Abstract
Purpose of Review
Improving HIV testing uptake is essential to ending the HIV pandemic. HIV testing approaches can be opt-in, opt-out or risk-based. This systematic review examines and compares the uptake of HIV testing in opt-in, opt-out and risk-based testing approaches.
Recent Findings
There remain missed opportunities for HIV testing in a variety of settings using different approaches: opt-in (a person actively accepts to be tested for HIV), opt-out (a person is informed that HIV testing is routine/standard of care, and they actively decline if they do not wish to be tested for HIV) or risk-based (using risk-based screening tools to focus testing on certain individuals or sub-populations at greater risk of HIV). It is not clear how the approach could impact HIV test uptake when adjusted for other factors (e.g. rapid testing, country-income level, test setting and population tested).
Summary
We searched four databases for studies reporting on HIV test uptake. In total, 18,238 records were screened, and 150 studies were included in the review. Most studies described an opt-in approach (87 estimates), followed by opt-out (76) and risk-based (19). Opt-out testing was associated with 64.3% test uptake (I2 = 99.9%), opt-in testing with 59.8% (I2 = 99.9%) and risk-based testing with 54.4% (I2 = 99.9%). When adjusted for settings that offered rapid testing, country income level, setting and population tested, opt-out testing had a significantly higher uptake (+ 12% (95% confidence intervals: 3–21), p = 0.007) than opt-in testing. We also found that emergency department patients and hospital outpatients had significantly lower HIV test uptake than other populations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11904-022-00614-0.
Introduction
Optimising HIV testing services is critical for ending the HIV/AIDS pandemic. Testing informs people living with HIV (PLHIV) of their status, preferably during the early stages of infection [1]. Earlier HIV detection and management have many benefits, including reducing morbidity and mortality, and preventing onward transmission [2]. It is more cost-effective to detect HIV infection early, as late presentations result in significantly higher medical costs and incur more public health expenditure [1]. Knowing one’s HIV-negative status also enables use of effective biomedical prevention strategies like pre-exposure prophylaxis [3].
Despite the importance of HIV testing, many countries are not on track to meet the Joint United Nations Programme on HIV/AIDS 95–95-95 targets where 95% of PLHIV know their HIV status, 95% of people who know their status are receiving treatment and 95% of people on treatment have a supressed viral load [4]. Globally, it is estimated that 84% of PLHIV were aware of their HIV status, with 87% of these receiving treatment and 90% of these virologically suppressed in 2020 [5]. HIV/AIDS-related deaths have only declined by 57.5%, from 1.9 million in 2010 to ~ 680,000 in 2020 [5]. Even in well-resourced health systems, a significant proportion of PLHIV are still diagnosed late [6]. In particular, the uptake of HIV testing services remains low in key populations, resulting from structural issues that limit access and fear of stigmatisation and breach of confidentiality [7]. Discriminatory attitudes towards PLHIV persist and negatively impact the use of HIV services [8]. Further, the fear of HIV-related stigma has led to PLHIV avoiding disclosure of HIV status and delaying or staying in treatment [9].
HIV testing services should always be voluntary and can take several approaches: opt-in (a person actively accepts to be tested for HIV), opt-out (a person is informed that HIV testing is routine/standard of care, and they actively decline if they do not wish to be tested for HIV) or risk-based (using risk-based screening tools to focus testing on certain individuals or sub-populations at greater risk of HIV) [10••]. Since 2006, the United States Centers for Disease Control and Prevention (CDC) has recommended an ‘opt-out’ approach, in which voluntary HIV testing is a part of routine health care for individuals between the ages of 13 and 64 [11]. Previous studies have suggested that this screening policy might reduce stigma by normalising HIV testing and making it a common behaviour [12–14]. Similarly, since 2007, the WHO recommends an opt-out approach to offer provider-initiated HIV testing service in health facilities for: (1) all patients, irrespective of epidemic setting, whose clinical presentation might result from underlying HIV infection; (2) as a standard part of medical care for all patients attending health facilities in high HIV prevalence settings; and (3) more selectively in low HIV prevalence settings [15]. Alternatively, a risk-based approach uses a set of criteria to either identify at-risk individuals for HIV testing who would not otherwise be offered a test (‘screen in’) or exclude people from a routine offer of a test (‘screen out’) [10••].
This systematic review examined the uptake of HIV testing by comparing opt-in, opt-out or risk-based testing approaches.
Methods
Search Strategy and Selection Criteria for the Systematic Literature Review
Ovid MEDLINE, Ovid EMBASE, Web of Science and Global Health were searched between 1st January 2010 and 9th July 2020. The search terminology revolved around two key aspects: ‘HIV’ and ‘Risk assessments or screening’. Appendix 1 shows the full search strategy. The inclusion criteria were any study that contained primary data on the uptake of HIV testing amongst those offered testing; we then grouped this according to opt-in, opt-out and risk-based testing. Systematic literature reviews, editorials, duplicated results from the same study, laboratory studies about HIV diagnostic performance and studies restricting study populations by clinical outcomes (e.g. men with urethritis or women with cervicitis) were excluded. The primary outcome of interest was the uptake of HIV testing amongst those offered testing.
Titles and abstracts were independently assessed for eligibility by two reviewers (QS, LO). Another reviewer (JO) resolved any discrepancies. This systematic review has been registered at the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020187838).
Data Analysis
An extraction file was created in Microsoft Excel, and the following information was collected: country income level, setting of the study, population tested, whether testing was opt-in/opt-out/risk-based and presence of rapid testing. Data extraction was conducted by two reviewers (QS, LO), and another reviewer (JO) resolved any discrepancies. The quality of each study was also assessed by two reviewers (QS, LO) using the relevant critical appraisal tool from Johanna Briggs Institute [16].
Statistical Analysis
We used descriptive analysis to summarise the characteristics of the studies included. We used the Fisher exact probability test to assess for statistically significant differences according to the testing approach. A country with a high HIV prevalence was defined as having a national prevalence above 5%, as reported by UNAIDS [17]. We used random effects meta-analysis to calculate the pooled proportion of people tested for HIV according to the type of HIV testing approach (opt-in, opt-out, risk-based). Inter-study heterogeneity was assessed using the I2 statistic. We explored heterogeneity using subgroup analysis and meta-regression according to availability of rapid HIV testing, country-income level, study setting, population targeted and the latest study year. Publication bias was assessed using funnel plot and Egger’s test. STATA version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC) was used to perform all statistical analyses. This review is reported per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18].
Role of the Funding Source
The funders did not have any role in the study design; collection, analysis or interpretation of the data; writing the report or decision to submit the paper for publication.
Results
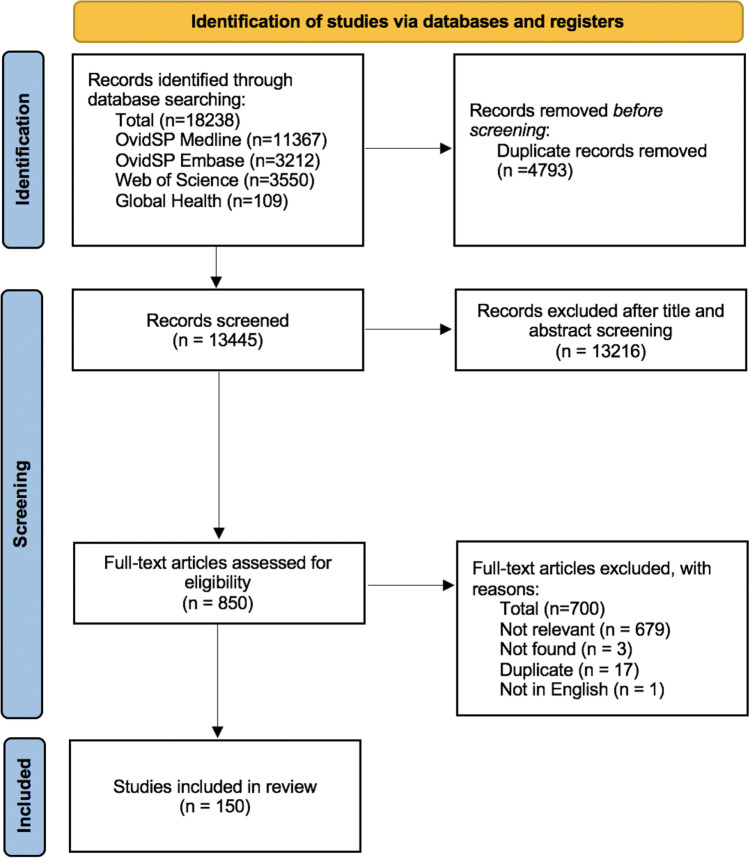
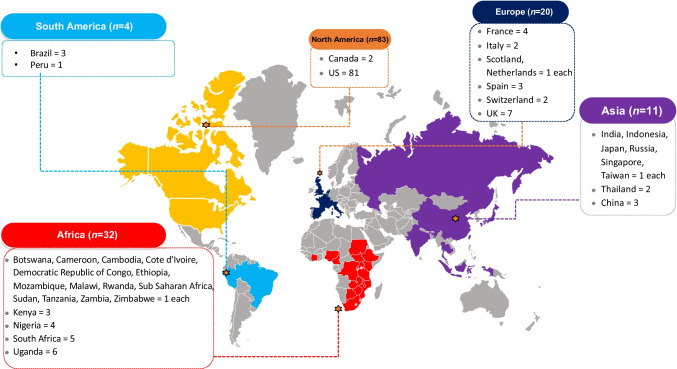
The initial search identified 18,238 potential articles, and 150 were included in this systematic review (Fig. 1). Figure 2 summarises the country of origin of the studies. Majority of studies arose from North America (n = 83), followed by Africa (n = 32) and Europe (n = 20).
Fig. 1.
PRISMA Flow diagram
Fig. 2.
Countries of included studies (N = 150)
Table 1 summarises the characteristics of the included studies according to the country’s HIV prevalence. Most studies were from high- (71%) and middle-income countries (22%), conducted in the emergency department (ED) (39%), for ED patients (41%) and involved settings with rapid testing (58%).
Table 1.
Study characteristics, according to low and high (≥ 5%) HIV prevalence [19]
| Total (N = 150) |
Low HIVprevalence (N = 133) |
HighHIVprevalence (N = 17) |
|
|---|---|---|---|
| Country income level | n (%) | n (%) | n (%) |
| High | 106 (71) | 106 (80) | 0 (0) |
| Middle | 33 (22) | 24 (18) | 9 (53) |
| Low | 11 (7) | 3 (2) | 8 (47) |
| Settings | |||
| Primary care/GP | 10 (7) | 9 (6) | 1 (6) |
| Pharmacy | 1 (0.7) | 1(1) | 0 (0) |
| Hospital | 20 (13) | 15 (11) | 5 (29) |
| Emergency department | 58 (39) | 58 (44) | 0 (0) |
| Community | 25 (16) | 17 (13) | 8 (47) |
| Dental/outpatient clinic | 29 (19) | 26 (19) | 3 (18) |
| Prisons | 7 (5) | 7 (6) | 0 (0) |
| Populations | |||
| ED patients | 60 (41) | 60 (46) | 0 (0) |
| Paediatrics | 5 (3) | 1 (1) | 4 (23) |
| Outpatients | 36 (24) | 34 (25) | 2 (12) |
| Hospital inpatients | 14 (9) | 11 (8) | 3(18) |
| General public | 28 (18) | 20 (14) | 8 (47) |
| Incarcerated persons | 7 (5) | 7 (6) | 0 (0) |
| Availability of rapid HIV testing | |||
| Yes | 86 (58) | 75 (57) | 11 (65) |
| No | 64 (42) | 58 (43) | 6 (35) |
| Study year | |||
| 2016–2020 | 27 (18) | 21 (16) | 6 (35) |
| 2011–2015 | 64 (43) | 57 (43) | 7 (41) |
| 2006–2010 | 54 (36) | 50 (37) | 4 (24) |
| 2001–2005 | 5 (3) | 5 (4) | 0 (0) |
ED emergency department, GP general practice
Table 2 compares the study characteristics of opt-in, opt-out and risk-based testing. We found that more studies from high-income countries used opt-out or risk-based approaches, and more studies from community-based settings and those targeting the general public used the opt-in approach.
Table 2.
Study characteristics according to opt-in testing, opt-out testing or risk-based testing approaches
| Opt-in (N = 87) |
Opt-out (N = 76) |
Risk-based (N = 19) |
p value | |
|---|---|---|---|---|
| Country income level | n (%) | n (%) | n (%) | |
| High | 52 (60) | 62 (82) | 15 (79) | 0.007 |
| Middle | 25 (29) | 11 (14) | 4 (21) | 0.083 |
| Low | 10 (11) | 3 (4) | 0 (0) | 0.104 |
| Settings | ||||
| Primary care/GP | 4 (5) | 6 (8) | 4 (21) | 0.050 |
| Pharmacy | 1 (1) | 0 (0) | 0 (0) | 1.000 |
| Hospital | 7 (8) | 12 (16) | 4 (21) | 0.131 |
| Emergency department | 29 (33) | 34 (45) | 7 (38) | 0.311 |
| Community | 23 (26) | 5 (6) | 2 (10) | 0.002 |
| Dental/outpatient clinic | 18 (20) | 12 (16) | 1 (5) | 0.281 |
| Prisons | 5 (7) | 7 (9) | 1 (5) | 0.769 |
| Populations | ||||
| ED patients | 30 (34) | 33 (44) | 6 (32) | 0.432 |
| Paediatrics | 1 (1) | 3 (4) | 2 (10) | 0.090 |
| Outpatients | 18 (20) | 19 (25) | 7 (38) | 0.309 |
| Hospital inpatients | 5 (6) | 10 (13) | 1 (5) | 0.218 |
| General public | 28 (32) | 4 (5) | 2 (10) | < 0.001 |
| Incarcerated persons | 5 (7) | 7 (9) | 1 (5) | 0.769 |
| Rapid HIV testing | ||||
| Yes | 57 (65) | 39 (52) | 9 (47) | 0.135 |
| No | 30 (35) | 37 (48) | 10 (53) | - |
Of 150 unique studies, some evaluated more than one approach and thus will appear more than once in the columns
Table 3 summarises the pooled proportion of people testing for HIV according to various settings. It demonstrates that opt-out testing had higher uptake of people testing for HIV compared with opt-in and risk-based testing (64.3% vs. 59.8%), although it was not statistically significantly different. However, in the meta-regression analysis (Table 4), when we adjusted for rapid HIV testing, country income level, test setting, population tested and the year of study, opt-out testing had a significantly higher HIV test uptake compared with opt-in and risk-based testing (additional 12% and 15%, respectively).
Table 3.
Pooled proportion of people testing for HIV
| Number of studies | Pooled proportion of people testing for HIV (%) | 95% confidence interval | I2 (p value) | |
|---|---|---|---|---|
| Total (N = 182) | 61.2 | 57.4–64.9 | 99.9 (< 0.001) | |
| Type of HIV testing service | ||||
| Opt-in | 87 | 59.8 | 52.2–67.3 | 99.9 (< 0.001) |
| Opt-out | 76 | 64.3 | 57.4–70.9 | 99.9 (< 0.001) |
| Risk-based | 19 | 54.4 | 41.2–67.4 | 99.9 (< 0.001) |
| Rapid testing | ||||
| Available | 86 | 62.2 | 56.1–68.0 | 100.0 (< 0.001) |
| Not available | 64 | 60.0 | 54.4–65.5 | 100.0 (< 0.001) |
| Country income level | ||||
| High | 106 | 53.7 | 49.5–57.8 | 100.0 (< 0.001) |
| Middle | 33 | 80.4 | 73.9–86.2 | 99.9 (< 0.001) |
| Low | 11 | 69.1 | 57.2–79.9 | 99.6 (< 0.001) |
| Setting | ||||
| Hospital | 22 | 72.0 | 56.4–85.2 | 100.0 (< 0.001) |
| GP/primary care | 14 | 81.4 | 72.0–89.3 | 98.5 (< 0.001) |
| Pharmacy | 1 | 39.5 | 33.2–46.1 | - |
| Community-based | 30 | 79.2 | 73.6–84.3 | 100.0 (< 0.001) |
| Emergency department | 71 | 46.6 | 40.1–53.3 | 100.0 (< 0.001) |
| Prison | 14 | 68.8 | 49.9–84.9 | 100.0 (< 0.001) |
| Outpatients | 29 | 55.9 | 44.9–66.7 | 100.0 (< 0.001) |
| Mixed | 3 | 46.9 | 16.1–79.1 | - |
| Populations | ||||
| Inpatients | 15 | 68.4 | 45.7–87.3 | 100.0 (< 0.001) |
| Emergency patients | 69 | 47.2 | 40.6–53.9 | 100.0 (< 0.001) |
| Paediatrics | 6 | 75.7 | 63.1–86.4 | 99.7 (< 0.001) |
| General public | 34 | 76.4 | 71.1–81.3 | 100.0 (< 0.001) |
| Outpatients | 41 | 64.8 | 50.1–78.2 | 100.0 (< 0.001) |
| Incarcerated persons | 11 | 68.8 | 49.9–84.9 | 100.0 (< 0.001) |
| Mixed | 3 | 46.9 | 16.1–79.1 | - |
| Latest study year | ||||
| 2016–2020 | 27 | 69.2 | 57.8–79.5 | 100.0 (< 0.001) |
| 2011–2015 | 64 | 59.8 | 54.7–64.8 | 100.0 (< 0.001) |
| 2006–2010 | 54 | 58.2 | 48.7–67.3 | 100.0 (< 0.001) |
| 2001–2005 | 5 | 63.2 | 49.7–75.7 | 99.8 (< 0.001) |
Table 4.
Meta-regression of HIV test uptake
| Variable | Univariable | Multivariable1 | |||
|---|---|---|---|---|---|
| β (95% CI) | P-value | Adjusted R2 | β (95% CI) | P-value | |
| Type of HIV testing service approach | -0.15% | ||||
| Opt-in | Reference | Reference | |||
| Opt-out | 0.04 (-0.05 to 0.12) | 0.413 | 0.12 (0.03 to 0.21) | 0.007 | |
| Risk-based | -0.05 (-0.19 to 0.09) | 0.506 | -0.03 (-0.17 to 0.11) | 0.685 | |
| Rapid testing | -0.51% | ||||
| Available | Reference | Reference | |||
| Not available | -0.02 (-0.10 to 0.07) | 0.674 | -0.03 (-0.11 to 0.06) | 0.542 | |
| Country income level | 12.1% | ||||
| High | Reference | Reference | |||
| Middle | 0.24 (0.15 to 0.33) | < 0.001 | 0.21 (0.09 to 0.32) | 0.001 | |
| Low | 0.14 (-0.01 to 0.30) | 0.067 | 0.19 (0.00 to 0.38) | 0.051 | |
| Mixed | 0.28 (-0.23 to 0.79) | 0.273 | 0.17 (-0.41 to 0.75) | 0.560 | |
| Setting | 17.9% | ||||
| Hospital | Reference | Reference | |||
| GP/primary care | 0.10 (-0.07 to 0.28) | 0.251 | 0.04 (-0.22 to 0.29) | 0.779 | |
| Pharmacy | -0.30 (-0.82 to 0.22) | 0.256 | -0.20 (-0.76 to 0.36) | 0.487 | |
| Community-based | 0.06 (-0.08 to 0.21) | 0.371 | 0.03 (-0.22 to 0.29) | 0.795 | |
| Emergency Department | -0.23 (-0.35 to -0.10) | < 0.001 | -0.35 (-0.68 to -0.03) | 0.032 | |
| Prison | -0.03 (-0.20 to 14.4) | 0.761 | 0.09 (-0.11 to 0.28) | 0.373 | |
| Outpatients | -0.15 (-0.29 to 0.0) | 0.044 | -0.29 (-0.54 to -0.04) | 0.021 | |
| Mixed | -0.23 (-0.54 to 0.08) | 0.141 | -0.32 (-0.67 to 0.03) | 0.072 | |
| Populations | 11.5% | ||||
| Inpatients | Reference | Reference | |||
| Emergency patients | -0.18 (-0.33 to -0.03) | 0.017 | 0.26 (-0.07 to 0.59) | 0.124 | |
| Paediatrics | 0.09 (-0.16 to 0.34) | 0.495 | -0.01 (-0.18 to 0.38) | 0.958 | |
| General public | 0.07 (-0.09 to 0.23) | 0.383 | 0.10 (-0.18 to 0.38) | 0.487 | |
| Outpatients | -0.03 (-0.19 to 0.13) | 0.710 | 0.22 (-0.05 to 0.48) | 0.108 | |
| Incarcerated persons | 0.01 (-0.18 to 0.21) | 0.900 | * | ||
| Mixed | -0.19 (-0.52 to 0.14) | 0.251 | * | ||
| Latest study year | -0.16% | ||||
| 2016–2020 | Reference | Reference | |||
| 2011–2015 | -0.07 (-0.19 to 0.04) | 0.194 | 0.01 (-0.10 to 0.12) | 0.818 | |
| 2006–2010 | -0.09 (-0.20 to 0.02) | 0.114 | 0.04 (-0.08 to 0.15) | 0.552 | |
| 2001–2005 | -0.03 (-0.29 to 0.23) | 0.812 | 0.19 (-0.06 to 0.44) | 0.135 |
*omitted because of collinearity
1Adjusted R2 23.3%
Supplementary Fig. 1 shows the funnel plot which demonstrates a possibility for publication bias with under-reporting of studies with lower HIV test uptake. The quality assessment for each paper is presented in Supplementary Tables 1–3.
Discussion
This systematic review aimed to understand the uptake of HIV testing by comparing opt-in, opt-out and risk-based testing approaches. This study adds to the evidence base regarding HIV testing approaches. We found that opt-out testing (when adjusted for rapid testing, country income level, setting and population tested) had higher uptake than opt-in and risk-based testing. We also found that the population of emergency department patients and hospital outpatients had significantly lower HIV test uptake than other populations.
Our finding that opt-out testing for HIV was associated with a higher proportion of people testing than opt-in testing is consistent with other studies. For example, a 2017 systematic review and meta-analysis comparing HIV opt-out testing and opt-in testing amongst patients attending emergency departments found that the opt-out strategies had higher uptake (44%) than the opt-in strategies (19%) [20••]. We extend the evidence base for the value of opt-out testing, as we included studies from various settings beyond emergency departments. The value of opt-out testing is exemplified by a 2021 study in Kenya that reported a 2.2-fold greater odds of new HIV diagnosis using opt-out point of care than opt-in testing [21]. The study reported higher refusal rates for opt-in testing, whilst a higher proportion of participants in the opt-out testing were willing to disclose risky sexual practices, suggesting that they were more likely to participate if testing were presented as part of standard care [21]. The study also reported that physicians were more likely to offer tests to patients who are at a higher risk of HIV (i.e. never tested, tested > 1 year ago, older men) and therefore were likely to miss a substantial proportion during opt-in testing [21].
Our review found that opt-out testing was mostly implemented in the emergency department setting. Yet, HIV test uptake was the lowest in emergency departments compared with other settings where opt-out testing was available. Whilst there could be value in HIV testing in emergency departments, studies have shown HIV testing in emergency departments could have poor linkage to care [22], low test acceptance rates amongst marginalised populations [23], high cost per positive diagnosis [24] and lack of cultural competency being integrated [25]. This could also be due to the transient nature of conditions and acute care needed in the emergency department, where the focus is on the patient’s current issue and less on peripheral issues like HIV testing. Furthermore, HIV testing uptake could be higher when a physician offers the test [26–28] which may not always be the case in a busy emergency department. Nevertheless, an ED-based HIV screening program remains an integral component of the overall HIV screening strategy to reduce the current HIV testing gap and complement existing community-based HIV screening programs. Therefore, our study highlights the need for further improvements for HIV testing beyond opt-out testing strategies for the emergency department setting.
These are missed opportunities for HIV testing in certain settings. Our review uncovered that pharmacies, followed by primary care clinics, had the lowest uptake of HIV testing. In many countries, the majority of the population sees a primary care practitioner at least once a year [29, 30]. The literature surrounding insights into GPs’ current HIV testing practices reveal the barriers GPs face to routinely offering testing, including being worried about potentially harming patient relationships [31] and feeling incapable of offering HIV tests due to perceived poor knowledge [32]. Steps should be taken to address barriers around HIV testing in primary care to improve HIV detection rates [33, 34]. In addition, pharmacies can provide point-of-care HIV testing [35] and participate in HIV prevention related to pre-exposure prophylaxis and post-exposure prophylaxis [36].
One unexpected finding from our review was in settings where rapid testing was available, there was no significant difference in HIV test uptake compared to settings without rapid testing. This observation should be interpreted with caution. One possibility could be because the majority of studies with rapid testing were conducted in emergency department settings, a setting with the lowest testing uptake in this review. Another possibility is that unlike other studies which specifically assessed the impact of rapid testing compared with venepuncture, our systematic review examined the difference in HIV test uptake between settings where rapid testing was available compared with settings that did not have rapid testing uptake. Therefore, there could be other confounders related to sub-populations attending these settings [37, 38]. There is evidence of greater appeal of rapid testing compared with venepuncture. For example, a 2013 systematic review on rapid point-of-care HIV testing found that youth preferred rapid point-of-care tests compared to traditional testing methods [39]. Similarly, a study of adults attending general practices in France reported higher acceptability of a rapid test (92%) compared with venepuncture (64%) [40]. Studies report that patients prefer to receive their results quickly and would recommend rapid testing to their peers [41, 42]. Rapid testing can reach high-risk populations in clinical and community settings, which is critical in testing untested individuals [43, 44]. However, there is evidence that some patients may have concerns regarding the reliability of the rapid test and having their clinical visits prolonged [33]. Further research is warranted to understand how rapid testing (including HIV self-testing) could improve HIV testing rates using an opt-out approach.
Our findings have implications for policy and practice for HIV testing. First, congruent with WHO and US CDC recommendations, our review strengthens the evidence base that an opt out testing approach could further improve HIV testing. However, this should not be a one-size-fits all recommendation as evidenced by the high heterogeneity between studies. This underscores the importance to consider the type of clinical service and the unique socio-cultural contexts of different regions and countries. Nevertheless, our review provides a useful compendium of studies where opt-out testing has worked well and where it has not. Second, we highlight the settings where more work is needed to improve HIV testing rates, for example, in emergency departments and hospital outpatients. Whilst opt-out testing in these settings may reduce stigma associated with HIV by normalising testing, further implementation research is needed to understand ongoing barriers and focus on strategies to better integrate HIV testing into the clinical workflow.
There are a few limitations of this systematic review. First, many studies included were from high-income countries, specifically, more than half were from the USA. As such, the results may not be easily generalisable to other settings and/or in lower-income settings. A large proportion (71 of 150) of studies were from an emergency department. Therefore, our findings could be skewed by the large proportion of studies from the USA, emergency department settings and/or high-income countries. Second, we found a low number of studies using the risk-based HIV testing approach (19 of 150 articles), thus exposing a gap in the literature for future studies to evaluate the value of this approach [10••]. Third, we found high heterogeneity between studies, highlighting the importance of the need for local, contextualised evidence when deciding between an opt-in, opt-out or risk-based testing approach. We explored this heterogeneity in our meta-regression analyses and found that country-income level, settings and type of population could explain some of this variability, but there remain unexplained confounders.
In conclusion, this review adds to the current literature that opt-out testing can significantly improve HIV test uptake compared to opt-in in various settings and across different populations. We also uncovered settings (emergency department, primary care, pharmacy) where HIV test uptake remains poor, highlighting the need to implement new strategies in those settings to improve HIV test uptake if we are to end the HIV/AIDS pandemic.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
JJO, CJ, MSJ conceptualised the idea. LO and QS performed the screening and extraction of data. JJO analysed the data. LO and QS wrote the original draft. All authors contributed to the writing of the manuscript and approved the final version for submission.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was supported by funding from the World Health Organization through the following grants: USAID GHA‐G‐00‐09‐00003 and the Bill and Melinda Gates Foundation OPP1177903. EPFC and JJO are supported by an Australian National Health and Medical Research Council Emerging Leadership Fellowship (GNT1172873, GNT1193955, respectively).
Data Availability
All relevant data are presented in the manuscript and online supplementary materials. Any further details can be obtained by contacting the corresponding author.
Code Availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests. The contents in this article are those of the authors alone and do not necessarily reflect the view of the World Health Organization.
Footnotes
Qi Rui Soh and Leon Y. J. Oh are equal first co-authors
This article is part of the Topical Collection on The Science of Prevention
The Editors would like to thank Dr. Juliet Iwelunmor for taking the time to review this manuscript
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Apers H, Nöstlinger C, Van Beckhoven D, Deblonde J, Apers L, Verheyen K, et al. Identifying key elements to inform HIV-testing interventions for primary care in Belgium. Health Promot Int. 2020;35(2):301–311. doi: 10.1093/heapro/daz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brault MA, Spiegelman D, Hargreaves J, Nash D, Vermund SH. Treatment as prevention: concepts and challenges for reducing HIV incidence. J Acquir Immune Defic Syndr. 2019;82(Suppl 2):S104–S112. doi: 10.1097/QAI.0000000000002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou R, Evans C, Hoverman A, Sun C, Dana T, Bougatsos C, et al. Preexposure prophylaxis for the prevention of HIV infection: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(22):2214–2230. doi: 10.1001/jama.2019.2591. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. Understanding Fast-Track. Accelerating action to end the AIDS epidemic by 2030 [Available from: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf. Accessed 1 July 2022
- 5.UNAIDS. Factsheet: World AIDS Day 2021. Available from https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- 6.Late Presentation Working Groups in Euro S, Cohere Estimating the burden of HIV late presentation and its attributable morbidity and mortality across Europe 2010–2016. BMC Infect Dis. 2020;20(1):728. doi: 10.1186/s12879-020-05261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause J, Subklew-Sehume F, Kenyon C, Colebunders R. Acceptability of HIV self-testing: a systematic literature review. BMC Public Health. 2013;13(1):1–9. doi: 10.1186/1471-2458-13-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesney MA, Smith AW. Critical delays in HIV testing and care: the potential role of stigma. Am Behav Sci. 1999;42(7):1162–1174. doi: 10.1177/00027649921954822. [DOI] [Google Scholar]
- 9.Tee Y, Huang M. Knowledge of HIV/AIDS and attitudes towards people living with HIV among the general staff of a public university in Malaysia. SAHARA-J: Journal of Social Aspects of HIV/AIDS. 2009;6(4):179–87. doi: 10.1080/17290376.2009.9724946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••. Ong JJ, Coulthard K, Quinn C, Tang MJ, Huynh T, Jamil MS, et al. Risk-based screening tools to optimise HIV testing services: a systematic review. Curr HIV/AIDS Rep. 2022. This systematic review summarizes the accuracy of risk-based screening tools for HIV testing. [DOI] [PMC free article] [PubMed]
- 11.Centers for Disease Control and Prevention. HIV testing. Available from https://www.cdc.gov/hiv/testing/index.html. Accessed 1 July 2022
- 12.Copenhaver MM, Fisher JD. Experts outline ways to decrease the decade-long yearly rate of 40,000 new HIV infections in the US. AIDS Behav. 2006;10(1):105–114. doi: 10.1007/s10461-005-9034-x. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson AB, Corbie-Smith G, Thomas SB, Mohanan S, Del Rio C. Understanding the patient’s perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Educ Prev. 2004;16(2):101–114. doi: 10.1521/aeap.16.2.101.29394. [DOI] [PubMed] [Google Scholar]
- 14.Irwin KL, Valdiserri RO, Holmberg SD. The acceptability of voluntary HIV antibody testing in the United States: a decade of lessons learned. AIDS 1996 Dec;10(14):1707–17. [DOI] [PubMed]
- 15.UNAIDS, WHO HIV/AIDS Programme. Guidance on Provider-Initiated Testing and Counseling in Health Facilities. World Health Organization; May, 2007. [Available from: http://apps.who.int/iris/bitstream/handle/10665/43688/9789241595568_eng.pdf?sequence=1. Accessed 1 July 2022
- 16.Johanna Briggs Institute Critical Appraisal Tools [Available from: https://jbi.global/critical-appraisal-tools. Accessed 1 July 2022
- 17.UNAIDS. Global HIV & AIDS statistics—fact sheet. [Available from: https://www.unaids.org/en/resources/fact-sheet#:~:text=In%202020%2C%20there%20were%2037.7,HIV%20were%20women%20and%20girls. Accessed 1 July 2022
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UNAIDS. Global HIV & AIDS statistics—2020 fact sheet 2020. Available from https://www.unaids.org/en/resources/fact-sheet. Accessed 1 July 2022
- 20••. Henriquez-Camacho C, Villafuerte-Gutierrez P, Perez-Molina JA, Losa J, Gotuzzo E, Cheyne N. Opt-out screening strategy for HIV infection among patients attending emergency departments: systematic review and meta-analysis. HIV Med. 2017;18(6):419–29. This systematic review compares opt-out versus opt-in screening in the emergency department. [DOI] [PubMed]
- 21.Sanders EJ, Agutu C, van der Elst E, Hassan A, Gichuru E, Mugo P, et al. Effect of an opt‐out point‐of‐care HIV‐1 nucleic acid testing intervention to detect acute and prevalent HIV infection in symptomatic adult outpatients and reduce HIV transmission in Kenya: a randomized controlled trial. HIV Medicine. 2022 Jan;23(1):16–28. [DOI] [PMC free article] [PubMed]
- 22.Menon AA, Nganga-Good C, Martis M, Wicken C, Lobner K, Rothman RE, et al. Linkage-to-care methods and rates in US emergency department–based HIV testing programs: a systematic literature review brief report. Acad Emerg Med. 2016;23(7):835–842. doi: 10.1111/acem.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merchant RC, Seage GR, III, Mayer KH, Clark MA, DeGruttola VG, Becker BM. Emergency department patient acceptance of opt-in, universal, rapid HIV screening. Public Health Reports. 2008;123(3_suppl):27–40. doi: 10.1177/00333549081230S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowan E, Herman HS, Rahman S, Zahn J, Leider J, Calderon Y. Bundled HIV and hepatitis C testing in the emergency department: a randomized controlled trial. Western Journal of Emergency Medicine. 2018;19(6):1049. doi: 10.5811/westjem.2018.8.37827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merchant RC, Liu T, Clark MA, Carey MP. Facilitating HIV/AIDS and HIV testing literacy for emergency department patients: a randomized, controlled, trial. BMC Emerg Med. 2018;18(1):1–18. doi: 10.1186/s12873-018-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White DA, Scribner AN, Vahidnia F, Dideum PJ, Gordon DM, Frazee BW, et al. HIV screening in an urban emergency department: comparison of screening using an opt-in versus an opt-out approach. Ann Emerg Med. 2011;58(1 Suppl 1):S89–95. doi: 10.1016/j.annemergmed.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 27.Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Prescott RJ. Uptake and acceptability of antenatal HIV testing: randomised controlled trial of different methods of offering the test. BMJ. 1998;316(7127):262–267. doi: 10.1136/bmj.316.7127.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haukoos JS, Hopkins E, Eliopoulos VT, Byyny RL, LaPerriere KA, Mendoza MX, et al. Development and implementation of a model to improve identification of patients infected with HIV using diagnostic rapid testing in the emergency department. Acad Emerg Med. 2007;14(12):1149–1157. doi: 10.1197/j.aem.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Australian Bureau of Statistics. Patient experiences in Australia: summary of findings. Available from https://www.abs.gov.au/statistics/health/health-services/patient-experiences-australia-summary-findings/latest-release. Accessed 1 July 2022
- 30.Irving G, Neves AL, Dambha-Miller H, Oishi A, Tagashira H, Verho A, et al. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open. 2017;7(10):e017902. doi: 10.1136/bmjopen-2017-017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hindocha S, Charlton T, Rayment M, Theobald N. Feasibility and acceptability of routine human immunodeficiency virus testing in general practice: your views. Primary health care research & development. 2013;14(2):212–216. doi: 10.1017/S1463423612000436. [DOI] [PubMed] [Google Scholar]
- 32.Manirankunda L, Loos J, Debackaere P, Nöstlinger C. “It is not easy”: challenges for provider-initiated HIV testing and counseling in Flanders. Belgium AIDS Education and Prevention. 2012;24(5):456–468. doi: 10.1521/aeap.2012.24.5.456. [DOI] [PubMed] [Google Scholar]
- 33.Avery AK, Del Toro M, Caron A. Increases in HIV screening in primary care clinics through an electronic reminder: an interrupted time series. BMJ Qual Saf. 2014;23(3):250–256. doi: 10.1136/bmjqs-2012-001775. [DOI] [PubMed] [Google Scholar]
- 34.Glew S, Pollard A, Hughes L, Llewellyn C. Public attitudes towards opt-out testing for HIV in primary care: a qualitative study. Br J Gen Pract. 2014;64(619):e60–e66. doi: 10.3399/bjgp14X677103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKeirnan K, Kherghehpoush S, Gladchuk A, Patterson S. Addressing barriers to HIV point-of-care testing in community pharmacies. Pharmacy. 2021;9(84). [DOI] [PMC free article] [PubMed]
- 36.Myers JE, Farhat D, Guzman A, Arya V. Pharmacists in HIV prevention: an untapped potential. Am J Public Health. 2019;109(6):859–861. doi: 10.2105/AJPH.2019.305057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma M, Ong JJ, Celum C, Terris-Prestholt F. Heterogeneity in individual preferences for HIV testing: a systematic literature review of discrete choice experiments. EClinicalMedicine. 2020;29–30:100653. doi: 10.1016/j.eclinm.2020.100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong JJ, Nwaozuru U, Obiezu-Umeh C, Airhihenbuwa C, Xian H, Terris-Prestholt F, et al. Designing HIV testing and self-testing services for young people in Nigeria: a discrete choice experiment. Patient. 2021;14(6):815–826. doi: 10.1007/s40271-021-00522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner SD, Anderson K, Slater M, Quigley L, Dyck M, Guiang CB. Rapid point-of-care HIV testing in youth: a systematic review. J Adolesc Health. 2013;53(6):683–691. doi: 10.1016/j.jadohealth.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Demorat H, Lopes A, Chopin D, Delcey V, Clevenbergh P, Simoneau G, et al. Acceptability and feasibility of HIV testing in general medicine by ELISA or rapid test from finger-stick whole blood. Presse Med. 2018;47(2):e15–e23. doi: 10.1016/j.lpm.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Merchant RC, Clark MA, Seage GR, III, Mayer KH, Degruttola VG, Becker BM. Emergency department patient perceptions and preferences on opt-in rapid HIV screening program components. AIDS Care. 2009;21(4):490–500. doi: 10.1080/09540120802270284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith LV, Rudy ET, Javanbakht M, Uniyal A, Sy LS, Horton T, et al. Client satisfaction with rapid HIV testing: comparison between an urban sexually transmitted disease clinic and a community-based testing center. AIDS Patient Care STDS. 2006;20(10):693–700. doi: 10.1089/apc.2006.20.693. [DOI] [PubMed] [Google Scholar]
- 43.Mutch AJ, Lui C-W, Dean J, Mao L, Lemoire J, Debattista J, et al. Increasing HIV testing among hard-to-reach groups: examination of RAPID, a community-based testing service in Queensland. Australia BMC health services research. 2017;17(1):1–7. doi: 10.1186/s12913-017-2249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pottie K, Medu O, Welch V, Dahal GP, Tyndall M, Rader T, et al. Effect of rapid HIV testing on HIV incidence and services in populations at high risk for HIV exposure: an equity-focused systematic review. BMJ Open. 2014;4(12):e006859. doi: 10.1136/bmjopen-2014-006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are presented in the manuscript and online supplementary materials. Any further details can be obtained by contacting the corresponding author.
Not applicable.