Abstract
Osteoporosis (OP) is one of the most commonly known extra-articular complications of rheumatoid arthritis (RA). Since the prevalence of OP is diverse in different studies and there is no general consensus about it, in this systematic review, we aimed to investigate the global prevalence of OP among RA patients. In this review, three databases including Medline via PubMed, Scopus, and Web of Science (Clarivate analytics) were searched by various keywords. After screening of retrieved papers, the related data of included papers were extracted and analyzed. To assess the risk of methodological bias of included studies, quality assessment checklist for prevalence studies was used. Because of heterogeneity among studies, random-effect model was used to pooled the results of primary studies. In this review, the results of 57 studies were summarized and the total included sample size was 227,812 cases of RA with 64,290 cases of OP. The summary point prevalence of OP among RA was estimated as 27.6% (95%CI 23.9–31.3%). Despite significant advances in prevention, treatment and diagnostic methods in these patients, it still seems that the prevalence of OP in these patients is high and requires better and more timely interventions.
Subject terms: Medical research, Rheumatology, Risk factors
Introduction
Rheumatoid arthritis (RA) is one of the most common autoimmune diseases that in the early stages of the disease begins with pain and symmetrical swelling of the small joints of the hands, feet, swelling of the soft tissue around the joint and morning stiffness and fatigue1–4 and it is characterized by persistent synovitis and progressive destruction of symmetrical multi-joints and intra-articular manifestations including subchondral lesions, decreased bone mass, and reduced generalized bone density4–7. The prevalence of RA in the general population is about 1%, but is more common in the 50 s and 60 s and is higher in women than men8,9.
Osteoporosis (OP) is one of the most known common extra-articular complications of RA10 and its prevalence in RA patients is almost twice that of the general population4,11,12. OP is a systemic skeletal disease characterized by decreased bone mineral density and its complication (increased fragility and fracture due to reduced resistance to torsion and compression)7,13. Bone fragility in people with RA includes a combination of systemic inflammation, circulating autoantibodies, and proinflammatory cytokines (IL1, IL6, TNF, etc.)11,14. Chronic inflammation in people with RA affects bone metabolism and disrupts the normal resorption cycle and reduces localized and generalized bone mineral density (BMD)15.
Decreased bone mass can also be affected by factors such as disease severity, gender, especially after menopause, decreased vitamin D levels, advanced age, using corticosteroids and disease-modifying anti-rheumatic drugs (DMRADs) and decreased mobility12,16. In the US, data show that osteoporotic fractures account for about one-third of RA-related mortality5. Fractures increase morbidity and mortality, reduce quality of life, reduce independent functioning of people, especially in old age, and increase economic burden6,17. Vertebral fracture is one of the most common fractures due to decreased BMD, which causes limitation of activity, disability, kyphosis and decreased pulmonary function10,18,19.
The diagnosis of OP is made by measuring bone marrow density by dual x ray absorptiometry of the lumbar vertebrae, which according to World Health Organization (WHO) classification: T > − 1 is normal, − 1 > T > − 2.5 is osteopenia and T < − 2.5 is OP20.
Despite advances in the identification of the destructive mechanism and pharmacological treatment of RA, the complications associated with this disease are still common. So, screening and assessing the prevalence of OP and proper management, especially in relation to timely identification, is essential to prevent fractures. For this reason, in this study, we systematically reviewed the international databases and the results of related papers were pooled regarding the prevalence of OP.
Methods
Study design
This is a systematic review and meta-analysis study. In this study, three international databases were systematically searched using different keywords. The “Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)”21 and “Cochrane Handbook for Systematic Reviews of Interventions”22 were used to report the results.
Search strategy
To find related articles, a combination of related keywords was used in three databases including Medline via PubMed, Scopus, and Web of Science (Clarivate analytics). The keywords used included a combination of the suggested words by Medical Subject Heading (MeSH) and other related words. The search query used in PubMed was as follows: ((("Arthritis, Rheumatoid"[Mesh] OR "Rheumatoid Arthritis"[tw] OR "Rheumatoid"[tw]) AND ("Osteoporosis"[Mesh] OR "Osteoporosis"[tw] OR "Osteoporo*"[tw] OR " Bone Loss"[tw] OR "Osteopenia"[tw] OR "Bone Density"[Mesh] OR "Bone Density"[tw] OR "Bone Mineral Density"[tw])) AND ("Prevalence"[Mesh] OR "Incidence"[tw] OR "Epidemiology"[Mesh] OR "epidemiology" [Subheading] OR "Incidence"[Mesh] OR "Incidence"[tw])) NOT ("Clinical Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] OR "Clinical Trial, Phase III" [Publication Type]). Finally, the search filtered to human studies and English language studies. The adapted keywords were used to search in Scopus and Web of Science databases. The detailed search strategy was presented in Box 1. Databases were searched by two authors (AAH and SM) on June 22, 2021, and to find gray literatures, Google Scholar, and references of remaining articles manually searched.
Box 1.
The search strategy in PubMed.
| Search | Query | Results |
|---|---|---|
| #5 | Search: ((("Arthritis, Rheumatoid"[Mesh] OR "Rheumatoid Arthritis"[tw] OR "Rheumatoid"[tw]) AND ("Osteoporosis"[Mesh] OR "Osteoporosis"[tw] OR "Osteoporo*"[tw] OR " Bone Loss"[tw] OR "Osteopenia"[tw] OR "Bone Density"[Mesh] OR "Bone Density"[tw] OR "Bone Mineral Density"[tw])) AND ("Prevalence"[Mesh] OR "Incidence"[tw] OR "Epidemiology"[Mesh] OR "epidemiology" [Subheading] OR "Incidence"[Mesh] OR "Incidence"[tw])) NOT ("Clinical Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] OR "Clinical Trial, Phase III" [Publication Type]) Filters: Humans, English Sort by: Most Recent | 527 |
| #4 | Search: "Clinical Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] OR "Clinical Trial, Phase III" [Publication Type] Sort by: Most Recent | 897,690 |
| #3 | Search: "Prevalence"[Mesh] OR "Incidence"[tw] OR "Epidemiology"[Mesh] OR "epidemiology" [Subheading] OR "Incidence"[Mesh] OR "Incidence"[tw] Sort by: Most Recent | 2,895,709 |
| #2 | Search: "Osteoporosis"[Mesh] OR "Osteoporosis"[tw] OR "Osteoporo*"[tw] OR " Bone Loss"[tw] OR "Osteopenia"[tw] OR "Bone Density"[Mesh] OR "Bone Density"[tw] OR "Bone Mineral Density"[tw] Sort by: Most Recent | 166,724 |
| #1 | Search: "Arthritis, Rheumatoid"[Mesh] OR "Rheumatoid Arthritis"[tw] OR "Rheumatoid"[tw] Sort by: Most Recent | 162,057 |
Study selection and screening
To find and screen related articles, all retrieved articles were entered into Endnote software, and duplicate articles were first identified and removed. Then, in the next step, the articles were screened in terms of title and abstract, and the irrelevant articles were deleted. In the next step, the full text of the related articles was screened, and the articles that met the inclusion criteria and related data were studied and the required information was extracted from them. All these steps were performed by two authors (SM and AAH) independently and in case of disagreement between the two authors, a decision was made after consultation.
Inclusion and exclusion criteria
Articles with English full-text that were indexed in desired databases up to June 22, 2021 (from 1962 to 2021) were searched and there was no publication time limit. All observational studies in which the prevalence of OP has been reported in patients with RA have been included in the study. All clinical trials, letter to editor, editorials, review articles, commentaries, case reports, case series studies and papers with no relevant data were excluded.
Data extraction
The required data were extracted from the articles by two authors (SM and AAH) and in case of disagreement, the final decision was made after consultation. The extracted data were entered into a designed checklist in Excel software. This data includes first author’s name, year of publication, duration of patient’s recruitment, mean age, mean of disease duration, countries, the score of risk of bias, sample size, number of cases with OP and prevalence of OP.
Risk of bias
To assess the risk of bias of included studies, quality assessment checklist for prevalence studies which was developed by Hoy et al.23 was used. This checklist consists of nine items, each item has a score of 0 or 1. The score of 0 indicates the low risk and score of 1 indicates the high risk. The total score of checklists ranges from 0 to 9, which categorized in three levels; 0–3, 4–6 and 7–9 as low, moderate and high risk, respectively.
Statistical analysis
The I2 statistic with as well as chi-square test was used to assess the heterogeneity across the included studies. The results revealed that there was noteworthy heterogeneity between studies, and a meta-regression to find the source of heterogeneity and a subgroup analysis were done, and because of heterogeneity, the random-effect model was used to pooled the extracted prevalence with “metaprop” command24. Egger’s linear regression and funnel plot were used to explore the publication bias and trim and fill method was used to estimate the prevalence in case of publication bias. To recognize the effect of each study on the pooled prevalence, a sensitivity analysis was conducted. All analyses were conducted using Stata software version 13 (Stata Corp, College Station, TX, USA).
Ethics approval and consent to participate
This study was approved by Ethical Committee of Arak University of Medical Sciences (Code: IR.ARAKMU.REC.1399.259).
Result
Study selection and study characteristics
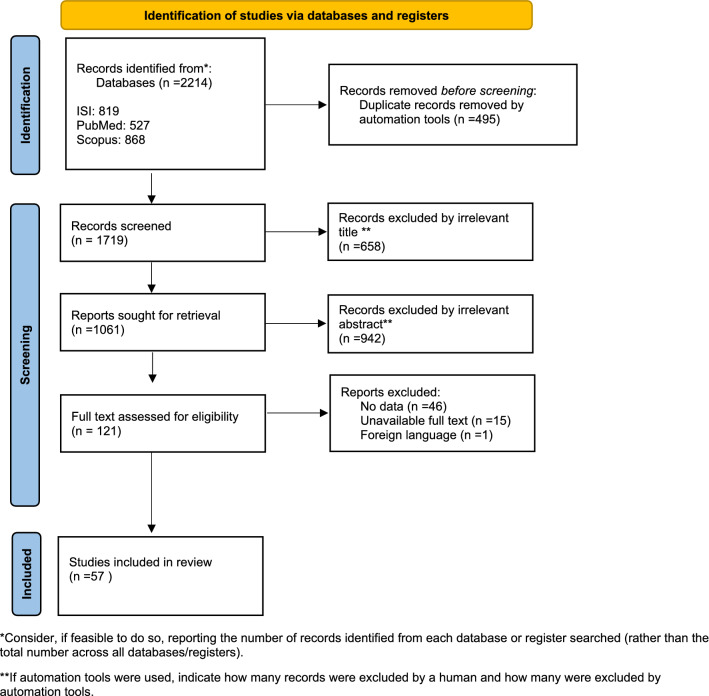
The process of study selection is presented in the PRISMA flow diagram25 (Fig. 1). First, after searching the desired databases, we retrieved 2214 primary studies (PubMed/Medline: 527, Scopus: 868, and Web of Science: 819). Then, 495 articles were removed due to duplication and 1719 studies were screened by title and abstract. Next, 658 papers were excluded by irrelevant title and 942 papers were excluded by irrelevant abstract. After that, the full text of 121 remained papers were assessed for eligibility and 62 papers were excluded (no data: 46 papers, unavailable full text: 15 papers and foreign language: 1 paper). Finally, data from 57 articles1,3,4,7,8,11,13,16,18,20,26–72 were entered into the meta-analysis.
Figure 1.
Flow diagram of the literature search for studies included in meta-analysis.
The sample size of imported articles ranged from 37 to 142,955. The oldest article was in 1962 and the most recent article was in 2021, and the reported prevalence of OP among RA patients varied from 3.7% to 62.2%. Further details regarding the selected studies are described in Table 1.
Table 1.
Characteristics of the primary studies included in the meta-analysis.
| Id | Author | Year | Countries | Prevalence | Sample size | Mean age | Disease duration | Risk score | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Venter G | 2021 | Australia | 14.7 | 109 | 59.5 | 204 | Moderate | 69 |
| 2 | Tavassoli S | 2021 | Iran | 8.5 | 129 | 56.33 | 83 | Low | 7 |
| 3 | Pierini F. S | 2021 | Argentina | 36.5 | 74 | 62.1 | 114 | Low | 13 |
| 4 | Hu Z | 2021 | China | 54.7 | 340 | 59.4 | 66 | Low | 4 |
| 5 | Tong J | 2019 | China | 33.6 | 865 | 55.6 | 113 | Low | 68 |
| 6 | Lindner L | 2020 | Germany | 6 | 5423 | 63 | 168 | Low | 51 |
| 7 | Hu Z | 2020 | China | 62.1 | 452 | 58 | 67 | Low | 43 |
| 8 | Yan S | 2019 | China | 4.19 | 788 | 56 | 48 | Low | 72 |
| 9 | Wafa H | 2018 | Tunisia | 48 | 173 | 54.1 | 98 | Low | 71 |
| 10 | Tong H | 2018 | China | 35 | 320 | 54.1 | 72 | Low | 67 |
| 11 | Luque Ramos A | 2019 | Germany | 25.9 | 2535 | 62.5 | Moderate | 52 | |
| 12 | Fauny M | 2019 | France | 26.7 | 105 | 61.1 | 144 | Low | 30 |
| 13 | Phuan-udom R | 2018 | Thailand | 5 | 232 | 61.1 | 155 | Moderate | 11 |
| 14 | Panopoulos S | 2018 | Greece | 21.4 | 182 | 61.6 | 108 | Low | 62 |
| 15 | Mohd-Tahir N. A | 2017 | Malaysia | 29 | 93 | 61.7 | 66 | Moderate | 59 |
| 16 | Kweon S. M | 2018 | South Korea | 19.7 | 76 | 64.5 | 37.5 | Low | 47 |
| 17 | Kim D | 2018 | South Korea | 33.8 | 142,955 | 54.2 | 24.5 | High | 45 |
| 18 | Heidari B | 2018 | Iran | 30.8 | 39 | 50.6 | 108 | Low | 41 |
| 19 | Gabdulina G | 2018 | Kazakhstan | 45.1 | 406 | 50.6 | 61.6 | Low | 31 |
| 20 | Ene C. G | 2018 | Romania | 32.2 | 62 | 49.43 | Low | 29 | |
| 21 | Choi S. T | 2018 | South Korea | 33.4 | 479 | 61.5 | 53 | Low | 27 |
| 22 | Rossini M | 2017 | Italian | 35 | 183 | 64 | 108 | Moderate | 65 |
| 23 | Meng J | 2017 | China | 41.07 | 168 | 54.3 | 146.5 | Low | 20 |
| 24 | Makhdoom A | 2017 | Pakistan | 40.6 | 229 | 46.4 | Low | 53 | |
| 25 | Galarza-Delgado D. A | 2017 | Mexico | 19.1 | 225 | 55.7 | 114 | Moderate | 32 |
| 26 | Singh S | 2016 | India | 5.9 | 51 | 45 | Low | 1 | |
| 27 | Lee J. H | 2016 | South Korea | 46.8 | 1322 | 63.7 | 145 | Low | 50 |
| 28 | Kim D | 2016 | South Korea | 5.5 | 5376 | 58.8 | 117.5 | Low | 44 |
| 29 | Innala L | 2016 | Sweden | 3.7 | 726 | 55.6 | 80.5 | Moderate | 3 |
| 30 | Garip Y | 2016 | Turkey | 21.2 | 160 | 53.6 | 145 | Low | 33 |
| 31 | Bautista-Molano W | 2016 | Colombia | 17.3 | 1652 | 58 | 110.5 | Low | 26 |
| 32 | Piao H. H | 2015 | China | 21.6 | 37 | 64.4 | Moderate | 63 | |
| 33 | Mohammad A | 2013 | Ireland | 59 | 603 | 57 | 180 | Low | 58 |
| 34 | Lee J. H | 2014 | South Korea | 59.1 | 545 | 57 | 135 | Low | 49 |
| 35 | Lee J. H | 2014 | South Korea | 51 | 100 | 61.2 | 78 | Low | 48 |
| 36 | Hauser B | 2014 | UK | 29.9 | 304 | 63.5 | 115 | Low | 40 |
| 37 | Gron K. L | 2014 | 34 countries | 17.6 | 9874 | 54.9 | 97 | Moderate | 37 |
| 38 | Mobini m | 2012 | Iran | 32.3 | 121 | 55.7 | 121 | Low | 57 |
| 39 | Lee S. G | 2012 | South Korea | 22.1 | 299 | 52.4 | 32 | Low | 16 |
| 40 | Gonzalez-Lopez L | 2012 | Mexico | 24.1 | 191 | 52 | 132 | Low | 36 |
| 41 | Ghazi M | 2012 | France | 55.4 | 101 | 56.1 | 179.5 | Low | 34 |
| 42 | Vis M | 2011 | (Norway, UK, Netherlands) | 35 | 102 | 61 | 204 | Moderate | 70 |
| 43 | Dao H. H | 2011 | Vietnam | 27.6 | 105 | 56.3 | 21 | Low | 28 |
| 44 | Kim S. Y | 2010 | USA | 18 | 47,034 | 55 | Low | 46 | |
| 45 | El Maghraoui A | 2010 | Morocco | 44.2 | 172 | 49.4 | 101 | Low | 18 |
| 46 | Shankar S | 2008 | India | 22 | 84 | 33.9 | 60 | Low | 66 |
| 47 | Sarkis K. S | 2009 | Brazil | 25.3 | 83 | 55 | 92.5 | Low | 8 |
| 48 | Richards J. S | 2009 | USA | 18 | 282 | 65.4 | 156 | Moderate | 64 |
| 49 | Oelzner P | 2008 | Germany | 47.8 | 551 | 58.4 | 144 | Low | 61 |
| 50 | Haugen I. K | 2007 | Norway | 19.4 | 194 | 60.9 | Low | 39 | |
| 51 | Nolla J. M | 2006 | Spain | 13 | 187 | 60.34 | 109 | Low | 60 |
| 52 | Mikuls T. R | 2005 | USA | 4.7 | 175 | 60 | 109 | Low | 56 |
| 53 | Heidari B | 2004 | Iran | 25 | 88 | 52.6 | 84 | Low | 42 |
| 54 | Manrique F | 2003 | Venezuela | 29.4 | 85 | 45.3 | 113 | Low | 54 |
| 55 | Haugeberg G | 2000 | Norway | 4.2 | 394 | 54.8 | 156 | Moderate | 38 |
| 56 | Gilboe I. M | 2000 | Norway | 5 | 75 | 45 | 95 | Low | 35 |
| 57 | Moconkey B | 1962 | 30.3 | 97 | 63.1 | 14.7 | Moderate | 55 |
Risk of bias within studies
The risk of bias of included studies was assessed by the quality assessment checklist for prevalence studies. The results showed that the risk of bias of 75.4% (n = 43), 22.8% (n = 13) and 1.75% (n = 1) of included papers were low, moderate and high, respectively.
Quantitative data synthesis
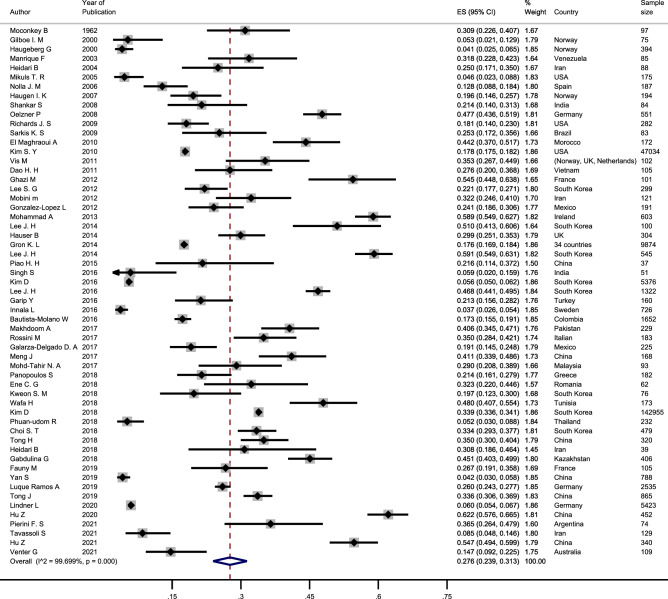
In this review, the results of 57 studies were summarized and the total included sample size was 227,812 cases of RA with 64,290 cases of OP. Due to the significant heterogeneity across studies, the random-effect model was used to pool the reported prevalence. The summary point prevalence was estimated as 27.6% (95%CI: 23.9–31.3%) (Table 2; Fig. 2).
Table 2.
Summary of meta-analysis results and subgroups analysis.
| Groups | No of studies | Prevalence rate | Heterogeneity | |||
|---|---|---|---|---|---|---|
| ES (95%CI) | Model | Chi square | P value | I square (%) | ||
| Date of publication | ||||||
| 1962–2010 | 14 | 21.6% (15.8–27.4) | Random | 553.1 | 0.001 | 97.6% |
| 2011–2015 | 12 | 36.2% (24.5–47.8) | Random | 875.9 | 0.001 | 98.7% |
| 2016–2021 | 31 | 27.1% (20.7–33.4) | Random | 15,203.5 | 0.001 | 99.8% |
| Study risk score | ||||||
| Low risk | 43 | 29.8% (26.2–33.5) | Random | 5504.0 | 0.001 | 99.2% |
| Moderate | 13 | 19.3% (13.9–24.7) | Random | 705.8 | 0.001 | 98.3% |
| High risk | 1 | 33.9% (33.6–34.1) | Random | – | – | – |
| Continents | ||||||
| Asia | 26 | 30.6% (23.2–38.0) | Random | 9508.0 | 0.001 | 99.7% |
| Europe | 17 | 25.6% (18.7–32.4) | Random | 1803.9 | 0.001 | 99.1% |
| America | 9 | 19.5% (15.9–23.1) | Random | 96.1 | 0.001 | 91.6% |
| Africa | 2 | 46.1% (40.8–51.3) | Random | – | – | – |
| Overall | 57 | 27.6% (23.9–31.3) | Random | 18,613.03 | 0.001 | 99.69% |
Figure 2.
Forest plot showing the prevalence of osteoporosis among rheumatoid arthritis patient.
Heterogeneity and meta-regression
The obtained results revealed a significant heterogeneity across primary included studies (heterogeneity chi-square = 18587.5, d.f = 56, p = 0.001, I-square (variation in prevalence attributable to heterogeneity) = 99.7%, estimate of between-study variance Tau-square = 0.019), for this reason, random-effect model was used to pool the reported prevalence. In addition, meta-regression method was used to find the heterogeneity source, and in meta-regression, we included sample size, study reign (continents), date of publication and risk score of studies and in the meta-regression model, none of these variables were significant. Finally, in addition to using a random effect model, subgroup analysis was performed based on study reigns (continents), date of publication and risk score of studies.
Sub-group analysis
As it was showed in Table 2, according to the subgroup analysis based on the data of publication, the highest prevalence was in studies conducted during 2011–2015 (36.2% (95%CI 24.5–47.8)), followed by 2016–2021 (27.1% (95%CI 20.7–33.4)) and before 2010 (21.6% (95%CI 15.8–27.4)). The prevalence in studies with low and moderate risk score was 29.8% (95%CI 26.2–33.5) and 36.2% (95%CI 24.5–47.8), respectively. Based on the study reign, the highest prevalence of OP was in Africa (46.1% (95%CI 40.8–51.3)), followed by Asia (30.6% (95%CI 23.2–38.0)), Europe (25.6% (95%CI 18.7–32.4)), and the Americas (19.5% (95%CI 15.9–23.1)).
Risk of bias across studies
Egger's test for small-study effects was performed to check for possibility of publication bias. The obtained results of Egger's test (z = 2.13, p = 0.033) suggested that there is an evidence of publication bias. In addition to Egger's test, the asymmetry in the funnel plot (Fig. 3) emphasized the existence of publication bias. For this reason, trim and fill method was used to estimate the OP prevalence and, the prevalence was estimated to be 23.3% (95%CI 19.7–26.8%) using random-effect model.
Figure 3.
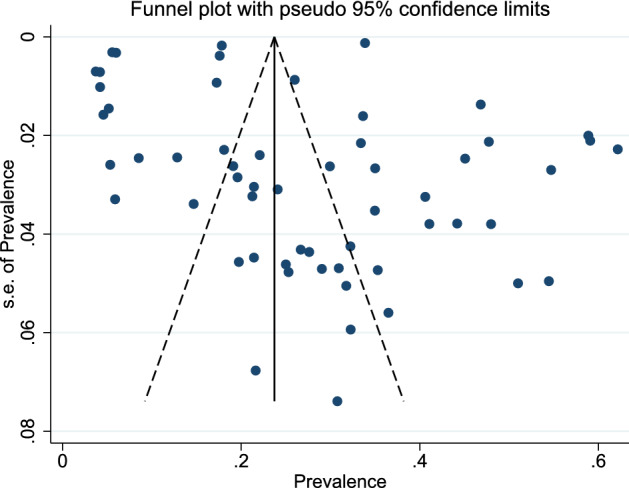
Funnel plot to check the publication bias.
Sensitivity analysis
To investigate the effect of each study on the pooled prevalence, we conducted a sensitivity analysis in which pooled prevalence are estimated omitting one study at a time. The highest pooled prevalence (28.1%, 95%CI 24.4–31.8%) was obtained by omitting the study of Innala et al.3 and the lowest pooled prevalence (27.0%, 95%CI 23.3–30.7%) was obtained by omitting the study of Hu et al.43.
Discussion
In this study, 57 primary studies with a total population of 227,812 cases were included in the meta-analysis, and according to the obtained results, OP prevalence among RA patients is 27.6%. The subgroup analysis based on the data of publication suggested that the highest prevalence was found in studies conducted during 2011–2015 (36.2%), followed by 2016–2021 (27.1%). The prevalence in studies with low and moderate risk score was 29.8% and 36.2%, respectively. Based on the study region, the highest prevalence of OP was in Africa (46.1%), followed by Asia (30.6%), Europe (25.6%), and the Americas (19.5%).
RA is a chronic inflammatory disease that, it leads to localized and generalized reduction in bone density and eventually causes OP73. Bone fractures are one of the most common complications in RA patients caused by OP and is associated with poor prognosis in old age and low quality of life74. According to the results, the prevalence of OP varies in different countries and continents, which can be attributed to the population density and different time of studies, age, economic situation and lack of government attention to the issue. In addition, difference in the quality of providing medical services, access to osteoporosis screening methods, and controlling the risk factors related to it and also preventing the disease play an important role.
A systematic review conducted by Salari et al.75 in 2021 to estimate the prevalence of OP in the general population. After review of 86 included studies, the worldwide prevalence of OP is estimated as 18.3% and in Asia, Europe, the Americas and Africa it was estimated as 16.7, 18.6, 12.4, and 39.5%, respectively. According to their study, the estimated prevalence was lower compared to our study, the reason is that people with RA have a higher risk of developing OP than the general population. In our study, similar to the study of Salari et al., the prevalence was lower in the Americas and higher in Africa followed by Asian and European countries.
In a meta-analysis, Ramírez et al.76 reviewed the results of 45 articles and found that the prevalence of OP in patients with axial spondylarthritis varies from 11.7 to 34.4%. In another meta-analysis study conducted on the general Chinese population, Chen et al. revealed that the prevalence of OP ranged from 1 to 85%77. The results of previous studies78 have shown that the prevalence of OP in people with RA is about 30%. The findings of our study had a similar estimate.
The results of our study and previous studies have shown that the prevalence of OP in people with RA is higher than the general population. Various factors play a role in increasing the prevalence of OP in patients with rheumatoid arthritis, the most important of which are continuous inflammation, glucocorticoid use, reduced physical activity due to old age and disability, and the use of DMARDs78.
In this study we investigated the results of 227,812 cases of RA with 64,290 cases of OP and it should be highlighted that 142,955 of these cases (63%) are related to the study conducted by Kim D et al.45 in South Korea, and the prevalence of OP reported as 33.8% in their study.
The incidence of OP is caused by several factors among RA patients. In the pathogenesis of inflammation and reduction of BMD, various factors in immune system, are involved such as hyper-expression and the effect of autoantibodies against citrullinated proteins, pro-inflammatory cytokine secretion, and receptor activator of NF-kappa B ligand derived from T-cell79. Immunosuppressive drugs such as glucocorticoids and DMARDs are used to treat RA. Glucocorticoids with their anti-inflammatory effects can prevent local and systemic decrease in BMD. Furthermore, DMARDs are used to achieve remission, and evidence suggests that DMARDs prevent structural damage to cartilage and bone80,81.
Decreased vitamin D intake is associated with an increased risk of RA, and also, vitamin D deficiency is associated with disease activity in patients with RA82. Therefore, vitamin D deficiency can be one of the common causes of RA and OP. The results of a meta-analysis study showed that vitamin D deficiency in RA patients is significantly higher than healthy individuals and serum vitamin D levels are inversely related with disease activity83.
The results suggest that the prevalence of RA has been declining in recent years, which may be attributed to the increase of human knowledge about drugs that suppress RA and timely imaging studies for early diagnosis and adequate treatment. Among the four continents (i.e., Africa, Asia, the Americas and Europe), Asia has the most prevalent of OP followed by Europe. In most studies, due to the higher risk of women with RA, the majority of the population was women and most of them were in menopausal ages and is associated with estrogen reduction, which is an important risk factor to increase prevalence of OP. It should be noted that because most studies used the DEXA method to evaluate OP, there is lower error in the diagnostic method. Although in some countries, limited studies have been conducted, but it can be said that the prevalence of OP in RA is high and it is necessary to have a decent platform for screening and timely use of medications and patients’ education to reduce modifiable risk factors to reduce the incidence of OP to minimize the complications.
One of the main limitations of the study is the lack of sufficient number of studies conducted in each area (for example only two studies from the African continent were included in this meta-analysis), which makes it difficult to generalize the results. Also, in other WHO regions, studies have been conducted in limited countries, which makes it impossible to show the true prevalence in each region. On the other hand, in a number of studies in which people were treated with corticosteroids and DMARDs, the rate of bone mass reduction was not examined separately, so it was not possible to compare between drug users and other people. Finally, due to the disparity of results in different continents and countries, more comprehensive studies are recommended to make a better conclusion.
Conclusion
Despite significant advances in prevention, treatment and diagnostic methods in RA patients, it still seems that the prevalence of OP in these patients is high and requires better and timelier interventions.
Supplementary Information
Acknowledgements
We would like to thank the vice chancellor for the research of Arak University of Medical Sciences for their financial and scientific supports.
Abbreviations
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- OP
Osteoporosis
- RA
Rheumatoid arthritis
- CI
Confidence interval
- DMARDs
Disease-modifying anti-rheumatic drugs
- MeSH
Medical subject headings
- BMD
Bone mineral density
- WHO
World Health Organization
Author contributions
S.M., B.T. and A.A.H. conceived the study. S.M. and A.A.H. contributed to the title and full-text screening. A.A.H. and S.M. extracted the data. All authors contributed equally to the initial draft of the manuscript. A.A.H. analyzed the data and all authors have read, revised and approved the final version of the manuscript.
Funding
The present study was funded Arak University of Medical Sciences (Project Number: 6232).
Data availability
All data for the analyses is available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20016-x.
References
- 1.Singh S, Lihite RJ, Baruah C, Lahkar M, Singh PK. A pilot study ofcomorbidities in patients with rheumatoid arthritis at a tertiary care hospital in Northeast India. Biomed. Res. Ther. 2016;3:454–459. doi: 10.7603/s40730-016-0001-0. [DOI] [Google Scholar]
- 2.Ziegelasch M, et al. Decrease in bone mineral density during three months after diagnosis of early rheumatoid arthritis measured by digital X-ray radiogrammetry predicts radiographic joint damage after one year. Arthritis Res. Ther. 2017;19:195. doi: 10.1186/s13075-017-1403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Innala L, et al. Co-morbidity in patients with early rheumatoid arthritis—Inflammation matters. Arthritis Res. Ther. 2016;18:33. doi: 10.1186/s13075-016-0928-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Z, et al. Prevalence and risk factors for bone loss in rheumatoid arthritis patients from South China: Modeled by three methods. BMC Musculoskelet. Disord. 2021;22:534. doi: 10.1186/s12891-021-04403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fardellone P, Salawati E, Le Monnier L, Goëb V. Bone loss, osteoporosis, and fractures in patients with rheumatoid arthritis: A review. J. Clin. Med. 2020;9:3361. doi: 10.3390/jcm9103361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin S, et al. Bone mineral density and microarchitecture among Chinese patients with rheumatoid arthritis: A cross-sectional study with HRpQCT. Arthritis Res. Ther. 2021;23:127. doi: 10.1186/s13075-021-02503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavassoli S, Rajaei A, Emam MM, Farsad F. Evaluating the value-added of the trabecular bone score in patients with rheumatoid arthritis. Arch. Iran. Med. 2021;24:193–198. doi: 10.34172/aim.2021.30. [DOI] [PubMed] [Google Scholar]
- 8.Sarkis KS, et al. Association between osteoporosis and rheumatoid arthritis in women: A cross-sectional study. Sao Paulo Med. J. 2009;127:216–222. doi: 10.1590/s1516-31802009000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raterman HG, Lems WF. Pharmacological management of osteoporosis in rheumatoid arthritis patients: A review of the literature and practical guide. Drugs Aging. 2019;36:1061–1072. doi: 10.1007/s40266-019-00714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera A, et al. Prevalence of osteoporotic vertebral fracture in Spanish women over age 45. Maturitas. 2015;80:288–295. doi: 10.1016/j.maturitas.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Phuan-Udom R, Lektrakul N, Katchamart W. The association between 10-year fracture risk by FRAX and osteoporotic fractures with disease activity in patients with rheumatoid arthritis. Clin. Rheumatol. 2018;37:2603–2610. doi: 10.1007/s10067-018-4218-8. [DOI] [PubMed] [Google Scholar]
- 12.Mazzucchelli R, et al. Trends in hip fracture in patients with rheumatoid arthritis: results from the Spanish National Inpatient Registry over a 17-year period (1999–2015). TREND-AR study. Rmd Open. 2018 doi: 10.1136/rmdopen-2018-000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierini FS, et al. Osteoporotic fractures in rheumatoid arthritis patients in Argentina: A matched retrospective cohort study. Adv. Rheumatol. 2021 doi: 10.1186/s42358-021-00179-3. [DOI] [PubMed] [Google Scholar]
- 14.Adami G, Saag KG. Osteoporosis pathophysiology, epidemiology, and screening in rheumatoid arthritis. Curr. Rheumatol. Rep. 2019;21:1–10. doi: 10.1007/s11926-019-0836-7. [DOI] [PubMed] [Google Scholar]
- 15.Lin YC, et al. Rheumatoid arthritis patients with hip fracture: A nationwide study. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2015;26:811–817. doi: 10.1007/s00198-014-2968-y. [DOI] [PubMed] [Google Scholar]
- 16.Lee SG, et al. Increased frequency of osteoporosis and BMD below the expected range for age among South Korean women with rheumatoid arthritis. Int. J. Rheum. Dis. 2012;15:289–296. doi: 10.1111/j.1756-185X.2012.01729.x. [DOI] [PubMed] [Google Scholar]
- 17.Nyhäll-Wåhlin BM, Ajeganova S, Petersson IF, Andersson M. Increased risk of osteoporotic fractures in Swedish patients with rheumatoid arthritis despite early treatment with potent disease-modifying anti-rheumatic drugs: a prospective general population-matched cohort study. Scand. J. Rheumatol. 2019;48:431–438. doi: 10.1080/03009742.2019.1611918. [DOI] [PubMed] [Google Scholar]
- 18.El Maghraoui A, et al. Prevalence and risk factors of vertebral fractures in women with rheumatoid arthritis using vertebral fracture assessment. Rheumatology (Oxford) 2010;49:1303–1310. doi: 10.1093/rheumatology/keq084. [DOI] [PubMed] [Google Scholar]
- 19.Chen B, Cheng GQ, Wang HT, Feng Y. Increased risk of vertebral fracture in patients with rheumatoid arthritis A meta-analysis. Medicine. 2016 doi: 10.1097/md.0000000000005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng J, Li Y, Yuan X, Lu Y. Evaluating osteoporotic fracture risk with the Fracture Risk Assessment Tool in Chinese patients with rheumatoid arthritis. Medicine (Baltimore) 2017;96:e6677. doi: 10.1097/md.0000000000006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Higgins, J. P. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions (2008).
- 23.Hoy D, et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Nyaga VN, Arbyn M, Aerts M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bautista-Molano W, et al. Epidemiological profile of colombian patients with rheumatoid arthritis in a specialized care clinic. Reumatologia Clinica. 2016;12:313–318. doi: 10.1016/j.reuma.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Choi ST, et al. Prevalence and fracture risk of osteoporosis in patients with rheumatoid arthritis: A multicenter comparative study of the FRAX and WHO criteria. J. Clin. Med. 2018 doi: 10.3390/jcm7120507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dao HH, Do QT, Sakamoto J. Bone mineral density and frequency of osteoporosis among Vietnamese women with early rheumatoid arthritis. Clin. Rheumatol. 2011;30:1353–1361. doi: 10.1007/s10067-011-1762-x. [DOI] [PubMed] [Google Scholar]
- 29.Ene CG, et al. Incidence of osteoporosis and the risk of fracture in patients with rheumatoid arthritis undergoing corticosteroid treatment. Rev. Chim. 2018;69:1851–1854. doi: 10.37358/RC.18.7.6430. [DOI] [Google Scholar]
- 30.Fauny M, et al. Study of vertebral fracture and Scanographic bone attenuation coefficient in rheumatoid arthritis and ankylosing spondylitis vs controls. Sci. Rep. 2019;9:13323. doi: 10.1038/s41598-019-49712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabdulina G, et al. An epidemiological analysis of osteoporotic characteristics in patients affected with rheumatoid arthritis in Kazakhstan. Arch. Osteoporos. 2018;13:99. doi: 10.1007/s11657-018-0514-9. [DOI] [PubMed] [Google Scholar]
- 32.Galarza-Delgado DA, et al. Prevalence of comorbidities in Mexican mestizo patients with rheumatoid arthritis. Rheumatol. Int. 2017;37:1507–1511. doi: 10.1007/s00296-017-3769-3. [DOI] [PubMed] [Google Scholar]
- 33.Garip Y, Eser F, Bodur H. Comorbidities in Turkish patients with rheumatoid arthritis: Association with the health-related quality of life in terms of disease activity, functional and radiological status, severity of pain, and social and emotional functioning. Acta Reumatologica Portuguesa. 2016;41:344–349. [PubMed] [Google Scholar]
- 34.Ghazi M, et al. Prevalence of vertebral fractures in patients with rheumatoid arthritis: Revisiting the role of glucocorticoids. Osteoporos. Int. 2012;23:581–587. doi: 10.1007/s00198-011-1584-3. [DOI] [PubMed] [Google Scholar]
- 35.Gilboe IM, Kvien TK, Haugeberg G, Husby G. Bone mineral density in systemic lupus erythematosus: Comparison with rheumatoid arthritis and healthy controls. Ann. Rheum. Dis. 2000;59:110–115. doi: 10.1136/ard.59.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Lopez L, et al. Performance of risk indices for identifying low bone mineral density and osteoporosis in Mexican Mestizo women with rheumatoid arthritis. J. Rheumatol. 2012;39:247–253. doi: 10.3899/jrheum.110467. [DOI] [PubMed] [Google Scholar]
- 37.Gron KL, et al. The association of fatigue, comorbidity burden, disease activity, disability and gross domestic product in patients with rheumatoid arthritis—Results from 34 countries participating in the Quest-RA programme. Clin. Exp. Rheumatol. 2014;32:869–877. [PubMed] [Google Scholar]
- 38.Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Reduced bone mineral density in male rheumatoid arthritis patients: frequencies and associations with demographic and disease variables in ninety-four patients in the Oslo County Rheumatoid Arthritis Register. Arthritis Rheum. 2000;43:2776–2784. doi: 10.1002/1529-0131(200012)43:12<2776::aid-anr18>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 39.Haugen IK, Slatkowsky-Christensen B, Orstavik R, Kvien TK. Bone mineral density in patients with hand osteoarthritis compared to population controls and patients with rheumatoid arthritis. Ann. Rheum. Dis. 2007;66:1594–1598. doi: 10.1136/ard.2006.068940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauser B, Riches PL, Wilson JF, Horne AE, Ralston SH. Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatology (Oxford) 2014;53:1759–1766. doi: 10.1093/rheumatology/keu162. [DOI] [PubMed] [Google Scholar]
- 41.Heidari B, Heidari P, Hajian-Tilaki K, Bayani MA, Babaei M. Effect of long-term low dose prednisolone administration on bone mineral density: Relating to non-compliant women with rheumatoid arthritis. Caspian J. Intern. Med. 2018;9:171–177. doi: 10.22088/cjim.9.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heidari B, Jalali F. Bone densitometry in patients with rheumatoid arthritis. Acta Med. Iran. 2005;43:99–104. [Google Scholar]
- 43.Hu Z, et al. Prevalence and risk factors for bone loss in Southern Chinese with rheumatic diseases. BMC Musculoskelet. Disord. 2020;21:416. doi: 10.1186/s12891-020-03403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim D, et al. Incidence and risk factors of fractures in patients with rheumatoid arthritis: an Asian prospective cohort study. Rheumatol. Int. 2016;36:1205–1214. doi: 10.1007/s00296-016-3453-z. [DOI] [PubMed] [Google Scholar]
- 45.Kim D, et al. Glucocorticoids are associated with an increased risk for vertebral fracture in patients with rheumatoid arthritis. J. Rheumatol. 2018;45:612–620. doi: 10.3899/jrheum.170054. [DOI] [PubMed] [Google Scholar]
- 46.Kim SY, et al. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res. Ther. 2010;12:R154. doi: 10.1186/ar3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kweon SM, et al. Male patients with rheumatoid arthritis have an increased risk of osteoporosis: Frequency and risk factors. Medicine (Baltimore) 2018;97:e11122. doi: 10.1097/md.0000000000011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, et al. Validity and role of vertebral fracture assessment in detecting prevalent vertebral fracture in patients with rheumatoid arthritis. Joint Bone Spine. 2014;81:149–153. doi: 10.1016/j.jbspin.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Lee JH, et al. The risk of osteoporotic fractures according to the FRAX model in Korean patients the rheumatoid arthritis. J. Korean Med. Sci. 2014;29:1082–1089. doi: 10.3346/jkms.2014.29.8.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JH, et al. The frequency of and risk factors for osteoporosis in Korean patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2016;17:98. doi: 10.1186/s12891-016-0952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindner L, et al. Osteoporosis in patients with rheumatoid arthritis: Trends in the German National Database 2007–2017. Rheumatol. Int. 2020;40:2005–2012. doi: 10.1007/s00296-020-04593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos L, et al. Comorbidities in patients with rheumatoid arthritis and their association with patient-reported outcomes. J. Rheumatol. 2019;46:564–571. doi: 10.3899/jrheum.180668. [DOI] [PubMed] [Google Scholar]
- 53.Makhdoom A, et al. Bone mineral density level by dual energy X-ray absorptiometry in rheumatoid arthritis. JPMA J. Pak. Med. Assoc. 2017;67:15–19. [PubMed] [Google Scholar]
- 54.Manrique F, et al. Abnormalities of bone mineral density and bone metabolism in Venezuelan patients with rheumatoid arthritis. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2003;9:219–227. doi: 10.1097/01.rhu.0000081262.36127.53. [DOI] [PubMed] [Google Scholar]
- 55.McConkey B, Fraser GM, Bligh AS. Osteoporosis and purpura in rheumatoid disease: Prevalence and relation to treatment with corticosteroids. QJM. 1962;31:419–427. doi: 10.1093/oxfordjournals.qjmed.a066977. [DOI] [Google Scholar]
- 56.Mikuls TR, et al. Prevalence of osteoporosis and osteopenia among African Americans with early rheumatoid arthritis: the impact of ethnic-specific normative data. J. Natl. Med. Assoc. 2005;97:1155–1160. [PMC free article] [PubMed] [Google Scholar]
- 57.Mobini M, Kashi Z, Ghobadifar A. Prevalence and associated factors of osteoporosis in female patients with rheumatoid arthritis. Casp. J. Intern. Med. 2012;3:447–450. [PMC free article] [PubMed] [Google Scholar]
- 58.Mohammad A, et al. The prevalence of vertebral fracture on vertebral fracture assessment imaging in a large cohort of patients with rheumatoid arthritis. Rheumatology (Oxford) 2014;53:821–827. doi: 10.1093/rheumatology/ket353. [DOI] [PubMed] [Google Scholar]
- 59.Mohd-Tahir NA, Thomas P, Mohamed-Said MS, Makmor-Bakry M, Li SC. Cost-effectiveness of bisphosphonates for prevention of fracture related to glucocorticoid-induced osteoporosis in Malaysia. Int. J. Rheum. Dis. 2018;21:647–655. doi: 10.1111/1756-185x.13206. [DOI] [PubMed] [Google Scholar]
- 60.Nolla JM, et al. Frequency of osteoporosis in 187 men with rheumatoid arthritis followed in a university hospital. J. Rheumatol. 2006;33:1472–1475. [PubMed] [Google Scholar]
- 61.Oelzner P, et al. Significance of risk factors for osteoporosis is dependent on gender and menopause in rheumatoid arthritis. Rheumatol. Int. 2008;28:1143–1150. doi: 10.1007/s00296-008-0576-x. [DOI] [PubMed] [Google Scholar]
- 62.Panopoulos S, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: A comparative, multicenter, matched-cohort study. Arthritis Res. Ther. 2018;20:267. doi: 10.1186/s13075-018-1771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piao HH, Zhang KQ, Tang ZH. Association between rheumatoid arthritics and osteoporosis among Chinese men, a community based study. Int. J. Clin. Exp. Med. 2015;8:16592–16598. [PMC free article] [PubMed] [Google Scholar]
- 64.Richards JS, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2009;12:434–440. doi: 10.1016/j.jocd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Rossini M, et al. Prevalence and incidence of osteoporotic fractures in patients on long-term glucocorticoid treatment for rheumatic diseases: the Glucocorticoid Induced OsTeoporosis TOol (GIOTTO) study. Reumatismo. 2017;69:30–39. doi: 10.4081/reumatismo.2017.922. [DOI] [PubMed] [Google Scholar]
- 66.Shankar S, et al. Bone mineral density in Indian women with rheumatoid arthritis. Rheumatol. Int. 2009;29:377–381. doi: 10.1007/s00296-008-0706-5. [DOI] [PubMed] [Google Scholar]
- 67.Tong H, et al. Osteoporosis self-assessment tool as a screening tool for predicting osteoporosis in elderly chinese patients with established rheumatoid arthritis. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2019;22:321–328. doi: 10.1016/j.jocd.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Tong JJ, et al. Prevalence and risk factors associated with vertebral osteoporotic fractures in patients with rheumatoid arthritis. Clin. Rheumatol. 2020;39:357–364. doi: 10.1007/s10067-019-04787-9. [DOI] [PubMed] [Google Scholar]
- 69.Venter G, et al. Perspectives of glucocorticoid use in patients with rheumatoid arthritis. ACR Open Rheumatol. 2021;3:231–238. doi: 10.1002/acr2.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vis M, et al. High incidence of vertebral and non-vertebral fractures in the OSTRA cohort study: A 5-year follow-up study in postmenopausal women with rheumatoid arthritis. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2011;22:2413–2419. doi: 10.1007/s00198-010-1517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wafa H, et al. Risk factors associated with bone loss and occurrence of fragility fractures in rheumatoid arthritis patients. Egyp. Rheumatol. 2019;41:1–5. doi: 10.1016/j.ejr.2018.01.004. [DOI] [Google Scholar]
- 72.Yan S, et al. The incidence of extra-articular manifestations in southern Chinese patients with inflammatory joint diseases. Int. J. Rheum. Dis. 2019;22:1686–1694. doi: 10.1111/1756-185x.13657. [DOI] [PubMed] [Google Scholar]
- 73.Sun T, Wang J, Zhang R, Li Y. A systematic review and meta-analysis: Effects of glucocorticoids on rheumatoid arthritis and systemic lupus erythematosus. Ann. Palliat. Med. 2021;10:7977. doi: 10.21037/apm-21-1485. [DOI] [PubMed] [Google Scholar]
- 74.Jin S, et al. Incidence of fractures among patients with rheumatoid arthritis: A systematic review and meta-analysis. Osteoporos. Int. 2018;29:1263–1275. doi: 10.1007/s00198-018-4473-1. [DOI] [PubMed] [Google Scholar]
- 75.Salari N, et al. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021;16:1–20. doi: 10.1186/s13018-021-02772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramírez J, Nieto-González JC, Curbelo Rodríguez R, Castañeda S, Carmona L. Prevalence and risk factors for osteoporosis and fractures in axial spondyloarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018;48:44–52. doi: 10.1016/j.semarthrit.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Chen P, Li Z, Hu Y. Prevalence of osteoporosis in China: A meta-analysis and systematic review. BMC Public Health. 2016;16:1039. doi: 10.1186/s12889-016-3712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Llorente I, García-Castañeda N, Valero C, González-Álvaro I, Castañeda S. Osteoporosis in rheumatoid arthritis: Dangerous liaisons. Front Med (Lausanne) 2020;7:601618–601618. doi: 10.3389/fmed.2020.601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rotta D, et al. Osteoporosis in inflammatory arthritides: New perspective on pathogenesis and treatment. Front. Med. 2020;7:896. doi: 10.3389/fmed.2020.613720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corrado A, et al. Influence of glucocorticoid treatment on trabecular bone score and bone remodeling regulators in early rheumatoid arthritis. Arthritis Res. Ther. 2021;23:1–9. doi: 10.1186/s13075-021-02562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanaka Y. Managing osteoporosis and joint damage in patients with rheumatoid arthritis: An overview. J. Clin. Med. 2021;10:1241. doi: 10.3390/jcm10061241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kostoglou-Athanassiou I, Athanassiou P, Lyraki A, Raftakis I, Antoniadis C. Vitamin D and rheumatoid arthritis. Ther. Adv. Endocrinol. Metab. 2012;3:181–187. doi: 10.1177/2042018812471070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee YH, Bae SC. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A meta-analysis. Clin. Exp. Rheumatol. 2016;34:827–833. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for the analyses is available from the corresponding author on request.