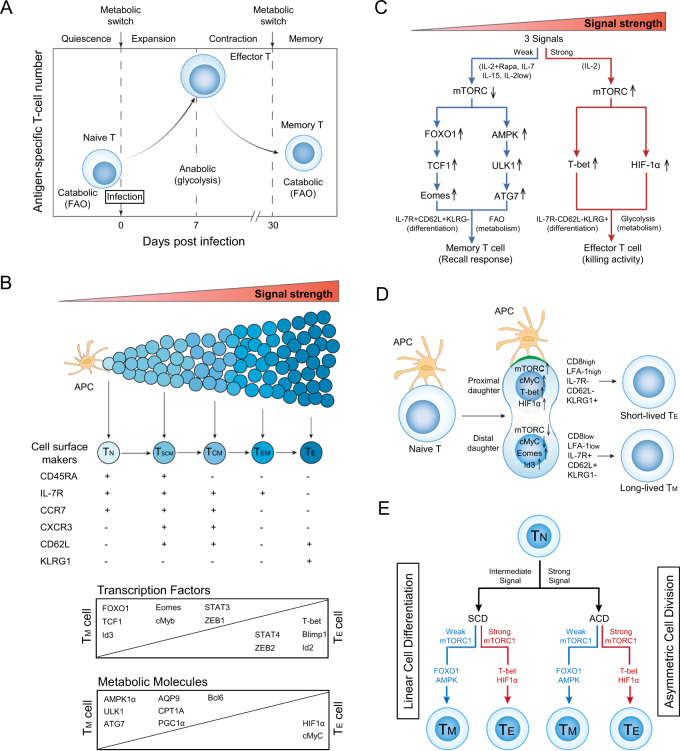
CD8+ effector T (TE) cells play a critical role in immunity against infections. In response to a pathogenic stimulus, antigen-presenting cells (APCs) deliver three signals [via T-cell receptor (TCR), costimulation, and cytokines] to naïve CD8+ T cells, stimulating their entry into a developmental program characterized by T-cell expansion followed by a contraction phase. During the contraction phase, 90–95% of IL-7R-CD62L-KLRG1+ TE cells undergo cell apoptosis, and the remaining 5–10% of T cells differentiate into IL-7R+CD62L+KLRG1- memory T (TM) cells (Fig. 1A) [1]. In this TM-cell population, the best characterized subsets are CD45RA+CCR7+IL-7R+CD62L+KLRG1- stem cell-like TM (TSCM), CCR7+IL-7R+CD62L+KLRG1- central TM (TCM), and CCR7-IL-7R+CD62L-KLRG1- effector TM (TEM) cells (Fig. 1B). A central question in fundamental immunology is the origin of the long-lived TM cells that confer protection against secondary infection. Two well-known models have been proposed to explain the origin of TM cells: the “linear cell differentiation” [2] and “asymmetric cell division” models [3].
Fig. 1.
mTORC1 signaling with distinct strengths controls T-cell memory via the transcriptional FOXO1 and metabolic AMPKα1 pathways in both the linear cell differentiation and asymmetric cell division models. A Metabolic changes in CD8+ T cells at various stages of an acute infection. In response to a pathogenic stimulus, naïve CD8+ T cells enter a developmental program characterized initially by T-cell expansion and then by a subsequent contraction phase. During this latter phase, the majority of effector T (TE) cells undergo apoptosis, while the remaining minority of T cells differentiate into long-lived memory T (TM) cells. To meet the bioenergetic demand during the expansion phase, naïve T cells switch from mitochondrial respiration to glycolysis. During contraction to the memory phase, the metabolic program reverts to catabolic fatty acid oxidation as TE cells gradually transition into TM cells. B In the linear cell differentiation model, signals provided at distinct strengths by the antigen-presenting cell (APC) control naïve T-cell differentiation into short-lived TE and long-lived TM cells, including stem cell-like TM (TSCM), central TM (TCM), and effector TM (TEM) cells. The phenotypic attributes and expression levels of transcription factors and metabolic molecules controlling these cellular phenotypes are illustrated. C Schematic diagram of the distinct strengths of cytokine signaling (strong and weak) that control naïve T-cell differentiation into TE and TM cells. A strong cytokine (IL-2, regular dose 100 U/ml) signal stimulates strong mTORC1 (mTORC1Strong) signaling, leading to the formation of TE cells via transcriptional T-bet and metabolic HIF-1α pathways. In contrast, weak cytokine [IL-7, IL-15, low dose (10 U/ml) of IL-2 (IL-2Low) and IL-2 (regular dose) plus rapamycin (IL-2+Rapa)] signals stimulate weak mTORC1 (mTORC1Weak) signaling, leading to the formation of TM cells via transcriptional FOXO1-TCF1-Eomes and metabolic AMPK-ULK1-ATG7 pathways. D In the asymmetric cell division model, the proximal CD8HighLFA-1HighKLRG1+IL-7R-CD62L- daughter cell displays upregulation of mTORC1, cMyC, T-bet, and HIF-1α and adopts a CD8+ TE-cell fate during the first cell division. The distal CD8LowLFA-1LowKLRG1-IL-7R+CD62L+ daughter cell shows up- and downregulation of Id3/Eomes and mTORC1/cMyC, respectively, and is destined to become a TM cell. The green region at the contact interface between the APC and engaged T cell represents the immunological synapse. E Schematic diagram illustrating in both the asymmetric cell division (ACD) model with ACD and linear cell differentiation (LCD) model with symmetric cell division (SCD), how the mTORC1Weak signal promotes T-cell memory via transcriptional FOXO1 and metabolic AMPK pathways and the mTORC1Strong signal induces TE cell formation via transcriptional T-bet and metabolic HIF-1α pathways
The “linear cell differentiation (LCD)” or “signal strength” model was originally proposed by Sallusto’s group in 2000 and posits that strong and weak strengths of the aforementioned three signals control T-cell differentiation into short-lived TE and long-lived TM cells, respectively (Fig. 1B) [2]. Subsequent evidence has accumulated in support of this model, with distinct strengths of TCR or antigen (high and low affinities) and IL-2 (high and low doses) signals favoring TE and TM cell differentiation, respectively [4, 5]. Various transcription factors crucial to controlling T-cell phenotypes have been identified, with forkhead box-O-1 (FOXO1), FOXO1-regulated T-cell factor-1 (TCF1), inhibition of DNA-binding protein-3 (Id3) and Eomes favoring TM cell differentiation and T-bet and Id2 favoring TE cell differentiation (Fig. 1B) [1]. Adenosine monophosphate-activated protein kinase-α1 (AMPKα1) is a conserved energy sensor that plays central role in controlling cellular metabolism and survival [6]. AMPKα1 stimulates mitochondrial biogenesis and fatty acid oxidation (FAO) to support TM-cell differentiation by increasing the abundance of Unc-51-like autophagy-activating kinase-1 (ULK1), autophagy-related gene-7 (ATG7), proliferator-activated receptor-γ coactivator-1α (PGC1α) and aquaporin-9 (AQP9). In contrast, mammalian target of rapamycin complex-1 (mTORC1) regulates the expression of hypoxia-inducible factor-1α (HIF-1α) and cMyC, which in turn promote glycolytic metabolism crucial for TE cell development (Fig. 1B) [6]. However, despite concerted efforts to identify the contributions of key transcription factors and metabolic profiles to T-cell memory, the underlying molecular mechanism(s) controlling distinct T-cell differentiation programs has yet to be discovered.
mTORC1 is an evolutionarily conserved protein complex that senses the strengths of all three signals and plays important role in T-cell proliferation, metabolism, and differentiation [1]. In 2009, Ahmed’s group provided the first evidence that rapamycin (Rapa)-mediated inhibition of mTORC1 promotes CD8+ TM-cell formation [7]. This finding was further supported by evidence that Rapa induces T-cell memory via a FOXO1-dependent transcriptional switch from T-bet to Eomes [8]. However, the molecular mechanism underlying Rapa-promoted T-cell memory is largely unknown.
The proinflammatory cytokine IL-2 and prosurvival IL-7 or IL-15 common γ-chain (γc)-family cytokines induce CD8+ TE-cell and TM-cell formation, respectively, by triggering a Janus kinase-3 (JAK3) signal, leading to activation of the PI3K-AKT-mTORC1 pathway [9]. To assess how activation of a single signaling pathway allows the formation of two distinct T-cell lineages, we genetically engineered IL-7R gene knockout (KO)/ovalbumin (OVA)-specific TCR transgenic OTI (IL-7R KO/OTI) mice and adoptively transferred CD8+ T cells derived from wild-type (WT) OTI or IL-7R KO/OTI mice into C57BL/6 mice. Following infection with recombinant Listeria monocytogenes rLmOVA, we demonstrated that, unlike T cells derived from WT OTI mice, IL-7R KO/OTI mouse-derived T cells downregulated the expression of FOXO1, TCF1, AMPKα1, and ULK1, which is necessary for TM-cell formation, and exhibited defective cell survival [10]. In addition, we prepared in vitro IL-2- and IL-7-stimulated T (IL-2/TE and IL-7/TM) cells derived from OTI mice, which approximated the in vivo TE- and TM-cell differentiation programs, for subsequent characterization [10]. Surprisingly, we showed that IL-2 and IL-7 stimulated strong and weak mTORC1 (IL-2/mTORC1Strong and IL-7/mTORC1weak) signaling due to persistent expression of cell-surface IL-2R, resulting in IL-2/mTORC1Strong signaling in IL-2/TE cells, while transient expression of cell-surface IL-7R resulted in IL-7/mTORC1weak signaling in IL-7/TM cells [10]. We also demonstrated that the IL-7/mTORC1weak signal upregulated the levels of the phenotypic markers IL-7R and CD62L; increased the expression of the transcription factors FOXO1, TCF1, and Id3 for TM-cell differentiation and the metabolic molecules AMPKα1, ULK1, ATG7, PGC1α, and AQP9 for stimulating mitochondrial biogenesis and FAO metabolism in IL-7R+CD62L+KLRG1- IL-7/TM cells; and promoted long-term T-cell survival and recall responses upon a secondary antigen boost [10]. In contrast, mTORC1Strong signaling in IL-7R-CD62L-KLRG1+ IL-2/TE cells reduced the collective activity of these pathway-related molecules and instead increased the abundance of the transcription factors T-bet and HIF-1α for TE-cell differentiation and glycolytic metabolism, respectively [10]. These data collectively indicate that IL-7/mTORC1Weak signaling induces T-cell memory via the transcriptional FOXO1 and metabolic AMPKα1 pathways (Fig. 1C). This conclusion is also supported by the findings that IL-15-stimulated prosurvival IL-15/TM cells and a low dose of inflammatory IL-2-stimulated IL-2Low/TM cells exhibited mTORC1Weak signaling, a TM-cell phenotype and long-term survival after adoptive transfer into C57BL/6 mice (Fig. 1C) [10].
To test molecular pathways crucial for Rapa-promoted T-cell memory, we prepared in vitro IL-2-stimulated OTI T cells in the absence or presence of Rapa to form IL-2(Rapa-)/T (IL-2/T) and IL-2(Rapa+)/T cells, respectively, for subsequent characterization [11]. We demonstrated that IL-2(Rapa+)/T cells with mTORCWeak signaling upregulated the expression of the transcription factors FOXO1, TCF1, and Eomes and the metabolic regulators AMPKα1, pULK1, and ATG7 to promote mitochondrial biogenesis and FAO and showed long-term survival after adoptive cell transfer into C57BL/6 mice compared to IL-2/TE cells with mTORC1Strong signaling [11]. These findings indicate that the Rapa-induced mTORCWeak signal promotes T-cell memory via the concerted activity of the transcriptional FOXO1-TCF1-Eomes and metabolic AMPKα1-ULK1-ATG7 networks in IL-2(Rapa+)/TM cells (Fig. 1C) [11] and further support the above finding that mTORC1Weak signaling induces T-cell memory via the transcriptional FOXO1 and metabolic AMPKα1 pathways [10].
In addition to the “LCD” model, Reiner’s group proposed an “asymmetric cell division (ACD)” or “bifurcative differentiation” model in 2007, in which stimulation of a single naïve CD8+ T-cell by an APC gives rise to two descendant daughter cells with distinct fates after the first cell division [3]. A special type of cellular apparatus called the immunological synapse (IS) is formed at the contact interface between the engaged naïve CD8+ T cell and APC. Numerous CD8, cytoskeletal talin, and T-cell/APC molecular conjugates, such as TCR/antigenic peptide/major histocompatibility complex-I (pMHCI), costimulatory CD28/CD80 and adhesive LFA-1/CD54, accumulate at the contact interface to form the IS. The CD8+ T cell then undergoes extensive cytoskeletal remodeling, leading to asymmetric partitioning of cell-surface molecules (CD8, CD62L, IL-7R, LFA-1, and KLRG1) [3, 12] and intracellular cell fate determinants (T-bet, Id3, Eomes, and Bcl6) [13]. Recently, it has been shown that this ACD process is also marked by differential segregation of key molecular components, such as mTORC1, cMyC, and amino acid transporters, into the daughter cells [14, 15]. This asymmetric molecular distribution pattern is maintained during mitosis and leads to the formation of two progenitor daughter cells with distinct fates after the first cell division [3]. As a result, the proximal CD8HighLFA-1HighKLRG1+IL-7R-CD62L- daughter cell displays upregulation of mTORC1, cMyC, T-bet, and HIF-1α and adopts a CD8+ TE-cell fate, while the distal CD8LowLFA-1LowKLRG1-IL-7R+CD62L+ daughter cell shows increased Id3/Eomes expression and decreased mTORC1/cMyC expression and is destined to become a TM cell (Fig. 1D). In addition, CD8+ T cells exposed to strong signals (high affinity for the antigenic peptide or higher levels of LFA-1 adhesion molecules) have been shown to undergo ACD, whereas T cells activated by below-threshold antigenic stimulation undergo symmetric cell division (SCD), indicating that strong stimuli preferentially lead to ACD, whereas intermediate (less strong) stimuli favor SCD [12]. Asymmetric inheritance of mTORC1 has an impact on lymphocyte metabolic fitness, with the mTORC1Weak distal daughter cell displaying elevated FAO and long-term survival [15].
The above evidence strongly supports the notion that the mTORC1Weak signal in the distal daughter cell promotes T-cell memory in the strong signal-stimulated “ACD” model, while mTORC1Weak signaling is able to promote T-cell memory development during SCD in the intermediate signal-stimulated “LCD” model (Fig. 1E). In both models, however, the mTORC1Weak signal promotes T-cell memory by coordinately regulating the expression of the transcriptional FOXO1 and metabolic AMPKα1 networks, indicating that the distinct strengths of mTORC1 signaling control T-cell memory via transcriptional FOXO1 and metabolic AMPKα1 pathways in the “LCD” and “ACD” models (Fig. 1E).
Understanding the molecular mechanism that governs T-cell memory is of great importance in vaccine or immunotherapy design. The above novel findings not only elucidate the molecular mechanism underlying the origin of TM cells in the “LCD” and “ACD” models but also have a great impact on the development of efficient immunotherapies and vaccines for cancer and infectious diseases.
Acknowledgements
This work was supported by a research grant (#PJT153314) from the Canadian Institute of Health Research (CIHR).
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: In the version of this article initially published, unintended typographical errors in the original version of Fig. 1B were made during manuscript preparation. The revised Fig. 1, including the correct Fig. 1B, is shown below.
Change history
1/25/2023
A Correction to this paper has been published: 10.1038/s41423-022-00970-2
References
- 1.Chen Y, Zander R, Khatun A, Schauder DM, Cui W. Transcriptional and epigenetic regulation of effector and memory CD8 T cell differentiation. Front Immunol. 2018;9:2826. doi: 10.3389/fimmu.2018.02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 3.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 4.Daniels MA, Teixeiro E. TCR signaling in T cell memory. Front Immunol. 2015;6:617. doi: 10.3389/fimmu.2015.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia V, Sarkar S. Regulation of effector and memory CD8 T cell differentiation by IL-2-A balancing act. Front Immunol. 2018;9:2987. doi: 10.3389/fimmu.2018.02987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung J, Zeng H, Horng T. Metabolism as a guiding force for immunity. Nat Cell Biol. 2019;21:85–93. doi: 10.1038/s41556-018-0217-x. [DOI] [PubMed] [Google Scholar]
- 7.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. Immunity. 2012;36:374–87. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–84. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 10.Xu A, Leary SC, Islam MF, Wu Z, Bhanumathy KK, Ara A, et al. Prosurvival IL-7-stimulated weak strength of mTORC1-S6K controls T cell memory via transcriptional FOXO1-TCF1-Id3 and metabolic AMPKα1-ULK1-ATG7 Pathways. J Immunol. 2022;208:155–68. doi: 10.4049/jimmunol.2100452. [DOI] [PubMed] [Google Scholar]
- 11.Ara A, Xu A, Ahmed KA, Leary SC, Islam MF, Wu Z, et al. The energy sensor AMPKα1 is critical in rapamycin-inhibition of mTORC1-S6K-induced T-cell memory. Int J Mol Sci. 2022;23:37. doi: 10.3390/ijms23010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King CG, Koehle S, Hausmann B, Schmaler M, Zehn D, Palmer E. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity. 2012;37:709–20. doi: 10.1016/j.immuni.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arsenio J, Kakaradov B, Metz PJ, Kim SH, Yeo GW, Chang JT. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat Immunol. 2014;15:365–72. doi: 10.1038/ni.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbist KC, Guy CS, Milasta S, Liedmann S, Kamiński MM, Wang R, et al. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532:389–93. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollizzi KN, Sun IH, Patel CH, Lo YC, Oh MH, Waickman AT, et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol. 2016;17:704–11. doi: 10.1038/ni.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]