Abstract
Formation of chlorate (ClO3−) and perchlorate (ClO4−) as by-products in electrooxidation process has raised concern. In the present study, the formation of ClO3− and ClO4− in the presence of 1.0 mM Cl− on boron doped diamond (BDD) and Magneli phase titanium suboxide (Ti4O7) anodes were evaluated. The Cl− was transformed to ClO3− (temporal maximum 276.2 μM) in the first 0.5 h on BDD anodes with a constant current density of 10 mA cm2, while approximately 1000 μM ClO4− was formed after 4.0 h. The formation of ClO3− on the Ti4O7 anode was slower, reaching a temporary maximum of approximately 350.6 μM in 4.0 h, and the formation of ClO4− was also slower on the Ti4O7 anode, taking 8.0 h to reach 780.0 μM. Compared with the BDD anode, the rate of ClO3− and ClO4− formation on the Ti4O7 anode were always slower, regardless of the supporting electrolytes used in the experiments, including Na2SO4, NaNO3, Na2B4O7, and Na2HPO4. It is interesting that the formation of ClO4− during electrooxidation was largely mitigated or even eliminated, when methanol, KI, and H2O2 were included in the reaction solutions. The mechanism of the inhibition on Cl− transformation by electrooxidation was explored.
Subject terms: Environmental chemistry, Pollution remediation
Introduction
Electrooxidation (EO) process is a promising technology in wastewater treatment1–4. EO process has been demonstrated to be a viable means to decompose a broad spectrum of recalcitrant organic pollutants that are not removable by conventional treatment processes, including pharmaceuticals, endocrine disruptors, phenolic compounds, and particularly per- and polyfluoroalkyl substances (PFASs)5–9. EO is a chemical destructive technology that promotes organic pollutants degradation by direct electron transfer from organic contaminants to the anode and attack by hydroxyl free radicals and other reactive oxygen species that are also generated on the anode surfaces during the EO process10.
Sufficiently stable and effective anode materials for EO water treatment have been developed in the last decades, including mixed oxides, such as iridium and/or ruthenium oxides11–13, titanium dioxide14, and doped diamond electrodes (BDD)15–17. This is one of the important reasons why the EO process has approached to technical maturity only recently18. Magnéli phase titanium sub-oxides, such as Ti4O7, have recently been explored as promising electrode materials for EO applications because of their high conductivity and chemical inertness. Ti4O7 anodes have been shown to oxidize recalcitrant contaminants by a combination of direct electron transfer (DET) and indirect reactions with HO· produced at the anode surface from water oxidation10. Our recent studies have demonstrated the degradation and mineralization of Perfluorooctanesulfonate (PFOS, the one most commonly used per-fluoroalkyl acids) on the Magneli phase Ti4O7 anode19,20.
One factor limiting the application of EO in water/wastewater treatment is that its strongly oxidizing conditions also result in the formation of toxic by-products in the presence of Cl−, such as chlorate (ClO3−) and perchlorate (ClO4−). In particular, ClO4− is difficult to remove from water and its consumption has been linked to health risks associated with disruption of the endocrine and reproductive systems21. These risks have caused the U.S. Environmental Protection Agency (EPA) to regulate perchlorate under the Safe Drinking Water Act, although an established federal limit has not yet been set22. The formation of ClO4− was reported during EO using several anode materials (e.g., BDD and Ti4O7)23. The presence of Cl− lead to the formation of free chlorine (HOCl) that is further converted to ClO3− and ClO4− in EO systems using both BDD and Ti4O7 anodes. This transformation process appeared much faster on BDD than Ti4O7 anode24. It is desirable to develop electrooxidation systems that minimize the formation of chlorine-related toxic by-products for water/wastewater treatment applications.
The purpose of this study was to systematically investigate the formation of ClO3− and ClO4− in solutions containing Cl− during electrooxidation on Magneli phase Ti4O7 anode and compares it to those on BDD anode. The experiments were performed in different supporting electrolytes at different electrochemical conditions. The effects of a few co-existing constituents were assessed to investigate the inhibition of ClO3− and ClO4− formation on the Ti4O7 anode. The findings provide a basis for devising strategies to reduce the formation of ClO3− and ClO4− in EO on Ti4O7 anode.
Materials and methods
Reagents and materials
All chemicals used in the experiments were of reagent grade or higher. ClO3− was purchased from Sigma-Aldrich (St. Louis, MO). ClO4−, NaCl, and HPLC grade methanol (MeOH) were obtained from Fisher Chemical. Na2SO4, NaNO3, Na2B4O7 Na2HPO4, NaH2PO4, H3PO4, H2O2, and KI were supplied by J.T. Baker. All stock solutions were prepared in ultrapure water (18.2 MΩ cm−1) produced by a Barnstead Nano pure water purification system.
Experimental procedures
EO experiment was carried out in an undivided rectangular cell (10 cm × 5 cm × 2.5 cm) made of acrylic materials. A ceramic plate Ti4O7 (10 cm × 5 cm) or a Si/BDD plate of the same size (both sides coated, NeoCoat, Switzerland) was used as the anode, and two 304 stainless steel plates of the same size as the anode, placed on both sides of the anode in parallel with an interval of about 2.5 cm, were used as the cathodes. The Ti4O7 electrodes were fabricated according to the method used in our previous study20,24, and information on their preparation and characterization is described in detail in Supporting Information (Text S1). During each experiment, the electrolytic cell contained a 200 mL solution containing Cl− (1.0 mM) and Na2SO4 (100 mM) or other salts (NaNO3, Na2B4O7, Na2HPO4, NaH2PO4, H3PO4) as supporting electrolytes stirred at 700 rpm unless otherwise specified. Some EO experiments were performed to explore the impact of pH with Na2HPO4 electrolyte as a buffer for pH 10–11, NaH2PO4 + Na2HPO4 for pH 6–7, and H3PO4 for pH 2–3. In some EO experiments, MeOH (10–1000 mM), KI (20–100 mM) or H2O2 (100–1000 mM) were spiked to the electrolyte solution to explore their impact on the formation of chlorate and perchlorate. All EO experiments were conducted at room temperature.
A constant electric current was supplied at the of 10 mA cm−2 density using a controllable DC power source (Electro Industries Inc., Monticello, MN), unless otherwise specified. The submerged surface area on both sides of the anode (total geometric surface area was 78 cm2) was used for calculating the electric current density. A CHI 660E electrochemical workstation (CH Instruments, Inc., Austin, TX) was used to measure the anodic potential using an Ag/AgCl reference electrode placed close to the anode, with the potential drop in solution (iRs) compensated. Triplicate samples (1.0 mL each) were withdrawn at pre-selected time points, with the power source paused and the solution continuously stirred to ensure homogeneity. The samples were stored at 4 °C until further analysis. The data were plotted with error bars representing the maximum and minimum of duplicated test results. The temperature of solution was monitored and no significant change was found during electrolysis process.
Analysis methods
Free chlorine, ClO3−, and ClO4− were quantified in selected samples. The Concentration of HClO was measured by spectrophotometer at 510 nm (Beckman Coulter DU 800, Brea, CA). A 2.5-mL aliquot sample was immediately mixed with 0.25-mL DPD solution (8.0 mM). DPD is oxidized by HOCl to show a red color. ClO3− and ClO4− were analyzed using a Waters (Milford, MA) ultra-high performance liquid chromatography with an electrospray ionization (ESI) source (UPLC-MS/MS). Detailed UPLC-MS/MS analytical parameters can be found in Text S2. Quantification of the ClO3− and ClO4− was based on multipoint standard calibration.
Results and discussion
Formation of ClO3− and ClO4− in EO systems
Cl− can be oxidized in EO systems to form reactive chlorine species that lead to ClO3− and ClO4−. It was shown that the presence of 1.0-mM Cl− resulted in increased HOCl, ClO3−, and ClO4− on BDD and Ti4O7 anodes (Fig. 1). Almost no appreciable HOCl was detected during the 4.0 h electrooxidation process on the BDD anode, while the concentration of HOCl increased continuously in the system with Ti4O7 anode and reached 103.2 μM at 8.0 h. In both systems, ClO3− concentration increased and then plateaued, while the concentration of ClO4− increased continuously. The transformation of Cl− was faster on BDD anode in general. The data (Fig. 1) indicate that the concentration of ClO3− reached 276.2 μM in the first 0.5 h and then decreased on BDD anode, while almost all Cl (about 1000 μM) was transformed to ClO4− within 4 h. The formation of ClO3− on Ti4O7 electrode was slower, reaching a plateau of ~ 350.6 μM in 4.0 h and then decreasing slowly. The formation of ClO4− also appeared to be more slowly on Ti4O7 electrode, taking about 8.0 h to reach 780.0 μM.
Figure 1.
Comparison of chlorine species during the electrochemical oxidation of Cl− on different anodes. Dashed lines show simulated results. Conditions: [Cl−]0 = 1.0 mM, [Na2SO4] = 100 mM, current density = 10 mA cm-2.
Cl− can be transformed in electrooxidation by direct electron transfer (DET) to ClO3− and ClO4− through a pathway of multiple steps (R1–R3). Direct oxidation of Cl− on BDD electrode generated Cl· and hypochlorite. However, unlike BDD electrode, the oxidation of Cl− due to DET on Ti4O7 anode was not as effective, thus resulting in slower formation of ClO3− and ClO4− than on BDD anode24. Note that indirect routes (R4–R5) can lead to Cl− generation on both Ti4O7 and BDD anode, which can further go through the reactions in R2 and R3 to form HOCl and chlorinated by-products. The conversion of Cl− to HOCl and the chlorinated byproducts via both DET and indirect routes involves the hydroxyl radicals (HO−) that are formed by water oxidation on anode.
| R1 |
| R2 |
| R3 |
| R4 |
| R5 |
The rate of Cl− conversion to chlorate and perchlorate in EO systems has been simulated using a model of two sequential steps by assuming each step as pseudo-first-order kinetics (R6–R7)23,25. The rate constants k1 and k2 in such sequential equations were obtained by fitting the data as shown in Fig. 1 a and b using the software Kintecus v6.8026. The values of k1 and k2 were fitted to be 5.40 × 10−4 and 7.16 × 10−4 s−1, respectively, on the BDD anode, while for Ti4O7 anode the values were 8.59 × 10−5 and 1.34 × 10−4 s−1, respectively. This indicates that Cl− is oxidized to ClO3− and ClO4− more easily on BDD, evidenced by the larger k1 and k2 on the BDD anode than on Ti4O7 anode. Retarded formation of ClO3− and ClO4− makes it advantageous to apply Ti4O7 anodes in water/wastewater treatment.
| R6 |
| R7 |
Effects of electrolytes on the formation of ClO3− and ClO4−
A set of experiments were performed to evaluate the ClO3− and ClO4− formation by EO with BDD and Ti4O7 anode in solutions containing different supporting electrolytes, including 100-mM Na2SO4, NaNO3, Na2B4O7, and Na2HPO4. The concentrations of ClO3− and ClO4− measured in different electrolyte solutions are summarized in Fig. 2. As shown in Fig. 2a and b, ClO4− concentration reached 990 μM after 4.0 h with BDD anode and Na2SO4 as supporting electrolyte, which accounts for about 99% of the total Cl− initially included in the solution. Almost no ClO3− was detected. At the same current density, the formation of ClO4− was slower with NaNO3, Na2B4O7, and Na2HPO4 as supporting electrolytes on the BDD electrode. It is known that sulfate radical (SO4·−) can be formed by one-electron oxidation of sulfate ion at the anode, which can participate in the oxidation of organics15 and chloride27. Occurrence of peroxodiphosphate was observed during the electrolysis of solutions containing phosphate with BDD anodes28. Hence, there could be competitive oxidation reactions from phosphate, although peroxodisulphate was also formed in sulfate containing solution29. The use of NaNO3 as an electrolyte can promote the formation of ammonium and other reduced nitrogen species by electrochemical reduction30. Ammonium can react with free chlorine, favoring the formation of chloramines and reducing the potential formation of chlorate and perchlorate31–33.
Figure 2.
Comparison of ClO3− and ClO4− formation by the electrochemical oxidation with Cl− in different electrolyte solutions. [Cl−]0 = 1.0 mM, electrolyte concentration = 100 mM, current density = 10 mA cm-2, reaction time = 4.0 h.
Overall, the transformation was more rapid on BDD anode in all the supporting electrolyte solutions. As shown in Fig. 2, the total ClO3− and ClO4− concentration was lower when Ti4O7 was used as the anode. For example, the ClO4− concentrations were 572.62 and 527.92 μM, respectively, after 4.0 h on the BDD anode with NaNO3 and Na2B4O7 as supporting electrolytes, while on Ti4O7 anode, they were 92.37 and 212.84 μM, respectively. In particular, the ClO4− concentration in BDD system was 572.6 μM after 4.0 h with NaNO3 as the supporting electrolyte, while it was only 92.4 μM at the same condition on the Ti4O7 anode.
Inhibitory effect of co-existing constituents
Experiments were performed to examine EO in the presence of Cl− as well as a few co-existing constituents, including MeOH, H2O2 and KI, so as to investigate the effect of the coexisting constituents on the formation of ClO3− and ClO4− with Ti4O7 anode.
MeOH
Ion exchange resin (IXR) exchange/adsorption has been shown effective to remove PFAS from water. Regeneration of PFAS-laden IXR generates a low-volume, high-concentration liquid waste known as still bottoms that contains high concentrations of PFASs, salts, and residual organic content, including MeOH that is often used as organic co-solvent for IXR regeneration. Our recent studies showed that the MeOH content in still bottoms may play a role in chloride oxidation20. In this section, we designed experiments to further explore the effects of MeOH during the transformation of Cl− by EO. As such, the EO experiment was performed in 100-mM Na2HPO4 solutions containing 1.0 mM Cl− and varying quantities of MeOH. The addition of MeOH appeared to impact the conductivity of the reaction solution slightly. The conductivity dropped from 10.51 mS cm−1 to 9.79 mS cm−1, but the anodic potential increased at the same current density (10 mA cm2) (Fig. S1a), from 2.93 V in the absence of MeOH increasing to 3.22 V with 100 mM MeOH. The presence of MeOH decreased the conductivity of the solution, and thus anodic potential increased at the same current density. The formation of ClO3− and ClO4− during EO treatment at 10 mA cm−2 is displayed in Fig. 3. In the absence of MeOH, ClO3− reached 117.8 μM in about 1.0 h and then decreased. The value decreased to 17.3 and 0.0 μM containing 10 mM and 100 mM MeOH, respectively. Such a time course profile indicates the further reaction of ClO3−. The formation of ClO4− increased monotonically, reaching 329.0 μM in 8.0 h in the absence of MeOH. When 10 mM and 100 mM MeOH were spiked, almost no ClO4− were formed for the first 2.0 h, after which ClO4− started to increase, reaching 300.0 μM and 251.8 μM in 8.0 h, respectively. The formation of ClO4− was completely inhibited when 1000 mM MeOH was added, indicating that MeOH inhibited the formation of ClO3− and ClO4−. Delayed formation of ClO4− in the presence of lower MeOH dosage (10 and 100 mM) may be caused by MeOH depletion over time. Formation of ClO3− and ClO4− was neither observed in acid or neutral conditions when 1000-mM MeOH was spiked, by respectively using 50-mM NaH2PO4 + 50-mM Na2HPO4 (pH 6–7) or 100-mM H3PO4 (pH 2–3) as electrolytes instead of Na2HPO4 (pH 10–11).
Figure 3.
Concentration of ClO3− and ClO4− during the electrochemical oxidation of Cl− in the presence of MeOH on Ti4O7 anodes. Conditions: [Cl−]0 = 1.0 mM, [Na2HPO4] = 100 mM, current density = 10 mA cm-2.
A prior study proved that Cl− was not oxidized to Cl· via DET on the Ti4O7 anode, while Cl· was formed mainly through the indirect pathways (R4−R5)24. Cl· reacts with another Cl− to form Cl2·−. Cl· and Cl2·− also combine with each other to form free chlorine (Cl2, HClO)21,34,35. These reactive chlorine species may accumulate and diffuse away from the anode surface, and finally convert into ClO3− and ClO4−. MeOH can transform HO· into perhydroxyl radicals (with a second-order rate constant is 2.1 × 109 M−1 s−1). Meanwhile, the reaction rate constant between MeOH and Cl· is 5.7 × 109 M−1 s−1. MeOH could consume Cl· in the bulk solution and HO· (if present). The formation of ClO3− and ClO4− was thus reduced with low concentrations of MeOH, while a high concentration of MeOH can rapidly transform Cl·, inhibiting the generation of ClO3− and ClO4−.
H2O2
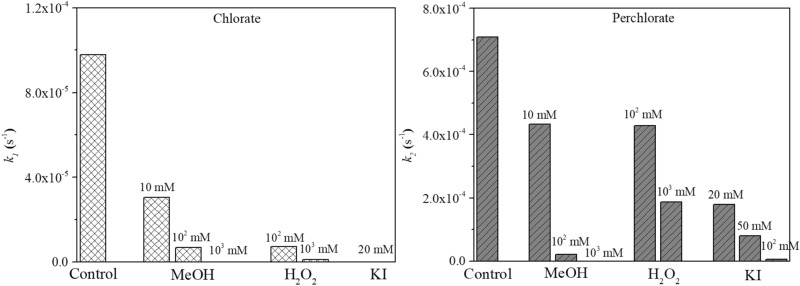
Yang et al. found that the formation of ClO4− during EO with BDD anode can be largely inhibited by adding H2O223. Therefore, H2O2, a commonly used quenchers were also investigated in this study. The time-course data of ClO3− and ClO4− formation in the presence of H2O2 are shown in Fig. S2. Using Kintecus v6.80, the data in Fig. S2 were fit to obtain k1 and k2 represented in equation R6−R7, and they were 9.78 × 10–5 and 7.09 × 10–4 s-1, respectively, in the absence of co-existing constituents (Fig. 4). The values of k1 and k2 decreased to 1.16 × 10–6 and 1.87 × 10–4 s-1 when 1000-mM H2O2 were spiked, respectively. The data shown in Fig. S2 and Fig. 4 also showed that addition of H2O2 at 1000 mM also significantly limited ClO3− and ClO4− formation during the EO.
Figure 4.
Comparison of fitted k1 (R6) and k2 (R7) during the electrochemical oxidation with the addition of MeOH, H2O2, and KI.
H2O2 is known to be both an oxidant (H2O2/H2O, E0 = 1.76 V) and a reductant (O2/H2O2, E0 = 0.68 V) depending on the composition of the reaction media. Thus, Earlier studies have demonstrated that HOCl can be reduced back to Cl− by H2O236,37 (R8–R9). In addition to free chlorine, H2O2 can also react with the chlorine radical species directly (R10-R11). Thus, it is presumed that the reduction of HOCl and chlorine radical species by H2O2 outweighed the oxidation of Cl− by H2O2 in the EO system, and thus decreased ClO3− and ClO4− formation. Moreover, H2O2 may react with ClO3− to form chlorine dioxide (R12)38,39, thus further reducing the formation of ClO3−.
| R8 |
| R9 |
| R10 |
| R11 |
| R12 |
KI
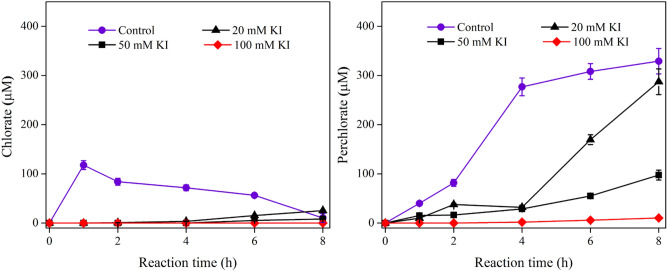
Cl− (Cl·/Cl−, 2.41 V) and Br−(Br·/Br−, 1.62 V) can be oxidized by HO· to form carcinogenic chlorate and bromate40, while I−, having a lower reduction potential of 1.33 V25,41, may be more readily oxidized than Cl− and Br− in theory42. It was also found in our previous studies that NaI may be used as a Cl− free salt to regenerate PFAS-laden ion exchange resin without compromised capability in PFAS recovery20. To evaluate the impact of I− on the formation of ClO3− and ClO4− during EO process, an EO experiment was performed in the presence of I−. It should be noted that the anodic potential was relatively constant at the same current density (10 mA cm2) with I− at different levels (Fig. S1b). The presence of I− inhibited the formation of ClO3− and ClO4− significantly as shown in Fig. 5. Almost no ClO3− was formed during the first 4.0 h and then increased to 25.5 μM after 8.0 h in the presence of 20-mM KI. Similarly, the formation of ClO4− increased slowly during the first 4.0 h and reached 287.2 μM at 8.0 h. Furthermore, near-complete inhibition of ClO3− and ClO4− formation was achieved when 100 mM KI was spiked, with the values of k1 and k2 decreased to 0 and 4.76 × 10–6 s-1, respectively (Fig. 4). This suggests that I− outcompetes CI− for reaction with HO·, leading to a slower generation of HOCl on Ti4O7, and thus inhibiting the formation of ClO3− and ClO4−. I− can be oxidized by common oxidants leading to reactive iodine species (e.g., hypoiodous acid (HOI), iodine (I2), and iodide radical (I·)), and then to iodate (IO3-), which is not considered carcinogenic because it is rapidly reduced to I− after being ingested43,44.
Figure 5.
Formation of ClO3− and ClO4− during the electrochemical oxidation of Cl− in the presence of KI on Ti4O7 anodes. Conditions: [Cl−]0 = 1.0 mM, [Na2HPO4] = 100 mM, current density = 10 mA cm-2.
Conclusions
In conclusion, oxidation of Cl− lead to the formation of ClO3− and ClO4− on both BDD and Magnéli phase Ti4O7 anode during EO. This transformation process was much faster on BDD than Ti4O7 anode in different supporting electrolytes, including Na2SO4, NaNO3, Na2B4O7, and Na2HPO4. The formation of ClO3− and ClO4− was easier with Na2SO4 as supporting electrolyte in both systems. Around 99% and 58% of the total Cl− was transformed to ClO4− after 4.0 h of EO with the BDD and Ti4O7 anode, respectively. When NaNO3 was used as electrolytes, ClO3− and ClO4− formation was decreased to some extent, with only 9% of the total Cl− transformed to ClO4− on Ti4O7 anode. Addition of MeOH, H2O2, and KI can effectively inhibit the formation of ClO3− and ClO4− during EO by Ti4O7 anode. Near complete inhibition of their formation was achieved with 1000-mM MeOH and 100-mM KI present. MeOH, H2O2, and KI appear to be ideal quenchers to mitigate ClO3− and ClO4− formation, because they are effective, accessible and inexpensive. In particular, KI is more stable and easier to be stored and transported than MeOH and H2O2. I− is oxidized to iodate ultimately in the EO system, while iodate is a relatively stable and benign chemical. In practice, the EO treatment can be designed to fully convert I−, or else a polishing step, such as IXR, has to be followed to remove remaining I−. The findings provide a basis for devising strategies to reduce the formation of ClO3− and ClO4− in the EO process.
Supplementary Information
Acknowledgements
This research was supported in part by U.S. Department of Defense SERDP ER-2717 and ER18-1320.
Author contributions
L.W. wrote the mani manuscript. Q.H. revised and edited. All authors reviewed the manuscript.
Data availability
The SEM collected during the current study is available in the NoMad repository, 8uLLHSolQ06aHg2roegwpg.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19310-5.
References
- 1.Barazesh JM, Prasse C, Sedlak DL. Electrochemical transformation of trace organic contaminants in the presence of halide and carbonate ions. Environ. Sci. Technol. 2016;50:10143–10152. doi: 10.1021/acs.est.6b02232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jasper JT, Shafaat OS, Hoffmann MR. Electrochemical transformation of trace organic contaminants in latrine wastewater. Environ. Sci. Technol. 2016;50:10198–10208. doi: 10.1021/acs.est.6b02912. [DOI] [PubMed] [Google Scholar]
- 3.Sires I, Brillas E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ Int. 2012;40:212–229. doi: 10.1016/j.envint.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Chaplin BP. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ Sci Process Impacts. 2014;16:1182–1203. doi: 10.1039/c3em00679d. [DOI] [PubMed] [Google Scholar]
- 5.Feng L, van Hullebusch ED, Rodrigo MA, Esposito G, Oturan MA. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013;228:944–964. [Google Scholar]
- 6.Soriano A, Gorri D, Biegler LT, Urtiaga A. An optimization model for the treatment of perfluorocarboxylic acids considering membrane preconcentration and BDD electrooxidation. Water Res. 2019;164:114954. doi: 10.1016/j.watres.2019.114954. [DOI] [PubMed] [Google Scholar]
- 7.Rahman MF, Peldszus S, Anderson WB. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res. 2014;50:318–340. doi: 10.1016/j.watres.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 8.Murugananthan M, Yoshihara S, Rakuma T, Shirakashi T. Mineralization of bisphenol A (BPA) by anodic oxidation with boron-doped diamond (BDD) electrode. J. Hazard Mater. 2008;154:213–220. doi: 10.1016/j.jhazmat.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, et al. Electrochemical oxidation characteristics of p-substituted phenols using a boron-doped diamond electrode. Environ. Sci. Technol. 2007;18:6541–6546. doi: 10.1021/es070955i. [DOI] [PubMed] [Google Scholar]
- 10.Lin H, et al. Development of macroporous Magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances. Chem. Eng. J. 2018;354:1058–1067. [Google Scholar]
- 11.Li M, Feng C, Hu W, Zhang Z, Sugiura N. Electrochemical degradation of phenol using electrodes of Ti/RuO2-Pt and Ti/IrO2-Pt. J. Hazard Mater. 2009;162:455–462. doi: 10.1016/j.jhazmat.2008.05.063. [DOI] [PubMed] [Google Scholar]
- 12.Radjenovic J, Escher BI, Rabaey K. Electrochemical degradation of the beta-blocker metoprolol by Ti/Ru0.7 Ir0.3O2 and Ti/SnO2-Sb electrodes. Water Res. 2011;45:3205–3214. doi: 10.1016/j.watres.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 13.FaridaYunus R, Zheng YM, Nadeeshani Nanayakkara KG, Chen JP. Electrochemical removal of rhodamine 6G by using RuO2 coated Ti DSA. Ind. Eng. Chem. Res. 2009;48:7466–7473. [Google Scholar]
- 14.Yang Y, et al. Cobalt-doped black TiO2 nanotube array as a stable anode for oxygen evolution and electrochemical wastewater treatment. ACS Catal. 2018;8:4278–4287. doi: 10.1021/acscatal.7b04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farhat A, Keller J, Tait S, Radjenovic J. Removal of persistent organic contaminants by electrochemically activated sulfate. Environ. Sci. Technol. 2015;49:14326–14333. doi: 10.1021/acs.est.5b02705. [DOI] [PubMed] [Google Scholar]
- 16.Schmalz V, Dittmar T, Haaken D, Worch E. Electrochemical disinfection of biologically treated wastewater from small treatment systems by using boron-doped diamond (BDD) electrodes-contribution for direct reuse of domestic wastewater. Water Res. 2009;43:5260–5266. doi: 10.1016/j.watres.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Soriano A, Gorri D, Urtiaga A. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Res. 2017;112:147–156. doi: 10.1016/j.watres.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 18.Kraft A. Electrochemical water disinfection: a short review. Platinum Met. Rev. 2008;52:177–185. [Google Scholar]
- 19.Shi H, Wang Y, Li C, Pierce R, Gao S, Huang Q. Degradation of perfluorooctanesulfonate by reactive electrochemical membrane composed of magneli phase titanium suboxide. Environ. Sci. Technol. 2019;53:14528–14537. doi: 10.1021/acs.est.9b04148. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, et al. Treatment of perfluoroalkyl acids in concentrated wastes from regeneration of spent ion exchange resin by electrochemical oxidation using Magnéli phase Ti4O7 anode. Chem. Eng. J. Adv. 2021;5:100078. [Google Scholar]
- 21.Bergmann MEH, Koparal AS, Iourtchouk T. Electrochemical advanced oxidation processes, formation of halogenate and perhalogenate species: A critical review. Crit. Rev. Environ. Sci. Technol. 2014;44:348–390. [Google Scholar]
- 22.Jawando W, Gayen P, Chaplin BP. The effects of surface oxidation and fluorination of boron-doped diamond anodes on perchlorate formation and organic compound oxidation. Electrochim Acta. 2015;174:1067–1078. [Google Scholar]
- 23.Yang S, Fernando S, Holsen TM, Yang Y. Inhibition of perchlorate formation during the electrochemical oxidation of perfluoroalkyl acid in groundwater. Environ. Sci. Technol. Lett. 2019;6:775–780. [Google Scholar]
- 24.Wang L, Lu J, Li L, Wang Y, Huang Q. Effects of chloride on electrochemical degradation of perfluorooctanesulfonate by Magnéli phase Ti4O7 and boron doped diamond anodes. Water Res. 2020;170:115254. doi: 10.1016/j.watres.2019.115254. [DOI] [PubMed] [Google Scholar]
- 25.Lin Z, et al. Perchlorate formation during the electro-peroxone treatment of chloride-containing water: Effects of operational parameters and control strategies. Water Res. 2016;88:691–702. doi: 10.1016/j.watres.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Ianni J.C. Kintecus V6.80. (2019).
- 27.Bagastyo AY, Batstone DJ, Rabaey K, Radjenovic J. Electrochemical oxidation of electrodialysed reverse osmosis concentrate on Ti/Pt-IrO2, Ti/SnO2-Sb and boron-doped diamond electrodes. Water Res. 2013;47:242–250. doi: 10.1016/j.watres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Cañizares P, Sáez C, Sánchez-Carretero A, Rodrigo MA. Synthesis of novel oxidants by electrochemical technology. J. Appl. Electrochem. 2009;39:2143–2149. [Google Scholar]
- 29.Moraleda I, et al. Can the substrate of the diamond anodes influence on the performance of the electrosynthesis of oxidants? J. Electroanal. Chem. 2019;850:113416. [Google Scholar]
- 30.Lacasa E, Cañizares P, Llanos J, Rodrigo MA. Removal of nitrates by electrolysis in non-chloride media: Effect of the anode material. Sep. Purif. Technol. 2011;80:592–599. [Google Scholar]
- 31.Lacasa E, Llanos J, Cañizares P, Rodrigo MA. Electrochemical denitrificacion with chlorides using DSA and BDD anodes. Chem. Eng. J. 2012;184:66–71. [Google Scholar]
- 32.Herraiz-Carbone M, et al. Improving the biodegradability of hospital urines polluted with chloramphenicol by the application of electrochemical oxidation. Sci. Total. Environ. 2020;725:138430. doi: 10.1016/j.scitotenv.2020.138430. [DOI] [PubMed] [Google Scholar]
- 33.Cotillas S, Lacasa E, Herraiz M, Sáez C, Cañizares P, Rodrigo MA. The role of the anode material in selective penicillin G oxidation in urine. ChemElectroChem. 2019;6:1376–1384. [Google Scholar]
- 34.Jung YJ, Baek KW, Oh BS, Kang JW. An investigation of the formation of chlorate and perchlorate during electrolysis using Pt/Ti electrodes: The effects of pH and reactive oxygen species and the results of kinetic studies. Water Res. 2010;44:5345–5355. doi: 10.1016/j.watres.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 35.Hou S, et al. Chlorate formation mechanism in the presence of sulfate radical, chloride, bromide and natural organic matter. Environ. Sci. Technol. 2018;52:6317–6325. doi: 10.1021/acs.est.8b00576. [DOI] [PubMed] [Google Scholar]
- 36.Sun P, Lee WN, Zhang R, Huang CH. Degradation of DEET and caffeine under UV/chlorine and simulated sunlight/chlorine conditions. Environ. Sci. Technol. 2016;50:13265–13273. doi: 10.1021/acs.est.6b02287. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Sun P, Boyer TH, Zhao L, Huang CH. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS. Environ. Sci. Technol. 2015;49:3056–3066. doi: 10.1021/es504799n. [DOI] [PubMed] [Google Scholar]
- 38.Cotillas S, Llanos J, Rodrigo MA, Cañizares P. Use of carbon felt cathodes for the electrochemical reclamation of urban treated wastewaters. Appl. Catal. B. 2015;162:252–259. [Google Scholar]
- 39.Crump B, Ernst WR, Neumann HM. Influence of H2O2 on a chloride-dependent reaction path to chlorine dioxide. AlChE J. 1998;44:2494–2500. [Google Scholar]
- 40.Huie RE, Clifton CL, Neta P. Electron transfer reaction rates and equilibria of the carbonate and sulfate radical anions. Int. J. Radiat. Appl. Instrum. C Radiat. Phys. Chem. 1991;38:477–481. [Google Scholar]
- 41.Yeo J, Choi W. Iodide-mediated photooxidation of arsenite under 254 nm irradiation. Environ. Sci. Technol. 2009;43:3784–3788. doi: 10.1021/es900602n. [DOI] [PubMed] [Google Scholar]
- 42.Ross I, et al. A review of emerging technologies for remediation of PFASs. Remediat. J. 2018;28:101–126. [Google Scholar]
- 43.von Gunten U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003;37:1469–1487. doi: 10.1016/S0043-1354(02)00458-X. [DOI] [PubMed] [Google Scholar]
- 44.Bürgi H., Schaffner T., Seiler J.P. The toxicology of iodate: A literature survey in view of its use in iodized salt. Thyroid, 909–918 (2001). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The SEM collected during the current study is available in the NoMad repository, 8uLLHSolQ06aHg2roegwpg.