Abstract
Long-range peripheral nerve defect is a severe and worldwide disease. With the increasing development of tissue engineering, the excellent ability of nerve extracellular matrix (ECM) in peripheral nerve injury (PNI) has been widely studied and verified. Here, we present a novel microtube that contains gradient decellularized porcine sciatic nerve ECM hydrogel (pDScNM-gel) from microfluidics for sciatic nerve regeneration. The pDScNM is confirmed to enhance cell proliferation and migration, and improve the axon growth of primary dorsal root ganglions (DRGs) in a concentration-related manner. These behaviors were also achieved when cells were co-cultured in a gradient pDScNM microtube. The in vivo sciatic nerve regeneration and functional recovery were also demonstrated by assembling the gradient pDScNM microtubes with a medical silicon tube. These results indicated that the microtubes with gradient pDScNM could act as a promising alternative for repairing peripheral nerve defects and showed great potential in clinical use.
Keywords: Microfiber, Peripheral nerve injury, Extracellular matrix, Microfluidics, Nerve regeneration
Graphical abstract
Highlights
-
•
A nerve microtube with controllable morphology and gradient ECM encapsulation was fabricated via microfluidics.
-
•
The gradient microtube could guide cell migration and axonal outgrowth.
-
•
Microtubes improved the sciatic nerve regeneration and functional recovery.
-
•
Microtubes could act as a promising alternative for repairing peripheral nerve defect.
1. Introduction
Peripheral nerve injury (PNI) caused by diseases or trauma is becoming increasingly common in modern society [1,2]. In many cases, lacking timely and effective treatment often results in restricted activity or further life-long disability in patients with severe PNI, especially with long segmental nerve defects [3,4]. To treat severe PNI, an autologous nerve graft is usually regarded as a golden standard in clinical practice despite the donor nerve shortage and secondary damage to the donor site [5]. Fortunately, the emergence of tissue engineering, which aims to repair and regenerate damaged tissues or organs by using bioactive materials, has provided patients with another choice [[6], [7], [8], [9], [10]]. Generally, nerve guidance conduit combined with nerve growth factors and stem cells successfully improve axon regeneration and nerve functional recovery [[11], [12], [13], [14], [15]]. Thus, several FDA-approved nerve guidance conduits such as Nerbridge™ have already been used in clinics and yielded good outcomes [16]. Although tissue engineering has achieved excellent advancements over the past 20 years in addressing PNI and other critical health issues, its wide applications in nerve regenerations are still restricted by the absence of the desired scaffold that meets the requirement of low immunogenicity, favorable biodegradability, and certain mechanical and nutrition support after PNI [[17], [18], [19]]. Therefore, the creation of scaffolds with the abilities to mimic peripheral nerve regeneration microenvironment and promote its reconstruction as well as functional recovery remains a huge challenge for researchers worldwide.
In this paper, we propose a novel microtube with gradient decellularized porcine sciatic nerve matrix for sciatic nerve regeneration by using a multi-injection microfluidic method, as schemed in Fig. 1. Decellularized porcine sciatic nerve extracellular matrix (ECM) is a three-dimensional (3D) network that provides biochemical and structural support to surrounding cells [20,21]. As the ECM is the natural template of scaffold derived from mammalian organs and tissues, the tissue-specific composition made it an ideal scaffold to keep the structural integrity of complex organisms [22,23]. In addition, the main components of ECM including collagen, laminin, and fibronectin can regulate cell behavior and support organization after transplantation [18,[24], [25], [26], [27], [28]]. In clinic, a variety of decellularized porcine tissues have been used and provide an appealing alternative to human tissues. Porcine nerve ECM also showed a comparable effect on neurite outgrowth to the rat nerve ECM, with the benefit of low immunogenicity and excellent biocompatibility [26,27]. Although the promising function of ECM has already been studied and confirmed in various fields [[29], [30], [31], [32]], the practical values of the raw ECM with bulk sizes are still limited as it was just simply sterilized after decellularization or further processed to ECM hydrogel, which was usually with a stubborn and fixed structure [33]. Therefore, the ECM with a controllable and designed shape that is suitable for in-depth use, especially in a blood vessel, nerve, and bone reconstruction, is helpful and necessary in clinical situations.
Fig. 1.
(a) Scheme of the fabrication process of gradient pDScNM microtube; (b) Scheme of gradient pDScNM microtube assembled with silicon conduit used in repairing sciatic nerve defect.
Herein, we employed microfluidics to generate microtubes that contain gradient decellularized porcine sciatic nerve ECM hydrogel (pDScNM-gel) for peripheral nerve regeneration (Fig. 1). The pDScNM-gel was obtained from digesting and neutralizing the decellularized porcine sciatic nerve ECM, which contained most structural proteins while removing nuclei and several nerve inhibitory components. To achieve gradient pDScNM encapsulation, the pDScNM was mixed with sodium alginate to prepare precursors of higher concentration and lower concentration, both of which were then introduced to different injection channels of the microfluidic device for the continuous generation of microtubes. Benefitting from the precise control of fluids in microfluidic channels, the generated microtubes were imparted with controllable morphology like diameters and gradient encapsulation of pDScNM-gel. It was demonstrated that this gradient pDScNM microtube could support cell growth and migration, and promote neurite extending for in vivo nerve regeneration. All of the results indicated that the microtubes with gradient decellularized porcine sciatic nerve matrix are valuable for providing a favorable microenvironment for cell growth and axon elongation and holding great promise for long-segment nerve defect repair.
2. Materials and methods
2.1. Preparation of pDScNM-gel
The preparation of pDScNM-gel was processed by mildly adjusting the reported protocol. Briefly, fresh porcine sciatic nerves were harvested from male or female miniature pigs (8–12 weeks) and immersed in 1% Triton X-100/PBS solution overnight. After washing three times with 0.01 M phosphate buffer (PBS), they were rinsed in 1% sodium dodecyl sulfate (SDS) for 48 h and changed solution every 12 h. Following another three times washing with PBS, the decellularized samples were lyophilized and smashed to powder. Next, the powder was digested with 1 mg/mL pepsin/0.01 M hydrochloride (HCl) solution under stirring for 48 h at room temperature. Finally, using HCl, NaOH, and 10 × PBS solution to adjust the pH value to ∼7.4. The whole process was conducted with agitation at 120 rpm and under 4 °C unless otherwise stated. The prepared pDScNM pre-gel solution was separated and stored at −80 °C.
2.2. Characterization of pDScNM-gel
The residual DNA in pDScNM was extracted and purified by a commercial E.Z.N.A.®Tissue DNA kit based on the recommended protocol. The residual dsDNA was then determined by agarose gel electrophoresis in contrast with standard DNA ladders. Meanwhile, major components of the sciatic nerve after decellularization were tested by histology and immunohistochemistry evaluation. Briefly, both fresh porcine sciatic nerve and pDScNM were fixed for 12 h at 4 °C, following cryoprotected with a graded series of sucrose solutions, and the samples were then embedded. 5-μm-thick sections were sliced by a freezing microtome. Sections were performed to H&E and Masson's trichrome staining. Then slides were immunostained according to the procedures described previously with PBS or several primary antibodies: mouse anti-Fibronectin (1:100, ab6328, Abcam), rabbit anti-Laminin (1:200, ab11575, Abcam), rabbit anti-Collagen IV (1:300, ab6586, Abcam), rabbit anti-Myelin associated glycoprotein (MAG) (1:100, DF7669, Affinity), mouse monoclonal anti-Chondroitin Sulfate (CS-56) (1:200, ab11570, Abcam). Following incubation with goat anti-rabbit or goat anti-mouse IgG H&L (HRP) (1:1000) secondary antibody for 1 h at 37 °C, sections were then treated with the DAB chromogen kit. All images were captured and the different antibody immunoreactivities were calculated from at least three images using ImageJ software. The micromorphology of the fresh porcine sciatic nerve, pDScNM, and pDScNM-gel was analyzed by a scanning electron microscope (SEM; Hitachi, SU8000, Japan). Fresh porcine sciatic nerve and pDScNM were fixed overnight with 2.5% glutaraldehyde, washed with saline solution, dehydrated, and stored in tert-butyl alcohol until subjected to critical point drying, whereas pDScNM-gel was freeze-dried immediately. All dried samples were immobilized on a carbon tape, sputter-coated with gold, and observed.
2.3. Fabrication of gradient pDScNM microtube
The assembling of the microfluidic device that can generate gradient pDScNM microtube was conducted according to the protocol reported earlier. The device was composed of a tapered injection capillary array of which several inner capillaries were inserted into each barrel of the tapered capillary to introduce the inner phases. The orifice of the tapered capillary was around 600 μm and that of the collection channel was 800 μm. In the process, different pDScNM solutions were pumped at a flow rate of 2 mL/h while the 10% polyvinyl alcohol (PVA) solution was at 0.5 mL/h. Additionally, the gradient pDScNM microtubes could be generated by switching different pDScNM and sodium alginate mixtures. The fast gelation of alginate and calcium ions allowed the continuous generation of the microtube and the programmable injection of inlet solutions imparted the resultant micro tube with concentration gradient.
2.4. Characterization of gradient pDScNM microtube
The morphology of the prepared microtube was observed and the outer diameters of these tubes were measured. The microstructure of the microtube was observed by SEM after dehydrating with a gradient series of ethanol. To make the concentration gradient more visible, red fluorescent nanoparticles and green fluorescent nanoparticles were added in two pDScNM solutions, respectively. Then the images of the achieved fluorescent microtubes were captured by a confocal laser microscope (Nikon).
2.5. Rat Schwann cell culture, proliferation, and migration assessment
Rat Schwann cells (RSC96) purchased from Sciencell Research Laboratories were cultured in recommended medium. We firstly assessed the cell viabilities in different concentrations of pDScNM-gel. The cell viability (CCK-8; Beyotime Biotechnology) assay was performed after co-culturing SCs with pDScNM-gel at different concentrations for 24 and 72 h. At each time point, the absorbance of 450 nm was assessed. For further confirmation, the cells co-cultured with different concentrations of pDScNM-gel were fixed and conducted to crystal violet staining. We then investigated the effect of different concentrations of pDScNM-gel on SCs migration using an optimal transwell migration assay. Briefly, 100 μL SCs (1 × 105 cells) were pipetted to the upper chambers, and 500 μL complete medium only or containing 1 mg/mL or 5 mg/mL pDScNM-gel were pipetted into the lower chambers. Following incubating for 24 h, cells on the bottom surface were fixed and stained. The number of migrated cells was measured after.
2.6. Rat pheochromocytoma cell culture, proliferation, and migration assessment
Rat pheochromocytoma cells (PC12) purchased from Sciencell Research Laboratories were cultured in recommended medium. The PC12 cells were co-cultured with different concentrations of pDScNM following the procedures performed above. Particularly, in the migration assay, the number of PC12 cells was changed to 1 × 104 cells.
2.7. Dorsal root ganglion (DRG) culture
The effect of different concentrations of pDScNM and different types of gels on the extension of neurites from the postnatal day 1 SD rat dorsal root ganglions (DRGs) was further investigated. All DRGs were isolated from the postnatal rat and cut nerve roots via sterile microdissection. Then, the DRGs were seeded onto the 35 mm2 cell culture dish (6 DRGs per dish) or different gel and cultured in a Neurobasal™ medium supplemented with 1% P/S only, additional with 1 mg/mL pDScNM, 5 mg/mL pDScNM, respectively. After incubation for 7 days, DRGs were fixed and incubated with beta III tubulin primary antibody (1:500, ab18270, Abcam) overnight. Following staining with donkey anti-rabbit IgG H&L (Alexa Fluor 488) (1:1000, ab150073, Abcam) and DAPI, the micrographs were captured using the inverted fluorescence microscope. The lengths of the extended neurites of DRGs in different groups were measured and calculated.
2.8. Growth and distribution of SCs in pDScNM microtube
To evaluate the survival and distribution of Schwann cells in microtubes, 100 μL cell suspension (2 × 105 cells) were injected into the tubes using a 1 mL syringe capable of 32G needle and cultured for 1, 3, and 5 days. On day 5, the cells in each microtube were stained with Calcein-AM to assess the live cells. Images were captured at each time point and the distance of SCs migration (length) and the diameter of cell fiber (width) on day 5 were measured and calculated using ImageJ software. The cell viability at each time point was also assessed by the CCK-8 assay. Additionally, to further confirm the growth of SCs in the tubes, SCs were stained by DiI cell-labeling solutions following the recommended protocol before being encapsulated in different microtubes. The day 5 DiI-labeled SCs were imaged after being stained with DAPI.
2.9. Animal surgery
All animal procedures were conducted under ethical approval from the Animal Experimental Committee of Wenzhou Medical University. 36 Male SD rats (200–230 g) were divided into six groups randomly: sham group; 1% Ca-Alginate group; 1 mg/mL pDScNM group; 5 mg/mL pDScNM group; gradient pDScNM group and Autograft group. The extra silicone tube was used to load the microtubes and stitched with the nerve ends to support the hydrogel-like microtubes in this study. The rats were housed in a specific-pathogen-free (SPF) breeding facility at a 12:12 light-dark cycle. The animals have free access to the chaw and water. Before the surgery, rats were fasted for 12 h without water deprivation. During surgery, after anesthetized by intraperitoneal injection of pentobarbital sodium, the right sciatic nerve of the rat was exposed and cut, leaving almost a 10-mm long defect. Different types of conduits were then used to bridge these nerve gaps using end-to-end epineurial suture with 8-0 nylon sutures under a surgical microscope. For the Autograft group, a 10-mm nerve was cut off, inverted and sutured with the nerve ends immediately. Make sure that the proximal and distal nerve stump has no torsion and is in its original position. Finally, the muscles and skin were sutured with 4-0 silk sutures and all groups received intramuscular injections of 800,000 units of penicillin to prevent post-surgery infection.
2.10. Walking track analysis and electrophysiological assessment
The analysis was conducted to evaluate the muscle reinnervation at 2, 4, and 8 weeks after surgical procedures. As illustrated in Fig. S6, there are several parameters needed to be assessed. The SFI was then calculated based on the SFI formula. The value of SFI close to 0 represented better recovery while approaching −100 indicated total impairment. At 8 weeks post-transplantation, the motor nerve function of the regenerated nerve was evaluated by electromyography (NeuroExam M − 800, medcomtech, China) according to the protocols recommended. The stimulating mode was set as follows: stimulus intensity 30 mV, frequency 1 Hz, and duration 1 ms. The compound muscle action potentials (CMAP) in each group were recorded and the amplitudes of CMAP were calculated.
2.11. Histological assessment of the regenerated nerve and gastrocnemius muscle
8 weeks after surgery, animals were sacrificed and the right regenerated nerves and bilateral gastrocnemius muscle were isolated. After fixing for 24 h in PFA, the nerve samples were embedded in OCT and cut into either longitudinal sections or transverse sections for future use. In the case of gastrocnemius muscles, one part of them was embedded in OCT and cut into longitudinal sections. For another part, they were embedded in paraffin and cut into transverse sections. The nerve and longitudinal muscle sections were then immunostained according to the procedures described previously. Briefly, after being antigen retrieved by 0.25% trypsin and blocked with 5% BSA, sections were incubated at 4 °C overnight with the primary antibodies: chicken anti-NF200 (1:1000, ab4680, Abcam), rabbit anti-MBP (1:300, ab40390, Abcam), rabbit anti-s100ꞵ (1:300, ab40390, Abcam), mouse anti-α SMA (1:500, ab7817, Abcam), rabbit anti-CD31 (1:100, AF6191, Affinity) and rabbit anti-CD68 (1:200, DF7518, Affinity). Following incubation with corresponding fluorescent secondary antibodies (1:1000), sections were mounted with DAPI Fluoromount-GTM. The cross-sections of muscle were performed with H&E staining. All pictures were captured either on a light microscope or an inverted confocal laser microscope (Nikon) and the different antibody immunoreactivity and the CSA of the muscle fibers were calculated from at least three images using ImageJ software.
2.12. Statistical analysis
The statistical analysis was performed with a one-way analysis of variance (ANOVA). The value of P less than 0.05 was considered to determine statistical significance.
3. Results and discussion
3.1. Preparation and characterization of pDScNM and pDScNM-gel
To prepare the pDScNM-gel, we firstly decellularized the porcine sciatic nerve (pScN) and characterized the microstructure and composition of the pDScNM. To achieve this, fresh sciatic nerves were cut into pieces, decellularized, and lyophilized (Fig. S1a, Supporting Information). Decellularization was identified by H&E staining. As displayed in Fig. 2a i, there were no nuclei remained in decellularized samples. Masson and immunohistochemistry staining were then conducted to determine the reservation of Fibronectin, Collagen IV, and Laminin after the decellularization, which was beneficial for cell adhesion, migration, and proliferation [20,23,27] (Fig. 2a ii-v). Furthermore, the removal of inhibitory components such as myelin-associated glycoprotein (MAG) and chondroitin sulfate were detected (Fig. 2a vi-vii). As demonstrated in Fig. 2b, pDScNM emitted higher immunochemistry against Collagen IV and Laminin, while extremely lower against MAG and chondroitin sulfate compared with pScN. Scanning electron microscopy (SEM) uncovered the micromorphology of pDScNM, showing its distinctive fibrous structure compared with pScN (Fig. 2c). Afterward, the pDScNM was digested with pepsin and then neutralized to form the pDScNM-gel (Fig. 2d i-ii). The SEM image of pDScNM-gel demonstrated its mesh-like microarchitecture (Fig. 2d iii). Additionally, the base-pair length of dsDNA in pScN and pDScNM were evaluated. The absence of dsDNA fragments greater than 200 bp in pDScNM revealed that the decellularization was complete, which is crucial since cellular remnants may provoke severe immunological reactions (Fig. S1b, Supporting Information).
Fig. 2.
(a) Representative images of porcine sciatic nerve characterization before and after decellularization including (i) H&E, black triangle indicates nucleus; (ii) Masson, the red star indicates collagen; immunostaining of (iii) Fibronectin, (iv) Laminin, (v) Collagen IV, (vi) MAG, (vii) Chondroitin Sulfate and negative controls, black arrow indicates positive expression, scale bar indicates 200 μm; (b) Quantification of positive area percentage (PAP) of (i) Fibronectin, (ii) Laminin, (iii) Collagen IV, (iv) MAG, (v) Chondroitin Sulfate (CS) immunoreactivity (%) as characterized in (a) for each group. The values are presented as the mean ± SD, n = 4; (c) SEM images of (i) porcine sciatic nerve and (ii) decellularized porcine sciatic nerve matrix, scale bar indicates 200 μm; (d) Photographs of decellularized sciatic nerve being (i) digested, (ii) neutralized to form pDScNM-gel, (iii) SEM image of the pDScNM-gel, scale bar indicates 50 μm ** and **** indicate statistical significance at P < 0.01 and P < 0.0001, respectively.
3.2. In vitro SCs, PC12 cells migration, and DRGs neurite outgrowth
Before it was used to generate microtubes, the in vitro cytocompatibility of pDScNM-gel, both on SCs and PC12 cells was studied. After co-culturing SCs and PC12 cells with different concentrations of pDScNM for 24 and 72 h, CCK-8 assay and 0.1% crystal violet staining showed a concentration-related manner between cell viability and concentration of pDScNM (Fig. S2 and Fig. S3, Supporting Information). Based on these, we selected 1 mg/mL and 5 mg/mL as the interested concentrations and evaluated their effect on cell migration. Following the experimental design (Fig. 3a), both the number of migration Schwann cells and PC12 cells per field in the pDScNM group went beyond that in the control group. Meanwhile, the cell number increased when the concentration of the pDScNM increased (Fig. 3b–c and f-g). This data revealed that pDScNM-gel can promote the migration of SCs and PC12 cells, in a concentration-related manner. To further confirm this effect of pDScNM-gel, we co-cultured primary DRGs with both 1 and 5 mg/mL pDScNM-gel (Fig. 3d). The immunostaining of ꞵ III tubulin, a neuronal marker, indicated that pDScNM-gel can enhance the axonal outgrowth compared to the control group (86.14 ± 47.73 μm) (Fig. 3e). Additionally, axon length in the 5 mg/mL group is longer than the 1 mg/mL group with the value of 2147.3 ± 477.1 μm and 1018.8 ± 390.3 μm, respectively (Fig. 3h).
Fig. 3.
(a) Scheme of experimental design for the SCs and PC12 cells migration assay; (b, c) Transwell assay of migration ability of SCs and PC12 cells in different concentrations of pDScNM-gel, (i) control group, (ii) 1 mg/mL pDScNM, (iii) 5 mg/mL pDScNM, scale bars indicate 200 μm and 50 μm; (d) Schematic illustration of DRGs co-culturing with pDScNM; (e) Immunofluorescence of ꞵ III tubulin and DAPI images of DRGs, (i) control group, (ii) 1 mg/mL pDScNM, (iii) 5 mg/mL pDScNM, scale bar indicates 500 μm; (f, g) Statistical analysis of the migration SCs and PC12 cells per field for each group. (h) Statistical analysis of the average axon length for each group. The values are presented as the mean ± SD, n = 3. *, **, *** and **** indicate statistical significance at P < 0.05, P < 0.01, P < 0.001 and P < 0.0001.
3.3. Preparation and characterization of gradient pDScNM microtube
Based on the results confirmed above, in this study, we fabricated a gradient pDScNM microtube to not only promote SCs proliferation and axonal elongation but also lead SCs migration and nerve regeneration. The device used for microtube generation was constructed of a tapered seven-barrel injection capillary and several inserted capillaries with spindle tips (Fig. 4a). During the microtube generation, the sacrificing core fluid of PVA flowed in the central channel of the injection capillary while different mixtures of sodium alginate with pDScNM flowed in six surrounding channels, respectively. Through controlling the appropriate flow ratio of sheath and core fluids, the microtubes with uniform inner and outer diameters were achieved (Fig. 4c). In addition, the inner diameter of the microtube increased in a flow rate-dependent manner of the PVA flow that varied from 0.2 to 1.7 mL/h for fixed sheath flow rates, showing about 200 μm increases (Fig. S4, Supporting Information). Furthermore, benefitting from the precise control of fluids in different microfluidic channels [8], the gradient pDScNM microtubes could be generated by switching different pDScNM and sodium alginate mixtures. To make it clear, we use different fluorescent nanoparticles and sodium alginate mixtures to prepare the gradient microtube for microstructure observation. The cross-sectional CLSM images of the microtube showed that it not only kept a homogeneous core-shell structure but also showed gradient compositions in different regions (Fig. 4d).
Fig. 4.
(a) Schematic illustration of the cross-section of capillary microfluidic chips for gradient pDScNM microtube; (b) SEM images of microtube, scale bars indicate 100 μm and 500 μm; (c) Statistical analysis of outer and inner diameter of the gradient pDScNM microfiber. The values are presented as the mean ± SD, n = 5; (d) Cross-sectional CLSM image of different parts of the gradient pDScNM microtube, scale bar indicates 200 μm.
3.4. In vitro DRGs neurite outgrowth and SCs growth and distribution in gradient pDScNM microtube
We prepared the gradient pDScNM gel on a 35 mm2 dish and seeded primary DRGs on it. 1% Ca-Alg, 1 mg/mL pDScNM, and 5 mg/mL pDScNM gels were also made for comparison. After incubating for 7 days, samples from each group were immunostained with ꞵ III tubulin and the lengths of axons were measured. As shown in Fig. S5a, neurites extended from both sides of the DRGs. The concentration-related manner of pDScNM on axon outgrowth was verified that the lengths of neurites were increased when DRGs were seeded on the 5 mg/mL gel compared to that on the 1 mg/mL (Fig. S5c, Supporting Information). Furthermore, the axon lengths on gradient gel were longer than that on the uniform gel, suggesting that the gradient distribution of pDScNM could accelerate the growth speed of the axon. It should be noted that when DRGs were cultivated on the gradient gel, there was a substantial variation in length between the left and right sides, indicating that the growth cones of the neurites were orientated to expand with the increased concentration of pDScNM. To corroborate our findings, we applied gradient gel to an electrospinning aligned nanofiber membrane to mimic the architecture guidance of the microtube. The staining micrographs and lengths evaluated 7 days after culture produced consistent outcomes to those on the dishes (Figs. S5b and S5d, Supporting Information). Taken together, our findings suggest that the gradient pDScNM may both accelerate and direct axonal development.
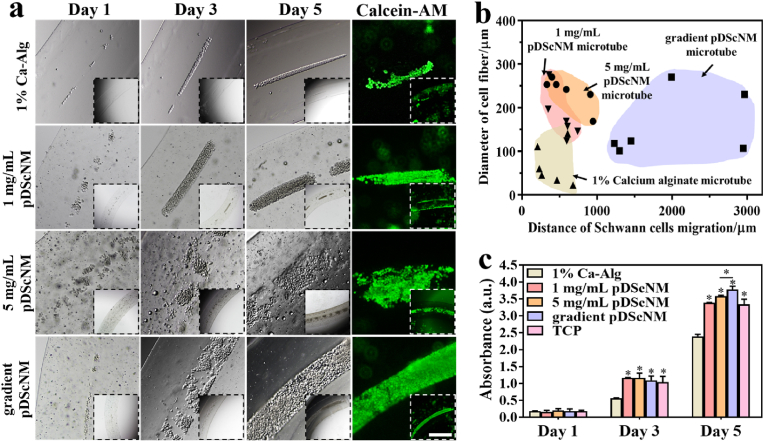
Because the proliferation and migration of Schwann cells (SCs) are two key factors for peripheral nerve regeneration [3], SCs suspension with a certain density was injected into the microtubes to demonstrate their feasibility when applied to nerve injury. The growth process of SCs in the microtube was first investigated and recorded continuously for 5 days, as shown in Fig. 5a. It was found that the SCs could proliferate and form cellular fiber gradually in pDScNM microtubes because pDScNM microtubes can provide an excellent three-dimensional microenvironment for the cells to grow. Further, we measured the diameter of the cellular fibers and the distance of cell migration. Fig. 5b showed that when SCs were cultured in the gradient pDScNM microtubes, both SCs proliferation and migration were significantly promoted, compared to the 1% Ca-Alginate microtube, 1 mg/mL pDScNM microtube, and 5 mg/mL pDScNM microtube (the light brown, pink and orange regions). Additionally, the result of DiI labeled SCs co-culturing with different microtubes for 5 days was consistent with the previous data (Fig. S6, Supporting Information). The proliferation of SCs seeded in the microtube was further investigated by CCK-8 assay (Fig. 5c). On day 1, the cell number in five groups displayed no apparent difference, proving the same initial cell seeding density. Further, the number of cells showed a significant increase in all pDScNM microtube groups compared to the 1% Ca-Alginate microtube group on day 3 and day 5, which indicated that the pDScNM may play a critical role in enhancing the SCs proliferation.
Fig. 5.
(a) Optical images and fluorescent images of SCs-laden microtubes during a 5-day culture, the live SCs were stained with Calcein-AM at day 5, scale bar indicates 200 μm; (b) Statistical significance of 1–5 mg/mL gradient pDScNM microtube in terms of SCs fiber formation and SCs migration after culturing for 5 days, compared to the 1% Ca-Alginate microtube, 1 mg/mL pDScNM microtube and 5 mg/mL pDScNM microtube; (c) Proliferation of SCs cultured on different microtubes during 5 days. The values are presented as the mean ± SD, n = 3. * indicates statistical significance at P < 0.05.
3.5. In vivo sciatic nerve regeneration
The practical value of the gradient pDScNM microtube was then realized by assembling them with a medical silicon tube to achieve a 3-channel conduit, whose peripheral nerve repair performance was checked after transplantation into the 1-cm sciatic nerve defect models (Fig. S7a and Fig. 6a, Supporting Information). The rats were divided into six groups, named sham group, 1% Ca-Alg group, 1 mg/mL pDScNM group, 5 mg/mL pDScNM group, gradient pDScNM group, and Autograft group, respectively (Fig. S7b, Supporting Information). After 8 weeks, the regenerated sciatic nerves in each group were collected. Both nerve regeneration and functional recovery were detected through several strategies. As displayed in fluorescence images of regenerated nerves in the middle of the graft, axons (NF-200 marker, green) and myelination (MBP marker, red) are arranged orderly in the gradient pDScNM group while in other groups the fluorescence distribution of axons and myelination were sparse and disordered (Fig. 6b). Furthermore, the distribution of two fluorescence in all pDScNM groups was far greater than that in the 1% Ca-Alg group, indicating a higher axonal regeneration and remyelination ability caused by pDScNM (Fig. 6c and d). Notably, there is no significant difference in the expression of NF-200 and MBP between the autograft and gradient groups. Specifically, regenerated nerve fibers in gradient pDScNM group had orientation angles from 0° to 30°, while 0° to 67°, 0° to 70°, 0° to 80° for 1% Ca-Alg, 1 mg/mL pDScNM and 5 mg/mL pDScNM group, suggesting a superior axonal directing by gradient pDScNM (Fig. 6e).
Fig. 6.
(a) Digital images of the nerve transplantation site; (b) Immunofluorescence images of NF-200, MBP, and DAPI of regenerated nerves in the middle of the graft 8 weeks post-surgery. Scale bar indicates 50 μm; (c, d) Quantification of positive area percentage (PAP) of NF-200 and MBP immunoreactivity (%) as characterized in (b) for each group; (e) Illustration of the calculation of orientation angle θ (i) and orientation angles of regenerated nerve fibers on (ii) sham, (iii) 1% Ca-Alg, (iv) 1 mg/mL pDScNM, (v) 5 mg/mL pDScNM, (vi) gradient pDScNM and (vii) Autograft groups. The values are presented as the mean ± SD, n = 3. * indicates statistical significance at P < 0.05 (versus 1% Ca-Alg).
As building blocks of glial bands of Büngner formation, the proliferation and migration of Schwann cells from both proximal and distal nerve stumps play a key role in nerve repair [15]. The fluorescence micrographs of s100 ꞵ and NF-200 stained sections uncovered that the host Schwann cells had migrated into the microtube and the regenerated axons had extended to different degrees in each group (Fig. S8, Supporting Information). Importantly, the expression of both s100 ꞵ and NF-200 were enhanced in the gradient pDScNM group, showing a better recovery level with chemotactic treatment. The represent immuno-stained photos of transverse nerve sections in the proximal, middle, and distal segments have further verified the promoting effect of gradient microtube on axonal regeneration and remyelination.
Angiogenesis is a vital process when wound healing and tissue reproduction [8,30]. After nerve injury, the newborn microvessels that offer oxygen and nutrients to the injured nerve are critical for nerve regeneration. Hence, the vascularization of the regenerated nerve in each group was evaluated. The more expression of CD31 found in the gradient pDScNM group revealed that the gradient microtube can promote nerve repair by stimulating angiogenesis and the effect is close to the autograft group (Fig. S9, Supporting Information). Additionally, the immune response in different groups was detected as well and the data showed no severe inflammation in all groups. To access the locomotor function recovery of the defected nerves, walking track analysis, electrophysiological assessment, and re-innervation and amyotrophy of target muscle were evaluated (Fig. S10 and Fig. S11, Supporting Information). The data indicated that the pDScNM microtube could promote nerve repairing and inhibit muscular atrophy. Meanwhile, using the gradient pDScNM microtube could further improve this therapeutic effect.
4. Conclusion
Herein, we developed a novel gradient pDScNM integrated microtube by using a programmable multiple-flow microfluidic method. After decellularization, digestion, and neutralization, the pDScNM hydrogel which retained most ECM components (Fibronectin, Laminin, and Collagen IV) and removed nuclei and nerve inhibitory components (MAG and chondroitin sulfate) were obtained. Additionally, the concentration-related manner of pDScNM in promoting the proliferation of SCs, PC12 cells, and axon outgrowth were observed in an in-vitro cell co-culturing study. Benefitting from the advanced control of fluids in microfluidic spinning, the ECM hydrogel microtubes were with tunable structures and sizes, especially the gradient dispersion of ECM, which enabled the SCs encapsulated in microtubes to form cellular fibers. The effect of the pDScNM microtube to improve nerve regeneration in vivo was assessed by bridging a 10-mm nerve defect. Consequently, the histomorphology of the regenerated nerve and the cross-section area (CSA) of the muscle fibers and SFI showed better axon regeneration and remyelination as well as functional recovery in the gradient pDScNM group than those in other groups. All of these attractive features convinced us that these gradient pDScNM microtubes will be a potential alternative nerve graft in repairing peripheral nerve defects.
Ethics approval and consent to participate
All animal procedures were conducted under ethical approval from the Animal Experimental Committee of Wenzhou Medical University.
CRediT authorship contribution statement
Binghui Jin: carried out the experiments, Writing – original draft. Yunru Yu: carried out the experiments, Writing – original draft. Xiangxiang Chen: contributed to the animal surgerycontributed to the animal surgery. Yanhong Yang: contributed to the animal surgery. Yushan Xiong: contributed to the animal surgery. Young Jun Im: assisted with the scientific discussion of the article. Yuanjin Zhao: Conceptualization, Methodology. Jian Xiao: Conceptualization, Methodology, All authors have given their consent to the final version of the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2020YFA0908200), the National Natural Science Foundation of China (81972150, 82172428 and 61927805), the Natural Science Foundation of Zhejiang (LR18H150001 and LQ22E030004), the Shenzhen Fundamental Research Program (JCYJ20190813152616459), the Key scientific and technological innovation projects of Wenzhou (ZY20200023), the Wenzhou Innovation Team (Growth factor drug development) (No. 201801) and CAMS Innovation Fund for Medical Sciences (2019-I2M-5-028).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.08.027.
Contributor Information
Yuanjin Zhao, Email: yjzhao@seu.edu.cn.
Jian Xiao, Email: xfxj2000@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dalakas M.C., Alexopoulos H., Spaeth P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020;16:601. doi: 10.1038/s41582-020-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sleigh J.N., Rossor A.M., Fellows A.D., Tosolini A.P., Schiavo G. Axonal transport and neurological disease. Nat. Rev. Neurol. 2019;15:691. doi: 10.1038/s41582-019-0257-2. [DOI] [PubMed] [Google Scholar]
- 3.Scheib J., Höke A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013;9:668. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 4.Roth J.G., Huang M.S., Li T.L., Feig V.R., Jiang Y., Cui B., Greely H.T., Bao Z., Paşca S.P., Heilshorn S.C. Advancing models of neural development with biomaterials. Nat. Rev. Neurosci. 2021;22:593. doi: 10.1038/s41583-021-00496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods. Acta Biomater. 2020;106:54. doi: 10.1016/j.actbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Gaharwar A.K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020;5:686. doi: 10.1038/s41578-020-0209-x. [DOI] [Google Scholar]
- 7.Spearman B.S., Desai V.H., Mobini S., McDermott M.D., Graham J.B., Otto K.J., Judy J.W., Schmidt C.E. Tissue-Engineered peripheral nerve interfaces. Adv. Funct. Mater. 2018;28 doi: 10.1002/adfm.201701713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G., Yu Y., Wu X., Wang G., Gu G., Wang F., Ren J., Zhang H., Zhao Y. Microfluidic electrospray niacin metal-organic frameworks encapsulated microcapsules for wound healing. Research. 2019;2019 doi: 10.34133/2019/6175398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W., Zhao L., Wang C., Zhou J. Conductive nanomaterials for cardiac tissues engineering - ScienceDirect. Eng. Regen. 2020;1:88. doi: 10.1016/j.engreg.2020.09.001. [DOI] [Google Scholar]
- 10.Sun L., Fan L., Bian F., Chen G., Wang Y., Zhao Y. MXene-integrated microneedle patches with innate molecule encapsulation for wound healing. Research. 2021;2021 doi: 10.34133/2021/9838490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou G., Chang W., Zhou X., Chen Y., Dai F., Anwar A., Yu X. Nanofibrous nerve conduits with nerve growth factors and bone marrow stromal cells pre-cultured in bioreactors for peripheral nerve regeneration. ACS Appl. Mater. Interfaces. 2020;12 doi: 10.1021/acsami.0c04191. [DOI] [PubMed] [Google Scholar]
- 12.Xi K., Gu Y., Tang J., Chen H., Xu Y., Wu L., Cai F., Deng L., Yang H., Shi Q., Cui W., Chen L. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat. Commun. 2020;11:4504. doi: 10.1038/s41467-021-23438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y.C., Chen M.H., Liao S.Y., Wu H.C., Kuan C.H., Sun J.S., Wang T.W. Multichanneled nerve guidance conduit with spatial gradients of neurotrophic factors and oriented nanotopography for repairing the peripheral nervous system. ACS Appl. Mater. Interfaces. 2017;9 doi: 10.1021/acsami.7b12567. [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Lv S., Yuan H., Ye G., Mu W., Fu Y., Zhang X., Feng Z., He Y., Chen W. Peripheral nerve regeneration with 3D printed bionic scaffolds loading neural crest stem cell derived schwann cell progenitors. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202010215. [DOI] [Google Scholar]
- 15.Dong X., Liu S., Yang Y., Gao S., Li W., Cao J., Wan Y., Huang Z., Fan G., Chen Q., Wang H., Zhu M., Kong D. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120767. [DOI] [PubMed] [Google Scholar]
- 16.Houshyar S., Bhattacharyya A., Shanks R. Peripheral nerve conduit: materials and structures. ACS Chem. Neurosci. 2019;10:3349. doi: 10.1021/acschemneuro.9b00203. [DOI] [PubMed] [Google Scholar]
- 17.Hassanzadeh P., Atyabi F., Dinarvand R. Tissue engineering: still facing a long way ahead. J. Contr. Release. 2018;279:181. doi: 10.1016/j.jconrel.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Zhu M., Li W., Dong X., Yuan X., Midgley A.C., Chang H., Wang Y., Wang H., Wang K., Ma P.X., Wang H., Kong D. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019;10:4620. doi: 10.1038/s41467-019-12545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasper M., Deister C., Beck F., Schmidt C.E. Bench-to-Bedside lessons learned: commercialization of an acellular nerve graft. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.202000174. [DOI] [PubMed] [Google Scholar]
- 20.Hussey G.S., Dziki J.L., Badylak S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018;3:159. doi: 10.1038/s41578-018-0023-x. [DOI] [Google Scholar]
- 21.Mayorca-Guiliani A.E., Willacy O., Madsen C.D., Rafaeva M., Elisabeth Heumüller S., Bock F., Sengle G., Koch M., Imhof T., Zaucke F., Wagener R., Sasaki T., Erler J.T., Reuten R. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat. Protoc. 2019;14:3395. doi: 10.1038/s41596-019-0225-8. [DOI] [PubMed] [Google Scholar]
- 22.Mouw J.K., Ou G., Weaver V.M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014;15:771. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J.-H., Li G., Wu T.-T., Lin Z.-J., Zou J.-L., Huang L.-J., Xu H.-Y., Wang J.-H., Ma Y.-H., Zeng Y.-S. Decellularization optimizes the inhibitory microenvironment of the optic nerve to support neurite growth. Biomaterials. 2020;258 doi: 10.1016/j.biomaterials.2020.120289. [DOI] [PubMed] [Google Scholar]
- 24.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giobbe G.G., Crowley C., Luni C., Campinoti S., Khedr M., Kretzschmar K., De Santis M.M., Zambaiti E., Michielin F., Meran L., Hu Q., van Son G., Urbani L., Manfredi A., Giomo M., Eaton S., Cacchiarelli D., Li V.S.W., Clevers H., Bonfanti P., Elvassore N., De Coppi P. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019;10:5658. doi: 10.1038/s41467-019-13605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin T., Liu S., Chen S., Qiu S., Rao Z., Liu J., Zhu S., Yan L., Mao H., Zhu Q., Quan D., Liu X. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects. Acta Biomater. 2018;73:326. doi: 10.1016/j.actbio.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Zou J.-L., Liu S., Sun J.-H., Yang W.-H., Xu Y.-W., Rao Z.-L., Jiang B., Zhu Q.-T., Liu X.-L., Wu J.-L., Chang C., Mao H.-Q., Ling E.-A., Quan D.-P., Zeng Y.-S. Peripheral nerve-derived matrix hydrogel promotes remyelination and inhibits synapse formation. Adv. Funct. Mater. 2018;28 doi: 10.1002/adfm.201705739. [DOI] [Google Scholar]
- 28.Zaokari Y., Persaud A., Ibrahim A. Biomaterials for adhesion in orthopedic applications: a review. Eng. Regen. 2020;1:51. doi: 10.1016/j.engreg.2020.07.002. [DOI] [Google Scholar]
- 29.Cerqueira S.R., Lee Y.S., Cornelison R.C., Mertz M.W., Wachs R.A., Schmidt C.E., Bunge M.B. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials. 2018;177:176. doi: 10.1016/j.biomaterials.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiakos G., Kuang Z., Lo E. Improved skin regeneration with acellular fish skin grafts. Eng. Regen. 2020;1:95. doi: 10.1016/j.engreg.2020.09.002. [DOI] [Google Scholar]
- 31.Xu Y., Zhou J., Liu C., Zhang S., Gao F., Guo W., Sun X., Zhang C., Li H., Rao Z., Qiu S., Zhu Q., Liu X., Guo X., Shao Z., Bai Y., Zhang X., Quan D. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120596. [DOI] [PubMed] [Google Scholar]
- 32.Zheng C., Yang Z., Chen S., Zhang F., Rao Z., Zhao C., Quan D., Bai Y., Shen J. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics. 2021;11:2917. doi: 10.7150/thno.50825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S., Du Z., Zou J., Qiu S., Rao Z., Liu S., Sun X., Xu Y., Zhu Q., Liu X., Mao H.Q., Bai Y., Quan D. Promoting neurite growth and schwann cell migration by the harnessing decellularized nerve matrix onto nanofibrous guidance. ACS Appl. Mater. Interfaces. 2019;11 doi: 10.1021/acsami.9b01066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.