment into the program and HIV care | [49–56] |
| Hospitals and well known/respected sites to improve adherence to treatment and care | [48, 57–74] | |
| Incentives | ||
| HIV prevention | Lottery-based incentives for HIV prevention | [24, 25, 28–30, 33, 39, 75–78] |
| Loss framing to promote HIV testing | [79–81] | |
| Matched savings accounts, deposit contracts, or micro-enterprise tools used to minimise HIV risk behaviours | [27, 31, 82–84] | |
| Incentives to reinforce HIV prevention education | [5, 27, 85–88] | |
| Financial incentives for peer and community-based recruiters to encourage HIV testing or VMMC | [16, 19, 23, 26, 36] | |
| Cash payment to encourage HIV testing | [22, 34, 37, 88–94] | |
| Financial incentive to take PrEP | [95] | |
| Cash payment to remain HIV negative | [96, 97] | |
| Travel costs reimbursement to engage in HIV prevention | [21, 88, 98] | |
| Non-cash incentives such as personal hygiene products, t-shirts, smartphone credits, gift cards, or food to engage in HIV prevention | [20, 35, 99–102] | |
| HIV management | Financial incentives to reduce HIV viral load or maintain viral load suppression | [38, 49, 55, 58, 62, 69, 103–113] |
| Lottery-based incentives contingent on maintaining viral suppression | [52, 53, 56, 59, 73, 114, 115] | |
| Loss framed whereby incentive was reset if viral load goals were not met | [57] | |
| Incentives to attend routine HIV management appointments | [51, 54, 63, 64, 116–119] | |
| Financial incentive for initiating ART/improving adherence | [48, 68, 72] | |
| Savings account for ART adherence | [66] | |
| Financial incentive contingent on non-reactive stimulant sample | [120] | |
|
Non-financial incentives: Mobile phone, data or minutes and other electronic devices for ART initiation, reduction of viral load, linkage, and retention in HIV care Food vouchers to improve adherence to treatment and retention in HIV care Culturally meaningful pillboxes to improve adherence to antiretroviral therapy and retention in care |
[128] |
|
| Norms | ||
| HIV prevention | Gender norms to encourage HIV testing and facilitate education | [5, 99, 101] |
| Social norms related to HIV prevention, including HIV testing and learning HIV results | [16, 22, 26, 28, 129–131] | |
| Social norms and gender norms to describe transactional sexual relationships and minimise HIV risk | [31] | |
| Subjective norms and workplace HIV counselling and testing | [132] | |
| HIV management | Social norms to improve adherence to antiretroviral therapy and retention in care | [58, 72, 128] |
| Gender norms and barriers to HIV management | [67] | |
| Health seeking norms to improve viral suppression | [53] | |
| Default | ||
| HIV prevention | Opt-out HIV screening | [41, 42, 44–47, 133] |
| Opt-in, opt-out, and active choice HIV screening | [43, 93] | |
| Cost-effectiveness of opt-in/out from a hospital perspective | [94] | |
| Salience | ||
| HIV prevention | Text-based messaging to facilitate HIV prevention and linkage to care | [28, 35, 82, 88, 95, 134] |
| Incentive-based HIV prevention and retention in care | [22, 31, 83, 111, 135] | |
| Respected messenger to increase engagement in HIV prevention, with/out financial incentives | [17, 21, 24, 25, 36, 100, 136] | |
| Culturally relevant and gender-specific messaging to engage in HIV prevention | [30, 101] | |
| Loss framed lottery intervention to encourage dual contraception methods to prevent HIV infection | [86] | |
| HIV management | Text messages to support linkage to care/medication adherence | [72, 116, 121, 126] |
| Lotteries and motivational SMS messages to encourage ART linkage/adherence/viral suppression | [59, 69, 119, 123, 124] | |
| Computer-based HIV education/motivational modules or mindfulness exercises to reduce HIV viral load | [50, 137, 138] | |
| Salience increased via incentives for ART initiation, viral suppression, or retention to services | [38, 48, 51, 53, 54, 57, 103, 106, 117, 135] | |
| Appointment/ART medication reminders | [70, 74, 105, 107, 128] | |
| Priming | ||
| HIV prevention | Use of images of peers in campaigns to encourage HIV testing | [139] |
| Contact details of local VCT clinics in banner ads on gay websites | [140] | |
| Use of prompt to encourage attending HIV testing clinic | [141] | |
| Daily phone-based PrEP and HIV education modules as well text reminders to take PrEP medication | [142] | |
| HIV management | Use of priming stimuli that is empowering or culturally meaningful to improve adherence to ART and retention in care | [143, 144] |
| Use of financial rewards as priming to improve adherence to ART and to suppress viral load count | [49, 145] | |
| Providing safe sex materials as priming to practice safe sex to prevent HIV transmission | [146] | |
| Personalised cues and reminder messages for remembering dose times to support ART adherence | [72, 74, 147, 148] | |
| Affect | ||
| HIV prevention | Creating positive emotion for HIV testing or accessing HIV services | [111, 139, 149, 150] |
| Peer-led group sessions to learn skills for self-efficacy and positive sexual health behaviours | [31] | |
| Increasing risk perception towards HIV to encourage HIV prevention behaviours | [99, 151] | |
| HIV management | Creating positive emotion for HIV care retention and ART adherence through social, financial, or non-financial support | [99, 111, 143, 146] |
| Group sessions targeting positive affect to increase skills for self-efficacy to encourage ART adherence and viral load suppression | [146, 152–154] | |
| Motivational messages to encourage ART adherence | [147, 150, 155] | |
| Commitment | ||
| HIV prevention | Use of binding contracts with financial deposits to encourage HIV testing or clinic visits | [139, 156–159] |
| Use of non-binding/ soft contracts that target the cognitive dissonance aspect to encourage HIV testing and clinic visits | [141, 160] | |
| Use of non-explicit commitment devices, such as rewards, for meeting a specified HIV prevention health behaviour in the future (e.g. maintaining HIV negative status) | [161–168] | |
| HIV management | Use of non-explicit commitment devices in the form of financial incentives for ART adherence and viral load suppression goal in the future | [108, 144, 147, 154, 169–181] |
| Use of non-explicit commitment devices in the form of financial incentives to meet HIV testing, linkage to HIV care and clinic attendance goal in the future | [172, 182, 183] | |
| Use of non-explicit commitment devices in the form of non-financial incentives for ART adherence and viral load suppression goal in the future | [74, 147, 155] | |
| Use of non-explicit commitment devices in the form of non-financial incentives to meet HIV testing, linkage to HIV care and clinic attendance goal in the future | [117, 143, 146, 184–186] | |
| Ego | ||
| HIV prevention | HIV education sessions alongside financial education programmes to target ego and self-efficacy | [18, 31, 99, 187–189] |
| HIV education sessions and peer support to target ego and increase HIV risk perception | [151, 190] | |
| Economic intervention to target ego and promote HIV prevention behaviours | [97, 158, 191] | |
| Peer support and positive messaging to target ego and encourage HIV prevention behaviours | [139, 149] | |
| HIV management | HIV education or personalised HIV support to encourage HIV linkage and care | [98, 99, 137, 184, 189] |
| Financial intervention to target ego and encourage viral load suppression | [66, 145, 152, 154, 178–180, 192, 193] | |
| Positive messaging, reminders, or motivational interviewing to target ego and encourage viral load suppression | [74, 155, 169, 182, 194] | |
Results
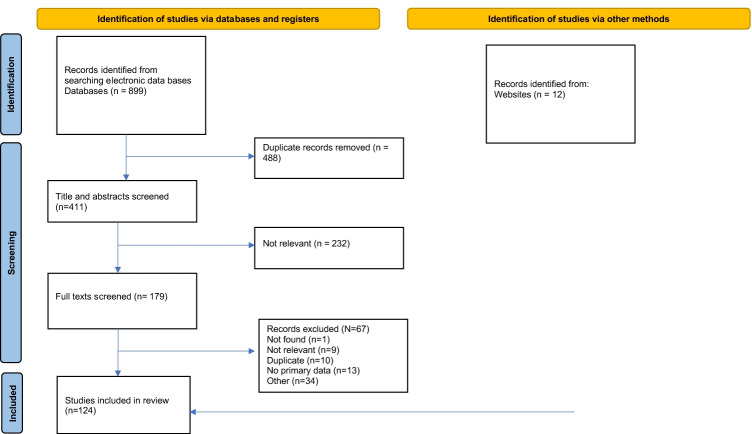
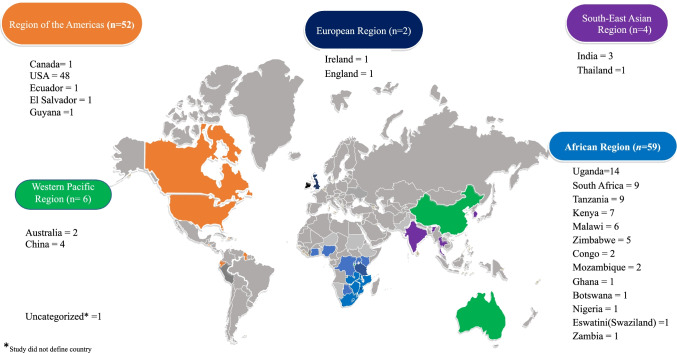
Figure 1 depicts the study screening process and article selection as a PRISMA flowchart. We found 899 studies and included 124 in this scoping review. The characteristics of the studies are summarised in Table 1. In total, 63 studies were related to HIV prevention, and 63 were related to HIV management (with two studies related to both prevention and management). Most were from high-income countries (n = 54) and most HIV prevention (n = 36) and management (n = 41) interventions targeted the general population. Figure 2 categorises the countries according to the World Health Organization (WHO) regions, with most studies taking place in Africa (n = 60) and the Americas (n = 52).
Fig. 1.
PRISMA flowchart. This figure has been created by the authors for the purposes of this research. No permission is needed
Table 1.
Characteristics of studies included in the scoping review
| n | % | |
|---|---|---|
| Country income level (N = 124) | ||
| High | 54 | 43.5 |
| Middle | 47 | 37.9 |
| Low | 21 | 16.9 |
| Populations targeted in HIV prevention studies (N = 63) | ||
| Female sex workers | 2 | 3.2 |
| Men who have sex with men | 12 | 19.0 |
| Transgender women* | 4 | 6.3 |
| Substance users | 5 | 7.9 |
| Children and young adults** | 10 | 15.9 |
| Mothers/pregnant women | 5 | 7.9 |
| General population | 36 | 57.1 |
| Populations targeted in HIV management studies (N = 63) | ||
| Female sex workers | 1 | 1.6 |
| Men who have sex with men | 4 | 6.3 |
| Transgender women* | 1 | 1.6 |
| Substance users | 8 | 12.7 |
| Children and young adults** | 9 | 14.3 |
| Mothers/pregnant women | 2 | 3.2 |
| General population | 41 | 65.1 |
| MINDSPACE | ||
| Messenger | 59 | 47.6 |
| Incentive | 100 | 80.6 |
| Norms | 17 | 13.7 |
| Default | 10 | 8.1 |
| Salience | 48 | 38.7 |
| Priming | 13 | 10.5 |
| Affect | 14 | 11.3 |
| Commitment | 50 | 40.3 |
| Ego | 34 | 27.4 |
*Studies in this category may also include MSM as they did not distinguish TGW and MSM
**Studies in this category aimed to recruit 10–25-year-old
Note: Some studies consisted of more than one category so the numbers may not align with total
Fig. 2.
Studies categorised by countries in WHO regions (N = 124). This figure has been created by the authors for the purposes of this research. No permission is needed
MINDSPACE Effects
Incentives were the most popular application of MINDSPACE (n = 100) followed by messenger (n = 59). A complete list of included HIV prevention and management studies can be found in Supplementary Tables 2 and 3. The summary of included studies categorised as each MINDSPACE effect can be found in Table 2, split by those aimed at HIV prevention and those aimed at HIV management. The studies utilizing each effect are now summarised.
Messenger
Thirty-one HIV prevention studies drew on the messenger effect, using a wide range of messengers and messages. For example, one multi-level community-based intervention in El Salvador utilised peers as messengers, both as recruiters to encourage hard-to-reach crack users to test for HIV and as counsellors to reduce sexual risk-taking behaviour [16]. A population-level intervention in Malawi used nurses as messengers. They offered HIV tests to participants in their home and simultaneously discussed HIV prevention strategies [29]. Other messengers included respected community HIV programmes involved in the recruitment of study participants (Table 2).
Twenty-six HIV management interventions drew on the messenger effect. This included a prospective RCT among non-adherent people living with HIV in the United States of America (USA). The intervention utilised a respected HIV/AIDS health care provider to provide individualised intensive case management [49]. Another example was a clinical trial to test the effect of a nurse-led motivational group intervention on adherence to ART and risk reduction behaviours among women living with HIV in the USA. The nurse facilitators used motivational interviewing techniques to explore discrepancies between current behaviours and values [50]. Other messengers included hospitals and well-known/respected sites to improve adherence to treatment, and care and case managers to facilitate recruitment into the programme and HIV care (Table 2).
Incentives
Fifty-one HIV prevention studies utilised incentives. For example, a study in rural Uganda used an RCT to explore the effectiveness of incentive strategies at high and low amounts to promote HIV testing among men. The study utilised loss framing and lotteries to promote HIV testing where participants were informed they had won a prize, asked to select a prize from various items, then told they would lose the prize if they did not obtain an HIV test. Those who got tested received a further opportunity to win larger prizes as part of a lottery [79]. Another example was a USA-based RCT for people who used drugs. The intervention consisted of a computer-based HIV prevention education programme designed to teach participants about PrEP. The intervention consisted of four modules each with 112 questions. Participants could earn $0.02 for each correct answer given [85]. Other incentive types, including lottery-based incentives, matched savings accounts, deposit contracts, or micro-enterprise tools to minimise HIV risk behaviours, remain HIV negative or participate in voluntary medical male circumcision (VMMC) (Table 2).
Forty-seven HIV management studies utilised incentives. This included an RCT in Uganda among men living with HIV. The financial incentives used contingency management principles to motivate participants in the intervention group to be virally suppressed. Specifically, incentives increased if they were virally suppressed but was reset to the initial value of 4 USD if participants were not virally suppressed [57]. An observational cohort study in rural Uganda utilised short message service (SMS) and travel reimbursements to improve HIV care following an abnormal CD4 test result. Participants with an abnormal CD4 test received SMS notification of their results daily, up to a maximum of 7 days. Participants who returned to the clinic within 7 days of the first message received a transportation reimbursement to cover the cost of transportation to the clinic [116]. Other incentives included lottery-based incentives contingent on maintaining viral suppression and non-financial incentives such as a mobile phone, data or minutes for ART initiation, reduction of viral load, linkage to care, and retention in HIV care (Table 2).
Norms
Twelve HIV prevention studies used gender, social, and subjective norms. This included a cluster RCT in South Africa that implemented a tablet-based app (EPIC-HIV) to provide a male-targeted intervention to support their HIV testing decision-making process. The content used local narrative and provided information about the likely outcomes of testing [99]. Another RCT provided funding for very small-scale businesses coupled with HIV education sessions targeting female sex workers in India. Group norms were developed at the onset via group rules for HIV prevention sessions which were interactive and didactic [5].
Five HIV management studies used gender, social, and health-seeking norms. Social norms were utilised in an intervention in Ghana. Participants received financial incentives based on group average viral loads and group average clinical attendance. The intervention also utilised peer support where group members chose the group name to provide a sense of identity [58]. A quasi-experimental pilot study in Tanzania explored social norms coupled with a positive priming image known to participants of a Baobab tree (the ‘tree of life’) to improve adherence to antiretroviral therapy and retention in care. This intervention utilised culturally relevant imaging paired with a widely known Tanzanian idiom to relay the group was working together to achieve a goal, implying the support available from other patients, staff, and the community [128].
Defaults
Ten HIV prevention studies used default options. This included an RCT where participants were designated into either an opt-in, active choice or opt-out HIV screening arm in an emergency department (ED) in the USA. The following text was provided: Opt-in, ‘You can let me, your nurse, or your doctor know if you’d like a test today’; active choice, ‘Would you like a test today?’; or opt-out, ‘You will be tested unless you decline’ [195]. A prospective mixed-methods study in Australia examined healthcare providers’ acceptability of opt-out HIV testing and how it impacted HIV testing rates among homeless and marginalised patients [133]. Other studies using defaults in HIV screening can be found in Table 2.
We did not find any HIV management studies which used the default option.
Salience
Twenty-one HIV prevention studies used salience. This included an RCT for justice-involved MSM and transgender women (TGW) in the USA, which used peer mentor support and a client-driven approach to address and track preventative health care goals via app-based technology with in-built medication and appointment reminders, to encourage PrEP usage and HIV testing [17]. A pilot RCT among Latinx MSM and TGW in the USA used culturally specific text messaging providing sexual health information coupled with frequent feedback to the health information responses to keep participants engaged [30]. Further examples can be found in Table 2.
Twenty-seven HIV management studies used salience. This included a pilot RCT among adults living with HIV with unsuppressed viral loads in the USA. This intervention used daily lotteries tied to the opening of a wirelessly enabled electronic pill bottle. Participants received an additional incentive if they were virally suppressed at three months. Participants received 1 of 4 daily feedback depending on adherence the day before [59]. An RCT in Uganda provided escalating incentives of $4, $8, and $12.50 for being virally suppressed at 6, 12, and 24 weeks. These incentives aimed to offset the costs of retention in HIV care, as well as provide a reward for viral suppression [57]. Other examples include text-based ART medication reminders and mindfulness exercises to reduce HIV viral load (Table 2).
Priming
Four HIV prevention studies used priming. Priming stimuli included images of overseas-born MSM in posters and videos in a public health campaign in Australia to encourage international MSM students to seek HIV/STI testing at health facilities [139] or displaying contact details for local voluntary counselling and testing (VCT) clinics in banner ads on the front pages of national gay dating websites to encourage the website users to seek HIV testing [140]. Another study used PrEP education and provision of medication adherence feedback and reminders as priming stimuli to improve the rates of PrEP medication adherence among an at-risk young MSM population for HIV prevention [142] (Table 2).
Eleven HIV management studies used priming. An intervention in Tanzania incorporated an image of a Baobab tree and a widely known local idiom, ‘Together we can hug the Baobab tree’ on the calendar and the small plastic pillbox given to the patient receiving HIV care [143]. The Baobab tree, also known as the ‘tree of life’ locally, was a positive image known by residents where the trees are numerous in the community. Similarly, a social marketing campaign intervention in the USA involved utilised graphic novels using a group of inspirational superheroes called ‘The Undetectables’ as a priming stimulus to engage and motivate people living with HIV (PLWHIV) to manage their ART adherence [144]. Other examples included personalised reminder messages, cues, or feedback to inform the individual of their adherence or remind them of the medication times via text messages or phone calls (Table 2).
Affect
Seven HIV prevention studies used the affect nudge. For example, individuals were invited to celebrate testing negative HIV/STI with their favourite or an indulgent activity [139], or individuals were provided with positive automated feedback through an app and messages when set goals were achieved and with messages from peer mentors to motivate accessing testing services [149]. Some studies used negative emotions associated with increased risk perception of getting HIV to encourage healthy behaviours [99, 151].
Ten HIV management studies used the affect nudge. In one study, patients would receive congratulations for attending the clinic three consecutive on-time visits for HIV care, and they were given a sticker to proudly place on an interactive poster publicly displayed in the clinic to celebrate and acknowledge their achievement [143]. Another study involved a multi-component positive affect intervention for MSM who used drugs, eight core skills, and meditation exercises were delivered to them to increase positive affect among them and to improve their psychological adjustment to cope effectively with methamphetamine withdrawal, to reduce their HIV viral load ultimately through this mediation [152].
Commitment
Twenty HIV prevention studies used commitment. Binding commitment contracts, which involved financial deposits made by participants, were used in some studies to encourage participants to attend clinics regularly for HIV testing services [139, 156, 157]. When participants underwent HIV testing regularly for the agreed commitment period, their deposits were returned with interest paid. Otherwise, if they failed to regularly attend the HIV prevention services, they forfeited their deposits and incurred financial loss. Alternatively, some studies targeted cognitive dissonance by implementing soft or non-binding commitment on the individuals, such as having participants express their intention to get HIV tested on a sheet of paper or selected a date and time on a calendar to attend the community health campaign for HIV prevention services [141, 160]. Other examples included commitments that provide participants with financial rewards or incentives, lottery tickets, or prize draws for meeting a certain specified goal or health behaviour in the future [161–163] (Table 2).
Thirty-two HIV management studies used commitment. Provided participants met a specified health outcome in the future, they were promised financial incentives such as vouchers [169], prize draws [170], and lottery prizes [171], or non-financial incentives such as mobile airtime [155, 184], and food [117, 185]. These incentives were provided to participants for health outcomes such as suppressing the viral load to a specified level (i.e. HIV RNA < 200 copies/mL) for a period of time, for example for 6 or 12 months [169–171], maintaining a high level of ART adherence [155], attending HIV management services regularly [117, 172, 185], and having linkage to HIV care within 1 month of positive HIV testing and visiting treatment services regularly [184, 186].
Ego
Thirteen HIV prevention studies used ego. HIV prevention education programmes were used to increase self-efficacy, negotiation, and communication skills related to HIV risk behaviours and sexual relationships [31, 187, 188]. These programmes were usually paired with other training and programmes which taught financial knowledge and skills, such as tailored microenterprise training [187], financial education programmes [31, 158], and micro-business start-up grants [18, 188] (Table 2).
Twenty-three HIV management studies used ego. This included participants attending motivational interviewing sessions with a psychologist or a specialist nurse [169, 182, 194], and participants regularly receiving carefully crafted motivational messages to target their ego to influence their health behaviours, for example messages such as ‘Stay strong!’, ‘Have courage!’, or ‘Don’t give up!’ [147, 155]. A study aimed to evoke positive emotions in PLWHIV for maintaining their HIV care and management, for example by providing them with opportunities to participate in non-medical-related leisure activities, such as massage therapy and beauty consultancy, alongside their HIV intervention sessions [146]. Other examples involved participants receiving HIV education and cognitive behavioural skills-building sessions to affect their ego through empowering them with HIV knowledge and management skills to look after their health and wellbeing (Table 2).
Discussion
We contribute to the literature by synthesizing the range of HIV prevention and management interventions which can be described by behavioural economic principles. To the best of our knowledge, our study is the first of its kind to use the MINDSPACE framework for describing HIV-related interventions. A variety of study designs were used that took place in range of settings, e.g. from the controlled environment of a clinical trial to quasi-experimental designs to qualitative interviews. Most interventions relied heavily on financial incentives to encourage behaviour change and thus most of the MINDSPACE effects are potentially underutilised. Future HIV prevention and management interventions could consider incorporating more MINDSPACE components and evaluate their potential to optimise the interventions’ effectiveness.
Using behavioural economics to inform interventions can benefit the HIV sector by providing a framework to support micro- and macro-level decision-making and improve health outcomes. Based on our review, we observe that the different MINDSPACE effects are already in use in multiple HIV prevention and management interventions. This demonstrates the feasibility for implementing these nudge effects. However, most effects are potentially underutilised. There could be greater use of nudges, for example implementing a default opt-out approach for HIV testing [41, 42, 133] or utilizing social norms by sending text messages to inform participants of their medication adherence level and adherence level of their peers [196]. We recommend the use of the MINDSPACE framework to consider whether the range of effects could be used in the design of an intervention. It is also important to note more than one effect can be used in the same intervention, which may further optimise the intervention’s effectiveness [12].
There can be disadvantages to using nudges in the design of HIV interventions. First, the effectiveness of nudge interventions are often context-dependent [197]. Additionally, there is little information about long-term behavioural changes resulting from these nudge interventions [198]. Additionally, the effects of nudges may weaken when more widely used, as individuals could become more aware of the intended effect and of their own decision-making biases [199]. Third, marginalised populations with limited resources may be at higher risk of being negatively impacted by health policies and interventions using nudges, such as default, because they may not be able to opt out without significant burden or cost [200].
Our study should be read in light of some limitations. First, we limited our search to four databases and English publications and may have missed other relevant literature. However, we aimed to collate examples of how behavioural economic principles were applied in the HIV sector and not provide an exhaustive list of all relevant studies. Second, it was beyond the scope of this paper to explore the effectiveness of the MINDSPACE interventions, which we will explore in a future publication.
There are several lessons for the future application of behavioural economics in the design of HIV prevention and management interventions. First, we found interesting examples of how nudges are applied, but we must be wary of one-size-fits-all nudges. Each nudge must be evaluated and carefully adapted to the local cultural context. Second, using a framework, like MINDSPACE, helps consider how nudges could be applied to help people go with the flow of their automatic (system 1) decision-making patterns. Third, as nudge interventions move beyond academic interest to practical applications, they need to demonstrate and communicate its impact, particularly evaluating the value of multifaceted behavioural solutions. Finally, MINDSPACE centred interventions should be aligned with the individual’s and the community’s existing identity and goals to make the interventions acceptable and successful. Importantly, when designing MINDSPACE-centred interventions, its impact on the vulnerable subset of target populations should be considered foremostly to ensure fairness and equity, and that the interventions remain a nudge as opposed to being coercive and involuntary. The results of rigorous field evaluations of interventions should inform the design and implementation of future programmes to have optimal impact of the nudge interventions on target populations.
Conclusion
The key influences described by the MINDSPACE framework can describe aspects of HIV prevention and management interventions. Our study indicates that Messenger, Incentive, and Commitment were the most frequently applied nudges. Therefore, there is an opportunity for future interventions to explore the use of other nudges (e.g. default and priming) to gather further evidence to understand the feasibility and value of applying nudges in HIV prevention and management strategies. Further research, specifically about long-term behavioural changes regarding HIV health outcomes due to nudge interventions, is also warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
JJO conceptualised the idea. AA and JT performed the screening and extraction of data. AA, JT, and JJO wrote the original draft. All authors contributed to the writing of the manuscript and approved the final version for submission.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions JJO is supported by an Australian National Health and Medical Research Council Emerging Leadership Fellowship (GNT1193955).
Data Availability
All relevant data are presented in the manuscript and online supplementary materials. Any further details can be obtained by contacting the corresponding author.
Code Availability
Not applicable.
Declarations
Ethics Approval
As this is a scoping review, no human participants were involved and no ethics approval was required.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Alexsandra Andrawis and James Tapa are equal first co-authors.
This article is part of the Topical Collection on Implementation Science
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global HIV & AIDS statistics — fact sheet []. 2020 [Available from: https://www.unaids.org/en/resources/fact-sheet. Accessed 1 Aug 2022.
- 2.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on ART across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11(5):492–500. doi: 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaramillo J, Pagkas-Bather J, Waters K, Shackelford LB, Campbell RD, Henry J, et al. Perceptions of sexual risk, PrEP services, and peer navigation support among HIV-negative Latinx and Black men who have sex with men (MSM) residing in western Washington. Sex Res Soc Policy. 2021:1–11. [DOI] [PMC free article] [PubMed]
- 4.Rao A, Schwartz S, Sabin K, Wheeler T, Zhao J, Hargreaves J, et al. HIV-related data among key populations to inform evidence-based responses: protocol of a systematic review. Syst Rev. 2018;7(1):220. doi: 10.1186/s13643-018-0894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman SG, Srikrishnan AK, Rivett KA, Liu SH, Solomon S, Celentano DD. Acceptability of a microenterprise intervention among female sex workers in Chennai, India. AIDS Behav. 2010;14(3):649–657. doi: 10.1007/s10461-010-9686-z. [DOI] [PubMed] [Google Scholar]
- 6.Kahneman D. A perspective on judgment and choice: mapping bounded rationality. Am Psychol. 2003;58(9):697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- 7.Thaler R, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. Yale University Press; 2008.
- 8.Simon C, Tagliabue M. Feeding the behavioral revolution: contributions of behavior analysis to nudging and vice versa. J Behav Econ Policy. 2018;2(1):91–7.
- 9.Thaler Richard H, Benartzi S. Save More Tomorrow™: using behavioral economics to increase employee saving. J Polit Econ. 2004;112(S1):S164–S187. doi: 10.1086/380085. [DOI] [Google Scholar]
- 10.How Amsterdam Schipol reduced toilet cleaning costs by 8% with fake urinal bugs. Available from: https://simpleflying.com/how-amsterdam-schipol-reduced-toilet-cleaning-costs-by-8-with-fake-urinal-bugs/. Accessed 1 Aug 2022.
- 11.Applying behavioural insights to reduce fraud, error and debt. Available from: http://38r8om2xjhhl25mw24492dir.wpengine.netdna-cdn.com/wp-content/uploads/2015/07/BIT_FraudErrorDebt_accessible.pdf. Accessed 1 Aug 2022.
- 12.MINDSPACE: influencing behaviour through public policy. Available from: https://www.bi.team/wp-content/uploads/2015/07/MINDSPACE.pdf. Accessed 1 Aug 2022.
- 13.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 14.Transparent reporting of systematic reviews and meta-analyses. PRISMA for scoping reviews. Available from: http://www.prisma-statement.org/Extensions/ScopingReviews. Accessed 1 Aug 2022.
- 15.Data for high income, middle income, low income. Available from: https://data.worldbank.org/?locations=XD-XP-XM. Accessed 1 Aug 2022.
- 16.Glasman LR, Dickson-Gomez J, Lechuga J, Tarima S, Bodnar G, de Mendoza LR. Using peer-referral chains with incentives to promote HIV testing and identify undiagnosed HIV infections among crack users in San Salvador. AIDS Behav. 2016;20(6):1236–1243. doi: 10.1007/s10461-015-1267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards GG, Reback CJ, Cunningham WE, Hilliard CL, McWells C, Mukherjee S, et al. Mobile-enhanced prevention support study for men who have sex with men and transgender women leaving jail: protocol for a randomized controlled trial. JMIR Res Protoc. 2020;9(9):e18106. doi: 10.2196/18106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings Mayo-Wilson L, Coleman J, Timbo F, Latkin C, Torres Brown ER, Butler AI, et al. Acceptability of a feasibility randomized clinical trial of a microenterprise intervention to reduce sexual risk behaviors and increase employment and HIV preventive practices (EMERGE) in young adults: a mixed methods assessment. BMC Public Health. 2020;20(1):N.PAG-N.PAG. [DOI] [PMC free article] [PubMed]
- 19.Lu Y, Ni Y, Li X, He X, Huang S, Zhou Y, et al. Monetary incentives and peer referral in promoting digital network-based secondary distribution of HIV self-testing among men who have sex with men in China: study protocol for a three-arm randomized controlled trial. BMC Public Health. 2020;20(1):911. doi: 10.1186/s12889-020-09048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCoy SI, Shiu K, Martz TE, Smith CD, Mattox L, Gluth DR, et al. Improving the efficiency of HIV testing with peer recruitment, financial incentives, and the involvement of persons living with HIV infection. J Acquir Immune Defic Syndr. 2013;63(2):e56–63. doi: 10.1097/QAI.0b013e31828a7629. [DOI] [PubMed] [Google Scholar]
- 21.Tun W, Sebastian MP, Sharma V, Madan I, Souidi S, Lewis D, et al. Strategies for recruiting injection drug users for HIV prevention services in Delhi, India. Harm Reduct J. 2013;10:16. doi: 10.1186/1477-7517-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godlonton S, Thornton R. Peer effects in learning HIV results. J Dev Econ. 2012;97(1):118–129. doi: 10.1016/j.jdeveco.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das A, George B, Ranebennur V, Parthasarathy MR, Shreenivas GS, Todankar P, et al. Getting to the first 90: incentivized peer mobilizers promote HIV testing services to men who have sex with men using social media in Mumbai, India. Glob Health Sci Pract. 2019;7(3):469–477. doi: 10.9745/GHSP-D-19-00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazant E, Mahler H, Machaku M, Lemwayi R, Kulindwa Y, Gisenge Lija J, et al. A randomized evaluation of a demand creation lottery for voluntary medical male circumcision among adults in Tanzania. J Acquir Immune Defic Syndr. 2016;72 Suppl 4:S280–S287. doi: 10.1097/QAI.0000000000001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas R, Skovdal M, Galizzi MM, Schaefer R, Moorhouse L, Nyamukapa C, et al. Improving risk perception and uptake of voluntary medical male circumcision with peer-education sessions and incentives, in Manicaland, East Zimbabwe: study protocol for a pilot randomised trial. Trials. 2020;21(1):108. doi: 10.1186/s13063-020-4048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanolini A, Bolton C, Lyabola LL, Phiri G, Samona A, Kaonga A, et al. Feasibility and effectiveness of a peer referral incentive intervention to promote male circumcision uptake in Zambia. J Acquir Immune Defic Syndr. 2016;72 Suppl 4:S257–S263. doi: 10.1097/QAI.0000000000000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayo-Wilson LJ, Ssewamala FM. Financial and behavioral economic factors associated with HIV testing in AIDS-affected adolescents in Uganda: a cross-sectional analysis. J Health Care Poor Underserved. 2019;30(1):339–357. doi: 10.1353/hpu.2019.0025. [DOI] [PubMed] [Google Scholar]
- 28.Moorhouse L, Schaefer R, Thomas R, Nyamukapa C, Skovdal M, Hallett TB, et al. Application of the HIV prevention cascade to identify, develop and evaluate interventions to improve use of prevention methods: examples from a study in east Zimbabwe. J Int AIDS Soc. 2019;22 Suppl 4:e25309. doi: 10.1002/jia2.25309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton RL. The demand for, and impact of, learning HIV status. Am Econ Rev. 2008;98(5):1829–1863. doi: 10.1257/aer.98.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linnemayr S, MacCarthy S, Kim A, Giguere R, Carballo-Dieguez A, Barreras JL. Behavioral economics-based incentives supported by mobile technology on HIV knowledge and testing frequency among Latino/a men who have sex with men and transgender women: Protocol for a randomized pilot study to test intervention feasibility and acceptability. Trials. 2018;19(1):540. doi: 10.1186/s13063-018-2867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gichane MW, Wamoyi J, Atkins K, Balvanz P, Maman S, Majani E, et al. The influence of cash transfers on engagement in transactional sex and partner choice among adolescent girls and young women in Northwest Tanzania. Cult Health Sex. 2020:1–15. [DOI] [PubMed]
- 32.Beadnell B, Baker S, Knox K, Stielstra S, Morrison DM, DeGooyer E, et al. The influence of psychosocial difficulties on women’s attrition in an HIV/STD prevention programme. AIDS Care. 2003;15(6):807–820. doi: 10.1080/09540120310001618658. [DOI] [PubMed] [Google Scholar]
- 33.Choko AT, Corbett EL, Stallard N, Maheswaran H, Lepine A, Johnson CC, et al. HIV self-testing alone or with additional interventions, including financial incentives, and linkage to care or prevention among male partners of antenatal care clinic attendees in Malawi: an adaptive multi-arm, multi-stage cluster randomised trial. PLoS Med. 2019;16(1):e1002719. doi: 10.1371/journal.pmed.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choko AT, Fielding K, Johnson CC, Kumwenda MK, Chilongosi R, Baggaley RC, et al. Partner-delivered HIV self-test kits with and without financial incentives in antenatal care and index patients with HIV in Malawi: a three-arm, cluster-randomised controlled trial. Lancet Glob Health. 2021;9(7):e977–e988. doi: 10.1016/S2214-109X(21)00175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibanda EL, Tumushime M, Mufuka J, Mavedzenge SN, Gudukeya S, Bautista-Arredondo S, et al. Effect of non-monetary incentives on uptake of couples’ counselling and testing among clients attending mobile HIV services in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health. 2017;5(9):e907–e915. doi: 10.1016/S2214-109X(17)30296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibanda EL, Neuman M, Tumushime M, Mangenah C, Hatzold K, Watadzaushe C, et al. Community-based HIV self-testing: a cluster-randomised trial of supply-side financial incentives and time-trend analysis of linkage to antiretroviral therapy in Zimbabwe. BMJ Glob Health. 2021;6(Suppl 4):07. doi: 10.1136/bmjgh-2020-003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner AD, Njuguna IN, Neary J, Omondi VO, Otieno VA, Babigumira J, et al. Financial Incentives to Increase Uptake of Pediatric HIV Testing (FIT): study protocol for a randomised controlled trial in Kenya. BMJ Open. 2018;8(10):e024310. doi: 10.1136/bmjopen-2018-024310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gambone GF, Feldman MB, Thomas-Ferraioli AY, Shubert V, Ghose T. Integrating financial incentives for viral load suppression into HIV care coordination programs: considerations for development and implementation. J Public Health Manag Pract. 2020;26(5):471–480. doi: 10.1097/PHH.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 39.MacCarthy S, Mendoza-Graf A, Wagner Z, Barreras JL, Kim A, Giguere R, et al. The acceptability and feasibility of a pilot study examining the impact of a mobile technology-based intervention informed by behavioral economics to improve HIV knowledge and testing frequency among Latinx sexual minority men and transgender women. BMC Public Health. 2021;21(1):341. doi: 10.1186/s12889-021-10335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacCarthy S, Wagner Z, Barreras JL, Kim A, Menodza-Graf AC, Giguere R, et al. Brief report: Using behavioral economics to increase HIV knowledge and testing among Latinx sexual minority men and transgender women: a quasi-experimental pilot study. J Acquir Immune Defic Syndr. 2020;85(2):189–194. doi: 10.1097/QAI.0000000000002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connell S, Lillis D, Cotter A, O'Dea S, Tuite H, Fleming C, et al. Opt-out panel testing for HIV, hepatitis B and hepatitis C in an urban emergency department: a pilot study. PLoS ONE. 2016;11(3):e0150546. doi: 10.1371/journal.pone.0150546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam G, Wong SYS. A cross-sectional study comparing two opt-out HIV testing strategies in the out-patient setting. Front Public Health. 2021;9(664494):664494. doi: 10.3389/fpubh.2021.664494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montoy JCC, Dow WH, Kaplan BC. Patient choice in opt-in, active choice, and opt-out HIV screening: randomized clinical trial. BMJ. 2016;352:h6895. [DOI] [PMC free article] [PubMed]
- 44.Creek TL, Ntumy R, Seipone K, Smith M, Mogodi M, Smit M, et al. Successful introduction of routine opt-out HIV testing in antenatal care in Botswana. J Acquir Immune Defic Syndr. 2007;45(1):102–107. doi: 10.1097/QAI.0b013e318047df88. [DOI] [PubMed] [Google Scholar]
- 45.Haukoos JS, Hopkins E, Conroy AA, Silverman M, Byyny RL, Eisert S, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010;304(3):284–292. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 46.White DA, Scribner AN, Vahidnia F, Dideum PJ, Gordon DM, Frazee BW, et al. HIV screening in an urban emergency department: comparison of screening using an opt-in versus an opt-out approach. Ann Emerg Med. 2011;58(1 Suppl 1):S89–95. doi: 10.1016/j.annemergmed.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Christensen A, Russ S, Rambaran N, Wright SW. Patient perspectives on opt-out HIV screening in a Guyanese emergency department. Int Health. 2012;4(3):185–191. doi: 10.1016/j.inhe.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Maughan-Brown B, Smith P, Kuo C, Harrison A, Lurie MN, Bekker L-G, et al. A conditional economic incentive fails to improve linkage to care and antiretroviral therapy initiation among HIV-positive adults in Cape Town, South Africa. AIDS Patient Care STDS. 2018;32(2):70–78. doi: 10.1089/apc.2017.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Javanbakht M, Prosser P, Grimes T, Weinstein M, Farthing C. Efficacy of an individualized adherence support program with contingent reinforcement among nonadherent HIV-positive patients: results from a randomized trial. J Int Assoc Physicians AIDS Care (Chic) 2006;5(4):143–150. doi: 10.1177/1545109706291706. [DOI] [PubMed] [Google Scholar]
- 50.Holstad MM, DiIorio C, Magowe MK. Motivating HIV positive women to adhere to antiretroviral therapy and risk reduction behavior: the KHARMA Project. Online J Issues Nurs. 2006;11(1):5. doi: 10.3912/OJIN.Vol11No01Man04. [DOI] [PubMed] [Google Scholar]
- 51.Bamberger JD, Unick J, Klein P, Fraser M, Chesney M, Katz MH. Helping the urban poor stay with antiretroviral HIV drug therapy. Am J Public Health. 2000;90(5):699–701. doi: 10.2105/AJPH.90.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghose T, Shubert V, Chaudhuri S, Poitevien V, Updyke A. Are financial incentives appropriate means of encouraging medication adherence among people living with HIV? AMA J Ethics. 2021;23(5):E394–401. doi: 10.1001/amajethics.2021.394. [DOI] [PubMed] [Google Scholar]
- 53.Ghose T, Shubert V, Poitevien V, Choudhuri S, Gross R. Effectiveness of a viral load suppression intervention for highly vulnerable people living with HIV. AIDS Behav. 2019;23(9):2443–2452. doi: 10.1007/s10461-019-02509-5. [DOI] [PubMed] [Google Scholar]
- 54.Stitzer ML, Hammond AS, Matheson T, Sorensen JL, Feaster DJ, Duan R, et al. Enhancing patient navigation with contingent incentives to improve healthcare behaviors and viral load suppression of persons with HIV and substance use. AIDS Patient Care STDS. 2018;32(7):288–296. doi: 10.1089/apc.2018.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beima-Sofie K, Begnel ER, Golden MR, Moore A, Ramchandani M, Dombrowski JC. “It’s me as a person, not me the disease”: patient perceptions of an HIV care model designed to engage persons with complex needs. AIDS Patient Care STDS. 2020;34(6):267–274. doi: 10.1089/apc.2019.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greene E, Pack A, Stanton J, Shelus V, Tolley EE, Taylor J, et al. “It makes you feel like someone cares” acceptability of a financial incentive intervention for HIV viral suppression in the HPTN 065 (TLC-Plus) study. PLoS ONE. 2017;12(2):e0170686. doi: 10.1371/journal.pone.0170686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thirumurthy H, Ndyabakira A, Marson K, Emperador D, Kamya M, Havlir D, et al. Financial incentives for achieving and maintaining viral suppression among HIV-positive adults in Uganda: a randomised controlled trial. Lancet HIV. 2019;6(3):e155–e163. doi: 10.1016/S2352-3018(18)30330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galarraga O, Enimil A, Bosomtwe D, Cao W, Barker DH. Group-based economic incentives to improve adherence to antiretroviral therapy among youth living with HIV: safety and preliminary efficacy from a pilot trial. Vulnerable Child Youth Stud. 2020;15(3):257–268. doi: 10.1080/17450128.2019.1709678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bien-Gund CH, Ho JI, Bair EF, Marcus N, Choi RJ, Szep Z, et al. Brief report: Financial incentives and real-time adherence monitoring to promote daily adherence to HIV treatment and viral suppression among people living with HIV: a pilot study. J Acquir Immune Defic Syndr. 2021;87(1):688–692. doi: 10.1097/QAI.0000000000002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Czaicki NL, Mnyippembe A, Blodgett M, Njau P, McCoy SI. It helps me live, sends my children to school, and feeds me: a qualitative study of how food and cash incentives may improve adherence to treatment and care among adults living with HIV in Tanzania. AIDS Care. 2017;29(7):876–884. doi: 10.1080/09540121.2017.1287340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ekwunife OI, Anetoh MU, Kalu SO, Ele PU, Eleje GU. Conditional economic incentives and motivational interviewing to improve adolescents’ retention in HIV care and adherence to antiretroviral therapy in Southeast Nigeria: study protocol for a cluster randomised trial. Trials. 2018;19(1):710. doi: 10.1186/s13063-018-3095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Sadr WM, Donnell D, Beauchamp G, Hall HI, Torian LV, Zingman B, et al. Financial incentives for linkage to care and viral suppression among HIV-positive patients: a randomized clinical trial (HPTN 065) JAMA Intern Med. 2017;177(8):1083–1092. doi: 10.1001/jamainternmed.2017.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Sadr WM, Beauchamp G, Hall HI, Torian LV, Zingman BS, Lum G, et al. Brief report: Durability of the effect of financial incentives on HIV viral load suppression and continuity in care: HPTN 065 study. J Acquir Immune Defic Syndr. 2019;81(3):300–303. doi: 10.1097/QAI.0000000000001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fahey CA, Njau PF, Katabaro E, Mfaume RS, Ulenga N, Mwenda N, et al. Financial incentives to promote retention in care and viral suppression in adults with HIV initiating antiretroviral therapy in Tanzania: a three-arm randomised controlled trial. Lancet HIV. 2020;7(11):e762–e771. doi: 10.1016/S2352-3018(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haukoos JS, Witt MD, Coil CJ, Lewis RJ. The effect of financial incentives on adherence with outpatient human immunodeficiency virus testing referrals from the emergency department. Acad Emerg Med. 2005;12(7):617–621. doi: 10.1197/j.aem.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 66.Ssewamala FM, Sensoy Bahar O, Nabunya P, Thames AD, Neilands TB, Damulira C, et al. Suubi+Adherence-Round 2: a study protocol to examine the longitudinal HIV treatment adherence among youth living with HIV transitioning into young adulthood in Southern Uganda. BMC Public Health. 2021;21(1):179. doi: 10.1186/s12889-021-10202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fladseth K, Gafos M, Newell ML, McGrath N. The impact of gender norms on condom use among HIV-positive adults in KwaZulu-Natal, South Africa. PLoS ONE. 2015;10(4):e0122671. doi: 10.1371/journal.pone.0122671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swartz A, Maughan-Brown B, Perera S, Harrison A, Kuo C, Lurie MN, et al. “The money, it’s OK but it’s not OK”: patients’ and providers’ perceptions of the acceptability of cash incentives for HIV treatment initiation in Cape Town, South Africa. AIDS Behav. 2021;20:20. doi: 10.1007/s10461-021-03355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linnemayr S, Stecher C, Saya U, MacCarthy S, Wagner Z, Jennings L, et al. Behavioral Economics Incentives to Support HIV Treatment Adherence (BEST): protocol for a randomized controlled trial in Uganda. Trials. 2020;21(1):9. doi: 10.1186/s13063-019-3795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNairy ML, Lamb MR, Gachuhi AB, Nuwagaba-Biribonwoha H, Burke S, Mazibuko S, et al. Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: the Link4Health cluster randomized trial. PLoS Med. 2017;14(11):e1002420. doi: 10.1371/journal.pmed.1002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Czaicki NL, Dow WH, Njau PF, McCoy SI. Do incentives undermine intrinsic motivation? Increases in intrinsic motivation within an incentive-based intervention for people living with HIV in Tanzania. PLoS ONE. 2018;13(6):e0196616. doi: 10.1371/journal.pone.0196616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacCarthy S, Wagner Z, Mendoza-Graf A, Gutierrez CI, Samba C, Birungi J, …, Linnemayr S. A randomized controlled trial study of the acceptability, feasibility, and preliminary impact of SITA (SMS as an Incentive To Adhere): a mobile technology-based intervention informed by behavioral economics to improve ART adherence among youth in Uganda. BMC Infect Dis. 2020;20(1):1–10. [DOI] [PMC free article] [PubMed]
- 73.Maragh-Bass AC, Gamble T, Tolley EE. ‘Either you float or you drown:’ the role of social ties and stigma in lived experiences of the HIV care continuum in HPTN 065. AIDS Behav. 2020;24(9):2532–2545. doi: 10.1007/s10461-020-02811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spratt ES, Papa CE, Mueller M, Patel S, Killeen T, Maher E, et al. Using technology to improve adherence to HIV medications in transitional age youth: research reviewed, methods tried, lessons learned. J Gen Med (Dover) 2017;1(1):1002. [PMC free article] [PubMed] [Google Scholar]
- 75.Thirumurthy H, Masters SH, Rao S, Murray K, Prasad R, Zivin JG, et al. The effects of providing fixed compensation and lottery-based rewards on uptake of medical male circumcision in Kenya: a randomized trial. J Acquir Immune Defic Syndr. 2016;72 Suppl 4(Suppl 4):S299–305. doi: 10.1097/QAI.0000000000001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choko AT, Fielding K, Stallard N, Maheswaran H, Lepine A, Desmond N, et al. Investigating interventions to increase uptake of HIV testing and linkage into care or prevention for male partners of pregnant women in antenatal clinics in Blantyre, Malawi: study protocol for a cluster randomised trial. Trials. 2017;18(1):349. doi: 10.1186/s13063-017-2093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kranzer K, Simms V, Bandason T, Dauya E, McHugh G, Munyati S, et al. Economic incentives for HIV testing by adolescents in Zimbabwe: a randomised controlled trial. Lancet HIV. 2018;5(2):e79–e86. doi: 10.1016/S2352-3018(17)30176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ong JJ, Neke N, Wambura M, Kuringe E, Grund JM, Plotkin M, et al. Use of lotteries for the promotion of voluntary medical male circumcision service: a discrete-choice experiment among adult men in Tanzania. Med Decis Making. 2019;39(4):474–485. doi: 10.1177/0272989X19852095. [DOI] [PubMed] [Google Scholar]
- 79.Chamie G, Schaffer EM, Ndyabakira A, Emperador DM, Kwarisiima D, Camlin CS, et al. Comparative effectiveness of novel nonmonetary incentives to promote HIV testing. AIDS. 2018;32(11):1443–1451. doi: 10.1097/QAD.0000000000001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ndyabakira A, Getahun M, Byamukama A, Emperador D, Kabageni S, Marson K, et al. Leveraging incentives to increase HIV testing uptake among men: qualitative insights from rural Uganda. BMC Public Health. 2019;19(1):1763. doi: 10.1186/s12889-019-8073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kavanagh NM, Schaffer EM, Ndyabakira A, Marson K, Havlir DV, Kamya MR, et al. Planning prompts to promote uptake of HIV services among men: a randomised trial in rural Uganda. BMJ Glob Health. 2020;5(11):11. doi: 10.1136/bmjgh-2020-003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jennings-Mayo-Wilson L, Coleman J, Timbo F, Latkin C, Torres Brown ER, Butler AI, et al. Acceptability of a feasibility randomized clinical trial of a microenterprise intervention to reduce sexual risk behaviors and increase employment and HIV preventive practices (EMERGE) in young adults: a mixed methods assessment. BMC Public Health. 2020;20(1):1846. doi: 10.1186/s12889-020-09904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chamie G, Kwarisiima D, Ndyabakira A, Marson K, Camlin CS, Havlir DV, et al. Financial incentives and deposit contracts to promote HIV retesting in Uganda: a randomized trial. PLoS Med. 2021;18(5):e1003630. doi: 10.1371/journal.pmed.1003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chamie G, Ndyabakira A, Marson KG, Emperador DM, Kamya MR, Havlir DV, et al. A pilot randomized trial of incentive strategies to promote HIV retesting in rural Uganda. PLoS ONE. 2020;15(5):e0233600. doi: 10.1371/journal.pone.0233600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Getty CA, Subramaniam S, Holtyn AF, Jarvis BP, Rodewald A, Silverman K. Evaluation of a computer-based training program to teach adults at risk for HIV about pre-exposure prophylaxis. AIDS Educ Prev. 2018;30(4):287–300. doi: 10.1521/aeap.2018.30.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galarraga O, Harries J, Maughan-Brown B, Cooper D, Short SE, Lurie MN, et al. The Empower Nudge lottery to increase dual protection use: a proof-of-concept randomised pilot trial in South Africa. Reprod Health Matters. 2018;26(52):1510701. doi: 10.1080/09688080.2018.1510701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ssewamala FM, Ismayilova L, McKay M, Sperber E, Bannon W, Jr, Alicea S. Gender and the effects of an economic empowerment program on attitudes toward sexual risk-taking among AIDS-orphaned adolescent youth in Uganda. J Adolesc Health. 2010;46(4):372–378. doi: 10.1016/j.jadohealth.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inwani I, Chhun N, Agot K, Cleland CM, Buttolph J, Thirumurthy H, et al. High-yield HIV testing, facilitated linkage to care, and prevention for female youth in Kenya (GIRLS Study): implementation science protocol for a priority population. JMIR Res Protoc. 2017;6(12):e179. doi: 10.2196/resprot.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Macis M, Grunauer M, Gutierrez E, Izurieta R, Phan P, Reina Ortiz M, et al. Using incentives and nudging to improve non-targeted HIV testing in Ecuador: a randomized trial. AIDS Behav. 2021;25(8):2542–2550. doi: 10.1007/s10461-021-03215-x. [DOI] [PubMed] [Google Scholar]
- 90.Njuguna IN, Wagner AD, Omondi VO, Otieno VA, Neary J, Bosire R, et al. Financial incentives for pediatric HIV testing in Kenya. Pediatr Infect Dis J. 2018;37(11):1142–1144. doi: 10.1097/INF.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Njuguna IN, Wagner AD, Neary J, Omondi VO, Otieno VA, Orimba A, et al. Financial incentives to increase pediatric HIV testing: a randomized trial. AIDS. 2021;35(1):125–130. doi: 10.1097/QAD.0000000000002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Atkins DL, Wagner AD, Njuguna IN, Neary J, Omondi VO, et al. Financial incentives for pediatric HIV testing (FIT): caregiver insights on incentive mechanisms, focus populations, and acceptability for programmatic scale up. AIDS Behav. 2021;25(9):2661–2668. doi: 10.1007/s10461-021-03356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montoy JCC, Dow WH, Kaplan BC. Cash incentives versus defaults for HIV testing: a randomized clinical trial. PLoS ONE. 2018;13(7):e0199833. doi: 10.1371/journal.pone.0199833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagner Z, Montoy JCC, Drabo EF, Dow WH. Incentives versus defaults: cost-effectiveness of behavioral approaches for HIV screening. AIDS Behav. 2020;24(2):379–386. doi: 10.1007/s10461-019-02425-8. [DOI] [PubMed] [Google Scholar]
- 95.Mitchell JT, LeGrand S, Hightow-Weidman LB, McKellar MS, Kashuba AD, Cottrell M, et al. Smartphone-based contingency management intervention to improve pre-exposure prophylaxis adherence: pilot trial. JMIR Mhealth Uhealth. 2018;6(9):e10456. doi: 10.2196/10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kohler HP, Thornton R. Conditional cash transfers and HIV/AIDS prevention: unconditionally promising? World Bank Econ Rev. 2012;26(2):165–190. doi: 10.1093/wber/lhr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Walque D, Dow WH, Nathan R, Abdul R, Abilahi F, Gong E, et al. Incentivising safe sex: a randomised trial of conditional cash transfers for HIV and sexually transmitted infection prevention in rural Tanzania. BMJ Open. 2012;2:e000747. doi: 10.1136/bmjopen-2011-000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beadnell B, Baker S, Knox K, Stielstra S, Morrison DM, Degooyer E, et al. The influence of psychosocial difficulties on women’s attrition in an HIV/STD prevention program. AIDS Care. 2003;15(6):807–820. doi: 10.1080/09540120310001618658. [DOI] [PubMed] [Google Scholar]
- 99.Mathenjwa T, Kim HY, Zuma T, Shahmanesh M, Seeley J, Matthews P, et al. Home-based intervention to test and start (HITS) protocol: a cluster-randomized controlled trial to reduce HIV-related mortality in men and HIV incidence in women through increased coverage of HIV treatment. BMC Public Health. 2019;19(1):969. doi: 10.1186/s12889-019-7277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anand T, Nitpolprasert C, Ananworanich J, Pakam C, Nonenoy S, Jantarapakde J, et al. Innovative strategies using communications technologies to engage gay men and other men who have sex with men into early HIV testing and treatment in Thailand. J Virus Erad. 2015;1(2):111–115. doi: 10.1016/S2055-6640(20)30483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tanser FC, Kim HY, Mathenjwa T, Shahmanesh M, Seeley J, Matthews P, et al. Home-Based Intervention to Test and Start (HITS): a community-randomized controlled trial to increase HIV testing uptake among men in rural South Africa. J Int AIDS Soc. 2021;24(2):e25665. doi: 10.1002/jia2.25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thirumurthy H, Masters SH, Rao S, Bronson MA, Lanham M, Omanga E, et al. Effect of providing conditional economic compensation on uptake of voluntary medical male circumcision in Kenya: a randomized clinical trial. JAMA. 2014;312(7):703–711. doi: 10.1001/jama.2014.9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Silverman K, Holtyn AF, Rodewald AM, Siliciano RF, Jarvis BP, Subramaniam S, et al. Incentives for viral suppression in people living with HIV: a randomized clinical trial. AIDS Behav. 2019;23(9):2337–2346. doi: 10.1007/s10461-019-02592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shelus V, Taylor J, Greene E, Stanton J, Pack A, Tolley EE, et al. It’s all in the timing: acceptability of a financial incentive intervention for linkage to HIV care in the HPTN 065 (TLC-Plus) study. PLoS ONE. 2018;13(2):e0191638. doi: 10.1371/journal.pone.0191638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rigsby MO, Rosen MI, Beauvais JE, Cramer JA, Rainey PM, O'Malley SS, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15(12):841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farber S, Tate J, Frank C, Ardito D, Kozal M, Justice AC, et al. A study of financial incentives to reduce plasma HIV RNA among patients in care. AIDS Behav. 2013;17(7):2293–2300. doi: 10.1007/s10461-013-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DeFulio A, Devoto A, Traxler H, Cosottile D, Fingerhood M, Nuzzo P, et al. Smartphone-based incentives for promoting adherence to antiretroviral therapy: a randomized controlled trial. Prev Med Rep. 2021;21:101318. doi: 10.1016/j.pmedr.2021.101318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alsan M, Beshears J, Armstrong WS, Choi JJ, Madrian BC, Nguyen MLT, et al. A commitment contract to achieve virologic suppression in poorly adherent patients with HIV/AIDS. AIDS. 2017;31(12):1765–1769. doi: 10.1097/QAD.0000000000001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson S, Jenner E, Lass K, Burgess S. Perspectives of HIV clinic staff on the implementation of a client financial incentives program targeting viral suppression. J Assoc Nurses AIDS Care. 2017;28(5):770–783. doi: 10.1016/j.jana.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 110.Foster C, McDonald S, Frize G, Ayers S, Fidler S. “Payment by results”–financial incentives and motivational interviewing, adherence interventions in young adults with perinatally acquired HIV-1 infection: a pilot program. AIDS Patient Care STDS. 2014;28(1):28–32. doi: 10.1089/apc.2013.0262. [DOI] [PubMed] [Google Scholar]
- 111.Saleska JL, Turner AN, Gallo MF, Shoben A, Kawende B, Ravelomanana NLR, et al. Role of temporal discounting in a conditional cash transfer (CCT) intervention to improve engagement in the prevention of mother-to-child transmission (PMTCT) cascade. BMC Public Health. 2021;21(1):477. doi: 10.1186/s12889-021-10499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yotebieng M, Thirumurthy H, Moracco KE, Kawende B, Chalachala JL, Wenzi LK, et al. Conditional cash transfers and uptake of and retention in prevention of mother-to-child HIV transmission care: a randomised controlled trial. Lancet HIV. 2016;3(2):e85–93. doi: 10.1016/S2352-3018(15)00247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ekwunife OI, Ofomata CJ, Okafor CE, Anetoh MU, Kalu SO, Ele PU, et al. Cost-effectiveness and feasibility of conditional economic incentives and motivational interviewing to improve HIV health outcomes of adolescents living with HIV in Anambra State, Nigeria. BMC Health Serv Res. 2021;21(1):685. doi: 10.1186/s12913-021-06718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adamson B, El-Sadr W, Dimitrov D, Gamble T, Beauchamp G, Carlson JJ, et al. The cost-effectiveness of financial incentives for viral suppression: HPTN 065 study. Value Health. 2019;22(2):194–202. doi: 10.1016/j.jval.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tolley EE, Taylor J, Pack A, Greene E, Stanton J, Shelus V, et al. The role of financial incentives along the antiretroviral therapy adherence continuum: a qualitative sub-study of the HPTN 065 (TLC-Plus) study. AIDS Behav. 2018;22(1):245–257. doi: 10.1007/s10461-017-1821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siedner MJ, Santorino D, Lankowski AJ, Kanyesigye M, Bwana MB, Haberer JE, et al. A combination SMS and transportation reimbursement intervention to improve HIV care following abnormal CD4 test results in rural Uganda: a prospective observational cohort study. BMC Med. 2015;13(1):160. doi: 10.1186/s12916-015-0397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fahey CA, Njau PF, Dow WH, Kapologwe NA, McCoy SI. Effects of short-term cash and food incentives on food insecurity and nutrition among HIV-infected adults in Tanzania. AIDS. 2019;33(3):515–524. doi: 10.1097/QAD.0000000000002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brantley AD, Burgess S, Bickham J, Wendell D, Gruber D. Using financial incentives to improve rates of viral suppression and engagement in care of patients receiving HIV care at 3 health clinics in Louisiana: the Health Models Program, 2013–2016. Public Health Rep. 2018;133(2_suppl):75S–86S. doi: 10.1177/0033354918793096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Linnemayr S, Stecher C. Behavioral economics matters for HIV research: the impact of behavioral biases on adherence to antiretrovirals (ARVs) AIDS Behav. 2015;19(11):2069–2075. doi: 10.1007/s10461-015-1076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jemison D, Jackson S, Oni O, Cats-Baril D, Thomas-Smith S, Batchelder A, et al. Pilot randomized controlled trial of a syndemics intervention with HIV-positive, cocaine-using women. AIDS Behav. 2019;23(9):2467–2476. doi: 10.1007/s10461-019-02625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elul B, Lahuerta M, Abacassamo F, Lamb MR, Ahoua L, McNairy ML, et al. A combination strategy for enhancing linkage to and retention in HIV care among adults newly diagnosed with HIV in Mozambique: study protocol for a site-randomized implementation science study. BMC Infect Dis. 2014;14:549. doi: 10.1186/s12879-014-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sutton R, Lahuerta M, Abacassamo F, Ahoua L, Tomo M, Lamb MR, et al. Feasibility and acceptability of health communication interventions within a combination intervention strategy for improving linkage and retention in HIV care in Mozambique. J Acquir Immune Defic Syndr. 2017;74 Suppl 1:S29–S36. doi: 10.1097/QAI.0000000000001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnabas RV, van Heerden A, McConnell M, Szpiro AA, Krows ML, Schaafsma TT, et al. Lottery incentives have short-term impact on ART initiation among men: results from a randomized pilot study. J Int AIDS Soc. 2020;23 Suppl 2:e25519. doi: 10.1002/jia2.25519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MacCarthy S, Mendoza-Graf A, Huang H, Mukasa B, Linnemayr S. Supporting Adolescents to Adhere (SATA): lessons learned from an intervention to achieve medication adherence targets among youth living with HIV in Uganda. Child Youth Serv Rev. 2019;102:56–62. doi: 10.1016/j.childyouth.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carrico AW, Neilands TB, Dilworth SE, Evans JL, Gomicronmez W, Jain JP, et al. Randomized controlled trial of a positive affect intervention to reduce HIV viral load among sexual minority men who use methamphetamine. J Int AIDS Soc. 2019;22(12):e25436. doi: 10.1002/jia2.25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clouse K, Mongwenyana C, Musina M, Bokaba D, Long L, Maskew M, et al. Acceptability and feasibility of a financial incentive intervention to improve retention in HIV care among pregnant women in Johannesburg, South Africa. AIDS Care. 2018;30(4):453–460. doi: 10.1080/09540121.2017.1394436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Packel L, Fahey C, Njau P, McCoy SI. Implementation science using proctor’s framework and an adaptation of the multiphase optimization strategy: optimizing a financial incentive intervention for HIV treatment adherence in Tanzania. J Acquir Immune Defic Syndr. 2019;82 Suppl 3:S332–S338. doi: 10.1097/QAI.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCoy SI, Fahey C, Rao A, Kapologwe N, Njau PF, Bautista-Arredondo S. Pilot study of a multi-pronged intervention using social norms and priming to improve adherence to antiretroviral therapy and retention in care among adults living with HIV in Tanzania. PLoS ONE. 2017;12(5):e0177394. doi: 10.1371/journal.pone.0177394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang C, Tucker JD, Liu C, Zheng H, Tang W, Ling L. Condom use social norms and self-efficacy with different kinds of male partners among Chinese men who have sex with men: results from an online survey. BMC Public Health. 2018;18(1):1175. doi: 10.1186/s12889-018-6090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Latkin CA, Kuramoto SJ, Davey-Rothwell MA, Tobin KE. Social norms, social networks, and HIV risk behavior among injection drug users. AIDS Behav. 2010;14(5):1159–1168. doi: 10.1007/s10461-009-9576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Latkin CA, Forman V, Knowlton A, Sherman S. Norms, social networks, and HIV-related risk behaviors among urban disadvantaged drug users. Soc Sci Med. 2003;56(3):465–476. doi: 10.1016/S0277-9536(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 132.Weihs M, Meyer-Weitz A, Baasner-Weihs F. The influence of lotteries on employees’ workplace HIV testing behaviour. Afr J AIDS Res. 2018;17(1):9–21. doi: 10.2989/16085906.2017.1377266. [DOI] [PubMed] [Google Scholar]
- 133.Leidel S, Leslie G, Boldy D, Davies A, Girdler S. ‘We didn’t have to dance around it’: opt-out HIV testing among homeless and marginalised patients. Aust J Prim Health. 2017;23(3):278–283. doi: 10.1071/PY16120. [DOI] [PubMed] [Google Scholar]
- 134.Mayo-Wilson LJ, Glass NE, Ssewamala FM, Linnemayr S, Coleman J, Timbo F, et al. Microenterprise intervention to reduce sexual risk behaviors and increase employment and HIV preventive practices in economically-vulnerable African-American young adults (EMERGE): protocol for a feasibility randomized clinical trial. Trials. 2019;20(1):439. doi: 10.1186/s13063-019-3529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yotebieng M, Moracco KE, Thirumurthy H, Edmonds A, Tabala M, Kawende B, et al. Conditional cash transfers improve retention in PMTCT services by mitigating the negative effect of not having money to come to the clinic. J Acquir Immune Defic Syndr. 2017;74(2):150–157. doi: 10.1097/QAI.0000000000001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zou H, Wu Z, Yu J, Li M, Ablimit M, Li F, et al. Internet-facilitated, voluntary counseling and testing (VCT) clinic-based HIV testing among men who have sex with men in China. PLoS ONE. 2013;8(2):e51919. doi: 10.1371/journal.pone.0051919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Subramaniam S, Getty C-A, Holtyn AF, Rodewald A, Katz B, Jarvis BP, et al. Evaluation of a computer-based HIV education program for adults living with HIV. AIDS Behav. 2019;23(11):3152–3164. doi: 10.1007/s10461-019-02474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee, Lee JE, Moskowitz JT, Feaster DJ, Neilands TB, Dilworth SE, et al. An autoregressive cross-lagged model unraveling co-occurring stimulant use and HIV: results from a randomized controlled trial. Drug Alcohol Depend. 2021;225:108752. doi: 10.1016/j.drugalcdep.2021.108752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ong JJ, Chow EPF, Read D, Taj U, Lee D, Vlaev I. Nudgeathons to control HIV: designing strategies using behavioural economics. AIDS. 2020;34(15):2337–2340. doi: 10.1097/QAD.0000000000002693. [DOI] [PubMed] [Google Scholar]
- 140.Zou H, Wu Z, Yu J, Li M, Ablimit M, Li F, et al. Internet-facilitated, voluntary counseling and testing (VCT) clinic-based HIV testing among men who have sex with men in China. PLoS ONE. 2013;8(2):e51919. doi: 10.1371/journal.pone.0051919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kavanagh NM, Schaffer EM, Ndyabakira A, Marson K, Havlir DV, Kamya MR, et al. Planning prompts to promote uptake of HIV services among men: a randomised trial in rural Uganda. BMJ Glob Health. 2020;5(11):11. doi: 10.1136/bmjgh-2020-003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mitchell JT, LeGrand S, Hightow-Weidman LB, McKellar MS, Kashuba AD, Cottrell M, et al. Smartphone-based contingency management intervention to improve pre-exposure prophylaxis adherence: pilot trial. JMIR Mhealth Uhealth. 2018;6(9):e10456. doi: 10.2196/10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCoy SI, Fahey C, Rao A, Kapologwe N, Njau PF, Bautista-Arredondo S. Pilot study of a multi-pronged intervention using social norms and priming to improve adherence to antiretroviral therapy and retention in care among adults living with HIV in Tanzania. PLoS ONE. 2017;12(5):e0177394. doi: 10.1371/journal.pone.0177394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gambone GF, Feldman MB, Thomas-Ferraioli AY, Shubert V, Ghose T. Integrating financial incentives for viral load suppression into HIV care coordination programs: considerations for development and implementation. J Public Health Manag Pract. 2020;26(5):471–480. doi: 10.1097/PHH.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 145.Ghose T, Shubert V, Chaudhuri S, Poitevien V, Updyke A. Are financial incentives appropriate means of encouraging medication adherence among people living with HIV? AMA J Ethics. 2021;23(5):E394–401. doi: 10.1001/amajethics.2021.394. [DOI] [PubMed] [Google Scholar]
- 146.Holstad MM, DiIorio C, Magowe MK. Motivating HIV positive women to adhere to antiretroviral therapy and risk reduction behavior: the KHARMA Project. Online J Issues Nurs. 2006;11(1):5. doi: 10.3912/OJIN.Vol11No01Man04. [DOI] [PubMed] [Google Scholar]
- 147.Bien-Gund CH, Ho JI, Bair EF, Marcus N, Choi RJ, Szep Z, et al. Brief report: Financial incentives and real-time adherence monitoring to promote daily adherence to HIV treatment and viral suppression among people living with HIV: a pilot study. J Acquir Immune Defic Syndr. 2021;87(1):688–692. doi: 10.1097/QAI.0000000000002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rigsby MO, Rosen MI, Beauvais JE, Cramer JA, Rainey PM, O'Malley SS, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15(12):841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Edwards GG, Reback CJ, Cunningham WE, Hilliard CL, McWells C, Mukherjee S, et al. Mobile-enhanced prevention support study for men who have sex with men and transgender women leaving jail: protocol for a randomized controlled trial. JMIR Res Protoc. 2020;9(9):e18106. doi: 10.2196/18106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Inwani I, Chhun N, Agot K, Cleland CM, Buttolph J, Thirumurthy H, et al. High-yield HIV testing, facilitated linkage to care, and prevention for female youth in Kenya (GIRLS Study): implementation science protocol for a priority population. JMIR Res Protoc. 2017;6(12):e179. doi: 10.2196/resprot.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thomas R, Skovdal M, Galizzi MM, Schaefer R, Moorhouse L, Nyamukapa C, et al. Improving risk perception and uptake of voluntary medical male circumcision with peer-education sessions and incentives, in Manicaland, East Zimbabwe: study protocol for a pilot randomised trial. Trials. 2020;21(1):108. doi: 10.1186/s13063-020-4048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee J-Y, Lee JE, Moskowitz JT, Feaster DJ, Neilands TB, Dilworth SE, et al. An autoregressive cross-lagged model unraveling co-occurring stimulant use and HIV: results from a randomized controlled trial. Drug Alcohol Depend. 2021;225:N.PAG-N.PAG. [DOI] [PMC free article] [PubMed]
- 153.Carrico AW, Neilands TB, Dilworth SE, Evans JL, Gomez W, Jain JP, et al. Randomized controlled trial of a positive affect intervention to reduce HIV viral load among sexual minority men who use methamphetamine. J Int AIDS Soc. 2019;22(12):e25436. doi: 10.1002/jia2.25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Greene E, Pack A, Stanton J, Shelus V, Tolley EE, Taylor J, et al. “It makes you feel like someone cares” acceptability of a financial incentive intervention for HIV viral suppression in the HPTN 065 (TLC-Plus) study. PLoS ONE. 2017;12(2):e0170686. doi: 10.1371/journal.pone.0170686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.MacCarthy S, Mendoza-Graf A, Huang H, Mukasa B, Linnemayr S. Supporting Adolescents to Adhere (SATA): lessons learned from an intervention to achieve medication adherence targets among youth living with HIV in Uganda. Child Youth Serv Rev. 2019;102:56–62. doi: 10.1016/j.childyouth.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chamie G, Kwarisiima D, Ndyabakira A, Marson K, Camlin CS, Havlir DV, et al. Financial incentives and deposit contracts to promote HIV retesting in Uganda: a randomized trial. PLoS Med / Public Libr Sci. 2021;18(5):e1003630. doi: 10.1371/journal.pmed.1003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chamie G, Ndyabakira A, Marson KG, Emperador DM, Kamya MR, Havlir DV, et al. A pilot randomized trial of incentive strategies to promote HIV retesting in rural Uganda. PLoS ONE. 2020;15(5):e0233600. doi: 10.1371/journal.pone.0233600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ssewamala FM, Ismayilova L, McKay M, Sperber E, Bannon W, Jr, Alicea S. Gender and the effects of an economic empowerment program on attitudes toward sexual risk-taking among AIDS-orphaned adolescent youth in Uganda. J Adolesc Health. 2010;46(4):372–378. doi: 10.1016/j.jadohealth.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Taylor NK, Buttenheim AM. Improving utilization of and retention in PMTCT services: can behavioral economics help? BMC Health Serv Res. 2013;13:406. doi: 10.1186/1472-6963-13-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Macis M, Grunauer M, Gutierrez E, Izurieta R, Phan P, Reina Ortiz M, et al. Using incentives and nudging to improve non-targeted HIV testing in Ecuador: a randomized trial. AIDS Behav. 2021;25(8):2542–2550. doi: 10.1007/s10461-021-03215-x. [DOI] [PubMed] [Google Scholar]
- 161.Choko AT, Fielding K, Johnson CC, Kumwenda MK, Chilongosi R, Baggaley RC, et al. Partner-delivered HIV self-test kits with and without financial incentives in antenatal care and index patients with HIV in Malawi: a three-arm, cluster-randomised controlled trial. Lancet Glob Health. 2021;9(7):e977–e988. doi: 10.1016/S2214-109X(21)00175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Linnemayr S, MacCarthy S, Kim A, Giguere R, Carballo-Dieguez A, Barreras JL. Behavioral economics-based incentives supported by mobile technology on HIV knowledge and testing frequency among Latino/a men who have sex with men and transgender women: protocol for a randomized pilot study to test intervention feasibility and acceptability. Trials. 2018;19(1):540. doi: 10.1186/s13063-018-2867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Galarraga O, Harries J, Maughan-Brown B, Cooper D, Short SE, Lurie MN, et al. The Empower Nudge lottery to increase dual protection use: a proof-of-concept randomised pilot trial in South Africa. Reprod Health Matters. 2018;26(52):1510701. doi: 10.1080/09688080.2018.1510701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choko AT, Corbett EL, Stallard N, Maheswaran H, Lepine A, Johnson CC, et al. HIV self-testing alone or with additional interventions, including financial incentives, and linkage to care or prevention among male partners of antenatal care clinic attendees in Malawi: an adaptive multi-arm, multi-stage cluster randomised trial. PLoS Med / Public Libr Sci. 2019;16(1):e1002719. doi: 10.1371/journal.pmed.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thornton RL. The demand for, and impact of, learning HIV status. Am Econ Rev. 2008;98(5):1829–1863. doi: 10.1257/aer.98.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Das A, George B, Ranebennur V, Parthasarathy MR, Shreenivas GS, Todankar P, et al. Getting to the first 90: incentivized peer mobilizers promote HIV testing services to men who have sex with men using social media in Mumbai, India. Glob Health Sci Pract. 2019;7(3):469–477. doi: 10.9745/GHSP-D-19-00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kohler HP, Thornton R. Conditional cash transfers and HIV/AIDS prevention: unconditionally promising? World Bank Econ Rev. 2012;26(2):165–190. doi: 10.1093/wber/lhr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Choko AT, Fielding K, Stallard N, Maheswaran H, Lepine A, Desmond N, et al. Investigating interventions to increase uptake of HIV testing and linkage into care or prevention for male partners of pregnant women in antenatal clinics in Blantyre, Malawi: study protocol for a cluster randomised trial. Trials. 2017;18(1):349. doi: 10.1186/s13063-017-2093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Foster C, McDonald S, Frize G, Ayers S, Fidler S. “Payment by results”–financial incentives and motivational interviewing, adherence interventions in young adults with perinatally acquired HIV-1 infection: a pilot program. AIDS Patient Care STDS. 2014;28(1):28–32. doi: 10.1089/apc.2013.0262. [DOI] [PubMed] [Google Scholar]
- 170.Linnemayr S, Stecher C, Saya U, MacCarthy S, Wagner Z, Jennings L, et al. Behavioral Economics Incentives to Support HIV Treatment Adherence (BEST): protocol for a randomized controlled trial in Uganda. Trials. 2020;21(1):9. doi: 10.1186/s13063-019-3795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thirumurthy H, Ndyabakira A, Marson K, Emperador D, Kamya M, Havlir D, et al. Financial incentives for achieving and maintaining viral suppression among HIV-positive adults in Uganda: a randomised controlled trial. Lancet HIV. 2019;6(3):e155–e163. doi: 10.1016/S2352-3018(18)30330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Galarraga O, Enimil A, Bosomtwe D, Cao W, Barker DH. Group-based economic incentives to improve adherence to antiretroviral therapy among youth living with HIV: safety and preliminary efficacy from a pilot trial. Vulnerable Child Youth Stud. 2020;15(3):257–268. doi: 10.1080/17450128.2019.1709678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.DeFulio A, Devoto A, Traxler H, Cosottile D, Fingerhood M, Nuzzo P, et al. Smartphone-based incentives for promoting adherence to antiretroviral therapy: a randomized controlled trial. Prev Med Rep. 2021;21:101318. doi: 10.1016/j.pmedr.2021.101318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.El-Sadr WM, Beauchamp G, Hall HI, Torian LV, Zingman BS, Lum G, et al. Brief report: Durability of the effect of financial incentives on HIV viral load suppression and continuity in care: HPTN 065 study. J Acquir Immune Defic Syndr. 2019;81(3):300–303. doi: 10.1097/QAI.0000000000001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.El-Sadr WM, Donnell D, Beauchamp G, Hall HI, Torian LV, Zingman B, et al. Financial incentives for linkage to care and viral suppression among HIV-positive patients: a randomized clinical trial (HPTN 065) JAMA Intern Med. 2017;177(8):1083–1092. doi: 10.1001/jamainternmed.2017.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farber S, Tate J, Frank C, Ardito D, Kozal M, Justice A, et al. A study of financial incentives to reduce plasma HIV RNA among patients in care. AIDS Behav. 2013;17(7):2293–2300. doi: 10.1007/s10461-013-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stitzer ML, Gukasyan N, Matheson T, Sorensen JL, Feaster DJ, Duan R, et al. Enhancing patient navigation with contingent financial incentives for substance use abatement in persons with HIV and substance use. Psychol Addict Behav. 2020;34(1):23–30. doi: 10.1037/adb0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gamble T, Branson B, Donnell D, Hall HI, King G, Cutler B, et al. Design of the HPTN 065 (TLC-Plus) study: a study to evaluate the feasibility of an enhanced test, link-to-care, plus treat approach for HIV prevention in the United States. Clin Trials. 2017;14(4):322–332. doi: 10.1177/1740774517711682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Javanbakht M, Prosser P, Grimes T, Weinstein M, Farthing C. Efficacy of an individualized adherence support program with contingent reinforcement among nonadherent HIV-positive patients: results from a randomized trial. J Int Assoc Phys AIDS Care. 2006;5(4):143–150. doi: 10.1177/1545109706291706. [DOI] [PubMed] [Google Scholar]
- 180.Jemison D, Jackson S, Oni O, Cats-Baril D, Thomas-Smith S, Batchelder A, et al. Pilot randomized controlled trial of a syndemics intervention with HIV-positive, cocaine-using women. AIDS Behav. 2019;23(9):2467–2476. doi: 10.1007/s10461-019-02625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Silverman K, Holtyn AF, Rodewald AM, Siliciano RF, Jarvis BP, Subramaniam S, et al. Incentives for viral suppression in people living with HIV: a randomized clinical trial. AIDS Behav. 2019;23(9):2337–2346. doi: 10.1007/s10461-019-02592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stitzer ML, Gukasyan N, Matheson T, Sorensen JL, Feaster DJ, Duan R, et al. Enhancing patient navigation with contingent financial incentives for substance use abatement in persons with HIV and substance use. Psychol Addict Behav. 2020;34(1):23–30. doi: 10.1037/adb0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Haukoos JS, Witt MD, Coil CJ, Lewis RJ. The effect of financial incentives on adherence with outpatient human immunodeficiency virus testing referrals from the emergency department. Acad Emerg Med. 2005;12(7):617–621. doi: 10.1197/j.aem.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 184.McNairy ML, Lamb MR, Gachuhi AB, Nuwagaba-Biribonwoha H, Burke S, Mazibuko S, et al. Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: the Link4Health cluster randomized trial. PLoS Med / Public Libr Sci. 2017;14(11):e1002420. doi: 10.1371/journal.pmed.1002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fahey CA, Njau PF, Katabaro E, Mfaume RS, Ulenga N, Mwenda N, et al. Financial incentives to promote retention in care and viral suppression in adults with HIV initiating antiretroviral therapy in Tanzania: a three-arm randomised controlled trial. Lancet HIV. 2020;7(11):e762–e771. doi: 10.1016/S2352-3018(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Elul B, Lahuerta M, Abacassamo F, Lamb MR, Ahoua L, McNairy ML, et al. A combination strategy for enhancing linkage to and retention in HIV care among adults newly diagnosed with HIV in Mozambique: study protocol for a site-randomized implementation science study. BMC Infect Dis. 2014;14:549. doi: 10.1186/s12879-014-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sherman SG, Srikrishnan AK, Rivett KA, Liu SH, Solomon S, Celentano DD. Acceptability of a microenterprise intervention among female sex workers in Chennai, India. AIDS Behav. 2010;14(3):649–657. doi: 10.1007/s10461-010-9686-z. [DOI] [PubMed] [Google Scholar]
- 188.Mayo-Wilson LJ, Glass NE, Ssewamala FM, Linnemayr S, Coleman J, Timbo F, et al. Microenterprise intervention to reduce sexual risk behaviors and increase employment and HIV preventive practices in economically-vulnerable African-American young adults (EMERGE): protocol for a feasibility randomized clinical trial. Trials. 2019;20(1):439. doi: 10.1186/s13063-019-3529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tanser FC, Kim HY, Mathenjwa T, Shahmanesh M, Seeley J, Matthews P, et al. Home-Based Intervention to Test and Start (HITS): a community-randomized controlled trial to increase HIV testing uptake among men in rural South Africa. J Int AIDS Soc. 2021;24(2):e25665. doi: 10.1002/jia2.25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Getty CA, Subramaniam S, Holtyn AF, Jarvis BP, Rodewald A, Silverman K. Evaluation of a computer-based training program to teach adults at risk for HIV about pre-exposure prophylaxis. AIDS Educ Prev. 2018;30(4):287–300. doi: 10.1521/aeap.2018.30.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Godlonton S, Thornton R. Peer effects in learning HIV results. J Dev Econ. 2012;97(1):118–129. doi: 10.1016/j.jdeveco.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Czaicki NL, Dow WH, Njau PF, McCoy SI. Do incentives undermine intrinsic motivation? Increases in intrinsic motivation within an incentive-based intervention for people living with HIV in Tanzania. PLoS ONE. 2018;13(6):e0196616. doi: 10.1371/journal.pone.0196616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beima-Sofie K, Begnel ER, Golden MR, Moore A, Ramchandani M, Dombrowski JC. “It’s me as a person, not me the disease”: patient perceptions of an HIV care model designed to engage persons with complex needs. AIDS Patient Care STDS. 2020;34(6):267–274. doi: 10.1089/apc.2019.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ekwunife OI, Ofomata CJ, Okafor CE, Anetoh MU, Kalu SO, Ele PU, et al. Cost-effectiveness and feasibility of conditional economic incentives and motivational interviewing to improve HIV health outcomes of adolescents living with HIV in Anambra State, Nigeria. BMC Health Serv Res. 2021;21(1):685. doi: 10.1186/s12913-021-06718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Montoy JC, Dow WH, Kaplan BC. Patient choice in opt-in, active choice, and opt-out HIV screening: randomized clinical trial. BMJ. 2016;532:h6895. doi: 10.1136/bmj.h6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.MacCarthy S, Wagner Z, Mendoza-Graf A, Gutierrez CI, Samba C, Birungi J, et al. A randomized controlled trial study of the acceptability, feasibility, and preliminary impact of SITA (SMS as an Incentive To Adhere): a mobile technology-based intervention informed by behavioral economics to improve ART adherence among youth in Uganda. BMC Infect Dis. 2020;20(1):173. doi: 10.1186/s12879-020-4896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lin Y, Osman M, Ashcroft R. Nudge: concept, effectiveness, and ethics. Basic Appl Soc Psychol. 2017;39(12):1–14. [Google Scholar]
- 198.Matjasko JL, Cawley JH, Yokum DV. Applying behavioral economics to public health policy. Am J Prev Med. 2016;50(5):13–19. doi: 10.1016/j.amepre.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Voyer BG. ‘Nudging’ behaviours in healthcare: insights from behavioural economics. Br J Healthc Manag. 2015;21(3):130–135. doi: 10.12968/bjhc.2015.21.3.130. [DOI] [Google Scholar]
- 200.Blumenthal-Barby JS, Burroughs H. Seeking better health care outcomes: the ethics of using the “nudge”. Am J Bioeth. 2012;12(2):1–10. doi: 10.1080/15265161.2011.634481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are presented in the manuscript and online supplementary materials. Any further details can be obtained by contacting the corresponding author.
Not applicable.