Abstract
The field of radiation oncology is rapidly advancing through technological and biomedical innovation backed by robust research evidence. However, cancer professionals are notoriously time-poor, meaning there is a need for high quality, accessible and tailored oncologic education programs. While traditional teaching methods including lectures and other in-person delivery formats remain important, digital learning (DL) has provided additional teaching options that can be delivered flexibly and on-demand from anywhere in the world.
While evidence of this digital migration has been evident for some time now, it has not always been met with the same enthusiasm by the teaching community, in part due to questions about its pedagogical effectiveness. Many of these reservations have been driven by a rudimentary utilisation of the medium and inexperience with digital best-practice. With increasing familiarity and understanding of the medium, increasingly sophisticated and pedagogically-driven learning solutions can be produced.
This article will review the application of immersive digital learning tools in radiation oncology education. This includes first and second-generation Virtual Reality (VR) environments and Augmented Reality (AR). It will explore the data behind, and best-practice application of, each of these tools as well as giving practical tips for educators who are looking to implement (or refine) their use of these learning methods. It includes a discussion of how to match the digital learning methods to the content being taught and ends with a horizon scan of where the digital medium may take us in the future. This article is the second in a two-part series, with the companion piece being on Screen-Based Digital Learning Methods in Radiation Oncology.
Overall, the digital space is well-placed to cater to the evolving educational needs of oncology learners. Further uptake over the next decade is likely to be driven by the desire for flexible on demand delivery, high-yield products, engaging delivery methods and programs that are tailored to individual learning needs. Educational programs that embrace these principles will have unique opportunities to thrive in this space.
Keywords: Digital learning, Radiotherapy, Medical education, Virtual reality, Augmented reality, VR, Radiation Oncology, Cancer education, Online learning, Immersive learning, Simulated Learning Environment
Abbreviations: AR, Augmented Reality; VR, Virtual Reality; DL, Digital Learning; SLE, Simulated Learning Environment
Introduction
Radiation oncology practitioners are required to possess knowledge spanning a broad range of scientific domains including physics, cell biology, anatomy and pathology in addition to a range of technical and procedural skills [1], [2]. Given the breadth and complexity of the information involved, novel pedagogical methods that stimulate active processing and meaningful retention of information are particularly worthwhile. Contemporary Digital Learning (DL) provides a number of cutting-edge, immersive delivery methods that can help to meet this need.
While the majority of DL is screen-based (see our companion piece for a detailed discussion of these[3]) fully immersive, 3D learning environments have recently become available. This includes both Virtual Reality (VR) and Augmented Reality (AR) Environments. They provide the new, qualitatively distinct learning experiences of being placed in a first-person scenario for oneself and often include the ability to manipulate the environment as one would in real life [4], [5]. In addition, most implementations inherently apply principles of gamification in their delivery through strategic design elements to motivate and engage users, such as awarding badges, showing leaderboards, or performance graphs. As a result, they are generally highly engaging experiences and, when designed correctly, can be effective educational tools which can be utilised as an adjunct, or alternative, to conventional teaching methods.
While both the hardware, and educational science, behind immersive learning techniques are still maturing – the advances in both these areas in the past decade has already been significant [6], [7]. In particular, there is now widespread, relatively low-cost access to VR hardware which has resulted in increasing uptake in first-world markets. A 2021 survey of United States households found that 22 % were using VR on at least a monthly basis [8]. In 2020, a survey of 1781 clinicians in Europe showed 5 % were using virtual reality technology to support care delivery [9]. While this may be a relatively small proportion overall, when one considers the total number of clinicians in Europe, it is not an insignificant number. Furthermore, this number is only expected to grow in time [9] and, like many aspects of digital education, already received a significant boost in uptake as a result of the COVID-19 pandemic [10].
Given the application of these tools to medical education are relatively new, there remains a level of unfamiliarity with them in the cancer education teaching space. This article will review the application and best-practice use of the immersive DL tools. It will explore their application, and the data behind these, while also giving advice for educators who are looking to implement (or refine) their use of these learning methods and will end with a horizon scan of where digital education techniques may take us in the near-future.
Virtual reality
Virtual reality environments are the most common immersive learning environment currently in use. Virtual reality is defined as ‘a virtual environment that is designed to be perceived as reality’. VR has been utilised in medical education for over a decade now, with two different generations of technology.
First generation simulated learning environments (SLE’s) using VR
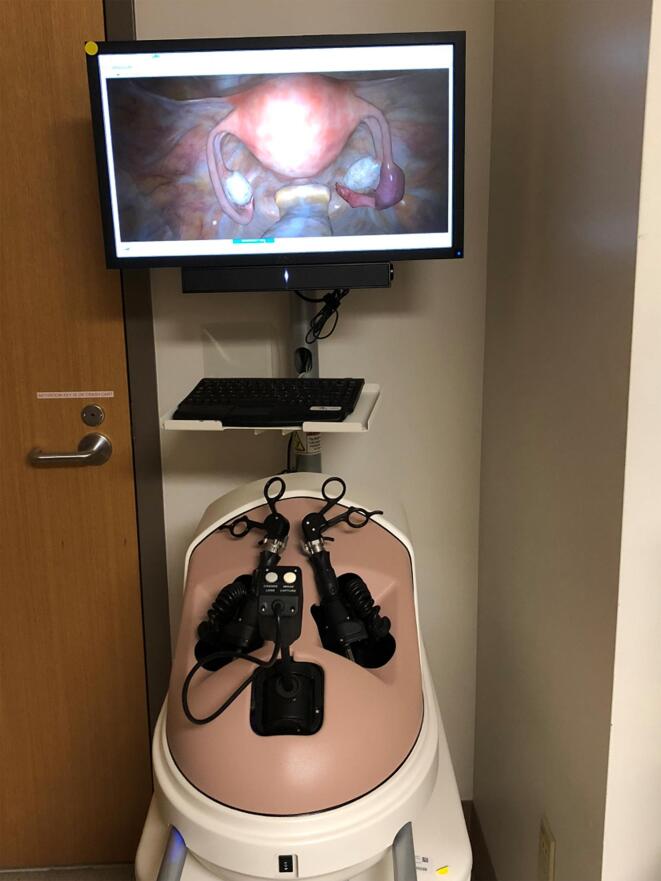
The first generation of SLE’s using VR were simulated environments where machines or devices were constructed to mimic or recreate an experience in real-life. The earliest first-generation VR simulators for medical education were created in the surgical field, which had a particularly strong need for improved laparoscopic surgery training techniques [11]. The VR stations created comprised a physical laparoscopic instrument interface, the VR software, and a screen. Used together they closely replicate the experience of performing laparoscopic surgery as depicted in Fig. 1.
Fig. 1.
Laparoscopy simulator comprising facsimile instruments and 2D screen visualisation (reproduced from Molina et al [12] under Creative Commons Licence).
The efficacy of this intervention was shown in a landmark study by Larsen et al., who randomly compared the real life surgical performance of surgical resident staff who trained on the VR simulator versus a group who had only trained on real patients. They demonstrated a substantial objective superiority of the VR simulator trained doctors in both technical performance, and speed, over those who had trained only on real patients [13].
Radiotherapy similarly pioneered a first generation VR solution for training of radiation therapists (RTTs) in the early 2010s. Known as Virtual Reality for Radiation Training (VERT), this VR experience comprises a (typically life-size) projection onto a screen or wall that, when viewed with 3D shutter glasses recreates the experience of being in a treatment bunker with a radiotherapy linear accelerator, complete with controls to move the bed, and gantry as in real life [14] (it can also be used without the 3D effect simply as a 2D projection as depicted in Fig. 2).
Fig. 2.
Students using VERT to view a projection of a linear accelerator, complete with controls that be used to operate it (Used with permission from Wijeysingha et al [15]).
VERT has had global uptake and currently more than 30 countries utilise the VERT system worldwide [14]. One of its key advantages is providing access to what are otherwise extremely in-demand pieces of equipment. For example its introduction to a clinical department in Aarhus, Denmark, allowed for a doubling in capacity of clinical training places for radiotherapy staff [16]. In addition it enables students to practice it in a risk-free environment so they may feel fully free to acquaint themselves with all aspects of the technology [17]. It also demonstrated particular benefits in improving students understanding and awareness of spatial concepts that would be otherwise difficult to convey, [18] such as in radiotherapy planning [19].
Some of VERT’s limitations are that it is still primarily a group-training rather than individual-training device, and it still requires centralised access to the equipment, meaning students must make themselves available to attend training at certain times and locations. In addition, the system allows only one user to practice using the linear accelerator ‘controls’ at a time. Finally, it still requires a moderately spacious room to be operated in and has limited portability. In short, while it is an excellent training experience, it does share significant scalability limitations that are typical of other first generation VR solutions [20].
Second generation SLE’s using fully immersive VR
In recent years, commercial VR headsets have become increasingly available and widespread. The two most utilised devices are the Oculus (Meta) and HTC Vive lines of hardware. Both are headsets that, when worn, completely replace the field of vision in both eyes with stereoscopic screens. Combined with built-in processing hardware, they generate imagery that the brain interprets in 3 dimensions. Since it obscures any ability to see outside of the headset, the user is completely immersed and transported in first-person to an entirely different virtual environment [21]. In addition, newer systems allow for hand, body and even eye tracking in an effort to allow users to simulate a level of interaction within the virtual environment in a safe, reproducible and deterministic manner [22].
This means the range of experiences that a learner can experience in this virtual environment are only limited by the technical expertise – and imagination – of the programmer. Re-creation of complete radiation oncology clinical environments with dynamic interactive patients, including clinic spaces, radiotherapy computerized tomography (CT) simulation machines and linear accelerators have all been created [23], [24], [25], although only one of these is commercially available currently, the Clinical Education Training Solution (CETSOL) immersive VR environment, depicted in Fig. 3 [23], [26].
Fig. 3.
Fully immersive radiation therapy environment. Top – Learner wearing a 3D stereoscopic headset (with on-screen image showing indicative view of what the learner is seeing). Bottom – Radiotherapy Linear accelerator as depicted in the 3D environment.
Utilising fully immersive VR environments for radiotherapy training is relatively new, but there are several clear qualitative and quantitative benefits [7]. Qualitatively, fully immersive first-person solutions are not limited by the scalability issues inherent in most first-generation solutions. If a user has a headset and a few metres of floorspace, they will be able to undertake the learning experience at any time and in any locale. In addition, there is a much wider range of potential interaction with the environment (and/or patient) given it is a first-person experience.
The data for its educational utility is nascent, but growing. In an initial study utilising the CETSOL system, we trained radiotherapy students in patient positioning using both an immersive VR environment and a conventional screen-based simulated environment. Students self-rated competence levels following the training were on average 24 % higher for using the immersive VR environment [23]. Also, when the same immersive VR software was compared to clinical role-play, considered to be the current gold-standard for clinical training, there was no statistically significant difference in students perception in their final ability [26]. Moreover, when assessing real world clinical radiotherapy positioning of actual patients, students trained using the fully immersive VR clinic SLE performed significantly better than the cohort who had been trained using real-life clinical role play counterparts [27].
Thus, the empirical data suggests that immersive second-generation VR experiences have a role to play in oncology education moving forward, as an effective teaching tool. They do, however, suffer from production issues similar to educational videos and other immersive teaching tools; namely being highly time-consuming and expensive to produce and generally requiring third-party expertise to develop. In addition, there is currently limited evidence to guide best-practice pedagogical design of immersive VR learning experiences. A discussion and commentary around specific design principles that could be applied to second generation VR learning are detailed in the section ‘Choosing the right learning tool for the right purpose’.
Augmented reality
Augmented reality (AR) is an emerging learning environment where there is real-time integration of digital objects on top of the real-world environment. In doing so, it provides the opportunity to embellish or re-frame real world experiences. For augmented reality to be possible, it requires a device to sit as an intermediary between the learner’s vision and the real world. Then, instead of simply displaying what is already visible, it actively processes and overlays objects on top of the ‘real-world’ view so that they appear to be there in actuality [5], [28].
The capability, and public appetite, for AR for everyday hand-held devices like smartphones and tablets to create an augmented reality was demonstrated with the viral phenomenon PokemonGo [29]. In this – fairly simple – implementation of augmented reality, players use their phone cameras to view real-world locations. Pokemon characters are added so as they appear to be in real world locations.
The evidence for the benefit of educational AR environments is currently maturing. However, in general it is believed to share many of the same benefits as VR including giving unlimited time to practice on what is usually a difficult to access resource, enhancing learning through immediate feedback [30], [31], and providing spatial conceptualisation of otherwise difficult to represent structures [32].
While there are no currently available commercial AR environments for radiotherapy training, there are working prototypes of such software which would allow a scaled, fully 3D-version of a radiotherapy medical linear accelerator (LINAC) to be inserted into a real-life locality [33] – whether that is the radiotherapy clinic or the patient’s living room. This would theoretically enable a teaching experience for both professional staff and patients who have never seen a radiotherapy machine to be become acquainted with it, understand how it works and interact with it. Images of a prototype in action are depicted in Fig. 4 & Video 1.
Fig. 4.
AR representation of linear accelerator overlayed on a kitchen table.
As AR capability becomes more advanced, even more sophisticated applications of AR may be able to be brought into play. Instead of simply inserting objects into reality, one can insert information into reality. This allows a seamless implementation of ‘just-in-time-learning’ - the emerging pedagogical technique where information is delivered to learner exactly at the exact moment of need, rather than in a bolus format, weeks or months removed from when the learner is actually going to utilise it [34].
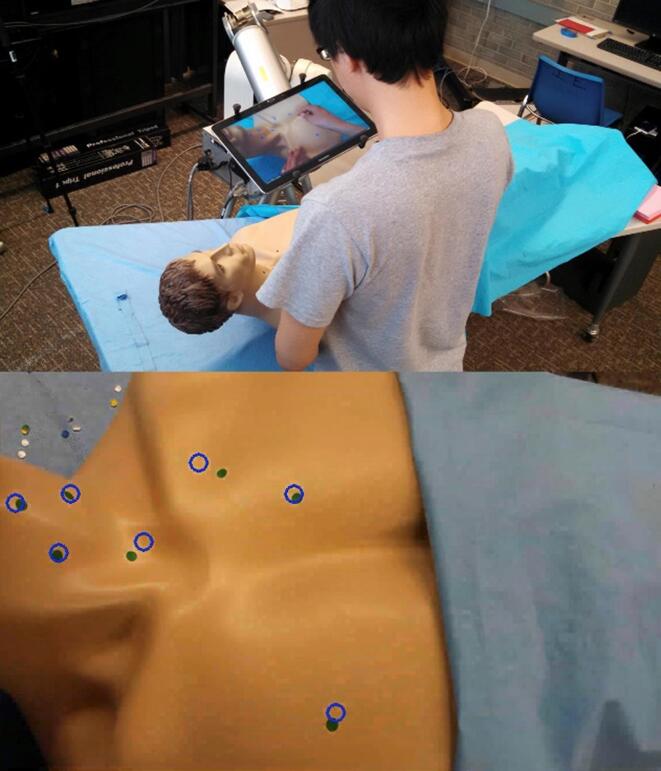
Simple 2D screen-based applications of this already exist and have been trialled in surgical training, with a remote mentor able to provide visual annotation to assist with correct anatomical locations to perform procedures (Fig. 5) [35]. This tool improved learner proficiency in the specific task at hand, although at the trade-off of performing the procedure more slowly.
Fig. 5.
Example of an existing 2D Augmented Reality visual overlay where a remote mentor can provide visual annotations so that a trainee knows the appropriate locations to perform procedures. (Used with permission from Anderson et al [35]).
No radiotherapy application of this currently exists, but a theoretical application of the same 2D technology could be utilised in brachytherapy or fibreoptic laryngoscopy, as depicted in Fig. 6. Here, a junior oncology staff member is performing the fibreoptic laryngoscopy, with the laryngoscope image being viewed by a remote mentor(s) who annotates the view in real time and can also discuss with the trainee what they are seeing via a phone linkup.
Fig. 6.
Theoretical implementation of a simple 2D Augmented Reality setup for fibreoptic laryngoscopy. The laryngoscope image is simultaneously being viewed by a remote mentor(s) who annotates the view in real time and can also discuss with the trainee via a phone linkup.
In the near-future, AR headsets may be able to seamlessly bring information into any clinical space. They would allow a wearer to view pertinent information on a medical procedure as they were performing it, without ever needing step or look-away from the procedure.
Choosing the right learning tool for the right purpose
As this review demonstrates, when designing an educational experience, there are a multitude of advanced digital learning techniques at the educator’s disposal; no single learning technique or environment is going to be superior in all circumstances. Different learning tools have specific affinities for teaching different types of material. Education designers must ensure there is alignment between intended learning outcomes, learning activities and assessment [36].
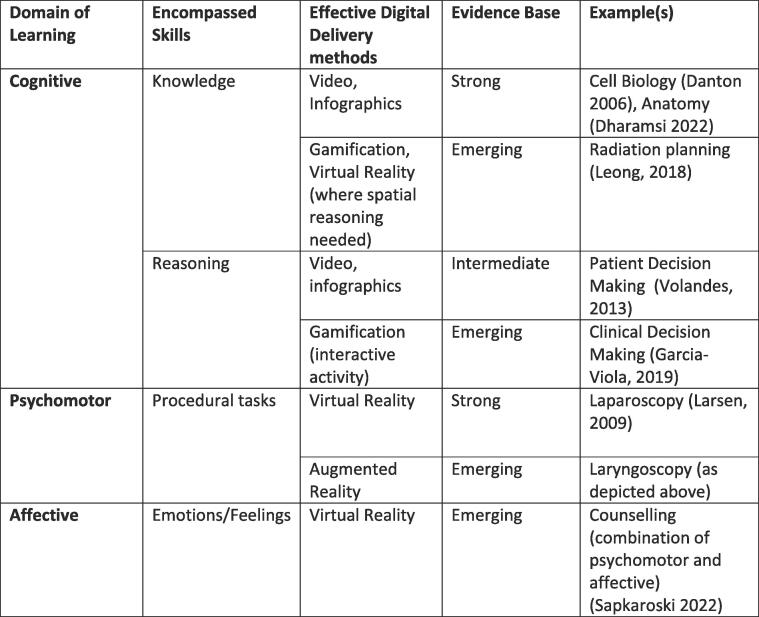
Therefore, choosing the appropriate tool for the right purpose is often as important as the design of the experience itself. To do this effectively, it is worth considering the three major domains of learning: Cognitive – which comprises acquired knowledge and reasoning skills; Affective – which includes emotions; and the Psychomotor – which includes procedural tasks and actions [37] (Fig. 7).
Fig. 7.
Effective Digital Delivery Methods and the Domains of Learning [13], [19], [38], [39], [40], [41], [42].
Thus far, the vast majority of research into Immersive Learning Environments has focused around its effectiveness for cognitive learning [4]. While VR is indeed effective for teaching knowledge based material [43], immersive environment instruction does not necessarily outperform 2D video based instruction, with numerous medical education studies proving comparable outcomes between the two methods [44], [45], [46].
When one considers some of the qualitative benefits afforded by immersive 3D environments, it is likely that over time, immersive 3D environments will outperform 2D methods for cognitive learning that inherently includes the need to visualise or organise information in a 3D-spatial manner. For other cognitive learning, a well-designed screen-based experience utilising best practice learning theory may produce an equally effective learning experience.
The 3D immersive environments do have a clear qualitative edge for procedural and affective learning. When teaching a procedure that requires psychomotor training, hand-eye coordination and familiarisiation with unique environments or equipment – the educational benefits of being placed into these environments in the first person, to practice/experiment without time constraints and in a low-stakes situation, are self-evident and are consistent with published medical education literature. It is in this domain that both VERT [18] and the CETSOL [27] VR simulations currently inhabit. It is likely there will be further growth in this area in years to come, with brachytherapy training being an obvious procedural technique that could benefit from an immersive teaching method.
Patient interviewing and counselling represents a combination of both affective and procedural skills. With a VR format, trainees can meet with multiple virtual patients facing various difficult scenarios such as a new cancer diagnosis, consenting for treatment, shifting goals of care, etc. This is important given the high stakes nature of these conversations in the clinic, as patient-reported anxiety and depression is linked to their provider’s communication skills [47], [48]. We have now produced two different VR-based interview training modules for oncology professionals [23], [49]. In these, trainees interact with virtual patients either through voice-activated dialogue or by choosing from a variety of pre-scripted responses. Each conversation is automatically mapped against pre-determined template responses and a transcript can be reviewed at the end of the consultation alongside expert feedback on the student’s chosen response. Preliminary data from these modules suggests VR-based training was effective in eliciting empathic communication skills - possibly even more so than those trained in real-life role play practice [39]. These benefits likely derive from VRs ability to provide unlimited practice with minimal pressure, both of which can be limiting factors in conventionally interview training with simulated patients. Therefore, interview and counselling training modules are likely to become more prevalent as the technology matures and more authentic, true-to-life interactions with virtual patients become possible.
All in all, there is great potential for immersive environments to be used for affective and procedural training, although further refinements of both hardware and software are still necessary to produce an optimal learning experience.
Conclusion
The digital space is well-placed to cater to the evolving educational needs of oncology learners. Novel, fully immersive learning environments such as VR and AR have the potential to provide first-person learning experiences that were previously impossible. By being able to simulate use of otherwise difficult to access machinery, and provide unlimited, risk-free practice of procedures, they have the potential to solve both pedagogical and logistical bottlenecks for educators. Evidence-based selection and design of these experiences is necessary to provide the best possible experience for learners. Educational programs that embrace these principles will have unique opportunities to thrive in this space.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Daniel Sapkaroski is the Research Development Lead of Clinical Education Training Solutions (CETSOL).].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2022.08.007.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.American Board of Radiology Initial Certification for Radiation Oncology - Studying for the Exam. 2022 20/08/2022]; Available from: https://www.theabr.org/radiation-oncology/initial-certification/the-qualifying-exam/studying-for-the-exam.
- 2.Radiation Oncology Curriculum Editorial Team Radiaition Oncology Training Program Curriculum. 2012, Royal Australian and New Zealand College of Radiologists.
- 3.Kok D.L., et al. Screen-Based Digital Learning Methods in Radiation Oncology Education - A Review and Expert Commentary. Technical Innovat Patient Support Radiat Oncol. 2022 doi: 10.1016/j.tipsro.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton D., et al. Immersive virtual reality as a pedagogical tool in education: a systematic literature review of quantitative learning outcomes and experimental design. J Computers Educ. 2021;8(1):1–32. [Google Scholar]
- 5.Lee K. Augmented Reality in Education and Training. TechTrends. 2012;56(2):13–21. [Google Scholar]
- 6.Rosenfeldt Nielsen M., et al. Clinical Ultrasound Education for Medical Students: Virtual Reality Versus e-Learning, a Randomized Controlled Pilot Trial. Ultrasound Q. 2021;37(3):292–296. doi: 10.1097/RUQ.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 7.van der Linde-van den Bor M., et al. The use of virtual reality in patient education related to medical somatic treatment: A scoping review. Patient Educ Couns. 2022;105(7):1828–1841. doi: 10.1016/j.pec.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Vorhaus, Digital Strategy Study 2022. 2022, Vorhaus.
- 9.Taylor K., Hall B., Siegel S. Deloitte Centre for Health Solutions; United Kingdom: 2020. Digital Transformation - Shaping the future of European Healthcare. [Google Scholar]
- 10.Ball C., Huang K.-T., Francis J. Virtual reality adoption during the COVID-19 pandemic: A uses and gratifications perspective. Telematics Inform. 2021;65:101728. doi: 10.1016/j.tele.2021.101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruparel R.K., et al. Assessment of Virtual Reality Robotic Simulation Performance by Urology Resident Trainees. J Surgical Educ. 2014;71(3):302–308. doi: 10.1016/j.jsurg.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Molina M.M., et al. Development of in-situ simulation lab for training gynecology residents in basic laparoscopic and hysteroscopic operative skills. Cureus. 2019;11(4) doi: 10.7759/cureus.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen C.R., et al. Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ. 2009;338:b1802. doi: 10.1136/bmj.b1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VertualLimited, VERT™ 2014.
- 15.Wijeysingha E.S., Chin V.Y., Lian C.P. Utilising virtual environments for radiation therapy teaching and learning. J Medical Imaging Radiat Sci. 2021;52(4):S83–S95. doi: 10.1016/j.jmir.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Boejen A., Grau C. Virtual reality in radiation therapy training. Surg Oncol. 2011;20(3):185–188. doi: 10.1016/j.suronc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Bridge P., et al. A virtual radiation therapy workflow training simulation. Radiography. 2016;22(1):e59–e63. [Google Scholar]
- 18.Jimenez Y.A., et al. Successful implementation of Virtual Environment for Radiotherapy Training (VERT) in Medical Physics education: The University of Sydney’s initial experience and recommendations. Australas Phys Eng Sci Med. 2017;40(4):909–916. doi: 10.1007/s13246-017-0592-9. [DOI] [PubMed] [Google Scholar]
- 19.Leong A., Herst P., Kane P. VERT, a virtual clinical environment, enhances understanding of radiation therapy planning concepts. J Med Radiat Sci. 2018;65(2):97–105. doi: 10.1002/jmrs.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wijeysingha E.S., Chin V.Y.W., Lian C.P.L. Utilising virtual environments for radiation therapy teaching and learning. J Med Imaging Radiat Sci. 2021;52(4, Supplement):S83–S95. doi: 10.1016/j.jmir.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Matthews T., Tian F., Dolby T. Interaction design for paediatric emergency VR training. Virtual Real Intell Hardware. 2020;2(4):330–344. [Google Scholar]
- 22.Whitmire E., et al. Proceedings of the 2016 ACM International Symposium on Wearable Computers. Association for Computing Machinery; Heidelberg, Germany: 2016. EyeContact: scleral coil eye tracking for virtual reality; pp. 184–191. [Google Scholar]
- 23.Sapkaroski D., et al. The implementation of a haptic feedback virtual reality simulation clinic with dynamic patient interaction and communication for medical imaging students. J Med Radiat Sci. 2018;65(3):218–225. doi: 10.1002/jmrs.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan V.S., et al. Computational Science – ICCS 2022. Springer International Publishing; Cham: 2022. Virtual Reality Prototype of a Linear Accelerator Simulator for Oncological Radiotherapy Training. [Google Scholar]
- 25.Bannister H., et al. 25th ACM Symposium on Virtual Reality Software and Technology. Association for Computing Machinery; Parramatta, NSW, Australia: 2019. LINACVR: VR Simulation for Radiation Therapy Education. p. Article 79. [Google Scholar]
- 26.Sapkaroski D., Mundy M., Dimmock M.R. Virtual reality versus conventional clinical role-play for radiographic positioning training: A students' perception study. Radiography. 2020;26(1):57–62. doi: 10.1016/j.radi.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Sapkaroski D., et al. Quantification of Student Radiographic Patient Positioning Using an Immersive Virtual Reality Simulation. Simul Healthcare. 2019;14(4) doi: 10.1097/SIH.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 28.Kidd S.H., Crompton H. In: Mobile Learning Design: Theories and Application. Churchill D., editor. Springer Singapore; Singapore: 2016. Augmented Learning with Augmented Reality; pp. 97–108. [Google Scholar]
- 29.Peyer RD. Pokemon Go: Huge crowd of players rush into Central Park to catch Vaporeon. In: The Evening Standard. United Kingdom; 2016.
- 30.Logishetty K., et al. Can an Augmented Reality Headset Improve Accuracy of Acetabular Cup Orientation in Simulated THA? A Randomized Trial. Clin Orthop Relat Res. 2019;477(5):1190–1199. doi: 10.1097/CORR.0000000000000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alismail A., et al. Augmented reality glasses improve adherence to evidence-based intubation practice. Adva Med Educ Practice. 2019;10:279. doi: 10.2147/AMEP.S201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons D., MacCallum K. Current Perspectives on Augmented Reality in Medical Education: Applications, Affordances and Limitations. Adv Med Educ Practice. 2021;12:77–91. doi: 10.2147/AMEP.S249891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopalakrishnan K., et al. 6th Kuala Lumpur International Conference on Biomedical Engineering 2021. Springer International Publishing; Cham: 2022.. Development of a Mobile Augmented Reality System for Radiotherapy Practitioner Training. [Google Scholar]
- 34.Kester L., et al. Just-in-time information presentation and the acquisition of complex cognitive skills. Comput Hum Behav. 2001;17(4):373–391. [Google Scholar]
- 35.Andersen D., et al. Medical telementoring using an augmented reality transparent display. Surgery. 2016;159(6):1646–1653. doi: 10.1016/j.surg.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Biggs J. Enhancing teaching through constructive alignment. High Educ. 1996;32(3):347–364. [Google Scholar]
- 37.Bloom B.S., et al. David McKay; New York: 1956. Handbook I: cognitive domain. [Google Scholar]
- 38.O'day D.H. Animated cell biology: A quick and easy method for making effective, high-quality teaching animations. CBE—Life Sci Educ. 2006;5(3):255–263. doi: 10.1187/cbe.05-11-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapkaroski D., Mundy M., Dimmock M.R. Immersive virtual reality simulated learning environment versus role-play for empathic clinical communication training. J Med Radiat Sci. 2022;69(1):56–65. doi: 10.1002/jmrs.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dharamsi M.S., et al. Efficacy of Video-Based Forearm Anatomy Model Instruction for a Virtual Education Environment. J Medical Educ Curricular Develop. 2022;9 doi: 10.1177/23821205211063287. 23821205211063287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volandes A.E., et al. Randomized controlled trial of a video decision support tool for cardiopulmonary resuscitation decision making in advanced cancer. J Clin Oncol. 2013;31(3):380. doi: 10.1200/JCO.2012.43.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Viola A., et al. The influence of gamification on decision making in nursing students. J Nurs Educ. 2019;58(12):718–722. doi: 10.3928/01484834-20191120-07. [DOI] [PubMed] [Google Scholar]
- 43.Johnston A.P.R., et al. Journey to the centre of the cell: Virtual reality immersion into scientific data. Traffic. 2018;19(2):105–110. doi: 10.1111/tra.12538. [DOI] [PubMed] [Google Scholar]
- 44.Greenwald S.W., et al. Comparing Learning in Virtual Reality with Learning on a 2D Screen Using Electrostatics Activities. J Univers Comput Sci. 2018;24(2):220–245. [Google Scholar]
- 45.Moro C., et al. The effectiveness of virtual and augmented reality in health sciences and medical anatomy. Anatomical Sci Educ. 2017;10(6):549–559. doi: 10.1002/ase.1696. [DOI] [PubMed] [Google Scholar]
- 46.Harrington C.M., et al. 360° Operative Videos: A Randomised Cross-Over Study Evaluating Attentiveness and Information Retention. J Surg Educ. 2018;75(4):993–1000. doi: 10.1016/j.jsurg.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Lobb E.A., et al. Communication and information-giving in high-risk breast cancer consultations: influence on patient outcomes. Br J Cancer. 2004;90(2):321–327. doi: 10.1038/sj.bjc.6601502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schofield P.E., et al. Psychological responses of patients receiving a diagnosis of cancer. Ann Oncol. 2003;14(1):48–56. doi: 10.1093/annonc/mdg010. [DOI] [PubMed] [Google Scholar]
- 49.Kok D.L., et al. The Development and Piloting of a Virtual Reality Patient Consultation Simulation to Improve Oncology Practitioners Communication and Counseling Skills. Int J Radiat Oncol Biol Phys. 2021;111(1):e18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.