Abstract
The immune-inflammatory response is associated with increased nitro-oxidative stress. The aim of this mechanistic review is to examine: (a) the role of redox-sensitive transcription factors and enzymes, ROS/RNS production, and the activity of cellular antioxidants in the activation and performance of macrophages, dendritic cells, neutrophils, T-cells, B-cells, and natural killer cells; (b) the involvement of high-density lipoprotein (HDL), apolipoprotein A1 (ApoA1), paraoxonase-1 (PON1), and oxidized phospholipids in regulating the immune response; and (c) the detrimental effects of hypernitrosylation and chronic nitro-oxidative stress on the immune response. The redox changes during immune-inflammatory responses are orchestrated by the actions of nuclear factor-κB, HIF1α, the mechanistic target of rapamycin, the phosphatidylinositol 3-kinase/protein kinase B signaling pathway, mitogen-activated protein kinases, 5' AMP-activated protein kinase, and peroxisome proliferator-activated receptor. The performance and survival of individual immune cells is under redox control and depends on intracellular and extracellular levels of ROS/RNS. They are heavily influenced by cellular antioxidants including the glutathione and thioredoxin systems, nuclear factor erythroid 2-related factor 2, and the HDL/ApoA1/PON1 complex. Chronic nitro-oxidative stress and hypernitrosylation inhibit the activity of those antioxidant systems, the tricarboxylic acid cycle, mitochondrial functions, and the metabolism of immune cells. In conclusion, redox-associated mechanisms modulate metabolic reprogramming of immune cells, macrophage and T helper cell polarization, phagocytosis, production of pro- versus anti-inflammatory cytokines, immune training and tolerance, chemotaxis, pathogen sensing, antiviral and antibacterial effects, Toll-like receptor activity, and endotoxin tolerance.
Keywords: Oxidative and nitrosative stress, Immune response, Inflammation, Antioxidants, Physiological stress
Subject terms: Chronic inflammation, Cell signalling
Introduction
The instigation of the innate immune response commences as a result of the recognition of an invading pathogen by organ-specific resident macrophages, dendritic cells (DCs), fibroblasts, pericytes, and in many cases endothelial cells [1–4]. This recognition is accomplished by cytosolic or membrane-bound Toll-like or NOD-like pattern-recognition receptors (PRR) that leads to the activation of these sentinel cells and the release of cytokines and chemokines [3–5]. Once secreted these molecules activate endothelial cells that then express chemokines and adhesion factors [6, 7]. Recruitment, binding, and activation of neutrophils, monocytes, macrophages, and platelets follow these processes in turn allowing the migration of myeloid cells into tissues that reach the sites of infection [8–10].
The multiple phenotypical and functional roles of myeloid cells are enabled by metabolic reprogramming comprising of changes in levels of glycolysis, fatty acid oxidation (FAO), the tricarboxylic acid (TCA) cycle activity, involvement of the pentose phosphate pathway (PPP), and mitochondrial respiration [11–13]. This is also true for neutrophils, T-cell activation and differentiation into helper, effector, and cytotoxic subsets [14], B-cell activation, differentiation and antibody production [15], and the activation and cytotoxic properties of natural killer (NK) cells [16].
These metabolic and redox changes are orchestrated and regulated by the cooperative and/or antagonistic actions of nuclear factor (NF-κB), HIF1α, the mechanistic target of rapamycin (mTOR), and the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway. Mitogen-activated protein (MAP) kinases, 5' AMP-activated protein kinase (AMPK), and peroxisome proliferator-activated receptor (PPAR) are also implicated. All these factors lead to the increase in reactive oxygen species (ROS) produced by mitochondria and to the upregulation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX). These transcription factors and enzymes are all redox-sensitive as is the performance of mitochondria [17–23].
In addition, the functioning of individual immune cells is under redox control. It is sensitive to intracellular and extracellular levels of nitric oxide (NO) [24, 25] and ROS [26–28] and is also heavily influenced by the activity of nuclear factor erythroid 2-related factor 2 (Nrf-2) and cellular antioxidants [29–31]. The action of individual immune cells is regulated by oxidized phospholipids [32–35], high-density lipoprotein (HDL), apolipoprotein A1 (ApoA1), paraoxonase-1 (PON1) activity [36–38], and indoleamine 2, 3-dioxygenase (IDO) [39, 40]. The levels and immune functions of these molecular players are under redox control as well [41].
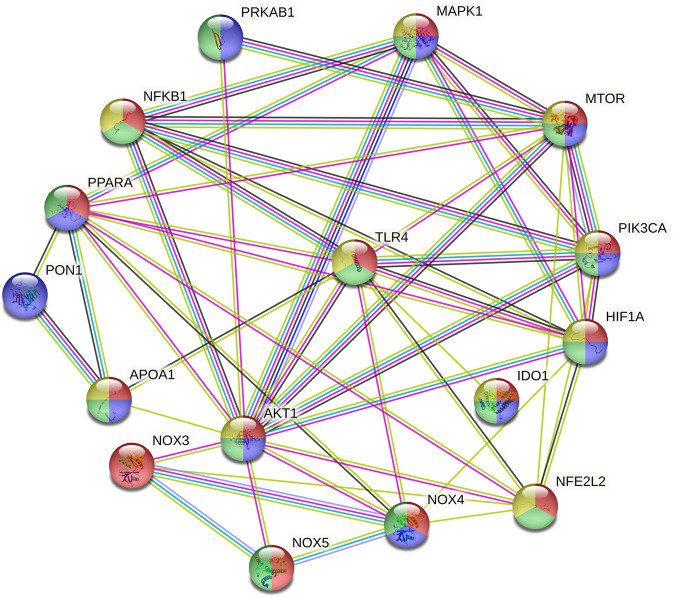
Figure 1 shows the outcome of a STRING (STRING version 11.0; https://string-db.org) protein–protein network analysis performed on the aforementioned proteins and enzymes, which are discussed in detail in this review. The zero-order network consists of 16 nodes. The number of edges (n = 50) exceeds the expected number of edges (n = 13) with p-enrichment value of 2.22E–15, average node degree = 6.25 and average local clustering coefficient = 0.78.
Fig. 1.
STRING protein–protein network analysis performed on the key proteins included in the present review. Nodes indicate proteins and edges indicate protein–protein interactions. Red colour of the nodes: reflects response to stress (p < 1.57E–05), blue node colour: small molecular metabolic process (p < 1.68E–05), green node colour: positive regulation of metabolic process (p < 2.17E–05), and yellow node colour: regulation of immune system process (p < 3.78E–05). Colours of the edges: see https://string-db.org for details. The figure displays the gene names and Table 1 specifies the names and functions of the proteins. NFKB1 nuclear factor (NF)-κB (NF-κB), HIF1A hypoxia-inducible factor 1-alpha (HIF1α), MTOR the mechanistic target of rapamycin (mTOR), PIK3CA phosphatidylinositol 3-kinase (PI3K), AKT1 protein kinase B, MAPK mitogen-activated protein kinases, PRKAB1 AMP-activated protein kinase (AMPK), PPARA peroxisome proliferator-activated receptor, NOX NADPH oxidase, NFE2L2 nuclear factor erythroid 2-related factor 2 (Nrf-2), APOA1 apolipoprotein A1 (ApoA1), PON1 paraoxonase-1, IDO1 indoleamine 2, 3-dioxygenase (IDO), TLR-4 Toll-like receptor-4
Table 1 summarizes the functions of the proteins in this highly interconnected protein interaction network.
Table 1.
Names and functions of the key proteins included in the present review
| ID | Names | Main functions (based on UniProt) | References |
|---|---|---|---|
| NF-κB | Nuclear factor NF-kappa-B | Pleiotropic transcription factor and endpoint of a series of signal transduction events including immune activation, differentiation, cell growth, and apoptosis | [42–44, 88, 89] |
| HIF1α | Hypoxia-inducible factor 1-alpha | Transcriptional regulator of the response to hypoxia. Activates over 40 genes, e.g., glycolytic enzymes, glucose transporters, vascular endothelial growth factor, and protein that increases oxygen delivery | [47, 49, 54–57] |
| mTOR | Mechanistic target of rapamycin | In response to stress, hormonal and energy signals, regulates cellular metabolism, survival, and growth | [72–78, 180, 228] |
| PI3K |
PI3-kinase Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform Phosphatidylinositol 3-kinase |
Group of signal transducer enzymes which regulate cellular functions including proliferation, differentiation, survival, motility, and morphology | [45, 47–49, 130, 230, 231] |
| AKT1 | RAC-alpha serine/threonine-protein kinase | Regulates metabolism, cell survival, proliferation, and growth | [22, 46, 48, 73, 331, 479] |
| MAPK1 | Mitogen-activated protein kinase | Mediates adhesion, cell growth, survival, and differentiation via transcription and translational processes and cytoskeletal rearrangements | [435] |
| AMPK | 5'-AMP-activated protein kinase | In response to lowered ATP, regulates energy metabolism and attenuates energy-consuming processes. AMPK reduces carbohydrate, lipid, and protein synthesis | [21, 133, 134, 293, 346, 448] |
| PPAR | Peroxisome proliferator-activated receptor | Regulates the beta-oxidation pathway and lipid metabolism | [41, 90, 138, 139] |
| NADPH oxidase (NOX) | Nicotinamide adenine dinucleotide phosphate oxidase | May constitutively produce superoxide | [144, 216, 470, 472] |
| TLR-4 | Toll-like receptor-4 | Mediates the immune response to lipopolysaccharides | [85, 87] |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 | Transcription activator that binds to antioxidant response elements in the promoter regions of (antioxidant) target genes | [160, 195, 196, 436] |
| PON1 | Paraoxonase/arylesterase 1 | Protects low-density lipoproteins against oxidative modification and consequent atherogenicity | [525, 526] |
| IDO | Indoleamine 2,3-dioxygenase 1 | Catalyzes the first step of the catabolism of tryptophan into kynurenine and other tryptophan catabolites | [535] |
| ApoA1 | Apolipoprotein A-I | Acts as a cofactor for lecithin cholesterol acyltransferase and participates in the reverse cholesterol transport | [522–524] |
This paper has three aims. Firstly, to detail the role of redox-sensitive transcription factors and enzymes, ROS, and reactive nitrogen species (RNS) production and the effect of cellular antioxidants on the activation and performance of macrophages, DCs, neutrophils, T-cells, B-cells, and NK-cells. Secondly, to explain the involvement of HDL, ApoA1, PON1, and oxidized phospholipids in regulating the immune-inflammatory response. Thirdly, to clarify the detrimental effects of chronic oxidative and nitrosative stress on the performance of individual immune cells and the immune-inflammatory response as a whole. We will begin with a discussion of the effects of these factors on macrophage activation and function, which offers a vehicle to illustrate many of the principles involved in metabolic reprogramming and the effects of individual signaling molecules, thus avoiding unnecessary repetition in later sections of the paper.
Metabolic reprogramming and redox factors involved in macrophage activation
Metabolic reprogramming in macrophages
Macrophages may be activated by cytokines, ROS, and PRR engagement by pathogen-associated molecular patterns, damage-associated molecular patterns, and commensal LPS leading to the activation of NF-κB [42–44] and the PI3K/AKT signaling pathway [45, 46]. Upregulated NF-κB results in increased transcription of proinflammatory cytokines and chemokines, inducible NO synthase (iNOS), and HIF1α [42–44]. Enhanced PI3K signaling also leads to the upregulation of mTOR [47–49] which in turn reinforces the upregulation of HIF1α [45, 46]. These signaling pathways, enzymes, and transcription factors play an essential role in maintaining macrophage activation and M1 polarization by driving metabolic reprogramming. It involves the downregulation of ATP production by mitochondrial oxidative phosphorylation (OXPHOS) and FAO [50, 51] to ATP production via aerobic glycolysis [52].
The shift to aerobic glycolysis is an indispensable metabolic event for M1 macrophages in terms of maintaining and increasing phagocytosis, production of ROS and proinflammatory cytokines and unsurprisingly, its inhibition may impair those functions [53–55]. Maintenance of this state is dependent on the activity of a range of transcription factors, most notably mTOR and HIF1α, with the latter playing a dominant role in enabling the continuance of glycolysis under normoxic conditions [49, 56].
HIF1α acts as a modulator of transcription by changing the methylation status of hypoxia-responsive elements in the promoter regions of target genes involved in the termination of OXPHOS and the instigation of aerobic glycolysis [57]. For example, HIF1α upregulation suppresses the activity of electron transport chain (ETC) enzymes [58, 59], decreases mitochondrial activity, and induces mitochondrial autophagy [60, 61]. Increased activity of this transcription factor also suppresses genes involved in FAO [62, 63]. HIF1α restrains metabolism by activating the gene for pyruvate dehydrogenase kinase 1, which in turn inhibits the TCA cycle [64] and inactivates pyruvate dehydrogenase [65]. In addition, HIF1α-regulated gene expression reduces the production of acetyl-CoA and succinyl-CoA [66].
HIF1α intensifies glycolytic flux, thereby augmenting the expression of glucose transporters (GLUT-1 and GLUT-3) [67]. Glycolysis is stimulated by the high levels of hexokinases [68], aldolase A, enolase 1 [69], and phosphoglycerate kinase 1 [70]. Finally, HIF1α also induces the transcription of lactate dehydrogenase A, which plays an indispensable role in maintaining a continuous supply of NAD+, thereby enabling the continuation of glycolysis [71]. HIF1α-regulated gene expression prevents acetyl-CoA from being synthesized from glucose and fatty acid-derived carbons [66].
While the role of HIF1α in instigating and regulating the transition between OXPHOS and aerobic glycolysis is of paramount importance, it should be emphasized that the activation of mTOR is involved. Firstly, mTOR stabilizes and enhances the activity of HIF1α and, secondly, it increases the rate of glycolysis, AKT, forkhead box transcription factors (FoxO), hexokinase II, and Myc proto-oncogene [72–74]. Upregulated mTOR participates in further reducing OXPHOS by enhancing NO and interferon (IFN)-γ production, thus compromising the activity of the mitochondrial ETC [75]. In total, the actions of mTOR inhibit M2 polarization [76] and stimulate M1 polarization [77, 78].
The PPP main role is to utilize the energy released from the metabolism of glucose-6-phosphate into ribulose-5-phosphate to form NADPH. The latter is used in the production of NADPH oxidase and as a reducing equivalent enabling the function of the glutathione (GSH) and thioredoxin antioxidant systems [13, 79]. The activation of M1 polarized macrophages also results in several other aspects of metabolic reprogramming in order to maintain the inflammatory status and prolong survival. Most notable are the upregulation of the cytosolic PPP [50, 80], increased lipid synthesis, and decreased lipid catabolism [62, 81], altered glutamine and arginine metabolism [81, 82], and a “broken” TCA cycle [83, 84]. These parameters are discussed below commencing with the Toll-like Receptor (TLR) and proinflammatory cytokine-mediated reprogramming of the lipidome [85].
The synthesis of lipids is a key component in membrane remodeling. In M1 macrophages the process depends on the production of acetyl-CoA from citrate ATP-citrate lyase [86]. The activity of this enzyme rapidly increases in activated macrophages. Intracellular fatty acids can also be used to synthesize triglycerides for energy storage, and sphingolipids for membrane synthesis, as well as eicosanoids for signaling [81]. The increase in lipid synthesis is largely enabled and regulated by the high activity of sterol regulatory element binding protein-1 (SREBP-1) by TLR-4 and PI3K-activated mTOR [73, 87]. It is also controlled by the enhanced expression of NF-κB and the presence of proinflammatory cytokines [88, 89]. SREBP-1 activation stimulates the synthesis of proinflammatory cytokines, ROS, and triggers the inflammasome [87–89]. M1 activation is accompanied by elevated iNOS, which induces the conversion of arginine to NO, so that the production of other RNS may be initiated [82, 90, 91].
M1 polarized macrophages accumulate cytosolic citrate stemming from the decreased activity of isocitrate dehydrogenase (IDH) [50] and the upregulation of the mitochondrial citrate carrier (CIC) [92, 93]. The increased activity of IDH is mediated by ADP levels [94]. CIC is upregulated by several inflammatory mediators such as tumor necrosis factor (TNF)-α, IFN-γ, or commensal LPS via the upregulation of NF-κB and or STAT-1 [92, 95]. In this scenario, citrate exerts a multiplicity of vital roles, enabling macrophage function and inflammatory status such as increasing NO, ROS, and prostaglandin E2 (PGE2) production [92, 96]. Cytosolic citrate can also act as a source of NADPH, either as a result of malate import into mitochondria via CIC, and the subsequent formation of pyruvate via malic enzyme, or the conversion of citrate into alpha-ketoglutarate via the action of cytosolic IDH [97, 98]. Cytosolic citrate is also a substrate of ACLY, producing acetyl-CoA and oxaloacetate and upregulating acetyl-CoA carboxylase (ACC) stimulating lipid synthesis [99].
Activated M1 polarized macrophages are characterized by high levels of cytosolic itaconate from cis-aconitate drawn from the Krebs cycle via a significant inflammation-mediated upregulation of macrophage aconitate decarboxylase 1 [100, 101]. Itaconate is involved in tolerance and suppression of inflammation [102, 103], inhibits mitochondrial respiration, stabilizes HIF1α, and activates Nrf-2 via alkylation of KEAP-1 [84, 104]. Finally, itaconate accumulation leads to the inhibition of succinate dehydrogenase, directing the accumulation of succinate and leading to numerous proinflammatory and prooxidative consequences [103, 105, 106]. For example, elevated succinate oxidation in a cellular environment of few or no ATP generation induces a phenomenon described as reverse electron transport whereby electrons flow “backwards” along the ETC to complex I. As a result, large increases in the genesis and release of ROS follow [107, 108]. High levels of cytosolic succinate may induce an increase in lysine group succinylation in the cellular proteome, which many influence protein activity via changes in charge and conformation [109]. The mechanisms involved are beyond the scope of this review, but it is important to note that this post-translational modification offers another route relaying subtle redox-mediated metabolic changes to protein function [110]. Finally, once externalized, succinate can bind to the G protein-coupled succinate receptor 1 (SUCNR1) that is expressed on the surface of activated M1 polarized macrophages [111, 112]. This is a mechanism involved in sustaining and amplifying their inflammatory effects [12, 113].
M2 polarized macrophages
In an environment of elevated IL-4 and or IL-13, activated M1 polarized macrophages may ultimately be driven toward a range of anti-inflammatory and tissue healing phenotypes classified as M2a, M2b, M2c, and M2d that for the purposes of this paper may be usefully described as “M2” [114–116]. Tyrosine phosphorylation and activation of the signal transducer/transcription activator 6 (STAT-6) are required for macrophage M2 polarization [117, 118]. The latter then triggers a wide range of M2-associated genes including GATA binding protein 3 (GATA3), CD36, arginase-1 (Arg1), matrix metalloproteases (MMPs), FIZZ1, and PPARγ [119, 120]. IL-4 and IL-13 also upregulate the activity of transforming growth factor (TGF)-β, suppressor of cytokine signaling 1 (SOCS-1), and insulin-like growth factor 1 (IGF-1) that act to suppress the production of proinflammatory cytokines and promotes tissue repair [114, 115, 121]. Unlike M1 polarization, M2 polarization is associated with a return to OXPHOS and increased FAO [114, 115]. In addition, M2 polarized macrophages possess an intact TCA cycle [114, 115].
M2 macrophages are also characterized by activation of the nuclear liver X receptor (LXR) thereby regulating lipid synthesis and cholesterol homeostasis [122]. Overexpression of LXR inhibits NF-κB and activator protein-1 (AP-1) to reduce M1 responses and inflammation [123, 124]. One major element reinforcing the transition from M1 to M2 polarization is the change in the metabolism of arginine. In M1 polarized macrophages, elevated activity of iNOS leads to the metabolism of arginine to produce citrulline and NO. The latter is a major element in maintaining the switch toward aerobic glycolysis as explained above [84]. However, in M2 polarized macrophages, the increased transcription of arginase-1 metabolizes arginine to ornithine and urea. They both play a vital role in M2 macrophage survival, proliferation, and tissue repair [120, 125]. Glutamine metabolism is also of particular importance in M2 macrophages for two main reasons. Firstly, oxidation of this amino acid is an essential source of acetyl-CoA in an inflammatory environment leading to depleted extracellular glucose levels thereby maintaining TCA activity [126–128]. Secondly, glutaminolysis-mediated increase in α-ketoglutarate and the activation of the glutamine–UDP-N-acetylglucosamine (GlcNAc) pathway reinforce M2 polarization [126].
There are major differences in the regulation of the metabolic bioenergetic pathways involved in the transition to M2 polarization compared to those governing M1 polarization. In the case of M2 polarization the main players are AMPK and PPARγ whose activities are briefly described below. AMPK stimulates OXPHOS and FAO while inhibiting NF-κB and mTOR. This, in turn, decreases inflammation, reduces the levels of HIF1α, and terminates aerobic glycolysis [129–132]. AMPK inhibits ACC, increases glycolytic flux, mitogenesis, lipases, autophagy, and lysosomal degradation [133, 134]. PPAR-γ upregulates FAO, maintains mitochondrial membrane potential, mitochondrial citrate synthase, and regulates numerous genes involved in mitochondrial function including transcription factor A (TFAM), and peroxisome proliferator-activated receptor-gamma (PGC)-1α [135–138]. It also downregulates NF-κB and upregulates Nrf-2 [135–137]. PPAR stimulates the activity of LXR [139], which controls cholesterol and lipid homeostasis. Thus, inflammation is reduced and glycolysis is blocked via the inhibition of NF-κB [123, 124]. Finally, PPAR-γ promotes the oxidation of glutamine [126] whose importance in M2 polarization has been discussed above [140].
Redox regulation of macrophage activation functions and survival
Macrophage ROS levels affect the activity of STAT-1, MAPKs, and NF-κB and lead to an overall increase in inflammatory signaling [141]. ROS levels also affect the assembly of NADPH oxidase subunits and regulate the formation of corrosive RNS species such as peroxynitrite, thereby influencing H2O2-mediated intracellular signaling and macromolecule damage [142]. Continually high ROS or NO levels are accompanied by the development of macrophage senescence [143–145]. The mechanisms driving this phenomenon appear to involve the persistent expression of NF-κB, STAT-3, IL-10, and TGF-β, and potentially the upregulation of PD-1 [144, 146, 147].
There is also ample evidence that macrophage functions and polarization patterns are influenced by GSH levels and the overall activity of the GSH system [148, 149]. For example, increased GSH oxidation compromises phagocytosis and macrophage survival [150, 151]. The GSH system also plays a key role in regulating M1 inflammatory status and the production of PGE2 and NO, while protecting macromolecules from oxidative damage [152, 153]. The antiviral responses initiated following M1 macrophage activation such as increased expression of STAT-1, Irf7, and Irf9 are also dependent on an optimally functioning GSH system and are compromised by GSH depletion [154].
Thioredoxin (TRX)-1 affects the inflammatory status of macrophages by modulating the activity of macrophage receptors, and the macrophage migration inhibiting factor (MIF) [155]. The latter effect reduces the proinflammatory status of M1 macrophages and encourages M2 polarization by lowering TNF-α and monocyte-chemoattractant protein (MCP)-1 production [156–159].
Nrf-2 upregulation also exerts an anti-inflammatory effect in activated macrophages by attenuating the activity of IL-1β and IL-6 [160, 161]. The mechanism involves Nrf-2 binding at the relevant gene promoter sites resulting in inhibition of the recruitment of RNA Polymerase II complex [162]. Nrf-2 upregulation also rises the expression of CD163 and Arg1 [161, 163]. It affects the transcription of a multitude of genes involved in the switch between M1 and M2 polarization [160, 161].
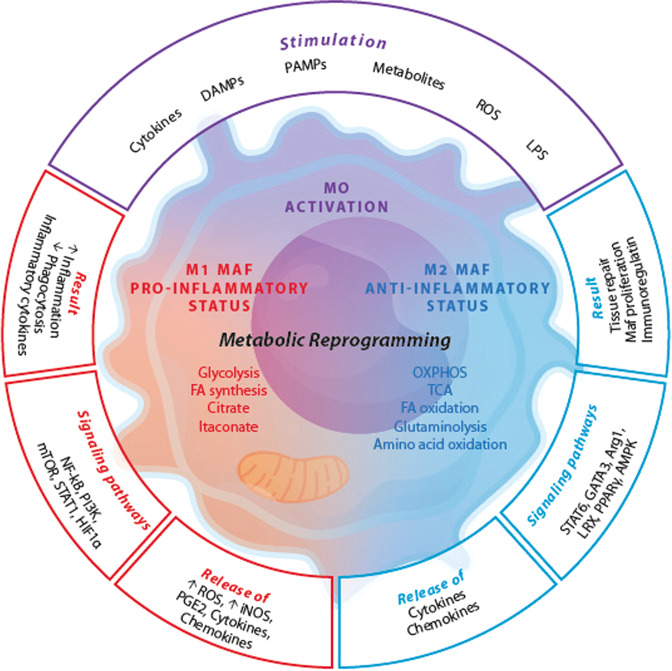
The metabolic reprogramming in macrophages is presented in Fig. 2 and Table 2 summarizes the effects of redox mechanisms on macrophage functions.
Fig. 2.
Metabolic reprogramming in macrophages (Maf). DAMPs damage-associated molecular patterns, PAMPs pathogen-associated molecular patterns, ROS reactive oxygen species, LPS lipopolysaccharide, STAT-6 signal transducer/transcription activator 6, GATA3 GATA binding protein 3, Arg1 Arginase-1, LXR liver X receptor, PPARγ peroxisome proliferator-activated receptor, AMPK AMP-activated protein kinase, iNOS inducible nitric oxide synthase, NO nitric oxide, PGE2 prostaglandin E2, OXPHOS oxidative phosphorylation, TCA tricarboxylic acid cycle, FA fatty acid, NF-kB nuclear factor NF-kappa-B, PI3K phosphatidylinositol 3-kinase, mTOR mechanistic target of rapamycin, STAT-1 signal transducer and activator оf transcription 1, HIF1α hypoxia-inducible factor 1-alpha
Table 2.
Redox mechanisms influencing macrophage functions
| Redox mechanisms | Macrophage functions | References |
|---|---|---|
| Reactive oxygen species (ROS) | Increase inflammatory signaling via STAT-1, MAPK, and NF-KB mechanisms | [141] |
| Modulate NADPH oxidase assembly thereby further increasing superoxide and ROS production as well as RNS with peroxynitrite formation | [142–147] | |
| Glutathione (GSH) | GSH oxidation compromises phagocytosis and leads to attenuated macrophage survival | [148–151] |
| Regulates M1 inflammatory status | [152, 153] | |
| As a ROS scavenger, protects against oxidative stress damage | [154] | |
| Thioredoxin (TRX) | Modulates MIF signaling thereby lowering inflammation and encouraging M2 polarization | [155–159] |
| Nuclear factor erythroid 2-related factor 2 (Nrf-2) | Anti-inflammatory effects through attenuating IL-1 and IL-6 | [160–163] |
| Transcription of a multitude of genes involved in the switch between M1 and M2 polarization | [160, 161] |
Metabolic reprogramming and redox factors involved in Dendritic cells activation
Metabolic reprogramming of DCs
DCs are archetypal antigen presenting cells (APCs) and play a dominant role in linking innate and humoral immunity [164]. In physiological conditions, tissue-resident DCs drain to the lymph nodes and, thereafter, present self-antigens to T-cells, thereby maintaining immune tolerance [165]. However, after pathogen invasion, TLR- mediated activation of DCs is followed by numerous changes in function and phenotype resulting in their active migration to lymph nodes and cytokine production [166].
Resting-state DCs rely on OXPHOS-driven TCA cycle activity fueled by glutaminolysis and FAO to meet their energy needs [167, 168]. Their overall metabolism is regulated by AMPK [168]. However, following pathogen recognition, TLR engagement results in activation of NF-κB, PI3K/AKT signaling, mTOR, and PPAR-γ and in a rapid shift to aerobic glycolysis and lactate production in a similar manner to M1 polarized macrophages discussed above [169, 170]. In addition, glycolytic intermediates are shunted into the PPP while increased NO production inhibits the ETC. Moreover, citrate is withdrawn from the TCA acting as a crucial player in FA synthesis that maintains and increases inflammatory cytokines, NO, and ROS production [171, 172]. The acute switch to glycolytic metabolism is facilitated by PI3K /AKT signaling [173]. However, chronic aerobic glycolysis is enabled and regulated by mTOR and HIF1α activation [174, 175]. In addition, upregulation of mTOR and the subsequent increase in HIF1α activity induces the transcription of iNOS [176, 177] leading to NO-mediated suppression of mitochondrial OXPHOS via reversible inhibition of ETC complex I, III, and IV [17, 178, 179]. mTOR activation initiates and controls lipid synthesis and mitochondrial biogenesis via the downstream upregulation of SREBPs and PPAR. It stimulates IL-6, IL-1, and TNF-α production, via the upregulation of AKT, FOXO3, and Myc [180]. mTOR activation serves as the enabler and master regulator of DC migration, maturation, and endocytosis [180].
Redox regulation of DC activation and function
Phagosomal ROS levels are involved in MH1-mediated presentation of digested antigens to CD8 T cells [181, 182]. In this context, it is noteworthy that the activation of CD8 T cells requires upregulation of mitochondrial reactive oxygen species (mtROS) production [183]. DC production of ROS following TLR activation also plays a major role in the maturation and priming of CD4 T cells [184, 185]. Many aspects of DC function are influenced by the GSH system activity. For example, GSH levels regulate DC differentiation and function as APCs [186]. DC GSH levels also determine T-cell polarization patterns by affecting IL-27 and IL-12 production [187, 188]. GSH depletion is associated with the differentiation of naive T cells [188] and inhibits DC maturation and inflammatory cytokine production leading to profound cellular dysfunction [189]. Moreover, DCs directly influence the redox state of activated T cells via the transfer of thioredoxin [190].
Redox homeostasis within activated DCs is regulated by Nrf-2 which acts to restrain T-cell proliferation by repressing IL-12 production and upregulating IL-10 [191, 192]. Conversely, DCs that lack Nrf-2 generate increased numbers of activated T helper (Th) cells and reduced numbers of T regulatory (Treg) cells [193]. Moreover, Nrf-2 depletion and the resultant prooxidative state in DCs encourage a Th-2 pattern of differentiation in naive T cells [194, 195]. Finally, Nrf-2 also plays an important role in the transition between glycolysis and OXPHOS in tolerogenic DCs that enables their long-term survival [196].
There is considerable evidence of DC dysfunction in diseases underpinned by chronic inflammation and oxidative stress [197, 198]. Such dysfunction may be directly or indirectly driven by increased inflammatory cytokines, RNS, and ROS. Direct effects involve damage to functional macromolecules and increased activation of apoptotic pathways [199, 200]. Indirect effects include enhanced Wnt signaling [90], epigenetic dysregulation, and compromised TLR activity [166, 201–203].
The metabolic reprogramming of DCs is shown in Fig. 3 and Table 3 summarizes the effects of redox mechanisms on DC functions.
Fig. 3.
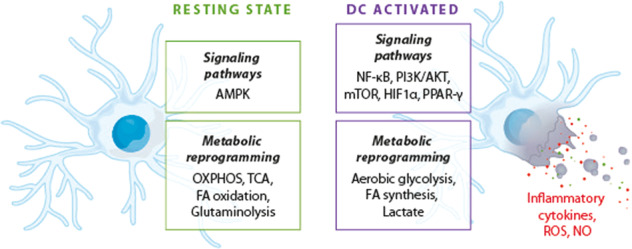
Metabolic reprogramming of dendritic cells (DCs). OXPHOS oxidative phosphorylation, TCA tricarboxylic acid cycle, FA fatty acid, NF-kB nuclear factor NF-kappa-B, mTOR mechanistic target of rapamycin, HIF1α hypoxia-inducible factor 1-alpha, PPARγ peroxisome proliferator-activated receptor, ROS reactive oxygen species, NO nitric oxide
Table 3.
Redox mechanisms influencing dendritic cell and neutrophil functions
| Redox mechanisms | Dendritic cell functions | References |
|---|---|---|
| Reactive oxygen species (ROS) | ROS due to NADPH oxidase (NOX-2) modulates the presentation of digested antigens to CD8 T cells | [181–183] |
| ROS due to TLR activation modulates maturation and the priming of CD4 T cells | [184, 185] | |
| Glutathione (GSH) | DC differentiation and function as APC | [186–189] |
| T-cell polarization | [188] | |
| DC maturation and inflammatory cytokine production | [189] | |
| Nuclear factor erythroid 2-related factor 2 (Nrf-2) | Redox homeostasis in DCs | [190–192] |
| Restrains T-cell proliferation by repressing IL-12 production and upregulating IL-10 | [191–195] | |
| Transition between glycolysis and OXPHOS in tolerogenic DCs | [196] | |
| Redox mechanisms | Neutrophil functions | |
| Reactive oxygen species (ROS) | Compromise initiation and outcome of phagocytosis | [235] |
| Dysregulate or decrease oxidative burst and NET production | [236, 237] | |
| Neutrophil sensing of pathogens | [238, 239] | |
| Activation of the NLRP3 inflammasome | [238, 239] | |
| Nitric oxide (NO) | Inhibits neutrophil migration, crawling, and adhesion | [240–243] |
| Downregulates adhesion molecules | [244] | |
| Compromises neutrophil binding to the endothelium | [244] | |
| Peroxynitrite | Compromises neutrophil migration | [245–248] |
| Nuclear factor erythroid 2-related factor 2 (Nrf-2) | Efficiency of neutrophil phagocytosis | [250] |
| Recruitment to inflammatory sites and survival | [251, 252] | |
| GSH reductase | Sustains neutrophil respiratory burst | [245, 253] |
| Sustains NET production | [253, 254] | |
| Influences optimal phagocytotic activity | [255, 256] | |
| Thioredoxin (TRX) | Neutrophil chemotaxis | [263, 264] |
| Desensitization of neutrophils toward monocyte-chemoattractant protein-1 | [264–266] |
Metabolic reprogramming and redox regulation of neutrophil activation
Metabolic reprogramming of neutrophils
Neutrophils are the first line responders of the innate immune system, which play a key role in the destruction of invading pathogens. However, these leucocytes also participate in humoral immunity via a sophisticated cross-talk with other immune cells [204–206]. Importantly, these regulatory functions extend beyond modulation of the activity of myeloid cells and also involve modifying the function of T-cells, marginal zone B-cells, and NK-cell homeostasis [204–206]. There is also considerable evidence of functionally distinct subsets and extensive cellular plasticity enabling a range of roles depending on cellular location and inflammatory status [207, 208]. These immune cells may be activated and/or primed by multiple stimuli such as inflammatory cytokines, chemokines, growth factors, PRRs (mainly c-type lectin receptors), opsonins (C3a and IgG), and G protein-coupled receptors [209, 210].
Glycolysis is the primary energy source for activated neutrophils under physiological conditions [211]. This is also true for inflammatory environments [212]. However, neutrophils adjust their metabolism to carry out their various effector functions such as phagocytosis, degranulation, oxidative burst, neutrophil extracellular traps (NET) formation, and chemotaxis [213]. The weight of evidence suggests that NET formation is reliant on glycolysis, with extensive involvement of lactate synthesis, the PPP, and glutamine metabolism as sources of NADPH [214, 215]. This metabolic reprogramming also supplies superoxide production, and induces ROS and hypochlorous acid, used in the neutrophil oxidative burst following phagocytosis of invading pathogens [211, 216–218]. The metabolic changes underpinning chemotaxis are somewhat more complicated, however, and involve mitochondrial contributions in addition to upregulated glycolysis [219–221]. This activity supplies ATP which activates membrane-bound P2Y2 receptors following the receipt of chemotactic stimuli (2019–2021). Mitochondrial activity provides the ATP required for neutrophil activity in regions of profound glucose deprivation. It occurs in an environment of extreme inflammation and also plays a dominant role in neutrophil autophagy and survival via FAO (2011) [222].
These metabolic changes underpinning neutrophil activity in inflammatory environments are primarily regulated by the cooperative action of NF-κB [43, 223], HIF1α [224, 225], and mTOR [211, 226]. The multiple and arguably pivotal roles of the latter include the regulation of NET production, autophagy, oxidative burst, phosphorylation, and stabilization of NOX and HIF1α [226, 227]. mTOR also increases the surface expression of GLUT-1 and intensifies mitochondrial biogenesis and FAO via the upregulation of PPARγ and SREBPs [72]. Elevated mTOR activity increases the production of leukotrienes, prostaglandins, resolving, and proinflammatory cytokines via phosphorylation of AKT [228]. mTORC1 also exerts an inhibitory effect on OXPHOS by upregulation of IFN-γ and NO which inhibits the activity of enzymes in the ETC [229].
While mTOR upregulation plays a key role in the optimal function of activated neutrophils, it should be stressed that other enzymes and transcription factors are also important regulatory elements enabling pathogen destruction. This in turn restrains extreme inflammation and prevents excessive survival. For example, PI3K enables chemotaxis and endothelial crawling via an intricate pattern of “cross-talk” with the Rho family GTPases [230, 231]. On the other hand, AMPK regulates and restrains NF-κB and the production of proinflammatory cytokines, limiting tissue inflammation and destruction while optimizing chemotaxis and phagocytosis [232, 233]. Finally, PPAR-γ also regulates migration and restrains inflammation by inhibiting NF-κB while stimulating IL-10 production [211, 234].
Redox regulation of neutrophil activation and function
The function of individual neutrophils is heavily influenced by cellular redox status in terms of cellular antioxidant system activity and or ROS/RNS production. For example, excessive ROS fabrication may compromise the initiation and outcome of phagocytosis [235], resulting in a dysregulated or decreased oxidative burst [236] and production of NETs [237]. In addition, intracellular and extracellular levels of ROS play a role in neutrophil “sensing “ of pathogens and consequent activation of the NLRP3 inflammasome and cytokine synthesis [238, 239]. Chronically upregulated ROS and cytokine production may also result in the internalization of membrane chemokine receptors, most notably CXCR2 [240], thereby decreasing neutrophil migration.
Upregulated NO inhibits neutrophil migration, crawling, and adhesion [241–243]. Mechanistically, this is achieved via the downregulation of adhesion factors such as E-selectin, P-selectin, ICAM-1, and VCAM-1. As a result, neutrophil binding to the endothelium is compromised, and subsequent crawling and transmigration to inflammatory centers are damaged [244]. Neutrophil migration may also be hampered by increased production of peroxynitrite due to the combination of NO and superoxide cations [245–248]. There is evidence suggesting that the tyrosine nitration mediates inhibition of P-selectins [245–247] and upregulation of haem oxygenase (HO-1)-1 [249].
A multitude of neutrophil functions is heavily affected by the cellular antioxidant system. For example, Nrf-2 activity influences the efficiency of neutrophil phagocytosis [250], recruitment to inflammatory sites [251], and prolonged survival [252]. The glutathione system regulates various functions displayed by activated neutrophils most notably the stimulation of glutathione reductase. It sustains the neutrophil respiratory burst and NET production [253, 254] influencing optimal phagocytic activity [255, 256]. It is noteworthy that the basal activity of the GSH system in neutrophils appears to be lower than that found in myeloid cells [257], rendering these immune cells vulnerable to depleted GSH levels [257]. This may result in compromised cytoskeletal reorganization, affecting chemotaxis and transmigration and leading to reduced recruitment to sites of inflammation, impaired degranulation, and early apoptosis [258, 259]. In this context, it should be noted that prolonged neutrophil activity depletes levels of GSH, likely due to excessive production of myeloperoxidase (MPO) during chronic nitro-oxidative stress and inflammation [260–262].
TRX plays an important role in the regulation of neutrophil chemotaxis as a result of its release from infected cells and/or inflamed tissues [263, 264]. This effect appears to be a result of the desensitization of neutrophils toward MCP-1 [264, 265], thereby restraining neutrophil recruitment into inflammatory tissues [266]. The mechanisms involved are not fully understood, but they appear to rely at least in part on the oxidation state of functional cysteine residues within the TRX protein [264].
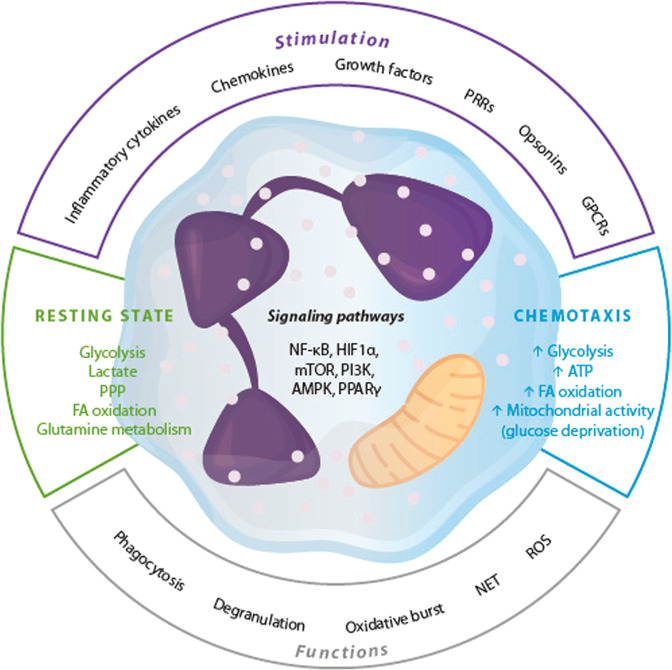
Table 3 summarizes the redox mechanisms that affect neutrophil functions, and the metabolic reprogramming of neutrophils is presented in Fig. 4.
Fig. 4.
Мodulation of effector functions of neutrophils. PRRs pattern-recognition receptors, GPCRs G protein-coupled receptors, NET neutrophil extracellular traps, ROS reactive oxygen species, PPP pentose phosphate pathway, FA fatty acid, ATP adenosine triphosphate, NF-kB nuclear factor NF-kappa-B, HIF1α hypoxia-inducible factor 1-alpha, mTOR mechanistic target of rapamycin, PI3K phosphatidylinositol 3-kinase, AMPK AMP-activated protein kinase, PPARγ peroxisome proliferator-activated receptor
Metabolic reprogramming and redox regulation of T-cell activation
Metabolic reprogramming of T-cells
Activation of T-cells follows the ligation of the T-cell receptor (TCR) and the major histocompatibility complex molecules by APC. Nuclear factor of activated T cell 1 (NFAT1), activation protein-1 (AP)-1, and NF-κB are triggered as a result of this signaling cascade [267]. When TCRs are ligated, ROS production increases by mitochondria and NOXs [268], which in turn regulates the signaling pathways required to enable and modulate T-cell activation, proliferation, and differentiation [268].
Unsurprisingly, T-cell activation and differentiation require extensive metabolic reprogramming [269–273]. In general, such reprogramming is regulated by the collaborative activity of PI3K/AKT, mTOR, HIF1α, and c-Myc [274–276]. However, it should be stressed that the metabolic reprogramming pathways of various T-cell subsets display important differences [277–279]. The metabolic needs of naive and memory T and Treg cells are relatively modest and are met by reliance on OXPHOS and FAO [274, 277, 279]. However, the differentiation and various effector functions of effector CD4 and CD8 cells require ATP obtained from aerobic glycolysis and NADPH. They are supplied by increased activity of the PPP and glutaminolysis, which is largely mediated by high levels of HIF1α and mTOR [278, 280–284].
Important differences exist between subsets when it comes to FA metabolism and T-cell activation and differentiation. For example, effector T-cell activity relies on FA uptake and FAS while T memory cells utilize stored FA [285, 286]. Uniquely, the relative reliance on FA uptake versus FA synthesis exerts a major influence on the differentiation of naive T cells into Tregs or Th-17 cells [286, 287]. In particular, uptake of environmental FA is a characteristic feature of Treg development, while Th-17 differentiation counts on ACC-mediated FA synthesis [276, 287].
TCR signaling also leads to the upregulation of amino acid transporters, facilitating the uptake of branch chain amino acids such as alanine, cysteine, leucine, glycine, and glutamine [288–290]. These amino acids, in combination with high PPP activity, promote the rapid increase of GSH needed for T-cell survival and function [284]. Augmented glutamine catabolism following T-cell activation, mediated by mitochondria-dependent oxidation, is of particular importance as the resultant increase in α-ketoglutarate production stimulates TCA activity and fuels increased OXPHOS [268, 291]. TCR-dependent uptake of glutamine, valine, and leucine is implicated in inflammatory T-cell responses, the differentiation of Th-1 and Th-17 cells, and the development of effector and memory CD8 cells [292–295].
Redox regulation of T-cells
ROS levels rise rapidly after TCR engagement and are critical in driving T-cell activation, proliferation, and differentiation [268, 291, 296, 297]. Unsurprisingly, given the information discussed above, ROS influences the differentiation patterns and the disparate effector functions of various T lymphocytes. For example, the Th-2 polarized phenotype is encouraged by excessive microenvironmental ROS [298]. Conversely, Th-1 and Th-17 polarizations occur at low microenvironmental levels of ROS [299]. Excessive ROS resulting from either high production or damaged cellular antioxidant defenses may lead to mitochondrial membrane polarization with fatal consequences for T-cell activation and survival following TCR engagement [300]. Similarly, prolonged or chronic ROS upregulation may result in T-cell hyperresponsiveness, exhaustion, and anergy [301–305]. Several mechanisms appear to underpin this phenomenon including compromised mitochondrial ETC activity and dynamics [302, 306], upregulation of PD-1 [307, 308], dysregulated NF-κB signaling, chronic IKKβ signaling [309–311], and oxidation of functional cysteine groups in proteins [312–314]. Finally, excessive ROS production may lead to dysregulated T-cell homeostasis by differential modulation of T-cell homeostasis as effector T cells are more susceptible to ROS-mediated cell death than Tregs [201, 315, 316].
Nrf-2 transcription is upregulated following TCR engagement on naive T cells and restrains inflammatory T-cell activity. Thus, a Th-2 pattern is activated following TCR stimulation [317, 318]. Animal studies show that the upregulation of Nrf-2 increases the proliferation of Tregs [319] and amplifies their immunosuppressive and cytotoxic functions [320].
As previously discussed, GSH synthesis rapidly escalates following TCR activation and affects T-cell survival and function [284]. Increased de novo GSH synthesis also suppresses Th-17 differentiation while encouraging the production of Tregs. Conversely, GSH depletion or loss of de novo GSH synthesis in a state of chronic nitro-oxidative stress [321] compromises mTOR, NFAT, and N-Myc function. Thus, the metabolic reprogramming is abrogated enabling the maintenance of aerobic glycolysis and leading to the termination of T-cell activation [322–324]. Tregs also appear to exert at least some of their cytotoxic and immunosuppressive functions on effector T cells by decreasing GSH synthesis [325].
The TRX system activity exerts a range of influences on T-cell proliferation and activation via increased TRX-1 production. This restrains their stimulation and encourages the development of Tregs from naive T cells, decreasing their differentiation down the Th-1 and Th-17 pathways [326]. TRX-1 upregulation is important in enabling T effector and Treg cell survival and function during chronic nitro-oxidative stress by protecting membrane protein thiols from oxidation [327, 328]. Increased TRX-1 activity is needed to maintain the production of IL-2 [329] and Th-mediated activation of B cells [330].
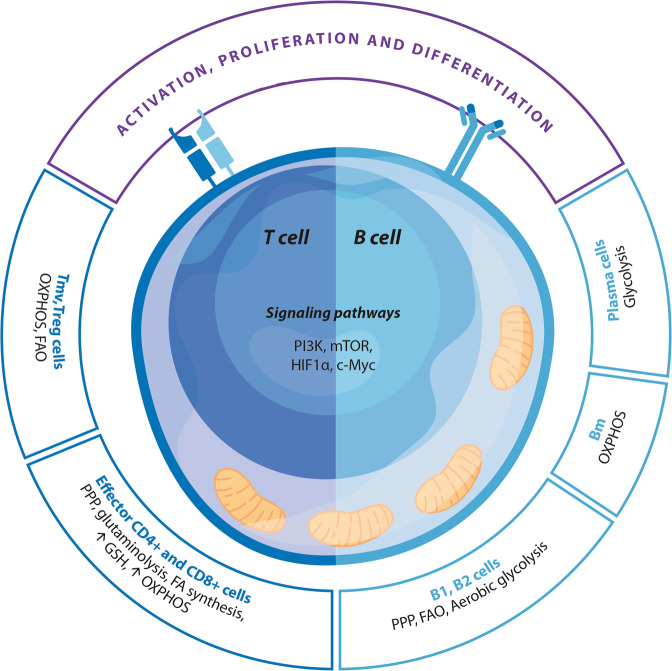
The metabolic reprogramming of T cells is depicted in Fig. 5 and Table 4 summarizes the redox mechanisms that affect T-cell functions.
Fig. 5.
Metabolic reprogramming of T and B cells. Tm cells memory T cells, Treg cells regulatory T cells, OXPHOS oxidative phosphorylation, FA fatty acid, PPP pentose phosphate pathway, GSH glutathione, PI3K phosphatidylinositol 3-kinase, mTOR mechanistic target of rapamycin, HIF1α hypoxia-inducible factor 1-alpha, c-Myc Myc proto-oncogenes, Pl cells plasma cells, Bm cells memory B cells, B1/B2 subclass of B-cells
Table 4.
Redox mechanisms influencing T-, B-, and NK-cell functions
| Redox mechanisms | T-cell function | References |
|---|---|---|
| Reactive oxygen species (ROS) | Encourage Th-2 polarized phenotype | [298] |
| Mitochondrial membrane polarization with fatal consequences for T-cell activation and survival following TCR engagement | [300] | |
| When chronic, may result in T-cell hyperresponsiveness, exhaustion, and anergy | [301–305] | |
| Dysregulated T-cell homeostasis | [201, 315, 316] | |
| Nuclear factor erythroid 2-related factor 2 (Nrf-2) | Restrains inflammatory T-cell activity | [319, 320] |
| Encourages a Th-2 pattern following TCR activation | [317, 318] | |
| Glutathione (GSH) | Suppresses Th-17 differentiation | [321] |
| Encourages the production of Tregs | [321] | |
| Thioredoxin (TRX) | Restrains T-cell activation | [326–328] |
| Encourages the development of Tregs | ||
| Enables T effector and Treg cell survival | ||
| Redox mechanisms | B-cell functions | |
| Mitochondrial reactive oxygen species (mtROS) | Increased ROS inhibit B-cell activation | [353] |
| Increased ROS inhibit the differentiation of B-cells into antibody-producing plasmablasts | [351, 352] | |
| Increased ROS inhibit the production of antibodies by downregulating CD19 expression | [358] | |
| Increased ROS upregulate the consumption of IgM antibodies | [355–360] | |
| GSH/TRX | Enables medium-term survival | [357] |
| Increased production of IgM | [352] | |
| Nuclear factor erythroid 2-related factor 2 (Nrf-2) | Increased survival and increased resistance of ROS-mediated apoptosis | [358–360] |
| Redox mechanisms | NK-cell functions | |
| Reactive oxygen species (ROS) | Enable NK-cell-mediated cytolysis | [381] |
| Enable NK-cell division and proliferation following pathogen invasion | [382] | |
| Nuclear factor erythroid 2-related factor 2 (Nrf-2) | Restrains activation and regulates effector functions | [383, 384] |
| Glutathione (GSH) | Enables the proliferation and cytotoxic functions of NK-cells | [385–387] |
| Thioredoxin 1 (TRX-1) | Maintains membrane cytoprotective sulfhydryl residues in a reduced state | [388, 389] |
| Protects cells from hydrogen peroxide-mediated NK-cell dysfunctions | [390–393] |
Metabolic reprogramming and redox regulation of B-cell activation
Metabolic reprogramming of B-cells
B-cell receptor (BCR) or cytokine-associated activation of naive B cells results in PI3K phospholipase C gamma 1 expression, leading to calcium mobilization and NF-κB activation and upregulation of c-Myc, HIF1α, AKT, mTOR, and STAT-6 [331]. Once activated, these lymphocytes migrate to germinal centers and display high rates of glycolysis and OXPHOS [332–334]. The short-term metabolic reprogramming and increased glycolysis are controlled by PI3K, HIF1α, AKT, and STAT-6 signaling [332–334]. The role of mTOR appears to be confined to the upregulation of GLUT-1 [335]. It is noteworthy that GSK-3 has a key role in regulating glycolysis in activated B cells and may also adjust ROS production and changes in mitochondrial dynamics [335, 336]. However, while mTOR may not be the primary player in the regulation of glycolysis, sustained germinal center B-cell BCR signaling requires activation of mTOR [337, 338]. mTOR is also involved in somatic hypermutation and in the formation of memory B cells [339–341].
The relative levels of OXPHOS and glycolysis differ in plasmablasts and memory B cells, with glycolysis being dominant in the former and OXPHOS being dominant in the latter to enable their long-term survival [342]. B1 and B2 subsets appear to display differing metabolic profiles, with PPP, FAO, and aerobic glycolysis being more active in B1 compared to B2 cells [342]. The production of high-affinity antibodies by plasmablasts is an energetically demanding process and requires rapid increases in glucose consumption and mitochondrial mass accompanied by significant changes in mitochondrial dynamics [336, 343, 344], reviewed in [342]. Unsurprisingly, functional mitochondria are an indispensable element in B-cell differentiation and effector functions [345]. The process of antibody synthesis is also regulated by AMPK, which enables memory B-cell formation and survival in part by regulating mitochondrial dynamics and suppressing the activation of mTOR [133, 346, 347].
Redox regulation of B-cell activation and function
High levels of hydrogen peroxide are required to initiate and maintain BCR signaling [348, 349]. This is primarily provided by the activity of NOX-2 [350], but in the longer term, the source of hydrogen peroxide is mtROS [348, 349]. In addition, the cellular redox state and mtROS release play a major role in B-cell survival and differentiation and IgM synthesis [351, 352]. However, excessive mitochondrial mtROS synthesis may inhibit B-cell activation and the differentiation of B cells into antibody-producing plasmablasts [353]. Increased concentrations of mtROS may also inhibit the production of antibodies by downregulating CD19 expression [354]. Finally, chronically upregulated ROS can upregulate the consumption of IgM antibodies [355, 356].
In this context, it is noteworthy that B-cell activation is accompanied by a concomitant stimulation of the TRX and GSH system, with the latter involving triggering of the cystine transporter xCT and higher uptake of cysteine [352]. Upregulation of GSH/TRX systems by activated B cells enables their medium-term survival [357]. The intensive function of both systems correlates with elevated production of IgM [352]. Finally, there is evidence associating increased Nrf-2 expression in activated B cells with prolonged survival and resistance to ROS-mediated apoptosis [358–360].
Table 4 summarizes the redox mechanisms that affect B-cell functions, and the metabolic reprogramming of B cells is depicted in Fig. 5.
Metabolic reprogramming and redox regulation of NK-cell activation
Metabolic reprogramming in NK-cells
The signaling mechanisms involved in NK-cell activation [361, 362] entail the engagement of multiple activation receptors such as natural cytotoxicity receptors [363–365] leading to the stimulation of AP-1, NFAT, and NF-κB [361, 366]. Cytoskeletal reorganization and release of chemokines, inflammatory cytokines, and lytic granules containing granzyme A, B, and perforin follows [367–369]. Unsurprisingly, the various effector and regulatory functions of activated NK-cells are enabled by metabolic programming, which is underpinned by the upregulation of glucose-driven glycolysis, OXPHOS, increased FA synthesis, and glutamine metabolism [370–373]. Metabolic reprogramming, glycolysis, and mitochondrial activity are controlled by mTOR that is upregulated in NK cells following stimulation by IL-15 and IL-3 [372, 374, 375]. The high expression of this kinase is also responsible for increased FA synthesis and glutamine metabolism by activated NK cells via the upregulation of SREBPs and N-Myc [370, 376].
In inflammatory conditions, PI3K/mTOR signaling, along with NF-κB and STAT-3 transcriptional activity, is responsible for triggering HIF1 protein synthesis [377, 378]. The importance of mTOR and HIF1α in NK-cell proliferation and function is difficult to overemphasize as reduced HIF1α and mTOR activity are associated with loss of cytotoxic effects. It is evidenced by decreased production of perforin and granzyme B, and premature apoptosis [372, 379, 380].
Redox regulation of NK-cell activation and function
Increased ROS production enables NK-cell-mediated cytolysis by promoting the release of perforin and granzyme B [381] and NK-cell division and proliferation after pathogen invasion [382]. Nrf-2 activation serves as an immunological checkpoint following NK-cell activation [383, 384].
The upregulation of GSH synthesis may enable the proliferation and cytotoxic functions of NK-cells and, conversely, GSH downregulation results in compromised functions and recruitment to sites of inflammation [385–387]. In an inflammatory environment, the upregulation of TRX-1 plays a role in NK-cell survival by maintaining membrane cytoprotective sulfhydryl residues in a reduced state [388, 389]. This phenomenon may protect those cells from hydrogen peroxide-mediated NK-cell dysfunctions [388, 389]. However, this level of protection is clearly limited as chronic nitro-oxidative stress may result in NK-cell hypofunction and loss of cytotoxic activity [390–393]. There is evidence suggesting that this is due to compromised hydrogen peroxide signaling following NOX-2 hyperactivity [390]. However, there is also proof that NK-cell function may be impaired by excessive production of NO [392].
Table 4 summarizes the redox mechanisms that affect NK-cell functions, while Fig. 6 shows the metabolic reprogramming in NK-cells.
Fig. 6.
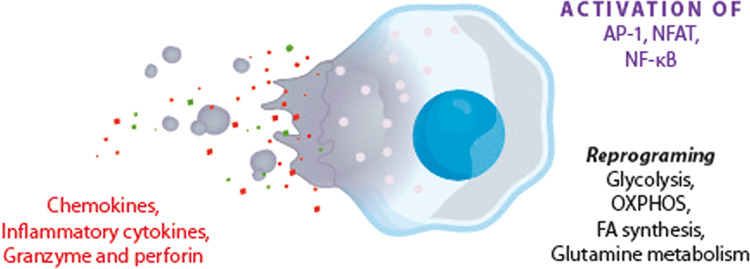
Metabolic reprograming in NK-cells. AP-1 activator protein-1, NFAT nuclear factor of activated T cell, NF-kB nuclear factor NF-kappa-B, OXPHOS oxidative phosphorylation, FA fatty acid
Role of the HDL complex and oxidized phospholipids in the immune response
Role of HDL, ApoA1, and PON1 in the regulation of the immune response
Previously, we have reviewed the important role of the HDL/ApoA1/PON1 complex in regulating immune responses [13, 41, 79, 90, 394]. In brief, HDL attenuates the activation of TLR-4 by stimulating cholesterol efflux from membrane lipid rafts (MLR), NF-κB activity, DC maturation and activation, and antigen presentation to T lymphocytes. It also affects Th-1 and Th-17 differentiation, T-cell and BCR activation, the complement system, and monocyte and macrophage chemotaxis [13, 41, 79, 90, 394]. HDL-mediated MLR disruption underpins anti-inflammatory and immunosuppressive effects. HDL exerts a unique immunoregulatory role by activating pentraxin 3, an immunosensory molecule. ApoA1 regulates the balance between Th-17 and Tregs, improves mitochondrial functions, increases the activity of the ETC, and stabilizes PON1 within the HDL particle, thereby maintaining PON1 activity. The latter protects against immune cell membrane lipid peroxidation, circulating oxidized lipoproteins, and oxidative damage to mitochondria. It positively affects glucose metabolism, PPP, FAO, PPAR-γ activity, and aerobic glycolysis via upregulation of GLUT-1 [41, 90].
Role of oxidized phospholipids in the regulation of the immune response
Evidence suggests that the bulk of oxidized phospholipids present in the circulation exists as immune complexes with natural IgM and IgG due to their status as oxidation-specific epitopes or neoantigens [395, 396]. It is also proposed that oxidized phospholipid complexes are proinflammatory [397, 398] using several routes, which include recruitment of the complement cascade [399] and production of inflammatory responses in human macrophages largely by engagement of the Fc gamma receptor 1 [400, 401]. These complexes may activate mature DCs leading to a primed inflammasome thereby exaggerating IFN-γ and IL-1 production [402–404]. Moreover, DCs activated and primed via this mechanism may trigger naive T cells and induce Th-17 polarization [404–406].
As a result of activating neutrophil PRR, oxidized phospholipids contribute significantly to inflammation and oxidative stress and the formation of NETs [407, 408]. In addition, oxidized phospholipid engagement with monocytes, macrophages, DCs, and NK cells may induce epigenetic and metabolic reprogramming leading to “immune training”. The process effectively endows these leucocytes with a de facto memory, resulting in an amplified inflammatory or anergic response to future antigenic challenges [409, 410]. The mechanisms driving the metabolic and epigenetic changes described above appear to depend, at least in part, on mTOR-induced assembly of NADPH oxidase and subsequent increases in ROS-mediated signaling [410, 411].
The final part of this review deals with the detrimental effects of chronic oxidative and nitrosative stress on the immune response as a whole. In physiological conditions, NOX-derived cytosolic hydrogen peroxide regulates redox-sensitive intracellular signaling pathways [412–416]. However, in conditions of excessive ROS production, hyperoxidation of thiolate anions to sulfonic acid essentially incapacitates reversible cysteine oxidation. It is an effective signaling mechanism, locking functional cysteines in the oxidized mode [90, 417].
The other signaling system involved in regulating the activity of redox-sensitive proteins and enzymes is reversible S-nitrosylation [17, 418]. However, pathological levels of ROS disable the mechanisms responsible for maintaining the reversibility of S-nitrosylation inducing a cellular state described as protein hypernitrosylation [202]. Hyperoxidation and S-nitrosylation can result in impaired function of the redox-sensitive transcription factors and enzymes regulating metabolic reprogramming in immune cells. Compromised mitochondrial functions and seriously suppressed immune cell activation and function may follow. Chronic nitro-oxidative stress also affects the activity of HDL, apoA1, and PON1 whilst increasing the density of oxidized phospholipids further dysregulates the immune response [41]. Finally, chronic nitro-oxidative stress and inflammation also stimulate IDO that may result in a state of profound immune suppression [419]. The section below deals with these processes, beginning with the effects of hypernitrosylation and hyperoxidation on transcription factors and enzymes.
The detrimental effects of chronic nitro-oxidative stress on the immune response
Chronic nitro-oxidative stress on transcription factors and enzymes
S-nitrosylation exerts a significant inhibition of NF-κB function by reducing the binding of its subunits to DNA thereby decreasing the activity of the complex as a transcription factor [420–422], as well as the expression of target effector genes [420, 423]. This consequence is largely due to S-nitrosylation-mediated conformational changes to crucial functional cysteine residues located on the p65 subunit of p50/p65 abrogating NF-κB DNA-binding capacity [420, 424]. The outcomes involve decreased levels of IL-12 [425], IL-1β [426], IL-6, IL-8, and iNOS [427, 428]. Moreover, S-nitrosylation may inhibit TLR-4 [429, 430] and TLR-2 signaling [431].
There is also in vivo evidence that S-nitrosylation leads to the inhibition of numerous MAPKs, most notably p38/MAPK [432, 433], Janus kinase [432, 434], and consequent STAT-3 and NF-κB activation [435]. S-nitrosylation is additionally involved in Nrf-2 triggering, which appears to be affected via the conformational modification of crucial thiol groups [436–438]. Hypernitrosylation is also accompanied by chronic activation of HIF1α via upregulation and/or stabilization of HIF1α [439–441]. In addition, irreversible nitrosylation of functional cysteine thiols may cause chronic upregulation of PI3K/AKT and mTOR signaling [442–445] thereby decreasing the capacity of immune cells to adapt to environmental conditions or changing metabolic needs. Moreover, mTOR may be directly activated following S-nitrosylation of the tuberous sclerosis complex 2 [445] and the nitrosylation of small GTPases [446]. Prolonged nitrosylation may also compromise immune cells via the chronic upregulation of GSK-3 [202]. Finally, by inhibiting AMPK activity, nitrosylation-mediated upregulation of PI3K/AKT and GSK-3 may introduce a further dimension of metabolic disorders [447, 448]. In addition, in an environment of chronic nitro-oxidative stress, mTOR may be inactivated by oxidation of Cys1483 [449] and AMPK activation [450, 451]. In an environment of increased ROS, several enzymes involved in regulating metabolic reprogramming in immune cells are triggered most notably via PPAR-γ [452, 453].
Detrimental effects on immune cells due to nitro-oxidative stress-mediated mitochondrial dysfunction
Chronically elevated ROS/RNS can impair mitochondrial structure and functions by injuring DNA, proteins, and lipids. The most prominent results are damage to the enzymes of the ETC [248, 454–456] and a range of structural and functional phospholipids, basically cardiolipin [457–459]. This ultimately leads to altered ATP production and accelerated ROS, provoking further impairement of macromolecules, forming the basis of self-amplifying pathology [248, 454–456]. Increased NO production by mitochondria in an environment of nitrosative stress may also be a source of dysfunction and damage [460–462]. In essence, two pathways are implicated. The first involves reversible inhibition of ETC enzymes by NO-mediated S-nitrosylation [17, 463, 464]. The second comprises irreversible nitration of functional enzymes and structural proteins by ONOO- [248, 465]. This pattern of pathology leads to a vicious circle of bioenergetic failure and elevated mtROS production [466–469].
Clearly compromised mitochondrial function has many direct adverse effects on the activity of immune cells, as discussed above. However, mitochondrial dysfunction may also lead to numerous indirect negative consequences related to depleted levels of NADPH, which results from the distorted activity of this organelle [470–472]. This is a significant source of metabolic dysfunction in immune cells as the GSH/TRX systems are wholly dependent on the presence of adequate levels of NADPH, which acts as an indispensable source of reducing equivalents [473–476]. The synthesis of NADPH from NADP [477, 478] and NAD+ kinases, which catalyze the production of NADP from NAD+ [479, 480], is dependent on mitochondrial respiration and on an adequate supply of ATP [470, 471, 481]. Mitochondrial dysfunction is associated with depleted levels of NAD+ [13] due to the fact that the enzyme nicotinamide mononucleotide adenylyl transferase, which catalyzes the formation of NAD+ synthesis from nicotinamide mononucleotide as part of the salvage pathway [482], is dependent on ample supplies of ATP [483–485].
An important adverse consequence of depleted NAD+ levels is the compromised mitochondrial NADPH production by malic enzyme 2, IDH, methylenetetrahydrofolate dehydrogenase 2, and aldehyde dehydrogenase, which are all NAD+ dependent [486, 487]. Lowered levels of malic enzyme 2 and IDH may affect the TCA cycle [488, 489]. NAD+ deficiency can impair the PPP's ability to produce NADPH via decreased hexokinase activity [490–492].
Chronic nitro-oxidative stress and the inhibition of antioxidant systems and TCA activity
Chronic nitro-oxidative stress may cause nitrosylation and hyperoxidation of the key cysteine residues within TRX and thioredoxin reductase thereby compromising or abrogating TRX activity [493–496]. Chronically elevated ROS/RNS decrease GSH system activity [497, 498]. Mechanistically, this is achieved via the oxidation and nitrosylation or tyrosine nitration or via inhibiting the activity of GSH, glutathione peroxidase, and glutathione reductase [13, 321, 499]. Increased production of radical species also raises the activity of multidrug resistance-associated proteins, resulting in extrusion of GSH and GSSH into the intercellular environment. The decreased importation of cysteine, which follows, leads to reduced synthesis of replacement GSH [500–503]. A state of persistent nitro-oxidative stress may also cause Nrf-2 inhibition via several mechanisms, including activation of MAPK kinase, decreased DJ-1 [459, 504], and reduced TRX system activity [505, 506].
Oxidation and/or nitrosylation of functional cysteine groups in several TCA enzymes may cause adverse effects on the metabolism of immune cells. Such inactivated enzymes are α-ketoglutarate dehydrogenase [507–509] and conitase, which catalyze the conversion of citrate to isocitrate [510, 511], IDH [512–514], ME2 [515, 516], and pyruvate dehydrogenase kinase [517]. The negative consequences of lowered α-ketoglutarate dehydrogenase and aconitase are of particular importance, and may lead to reduced TCA cycle activity and NADPH synthesis [518, 519] and accumulation of citrate [519]. The inactivation of pyruvate dehydrogenase kinase also results in adverse metabolic consequences by attenuating the conversion of pyruvate to acetyl-CoA [517].
Detrimental effects of chronic nitro-oxidative stress on the HDL complex
Chronically elevated ROS/RNS levels are a cause of depleted circulating HDL [520–522], ApoA1 [522–524], and PON1 [525, 526] levels. Chronic oxidative stress induces HDL [527–529] and ApoA1 [521, 530, 531] dysfunctions. PON1 is rendered dysfunctional in such an environment, which appears to be mediated by the high activity of MPO [525, 526, 532]. The mechanisms underpinning the development of a dysfunctional HDL particle and reduced activity of ApoA1 are complex and readers are referred to the work of Morris et al. [41].
Chronic nitro-oxidative stress and the advent of immunosuppression
Chronic nitro-oxidative stress can induce the development of endotoxin tolerance by provoking IDO activation [533, 534]. Increased IDO activity upregulates the tryptophan catabolite (TRYCAT) pathway, as well as TGF-β1 and IL-10 [535, 536], which exert multiple inhibitory effects on TLR signaling [537, 538]. Neutrophils with endotoxin tolerance are characterized by decreased oxidative burst, downregulated TLR-4 receptors, and impaired cell adhesion, rolling, and migration [539–541]. Macrophages with endotoxin tolerance display significant dysregulation of their function as APCs [542]. Impaired antigen presentation is also seen in DCs following IDO activation [542]. In this state, DC activation of naive T cells leads to Th-2 polarization [543, 544]. DCs may inhibit T memory and T effector cells and induce CD4 and CD8 T-cell anergy and activation of Tregs [545, 546]. This explains that prolonged endotoxin tolerance is typified by impaired proliferation and anergy of CD4 T and CD8 T cells and increased Treg cell numbers [547–549]. Finally, endotoxin tolerance is characterized by a reduced number and cytolytic function of NK cells [550–552].
Summary and conclusion
The functions, performance, and survival of immune cells are strongly regulated by redox mechanisms, including intracellular and extracellular ROS/RNS and oxidized phospholipids, cellular antioxidants such as glutathione, thioredoxin, the HDL complex, and Nrf-2. Hypernitrosylation and chronic nitro-oxidative stress may inhibit these antioxidant systems, thereby decreasing the activity levels of the TCA cycle, mitochondrial functions, and immune cell metabolism. As such, redox mechanisms regulate and modulate many different immune functions, including but not limited to macrophage and Th cell polarization, phagocytosis, production of pro- and anti-inflammatory cytokines, metabolic reprogramming of immune cells, immune training and tolerance, chemotaxis, pathogen sensing, antiviral and antibacterial effects, TLR activity, and endotoxin tolerance. ROS/RNS, oxidized phospholipids, and the key antioxidant systems could be regarded as new drug targets in the treatment and prevention of immune disorders.
Acknowledgements
The authors acknowledge the graphic work of Kristin Ozanian.
Author contributions
All authors contributed to the writing up of the paper. The work was designed by MM and GM. All authors revised and approved the final draft.
Competing interests
The authors declare no competing interests.
References
- 1.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 2.Zettel K, Korff S, Zamora R, Morelli AE, Darwiche S, Loughran PA, et al. Toll-like receptor 4 on both myeloid cells and dendritic cells is required for systemic inflammation and organ damage after hemorrhagic shock with tissue trauma in mice. Front Immunol. 2017;8:1672. [DOI] [PMC free article] [PubMed]
- 3.Kim ND, Luster AD. The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 2015;36:547–55. doi: 10.1016/j.it.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcinkiewicz J, Walczewska M. Neutrophils as sentinel cells of the immune system: a role of the MPO-halide-system in innate and adaptive immunity. Curr Medicinal Chem. 2020;27:2840–51. doi: 10.2174/0929867326666190819123300. [DOI] [PubMed] [Google Scholar]
- 5.Morris G, Bortolasci CC, Puri BK, Olive L, Marx W, O'Neil A, et al. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2021;264:118617. doi: 10.1016/j.lfs.2020.118617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konradt C, Hunter CA. Pathogen interactions with endothelial cells and the induction of innate and adaptive immunity. Eur J Immunol. 2018;48:1607–20. doi: 10.1002/eji.201646789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao Y, Saredy J, Yang WY, Sun Y, Lu Y, Saaoud F, et al. Vascular endothelial cells and innate immunity. Arterioscler Thromb Vasc Biol. 2020;40:e138–52. doi: 10.1161/ATVBAHA.120.314330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dib PRB, Quirino-Teixeira AC, Merij LB, Pinheiro MBM, Rozini SV, Andrade FB, et al. Innate immune receptors in platelets and platelet-leukocyte interactions. J Leukoc Biol. 2020;108:1157–82. doi: 10.1002/JLB.4MR0620-701R. [DOI] [PubMed] [Google Scholar]
- 9.Guo L, Rondina MT. The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Front Immunol. 2019;10:2204. doi: 10.3389/fimmu.2019.02204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris G, Bortolasci CC, Puri BK, Olive L, Marx W, O'Neil A, et al. The pathophysiology of SARS-CoV-2: a suggested model and therapeutic approach. Life Sci. 2020;258:118166. doi: 10.1016/j.lfs.2020.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieow JL, Gun SY, Wong SC. The sweet surrender: how myeloid cell metabolic plasticity shapes the tumor microenvironment. Front Cell Dev Biol. 2018;6:168. [DOI] [PMC free article] [PubMed]
- 12.Kelly B, O'Neill LAJ. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–84. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris G, Walder KR, Berk M, Marx W, Walker AJ, Maes M, et al. The interplay between oxidative stress and bioenergetic failure in neuropsychiatric illnesses: can we explain it and can we treat it? Mol Biol Rep. 2020;47:5587–620. [DOI] [PubMed]
- 14.Shyer JA, Flavell RA, Bailis W. Metabolic signaling in T cells. Cell Res. 2020;30:649–59. doi: 10.1038/s41422-020-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters LR, Ahsan FM, Wolf DM, Shirihai O, Teitell MA. Initial B cell activation induces metabolic reprogramming and mitochondrial remodeling. iScience. 2018;5:99–109. doi: 10.1016/j.isci.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cong J. Metabolism of natural killer cells and other innate lymphoid cells. Front Immunol. 2020;11:1989. doi: 10.3389/fimmu.2020.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris G, Berk M, Klein H, Walder K, Galecki P, Maes M. Nitrosative stress, hypernitrosylation, and autoimmune responses to nitrosylated proteins: new pathways in neuroprogressive disorders including depression and chronic fatigue syndrome. Mol Neurobiol. 2017;54:4271–91. doi: 10.1007/s12035-016-9975-2. [DOI] [PubMed] [Google Scholar]
- 18.Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–9. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 21.Shao D, Oka S-I, Liu T, Zhai P, Ago T, Sciarretta S, et al. A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metab. 2014;19:232–45. doi: 10.1016/j.cmet.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koundouros N, Poulogiannis G. Phosphoinositide 3-kinase/Akt signaling and redox metabolism in cancer. Front Oncol. 2018;8:160. [DOI] [PMC free article] [PubMed]
- 23.Bonello S, Zähringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, et al. Reactive oxygen species activate the HIF1-α promoter via a functional NF-κB site. Arterioscler Thromb Vasc Biol. 2007;27:755–61. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 24.Wink DA, Hines HB, Cheng RYS, Switzer CH, Flores-Santana W, Vitek MP, et al. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89:873–91. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–78. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Tavassolifar MJ, Vodjgani M, Salehi Z, Izad M. The influence of reactive oxygen species in the immune system and pathogenesis of multiple sclerosis. Autoimmune Dis. 2020;2020:5793817. doi: 10.1155/2020/5793817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Bazhin AV, Werner J, Karakhanova S. Reactive oxygen species in the immune system. Int Rev Immunol. 2013;32:249–70. doi: 10.3109/08830185.2012.755176. [DOI] [PubMed] [Google Scholar]
- 28.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–61. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battino M, Giampieri F, Pistollato F, Sureda A, de Oliveira MR, Pittalà V, et al. Nrf2 as regulator of innate immunity: a molecular Swiss army knife! Biotechnol Adv. 2018;36:358–70. doi: 10.1016/j.biotechadv.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Investig. 2006;116:984–95. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Surh Y-J. The role of Nrf2 in cellular innate immune response to inflammatory injury. Toxicol Res. 2009;25:159–73. doi: 10.5487/TR.2009.25.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krönke G, Leitinger N. Oxidized phospholipids at the interface of innate and adaptive immunity. Future Lipidol. 2006;1:623–30. doi: 10.2217/17460875.1.5.623. [DOI] [Google Scholar]
- 33.Serbulea V, DeWeese D, Leitinger N. The effect of oxidized phospholipids on phenotypic polarization and function of macrophages. Free Radic Biol Med. 2017;111:156–68. doi: 10.1016/j.freeradbiomed.2017.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freigang S. The regulation of inflammation by oxidized phospholipids. Eur J Immunol. 2016;46:1818–25. doi: 10.1002/eji.201545676. [DOI] [PubMed] [Google Scholar]
- 35.Matt U, Sharif O, Martins R, Knapp S. Accumulating evidence for a role of oxidized phospholipids in infectious diseases. Cell Mol Life Sci. 2015;72:1059–71. doi: 10.1007/s00018-014-1780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creasy KT, Kane JP, Malloy MJ. Emerging roles of HDL in immune function. Curr Opin Lipidol. 2018;29:486–7. doi: 10.1097/MOL.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 37.Macpherson ME, Halvorsen B, Yndestad A, Ueland T, Mollnes TE, Berge RK, et al. Impaired HDL function amplifies systemic inflammation in common variable immunodeficiency. Sci Rep. 2019;9:9427. doi: 10.1038/s41598-019-45861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovascular Res. 2014;103:372–83. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Gong J, Liu Y. Indoleamine 2, 3-dioxygenase regulation of immune response (Review) Mol Med Rep. 2018;17:4867–73. doi: 10.3892/mmr.2018.8537. [DOI] [PubMed] [Google Scholar]
- 40.Nelp MT, Kates PA, Hunt JT, Newitt JA, Balog A, Maley D, et al. Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form. Proc Natl Acad Sci USA. 2018;115:3249–54. doi: 10.1073/pnas.1719190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris G, Puri BK, Bortolasci CC, Carvalho A, Berk M, Walder K, et al. The role of high-density lipoprotein cholesterol, apolipoprotein A and paraoxonase-1 in the pathophysiology of neuroprogressive disorders. Neurosci Biobehav Rev. 2021;125:244–63. doi: 10.1016/j.neubiorev.2021.02.037. [DOI] [PubMed] [Google Scholar]
- 42.Dorrington MG, Fraser IDC. NF-κB signaling in macrophages: dynamics, crosstalk, and signal integration. Front Immunol. 2019;10:705. [DOI] [PMC free article] [PubMed]
- 43.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ernst O, Vayttaden SJ, Fraser IDC. Measurement of NF-κB activation in TLR-activated macrophages. Methods Mol Biol (Clifton, NJ) 2018;1714:67–78. doi: 10.1007/978-1-4939-7519-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharif O, Brunner JS, Vogel A, Schabbauer G. Macrophage rewiring by nutrient associated PI3K dependent pathways. Front Immunol. 2019;10:2002. [DOI] [PMC free article] [PubMed]
- 46.Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198:1006–14. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 47.Joshi S, Singh AR, Zulcic M, Durden DL. A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1α and HIF2α stability and tumor growth, angiogenesis, and metastasis. Mol Cancer Res. 2014;12:1520–31. doi: 10.1158/1541-7786.MCR-13-0682. [DOI] [PubMed] [Google Scholar]
- 48.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci USA. 2012;109:9517–22. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1 -mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jha AK, Huang SC-C, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–30. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Feingold KR, Shigenaga JK, Kazemi MR, McDonald CM, Patzek SM, Cross AS, et al. Mechanisms of triglyceride accumulation in activated macrophages. J Leukoc Biol. 2012;92:829–39. doi: 10.1189/jlb.1111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Uden P, Kenneth Niall S, Rocha S. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochemical J. 2008;412:477–84. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, et al. Metabolic reprogramming of macrophages. J Biol Chem. 2014;289:7884–96. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang T, Liu H, Lian G, Zhang S-Y, Wang X, Jiang C. HIF1α-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediators Inflamm. 2017;2017:1–10. doi: 10.1155/2017/3102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavlou S, Wang L, Xu H, Chen M. Higher phagocytic activity of thioglycollate-elicited peritoneal macrophages is related to metabolic status of the cells. J Inflamm (Lond). 2017;14:4. [DOI] [PMC free article] [PubMed]
- 56.Blouin CC, Pagé EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1α. Blood. 2004;103:1124–30. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 57.Cimmino F, Avitabile M, Lasorsa VA, Montella A, Pezone L, Cantalupo S, et al. HIF-1 transcription activity: HIF1A driven response in normoxia and in hypoxia. BMC Med Genet. 2019;20:37. doi: 10.1186/s12881-019-0767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–8. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okamoto A, Sumi C, Tanaka H, Kusunoki M, Iwai T, Nishi K, et al. HIF-1-mediated suppression of mitochondria electron transport chain function confers resistance to lidocaine-induced cell death. Sci Rep. 2017;7:3816. doi: 10.1038/s41598-017-03980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol. 2021;599:23–37. doi: 10.1113/JP280572. [DOI] [PubMed] [Google Scholar]
- 61.Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. 2019;20:238. doi: 10.3390/ijms20020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Batista-Gonzalez A, Vidal R, Criollo A, Carreño LJ. New insights on the role of lipid metabolism in the metabolic reprogramming of macrophages. Front Immunol. 2020;10:2993. [DOI] [PMC free article] [PubMed]
- 63.Nomura M, Liu J, Rovira II, Gonzalez-Hurtado E, Lee J, Wolfgang MJ, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17:216–7. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui XG, Han ZT, He SH, Wu XD, Chen TR, Shao CH, et al. HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget. 2017;8:24840–52. doi: 10.18632/oncotarget.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T, et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem. 2006;281:37457–67. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 66.Thomas LW, Ashcroft M. Exploring the molecular interface between hypoxia-inducible factor signalling and mitochondria. Cell Mol Life Sci. 2019;76:1759–77. doi: 10.1007/s00018-019-03039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadlecki P, Bodnar M, Grabiec M, Marszalek A, Walentowicz P, Sokup A, et al. The role of hypoxia-inducible factor-1α, glucose transporter-1, (GLUT-1) and carbon anhydrase IX in endometrial cancer patients. BioMed Res Int. 2014;2014:616850. doi: 10.1155/2014/616850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharmaceutica Sin B. 2015;5:378–89. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suda T, Takubo K, Semenza, Gregg L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–37. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 71.Goda N, Kanai M. Hypoxia-inducible factors and their roles in energy metabolism. Int J Hematol. 2012;95:457–63. doi: 10.1007/s12185-012-1069-y. [DOI] [PubMed] [Google Scholar]
- 72.Weichhart T, Hengstschläger M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol. 2015;27:286–96. doi: 10.1016/j.smim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22:248–57. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linke M, Fritsch SD, Sukhbaatar N, Hengstschläger M, Weichhart T. mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett. 2017;591:3089–103. doi: 10.1002/1873-3468.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haloul M, Oliveira ERA, Kader M, Wells JZ, Tominello TR, El Andaloussi A, et al. mTORC1-mediated polarization of M1 macrophages and their accumulation in the liver correlate with immunopathology in fatal ehrlichiosis. Sci Rep. 2019;9:14050. doi: 10.1038/s41598-019-50320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Andaloussi A, Haloul MA, Kader M, Tominello T, Wells JZ, Ismail N. mTORC1-mediated macrophage polarization into M1 contributes to Ehrlichia-induced sepsis. J Immunol. 2019;202(1 Supplement):190.63. [Google Scholar]
- 79.Morris G, Walker AJ, Walder K, Berk M, Marx W, Carvalho AF, et al. Increasing Nrf2 activity as a treatment approach in neuropsychiatry. Mol Neurobiol. 2021;58:2158–82. [DOI] [PubMed]
- 80.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, et al. Metabolic reprogramming in macrophage responses. Biomark Res. 2021;9:1. doi: 10.1186/s40364-020-00251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. [DOI] [PMC free article] [PubMed]
- 83.O’Neill Luke AJ. A broken Krebs cycle in macrophages. Immunity. 2015;42:393–4. doi: 10.1016/j.immuni.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 84.Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1462. [DOI] [PMC free article] [PubMed]
- 85.Hsieh WY, Zhou QD, York AG, Williams KJ, Scumpia PO, Kronenberger EB, et al. Toll-like receptors induce signal-specific reprogramming of the macrophage lipidome. Cell Metab. 2020;32:128–43.e5. doi: 10.1016/j.cmet.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baardman J, Verberk SGS, van der Velden S, Gijbels MJJ, van Roomen CPPA, Sluimer JC, et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat Commun. 2020;11:6296. doi: 10.1038/s41467-020-20141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J-H, Phelan P, Shin M, Oh B-C, Han X, Im S-S, et al. SREBP-1a–stimulated lipid synthesis is required for macrophage phagocytosis downstream of TLR4-directed mTORC1. Proc Natl Acad Sci USA. 2018;115:E12228–E34. doi: 10.1073/pnas.1813458115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Posokhova EN, Khoshchenko OM, Chasovskikh MI, Pivovarova EN, Dushkin MI. Lipid synthesis in macrophages during inflammation in vivo: effect of agonists of peroxisome proliferator activated receptors α and γ and of retinoid X receptors. Biochemistry (Mosc) 2008;73:296–304. doi: 10.1134/S0006297908030097. [DOI] [PubMed] [Google Scholar]
- 89.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morris G, Puri BK, Olive L, Carvalho A, Berk M, Walder K, et al. Endothelial dysfunction in neuroprogressive disorders-causes and suggested treatments. BMC Med. 2020;18:305. doi: 10.1186/s12916-020-01749-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palmieri EM, Gonzalez-Cotto M, Baseler WA, Davies LC, Ghesquière B, Maio N, et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun. 2020;11:698. doi: 10.1038/s41467-020-14433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Infantino V, Iacobazzi V, Menga A, Avantaggiati ML, Palmieri F. A key role of the mitochondrial citrate carrier (SLC25A1) in TNFα- and IFNγ-triggered inflammation. Biochim Biophys Acta. 2014;1839:1217–25. doi: 10.1016/j.bbagrm.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gnoni GV, Priore P, Geelen MJH, Siculella L. The mitochondrial citrate carrier: Metabolic role and regulation of its activity and expression. IUBMB life. 2009;61:987–94. doi: 10.1002/iub.249. [DOI] [PubMed] [Google Scholar]
- 94.Ma T, Peng Y, Huang W, Ding J. Molecular mechanism of the allosteric regulation of the αγ heterodimer of human NAD-dependent isocitrate dehydrogenase. Sci Rep. 2017;7:40921. doi: 10.1038/srep40921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Infantino V, Convertini P, Cucci L, Panaro Maria A, Di Noia Maria A, Calvello R, et al. The mitochondrial citrate carrier: a new player in inflammation. Biochemical J. 2011;438:433–6. doi: 10.1042/BJ20111275. [DOI] [PubMed] [Google Scholar]
- 96.Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013;440:105–11. doi: 10.1016/j.bbrc.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 97.Oeggl R, Neumann T, Gätgens J, Romano D, Noack S, Rother D. Citrate as cost-efficient NADPH regenerating agent. Front Bioeng Biotechnol. 2018;6:196. [DOI] [PMC free article] [PubMed]
- 98.Palmieri EM, Spera I, Menga A, Infantino V, Porcelli V, Iacobazzi V, et al. Acetylation of human mitochondrial citrate carrier modulates mitochondrial citrate/malate exchange activity to sustain NADPH production during macrophage activation. Biochim Biophys Acta. 2015;1847:729–38. doi: 10.1016/j.bbabio.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 99.Williams NC, O’Neill LAJ. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol. 2018;9:141. [DOI] [PMC free article] [PubMed]
- 100.Strelko CL, Lu W, Dufort FJ, Seyfried TN, Chiles TC, Rabinowitz JD, et al. Itaconic acid is a mammalian metabolite induced during macrophage activation. J Am Chem Soc. 2011;133:16386–9. doi: 10.1021/ja2070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013;110:7820–5. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hooftman A, O'Neill LAJ. The immunomodulatory potential of the metabolite itaconate. Trends Immunol. 2019;40:687–98. doi: 10.1016/j.it.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 103.Ferreira AV, Netea MG, Domínguez-Andrés J. Itaconate as an immune modulator. Aging. 2019;11:3898–9. doi: 10.18632/aging.102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–7. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent Emma E, Loginicheva E, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24:158–66. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, et al. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem. 2016;291:14274–84. doi: 10.1074/jbc.M115.685792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–70.e13. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benmoussa K, Garaude J, Acín-Pérez R. How mitochondrial metabolism contributes to macrophage phenotype and functions. J Mol Biol. 2018;430:3906–21. doi: 10.1016/j.jmb.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Xie Z, Dai J, Dai L, Tan M, Cheng Z, Wu Y, et al. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteom. 2012;11:100–7. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Y, Gibson GE. Succinylation links metabolism to protein functions. Neurochem Res. 2019;44:2346–59. doi: 10.1007/s11064-019-02780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008;9:1261–9. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- 112.He W, Miao FJP, Lin DCH, Schwandner RT, Wang Z, Gao J, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–93. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 113.Littlewood-Evans A, Sarret S, Apfel V, Loesle P, Dawson J, Zhang J, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213:1655–62. doi: 10.1084/jem.20160061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 115.Boscá L, González-Ramos S, Prieto P, Fernández-Velasco M, Mojena M, Martín-Sanz P, et al. Metabolic signatures linked to macrophage polarization: from glucose metabolism to oxidative phosphorylation. Biochem Soc Trans. 2015;43:740–4. doi: 10.1042/BST20150107. [DOI] [PubMed] [Google Scholar]
- 116.Morris G, Puri BK, Maes M, Olive L, Berk M, Carvalho AF. The role of microglia in neuroprogressive disorders: mechanisms and possible neurotherapeutic effects of induced ketosis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109858. doi: 10.1016/j.pnpbp.2020.109858. [DOI] [PubMed] [Google Scholar]
- 117.Celik MÖ, Labuz D, Keye J, Glauben R, Machelska H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight. 2020;5:e133093. [DOI] [PMC free article] [PubMed]
- 118.Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S, Jimenez C, et al. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2018;100:1034–43. doi: 10.1016/j.ijrobp.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 119.Yu T, Gan S, Zhu Q, Dai D, Li N, Wang H, et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat Commun. 2019;10:4353. doi: 10.1038/s41467-019-12384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS–) vs. alternatively activated macrophages. Front Immunol. 2019;10:1084. [DOI] [PMC free article] [PubMed]
- 121.Ren W, Xia Y, Chen S, Wu G, Bazer FW, Zhou B, et al. Glutamine metabolism in macrophages: a novel target for obesity/type 2 diabetes. Adv Nutr. 2019;10:321–30. doi: 10.1093/advances/nmy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zelcer N. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Investig. 2006;116:607–14. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hong C, Walczak R, Dhamko H, Bradley MN, Marathe C, Boyadjian R, et al. Constitutive activation of LXR in macrophages regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target. J Lipid Res. 2011;52:531–9. doi: 10.1194/jlr.M010686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spann NJ, Glass CK. Sterols and oxysterols in immune cell function. Nat Immunol. 2013;14:893–900. doi: 10.1038/ni.2681. [DOI] [PubMed] [Google Scholar]
- 125.Ley K. M1 means kill; M2 means heal. J Immunol. 2017;199:2191–3. doi: 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- 126.Nelson VL, Nguyen HCB, Garcìa-Cañaveras JC, Briggs ER, Ho WY, DiSpirito JR, et al. PPARγ is a nexus controlling alternative activation of macrophages via glutamine metabolism. Genes Dev. 2018;32:1035–44. doi: 10.1101/gad.312355.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu P-S, Wang H, Li X, Chao T, Teav T, Christen S, et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18:985–94. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 128.Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 2018;28:463–75.e4. doi: 10.1016/j.cmet.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xiang H-C, Lin L-X, Hu X-F, Zhu H, Li H-P, Zhang R-Y, et al. AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J Neuroinflammation. 2019;16:34. doi: 10.1186/s12974-019-1411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang BP, Lin CH, Chen HM, Lin JT, Cheng YF, Kao SH. AMPK activation inhibits expression of proinflammatory mediators through downregulation of PI3K/p38 MAPK and NF-κB signaling in murine macrophages. DNA Cell Biol. 2015;34:133–41. doi: 10.1089/dna.2014.2630. [DOI] [PubMed] [Google Scholar]
- 131.Zhu YP, Brown JR, Sag D, Zhang L, Suttles J. Adenosine 5′-monophosphate–activated protein kinase regulates IL-10–mediated anti-inflammatory signaling pathways in macrophages. J Immunol. 2015;194:584–94. doi: 10.4049/jimmunol.1401024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–41. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–35. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ke R, Xu Q, Li C, Luo L, Huang D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol Int. 2018;42:384–92. doi: 10.1002/cbin.10915. [DOI] [PubMed] [Google Scholar]
- 135.Rigamonti E, Chinetti-Gbaguidi G, Staels B. Regulation of macrophage functions by PPAR-α, PPAR-γ, and LXRs in mice and men. Arterioscler Thromb Vasc Biol. 2008;28:1050–9. doi: 10.1161/ATVBAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 136.Leopold Wager CM, Arnett E, Schlesinger LS. Macrophage nuclear receptors: emerging key players in infectious diseases. PLoS Pathog. 2019;15:e1007585. doi: 10.1371/journal.ppat.1007585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Corona JC, Duchen MR. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic Biol Med. 2016;100:153–63. doi: 10.1016/j.freeradbiomed.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fan W, Evans RP. PPARs and ERRs: molecular mediators of mitochondrial metabolism. Curr Opin Cell Biol. 2015;33:49–54. doi: 10.1016/j.ceb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu P, Zhai Y, Wang J. The role of PPAR and its cross-talk with CAR and LXR in obesity and atherosclerosis. Int J Mol Sci. 2018;19:1260. doi: 10.3390/ijms19041260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, et al. Glutamine‐driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol. 2013;9:712. doi: 10.1038/msb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224:242–53. doi: 10.1016/j.imbio.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 142.Forman HJ, Torres M. Redox signaling in macrophages. Mol Asp Med. 2001;22:189–216. doi: 10.1016/S0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 143.Burova E, Borodkina A, Shatrova A, Nikolsky N. Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxid Med Cell Longev. 2013;2013:474931. doi: 10.1155/2013/474931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li H, Luo Y-F, Wang Y-S, Yang Q, Xiao Y-L, Cai H-R, et al. Using ROS as a second messenger, NADPH oxidase 2 mediates macrophage senescence via interaction with NF-κB during Pseudomonas aeruginosa infection. Oxid Med Cell Longev. 2018;2018:9741838. doi: 10.1155/2018/9741838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Elder SS, Emmerson E. Senescent cells and macrophages: key players for regeneration? Open Biol. 2020;10:200309. doi: 10.1098/rsob.200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N, et al. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun. 2015;6:7847. [DOI] [PMC free article] [PubMed]
- 147.Bally APR, Lu P, Tang Y, Austin JW, Scharer CD, Ahmed R, et al. NF-κB regulates PD-1 expression in macrophages. J Immunol. 2015;194:4545–54. doi: 10.4049/jimmunol.1402550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morris D, Guerra C, Khurasany M, Guilford F, Saviola B, Huang Y, et al. Glutathione supplementation improves macrophage functions in HIV. J Interferon Cytokine Res. 2013;33:270–9. doi: 10.1089/jir.2012.0103. [DOI] [PubMed] [Google Scholar]
- 149.Morris D, Khurasany M, Nguyen T, Kim J, Guilford F, Mehta R, et al. Glutathione and infection. Biochim Biophys Acta. 2013;1830:3329–49. doi: 10.1016/j.bbagen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 150.Fitzpatrick AM, Teague WG, Burwell L, Brown MS, Brown LAS. Glutathione oxidation is associated with airway macrophage functional impairment in children with severe asthma. Pediatr Res. 2011;69:154–9. doi: 10.1203/PDR.0b013e3182026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brown LAS, Ping X-D, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol. 2007;292:L824–32. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- 152.Rosenblat M, Coleman R, Aviram M. Increased macrophage glutathione content reduces cell-mediated oxidation of LDL and atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2002;163:17–28. doi: 10.1016/S0021-9150(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 153.Kwon DH, Lee H, Park C, Hong S-H, Hong SH, Kim G-Y, et al. Glutathione induced immune-stimulatory activity by promoting M1-like macrophages polarization via potential ROS scavenging capacity. Antioxidants (Basel) 2019;8:413. doi: 10.3390/antiox8090413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Diotallevi M, Checconi P, Palamara AT, Celestino I, Coppo L, Holmgren A, et al. Glutathione fine-tunes the innate immune response toward antiviral pathways in a macrophage cell line independently of its antioxidant properties. Front Immunol. 2017;8:1239. [DOI] [PMC free article] [PubMed]
- 155.Kondo A, Morita H, Nakamura H, Kotani K, Kobori K, Ito S, et al. Influence of fibrate treatment on malondialdehyde-modified LDL concentration. Clin Chim Acta. 2004;339:97–103. doi: 10.1016/j.cccn.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 156.Son A, Kato N, Horibe T, Matsuo Y, Mochizuki M, Mitsui A, et al. Direct association of thioredoxin-1 (TRX) with macrophage migration inhibitory factor (MIF): regulatory role of TRX on MIF internalization and signaling. Antioxid Redox Signal. 2009;11:2595–605. doi: 10.1089/ars.2009.2522. [DOI] [PubMed] [Google Scholar]
- 157.Tamaki H, Nakamura H, Nishio A, Nakase H, Ueno S, Uza N, et al. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131:1110–21. doi: 10.1053/j.gastro.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 158.El Hadri K, Mahmood DF, Couchie D, Jguirim-Souissi I, Genze F, Diderot V, et al. Thioredoxin-1 promotes anti-inflammatory macrophages of the M2 phenotype and antagonizes atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1445–52. doi: 10.1161/ATVBAHA.112.249334. [DOI] [PubMed] [Google Scholar]
- 159.Leaver SK, MacCallum NS, Pingle V, Hacking MB, Quinlan GJ, Evans TW, et al. Increased plasma thioredoxin levels in patients with sepsis: positive association with macrophage migration inhibitory factor. Intensive Care Med. 2010;36:336–41. doi: 10.1007/s00134-009-1640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Luo J-F, Shen X-Y, Lio CK, Dai Y, Cheng C-S, Liu J-X, et al. Activation of Nrf2/HO-1 pathway by nardochinoid C inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front Pharmacol. 2018;9:911. [DOI] [PMC free article] [PubMed]
- 161.Feng R, Morine Y, Ikemoto T, Imura S, Iwahashi S, Saito Y, et al. Nrf2 activation drive macrophages polarization and cancer cell epithelial-mesenchymal transition during interaction. Cell Commun Signal. 2018;16:54. doi: 10.1186/s12964-018-0262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. [DOI] [PMC free article] [PubMed]
- 163.Yang X, Gu J, Lv H, Li H, Cheng Y, Liu Y, et al. Uric acid induced inflammatory responses in endothelial cells via up-regulating(pro)renin receptor. Biomed Pharmacother. 2019;109:1163–70. doi: 10.1016/j.biopha.2018.10.129. [DOI] [PubMed] [Google Scholar]
- 164.Gordon J, Ma Y, Churchman L, Gordon S, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014;5:7. [DOI] [PMC free article] [PubMed]
- 165.Gallo P, Gallucci S. The dendritic cell response to classic, emerging, and homeostatic danger signals. implications for autoimmunity. Front Immunol. 2013;4:138. [DOI] [PMC free article] [PubMed]
- 166.Wu D-D, Li T, Ji X-Y. Dendritic cells in sepsis: pathological alterations and therapeutic implications. J Immunol Res. 2017;2017:3591248. doi: 10.1155/2017/3591248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thwe PM, Pelgrom LR, Cooper R, Beauchamp S, Reisz JA, D’Alessandro A, et al. Cell-intrinsic glycogen metabolism supports early glycolytic reprogramming required for dendritic cell immune responses. Cell Metab. 2017;26:558–67.e5. doi: 10.1016/j.cmet.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wculek SK, Khouili SC, Priego E, Heras-Murillo I, Sancho D. Metabolic control of dendritic cell functions: digesting information. Front Immunol. 2019;10:775. doi: 10.3389/fimmu.2019.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, et al. Toll-like receptor–induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–9. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sukhbaatar N, Hengstschläger M, Weichhart T. mTOR-mediated regulation of dendritic cell differentiation and function. Trends Immunol. 2016;37:778–89. doi: 10.1016/j.it.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.O’Neill LAJ, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2015;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ryan DG, O'Neill LAJ. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. 2017;591:2992–3006. doi: 10.1002/1873-3468.12744. [DOI] [PubMed] [Google Scholar]
- 173.Pearce EJ, Everts B. Dendritic cell metabolism. Nat Rev Immunol. 2014;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Perrin-Cocon L, Aublin-Gex A, Diaz O, Ramière C, Peri F, André P, et al. Toll-like receptor 4–induced glycolytic burst in human monocyte-derived dendritic cells results from p38-dependent stabilization of HIF-1α and increased hexokinase II expression. J Immunol. 2018;201:1510–21. doi: 10.4049/jimmunol.1701522. [DOI] [PubMed] [Google Scholar]
- 175.Fliesser M, Morton CO, Bonin M, Ebel F, Hünniger K, Kurzai O, et al. Hypoxia-inducible factor 1α modulates metabolic activity and cytokine release in anti- Aspergillus fumigatus immune responses initiated by human dendritic cells. Int J Med Microbiol. 2015;305:865–73. doi: 10.1016/j.ijmm.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 176.Harris AJ, Thompson AR, Whyte MK, Walmsley SR. HIF-mediated innate immune responses: cell signaling and therapeutic implications. Hypoxia (Auckl) 2014;2:47–58. doi: 10.2147/HP.S50269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lawless SJ, Kedia-Mehta N, Walls JF, McGarrigle R, Convery O, Sinclair LV, et al. Glucose represses dendritic cell-induced T cell responses. Nat Commun. 2017;8:15620. [DOI] [PMC free article] [PubMed]
- 178.Everts B, Amiel E, van der Windt GJW, Freitas TC, Chott R, Yarasheski KE, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–31. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Amiel E, Everts B, Fritz D, Beauchamp S, Ge B, Pearce EL, et al. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J Immunol. 2014;193:2821–30. doi: 10.4049/jimmunol.1302498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Snyder JP, Amiel E. Regulation of dendritic cell immune function and metabolism by cellular nutrient sensor mammalian target of rapamycin (mTOR) Front Immunol. 2018;9:3145. doi: 10.3389/fimmu.2018.03145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mantegazza AR, Savina A, Vermeulen M, Pérez L, Geffner J, Hermine O, et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–22. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Paardekooper LM, Dingjan I, Linders PTA, Staal AHJ, Cristescu SM, Verberk WCEP, et al. Human monocyte-derived dendritic cells produce millimolar concentrations of ROS in phagosomes per second. Front Immunol. 2019;10:1216. [DOI] [PMC free article] [PubMed]
- 183.Oberkampf M, Guillerey C, Mouriès J, Rosenbaum P, Fayolle C, Bobard A, et al. Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat Commun. 2018;9:2241. doi: 10.1038/s41467-018-04686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Götz A, Ty MC, Rodriguez A. Oxidative stress enhances dendritic cell responses to Plasmodium falciparum. ImmunoHorizons. 2019;3:511–8. doi: 10.4049/immunohorizons.1900076. [DOI] [PubMed] [Google Scholar]
- 185.Romero MM, Basile JI, Corra Feo L, López B, Ritacco V, Alemán M. Reactive oxygen species production by human dendritic cells involves TLR2 and dectin-1 and is essential for efficient immune response against Mycobacteria. Cell Microbiol. 2016;18:875–86. doi: 10.1111/cmi.12562. [DOI] [PubMed] [Google Scholar]
- 186.D'Angelo JA, Dehlink E, Platzer B, Dwyer P, Circu ML, Garay J, et al. The cystine/glutamate antiporter regulates dendritic cell differentiation and antigen presentation. J Immunol. 2010;185:3217–26. doi: 10.4049/jimmunol.1001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kamide Y, Utsugi M, Dobashi K, Ono A, Ishizuka T, Hisada T, et al. Intracellular glutathione redox status in human dendritic cells regulates IL-27 production and T-cell polarization. Allergy. 2011;66:1183–92. doi: 10.1111/j.1398-9995.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 188.Dobashi K, Kamide Y, Utsugi M, Ono A, Ishizuka T, Hisada T, et al. Intracellular glutathione redox status in human dendritic cells regulates Th1/Th2 balance through IL-12 and IL-27 production. Eur Respiratory J. 2012;40(Suppl 56):P2330. doi: 10.1111/j.1398-9995.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 189.Kim H-J, Barajas B, Chan RC-F, Nel AE. Glutathione depletion inhibits dendritic cell maturation and delayed-type hypersensitivity: implications for systemic disease and immunosenescence. J Allergy Clin Immunol. 2007;119:1225–33. doi: 10.1016/j.jaci.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 190.Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci USA. 2002;99:1491–6. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang J, Liu P, Xin S, Wang Z, Li J. Nrf2 suppresses the function of dendritic cells to facilitate the immune escape of glioma cells. Exp cell Res. 2017;360:66–73. doi: 10.1016/j.yexcr.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 192.Macoch M, Morzadec C, Génard R, Pallardy M, Kerdine-Römer S, Fardel O, et al. Nrf2-dependent repression of interleukin-12 expression in human dendritic cells exposed to inorganic arsenic. Free Radic Biol Med. 2015;88:381–90. doi: 10.1016/j.freeradbiomed.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 193.Hammer A, Waschbisch A, Knippertz I, Zinser E, Berg J, Jörg S, et al. Role of nuclear factor (erythroid-derived 2)-like 2 signaling for effects of fumaric acid esters on dendritic cells. Front Immunol. 2017;8:1922. [DOI] [PMC free article] [PubMed]
- 194.Li N, Wang M, Barajas B, Sioutas C, Williams MA, Nel AE. Nrf2 deficiency in dendritic cells enhances the adjuvant effect of ambient ultrafine particles on allergic sensitization. J innate Immun. 2013;5:543–54. doi: 10.1159/000347060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, et al. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–59. doi: 10.4049/jimmunol.181.7.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wei H-J, Pareek TK, Gupta A, Kao W, Almudallal O, Letterio JJ. Nrf2 mediated metabolic reprogramming of tolerogenic dendritic cells is protective against aplastic anemia. J Immunol. 2018;200(1 Supplement):176. doi: 10.1016/j.jaut.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 197.Said A, Weindl G. Regulation of dendritic cell function in inflammation. J Immunol Res. 2015;2015:743169. doi: 10.1155/2015/743169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Agrawal A, Agrawal S, Gupta S. Role of dendritic cells in inflammation and loss of tolerance in the elderly. Front Immunol. 2017;8:896. doi: 10.3389/fimmu.2017.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Carstensen LS, Lie-Andersen O, Obers A, Crowther MD, Svane IM, Hansen M. Long-term exposure to inflammation induces differential cytokine patterns and apoptosis in dendritic cells. Front Immunol. 2019;10:2702. [DOI] [PMC free article] [PubMed]
- 200.Chatterjee S, Lardinois O, Bhattacharjee S, Tucker J, Corbett J, Deterding L, et al. Oxidative stress induces protein and DNA radical formation in follicular dendritic cells of the germinal center and modulates its cell death patterns in late sepsis. Free Radic Biol Med. 2011;50:988–99. doi: 10.1016/j.freeradbiomed.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Morris G, Maes M, Murdjeva M, Puri BK. Do human endogenous retroviruses contribute to multiple sclerosis, and if so, how? Mol Neurobiol. 2019;56:2590–605. doi: 10.1007/s12035-018-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Morris G, Walder K, Carvalho AF, Tye SJ, Lucas K, Berk M, et al. The role of hypernitrosylation in the pathogenesis and pathophysiology of neuroprogressive diseases. Neurosci Biobehav Rev. 2018;84:453–69. doi: 10.1016/j.neubiorev.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 203.Fan X, Liu Z, Jin H, Yan J, Liang HP. Alterations of dendritic cells in sepsis: featured role in immunoparalysis. Biomed Res Int. 2015;2015:903720. doi: 10.1155/2015/903720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 205.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 206.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–99. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127:2173–81. doi: 10.1182/blood-2016-01-688887. [DOI] [PubMed] [Google Scholar]
- 208.Takashima A, Yao Y. Neutrophil plasticity: acquisition of phenotype and functionality of antigen-presenting cell. J Leukoc Biol. 2015;98:489–96. doi: 10.1189/jlb.1MR1014-502R. [DOI] [PubMed] [Google Scholar]
- 209.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34:317–28. doi: 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 211.Kumar S, Dikshit M. Metabolic insight of neutrophils in health and disease. Front Immunol. 2019;10:2099. [DOI] [PMC free article] [PubMed]
- 212.Sadiku P, Willson JA, Ryan EM, Sammut D, Coelho P, Watts ER, et al. Neutrophils fuel effective immune responses through gluconeogenesis and glycogenesis. Cell Metab. 2021;33:411–23.e4. doi: 10.1016/j.cmet.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jeon JH, Hong CW, Kim EY, Lee JM. Current understanding on the metabolism of neutrophils. Immune Netw. 2020;20:e46. doi: 10.4110/in.2020.20.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rodríguez-Espinosa O, Rojas-Espinosa O, Moreno-Altamirano MMB, López-Villegas EO, Sánchez-García FJ. Metabolic requirements for neutrophil extracellular traps formation. Immunology. 2015;145:213–24. doi: 10.1111/imm.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Azevedo EP, Rochael NC, Guimarães-Costa AB, de Souza-Vieira TS, Ganilho J, Saraiva EM, et al. A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and phorbol 12-myristate 13-acetate-induced neutrophil extracellular trap (NET) formation. J Biol Chem. 2015;290:22174–83. doi: 10.1074/jbc.M115.640094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017;7:373. [DOI] [PMC free article] [PubMed]
- 217.Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol (Clifton, NJ) 2012;844:115–24. doi: 10.1007/978-1-61779-527-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Injarabian L, Devin A, Ransac S, Marteyn BS. Neutrophil metabolic shift during their lifecycle: impact on their survival and activation. Int J Mol Sci. 2019;21:287. doi: 10.3390/ijms21010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–5. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 220.Bao Y, Ledderose C, Graf AF, Brix B, Birsak T, Lee A, et al. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J Cell Biol. 2015;210:1153–64. doi: 10.1083/jcb.201503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fossati G, Moulding DA, Spiller DG, Moots RJ, White MR, Edwards SW. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol. 2003;170:1964–72. doi: 10.4049/jimmunol.170.4.1964. [DOI] [PubMed] [Google Scholar]
- 222.Rice CM, Davies LC, Subleski JJ, Maio N, Gonzalez-Cotto M, Andrews C, et al. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat Commun. 2018;9:5099. doi: 10.1038/s41467-018-07505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. [DOI] [PMC free article] [PubMed]
- 224.Lin N, Simon MC. Hypoxia-inducible factors: key regulators of myeloid cells during inflammation. J Clin Investig. 2016;126:3661–71. doi: 10.1172/JCI84426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Branitzki-Heinemann K, Möllerherm H, Völlger L, Husein DM, de Buhr N, Blodkamp S, et al. Formation of neutrophil extracellular traps under low oxygen level. Front Immunol. 2016;7:518. [DOI] [PMC free article] [PubMed]
- 226.Itakura A, McCarty OJT. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am J Physiol Cell Physiol. 2013;305:C348–54. doi: 10.1152/ajpcell.00108.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sabbatini M, Magnelli V, Renò F. NETosis in wound healing: when enough is enough. Cells. 2021;10:494. doi: 10.3390/cells10030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Säemann MD, Haidinger M, Hecking M, Hörl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–61. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 229.Nouwen LV, Everts B. Pathogens MenTORing macrophages and dendritic cells: manipulation of mTOR and cellular metabolism to promote immune escape. Cells. 2020;9:161. doi: 10.3390/cells9010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gambardella L, Vermeren S. Molecular players in neutrophil chemotaxis—focus on PI3K and small GTPases. J Leukoc Biol. 2013;94:603–12. doi: 10.1189/jlb.1112564. [DOI] [PubMed] [Google Scholar]
- 231.McCormick B, Chu JY, Vermeren S. Cross-talk between Rho GTPases and PI3K in the neutrophil. Small GTPases. 2019;10:187–95. doi: 10.1080/21541248.2017.1304855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhao X, Zmijewski JW, Lorne E, Liu G, Park Y-J, Tsuruta Y, et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Park DW, Jiang S, Tadie JM, Stigler WS, Gao Y, Deshane J, et al. Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol Med (Camb, Mass) 2013;19:387–98. doi: 10.2119/molmed.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP. PPARγ and the innate immune system mediate the resolution of inflammation. PPAR Res. 2015;2015:549691. doi: 10.1155/2015/549691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tyurin VA, Balasubramanian K, Winnica D, Tyurina YY, Vikulina AS, He RR, et al. Oxidatively modified phosphatidylserines on the surface of apoptotic cells are essential phagocytic ‘eat-me’ signals: cleavage and inhibition of phagocytosis by Lp-PLA2. Cell Death Differ. 2014;21:825–35. doi: 10.1038/cdd.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fokam D. Instrumental role for reactive oxygen species in the inflammatory response. Front Biosci. 2020;25:1110–9. doi: 10.2741/4848. [DOI] [PubMed] [Google Scholar]
- 237.Araźna M, Pruchniak MP, Demkow U. Reactive oxygen species, granulocytes, and NETosis. Adv Exp Med Biol. 2015;836:1–7. [DOI] [PubMed]
- 238.Mullen L, Mengozzi M, Hanschmann E-M, Alberts B, Ghezzi P. How the redox state regulates immunity. Free Radic Biol Med. 2020;157:3–14. doi: 10.1016/j.freeradbiomed.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 239.Lorenzen I, Mullen L, Bekeschus S, Hanschmann E-M. Redox regulation of inflammatory processes is enzymatically controlled. Oxid Med Cell Longev. 2017;2017:1–23. doi: 10.1155/2017/8459402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sônego F, Castanheira FVES, Ferreira RG, Kanashiro A, Leite CAVG, Nascimento DC, et al. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front Immunol. 2016;7:155. [DOI] [PMC free article] [PubMed]
- 241.Spiller F, Oliveira Formiga R, Fernandes da Silva Coimbra J, Alves-Filho JC, Cunha TM, Cunha FQ. Targeting nitric oxide as a key modulator of sepsis, arthritis and pain. Nitric Oxide. 2019;89:32–40. doi: 10.1016/j.niox.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 242.Zhang F, Liu A-L, Gao S, Ma S, Guo S-B. Neutrophil dysfunction in sepsis. Chin Med J (Engl) 2016;129:2741–4. doi: 10.4103/0366-6999.193447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dal Secco D, Moreira AP, Freitas A, Silva JS, Rossi MA, Ferreira SH, et al. Nitric oxide inhibits neutrophil migration by a mechanism dependent on ICAM-1: role of soluble guanylate cyclase. Nitric Oxide. 2006;15:77–86. doi: 10.1016/j.niox.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 244.Alves-Filho JC, Spiller F, Cunha FQ. Neutrophil paralysis in sepsis. Shock. 2010;34:15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 245.Clements MK, Siemsen DW, Swain SD, Hanson AJ, Nelson-Overton LK, Rohn TT, et al. Inhibition of actin polymerization by peroxynitrite modulates neutrophil functional responses. J Leukoc Biol. 2003;73:344–55. doi: 10.1189/jlb.0802401. [DOI] [PubMed] [Google Scholar]
- 246.Torres-Dueñas D, Celes MRN, Freitas A, Alves-Filho JC, Spiller F, Dal-Secco D, et al. Peroxynitrite mediates the failure of neutrophil migration in severe polymicrobial sepsis in mice. Br J Pharmacol. 2007;152:341–52. doi: 10.1038/sj.bjp.0707393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lefer DJ, Scalia R, Campbell B, Nossuli T, Hayward R, Salamon M, et al. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J Clin Invest. 1997;99:684–91. doi: 10.1172/JCI119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Morris G, Maes M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) Curr Neuropharmacol. 2014;12:168–85. doi: 10.2174/1570159X11666131120224653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Freitas A, Alves-Filho JC, Secco DD, Neto AF, Ferreira SH, Barja-Fidalgo C, et al. Heme oxygenase/carbon monoxide-biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br J Pharm. 2006;149:345–54. doi: 10.1038/sj.bjp.0706882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Helou DG, Noël B, Gaudin F, Groux H, El Ali Z, Pallardy M, et al. Cutting edge: Nrf2 regulates neutrophil recruitment and accumulation in skin during contact hypersensitivity. J Immunol. 2019;202:2189–94. doi: 10.4049/jimmunol.1801065. [DOI] [PubMed] [Google Scholar]
- 251.Li Y-J, Shimizu T, Shinkai Y, Ihara T, Sugamata M, Kato K, et al. Nrf2 lowers the risk of lung injury via modulating the airway innate immune response induced by diesel exhaust in mice. Biomedicines. 2020;8:443. doi: 10.3390/biomedicines8100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Joshi N, Werner S. Nrf2 is highly expressed in neutrophils, but myeloid cell-derived Nrf2 is dispensable for wound healing in mice. PLoS One. 2017;12:e0187162. doi: 10.1371/journal.pone.0187162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Liu Y, Crowell SA, Chen Y, Rogers LK, Nelin LD. The role of glutathione reductase in neutrophil respiratory burst and ERK signaling. J Immunol. 2016;196(1 Supplement):60.19. [Google Scholar]
- 254.Yan J, Meng X, Wancket LM, Lintner K, Nelin LD, Chen B, et al. Glutathione reductase facilitates host defense by sustaining phagocytic oxidative burst and promoting the development of neutrophil extracellular traps. J Immunol. 2012;188:2316–27. doi: 10.4049/jimmunol.1102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yan J, Ralston MM, Meng X, Bongiovanni KD, Jones AL, Benndorf R, et al. Glutathione reductase is essential for host defense against bacterial infection. Free Radic Biol Med. 2013;61:320–32. doi: 10.1016/j.freeradbiomed.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kim VY, Batty A, Li J, Kirk SG, Crowell SA, Jin Y, et al. Glutathione reductase promotes fungal clearance and suppresses inflammation during systemic candida albicans infection in mice. J Immunol. 2019;203:2239–51. doi: 10.4049/jimmunol.1701686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kinnula VL, Soini Y, Kvist-Mäkelä K, Savolainen E-R, Koistinen P. Antioxidant defense mechanisms in human neutrophils. Antioxid Redox Signal. 2002;4:27–34. doi: 10.1089/152308602753625825. [DOI] [PubMed] [Google Scholar]
- 258.Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci USA. 2006;103:4628–33. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Santangelo F. [General articles] Intracellular thiol concentration modulating inflammatory response: influence on the regulation of cell functions through cysteine prodrug approach. Curr Med Chem. 2003;10:2599–610. doi: 10.2174/0929867033456567. [DOI] [PubMed] [Google Scholar]
- 260.Carr AC, Winterbourn CC. Oxidation of neutrophil glutathione and protein thiols by myeloperoxidase-derived hypochlorous acid. Biochemical J. 1997;327(Pt 1):275–81. doi: 10.1042/bj3270275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ogino T, Packer L, Maguire JJ. Neutrophil antioxidant capacity during the respiratory burst: loss of glutathione induced by chloramines. Free Radic Biol Med. 1997;23:445–52. doi: 10.1016/S0891-5849(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 262.Carrera-Quintanar L, Funes L, Herranz-López M, Martínez-Peinado P, Pascual-García S, Sempere JM, et al. Antioxidant supplementation modulates neutrophil inflammatory response to exercise-induced stress. Antioxidants (Basel). 2020;9:1242. [DOI] [PMC free article] [PubMed]
- 263.Bertini R, Zack Howard OM, Dong H-F, Oppenheim JJ, Bizzarri C, Sergi R, et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189:1783–9. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bizzarri C, Holmgren A, Pekkari K, Chang G, Colotta F, Ghezzi P, et al. Requirements for the different cysteines in the chemotactic and desensitizing activity of human thioredoxin. Antioxid Redox Signal. 2005;7:1189–94. doi: 10.1089/ars.2005.7.1189. [DOI] [PubMed] [Google Scholar]
- 265.Pagliei S, Ghezzi P, Bizzarri C, Sabbatini V, Frascaroli G, Sozzani S, et al. Thioredoxin specifically cross-desensitizes monocytes to MCP-1. Eur Cytokine Netw. 2002;13:261–7. [PubMed] [Google Scholar]
- 266.Nakamura H, Herzenberg LA, Bai J, Araya S, Kondo N, Nishinaka Y, et al. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc Natl Acad Sci USA. 2001;98:15143–8. doi: 10.1073/pnas.191498798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kamiński Marcin M, Sauer Sven W, Kamiński M, Opp S, Ruppert T, Grigaravičius P, et al. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep. 2012;2:1300–15. doi: 10.1016/j.celrep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 269.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–15. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 270.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–43. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ma EH, Poffenberger MC, Wong AHT, Jones RG. The role of AMPK in T cell metabolism and function. Curr Opin Immunol. 2017;46:45–52. doi: 10.1016/j.coi.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 272.Patel CH, Powell JD. Targeting T cell metabolism to regulate T cell activation, differentiation and function in disease. Curr Opin Immunol. 2017;46:82–8. doi: 10.1016/j.coi.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zeng H, Chi H. mTOR signaling in the differentiation and function of regulatory and effector T cells. Curr Opin Immunol. 2017;46:103–11. doi: 10.1016/j.coi.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang R, Dillon Christopher P, Shi Lewis Z, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–82. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zeng H, Chi H. mTOR signaling and transcriptional regulation in T lymphocytes. Transcription. 2014;5:e28263. doi: 10.4161/trns.28263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Le Bourgeois T, Strauss L, Aksoylar H-I, Daneshmandi S, Seth P, Patsoukis N, et al. Targeting T cell metabolism for improvement of cancer immunotherapy. Front Oncol. 2018;8:237. [DOI] [PMC free article] [PubMed]
- 277.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–53. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang L-S, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jacobs SR, Herman CE, MacIver NJ, Wofford JA, Wieman HL, Hammen JJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–86. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol. 2012;33:168–73. doi: 10.1016/j.it.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ray John P, Staron Matthew M, Shyer Justin A, Ho P-C, Marshall Heather D, Gray, Simon M, et al. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity. 2015;43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Angela M, Endo Y, Asou HK, Yamamoto T, Tumes DJ, Tokuyama H, et al. Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARγ directs early activation of T cells. Nat Commun. 2016;7:13683. [DOI] [PMC free article] [PubMed]
- 284.Rashida Gnanaprakasam JN, Wu R, Wang R. Metabolic reprogramming in modulating T cell reactive oxygen species generation and antioxidant capacity. Front Immunol. 2018;9:1075. [DOI] [PMC free article] [PubMed]
- 285.O’Sullivan D, van der Windt Gerritje JW, Huang Stanley C-C, Curtis Jonathan D, Chang C-H, Buck Michael D, et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36:81–91. doi: 10.1016/j.it.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 287.Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–33. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 288.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77. doi: 10.1016/S1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 289.Sinclair LV, Rolf J, Emslie E, Shi Y-B, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500–8. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ren W, Liu G, Yin J, Tan B, Wu G, Bazer FW, et al. Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis. 2017;8:e2655-e. doi: 10.1038/cddis.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sena Laura A, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman David A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–36. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Nakaya M, Xiao Y, Zhou X, Chang J-H, Chang M, Cheng X, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Blagih J, Coulombe F, Vincent Emma E, Dupuy F, Galicia-Vázquez G, Yurchenko E, et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42:41–54. doi: 10.1016/j.immuni.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 294.Wei J, Raynor J, Nguyen T-LM, Chi H. Nutrient and metabolic sensing in T cell responses. Front Immunol. 2017;8:247. [DOI] [PMC free article] [PubMed]
- 295.Wang W, Zou W. Amino acids and their transporters in T cell immunity and cancer therapy. Mol Cell. 2020;80:384–95. doi: 10.1016/j.molcel.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Franchina DG, Dostert C, Brenner D. Reactive oxygen species: involvement in T cell signaling and metabolism. Trends Immunol. 2018;39:489–502. doi: 10.1016/j.it.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 297.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–27. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 298.Frossi B, De Carli M, Piemonte M, Pucillo C. Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol Immunol. 2008;45:58–64. doi: 10.1016/j.molimm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 299.Abimannan T, Peroumal D, Parida JR, Barik PK, Padhan P, Devadas S. Oxidative stress modulates the cytokine response of differentiated Th17 and Th1 cells. Free Radic Biol Med. 2016;99:352–63. doi: 10.1016/j.freeradbiomed.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 300.Zhang B, Liu S-Q, Li C, Lykken E, Jiang S, Wong E, et al. MicroRNA-23a curbs necrosis during early T cell activation by enforcing intracellular reactive oxygen species equilibrium. Immunity. 2016;44:568–81. doi: 10.1016/j.immuni.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lee DH, Son DJ, Park MH, Yoon DY, Han SB, Hong JT. Glutathione peroxidase 1 deficiency attenuates concanavalin A-induced hepatic injury by modulation of T-cell activation. Cell Death Dis. 2016;7:e2208. doi: 10.1038/cddis.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217:e20200678. [DOI] [PMC free article] [PubMed]
- 303.Bettonville M, d'Aria S, Weatherly K, Porporato PE, Zhang J, Bousbata S, et al. Long-term antigen exposure irreversibly modifies metabolic requirements for T cell function. eLife. 2018;7:e30938. doi: 10.7554/eLife.30938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Deguit CDT, Hough M, Hoh R, Krone M, Pilcher CD, Martin JN, et al. Some aspects of CD8+ T-cell exhaustion are associated with altered T-cell mitochondrial features and ROS content in HIV infection. J Acquir Immune Defic Syndr. 2019;82:211–9. doi: 10.1097/QAI.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Morris G, Maes M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 2013;11:205. doi: 10.1186/1741-7015-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yu Y-R, Imrichova H, Wang H, Chao T, Xiao Z, Gao M, et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat Immunol. 2020;21:1540–51. doi: 10.1038/s41590-020-0793-3. [DOI] [PubMed] [Google Scholar]
- 307.Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85. doi: 10.1186/s12929-015-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2013;18:1497–534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Malmberg K-J, Arulampalam V, Ichihara F, Petersson M, Seki K, Andersson T, et al. Inhibition of activated/memory (CD45RO+) T cells by oxidative stress associated with block of NF-κB activation. J Immunol. 2001;167:2595–601. doi: 10.4049/jimmunol.167.5.2595. [DOI] [PubMed] [Google Scholar]
- 310.Lahdenpohja N, Savinainen K, Hurme M. Pre-exposure to oxidative stress decreases the nuclear factor-κB-dependent transcription in T lymphocytes. J Immunol. 1998;160:1354–8. [PubMed] [Google Scholar]
- 311.Krishna S, Xie D, Gorentla B, Shin J, Gao J, Zhong X-P. Chronic activation of the kinase IKKβ impairs T cell function and survival. J Immunol. 2012;189:1209–19. doi: 10.4049/jimmunol.1102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Cemerski S, Cantagrel A, Van Meerwijk JP, Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem. 2002;277:19585–93. doi: 10.1074/jbc.M111451200. [DOI] [PubMed] [Google Scholar]
- 313.Gringhuis SI, Papendrecht-van der Voort EAM, Leow A, Levarht EWN, Breedveld FC, Verweij CL. Effect of redox balance alterations on cellular localization of LAT and downstream T-cell receptor signaling pathways. Mol Cell Biol. 2002;22:400–11. doi: 10.1128/MCB.22.2.400-411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Morris G, Walder K, Puri BK, Berk M, Maes M. The deleterious effects of oxidative and nitrosative stress on palmitoylation, membrane lipid rafts and lipid-based cellular signalling: new drug targets in neuroimmune disorders. Mol Neurobiol. 2016;53:4638–58. doi: 10.1007/s12035-015-9392-y. [DOI] [PubMed] [Google Scholar]
- 315.Yarosz EL, Chang C-H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 2018;18:e14. doi: 10.4110/in.2018.18.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Morzadec C, Macoch M, Sparfel L, Kerdine-Römer S, Fardel O, Vernhet L. Nrf2 expression and activity in human T lymphocytes: stimulation by T cell receptor activation and priming by inorganic arsenic and tert-butylhydroquinone. Free Radic Biol Med. 2014;71:133–45. doi: 10.1016/j.freeradbiomed.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 318.Turley AE, Zagorski J, Rockwell CE. The role of Nrf2 in primary human CD4 T cell activation and differentiation. FASEB J. 2017;31(S1):lb621. [Google Scholar]
- 319.Noel S, Martina MN, Bandapalle S, Racusen LC, Potteti HR, Hamad ARA, et al. T lymphocyte–specific activation of Nrf2 protects from AKI. J Am Soc Nephrol. 2015;26:2989–3000. doi: 10.1681/ASN.2014100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Suzuki T, Murakami S, Biswal SS, Sakaguchi S, Harigae H, Yamamoto M, et al. Systemic activation of NRF2 alleviates lethal autoimmune inflammation in scurfy mice. Mol Cell Biol. 2017;37:e00063–17. doi: 10.1128/MCB.00063-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M, et al. The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol. 2014;50:1059–84. doi: 10.1007/s12035-014-8705-x. [DOI] [PubMed] [Google Scholar]
- 322.Mak TW, Grusdat M, Duncan GS, Dostert C, Nonnenmacher Y, Cox M, et al. Glutathione primes T cell metabolism for inflammation. Immunity. 2017;46:675–89. doi: 10.1016/j.immuni.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 323.Klein Geltink RI, O'Sullivan D, Pearce EL. Caught in the cROSsfire: GSH controls T cell metabolic reprogramming. Immunity. 2017;46:525–7. doi: 10.1016/j.immuni.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lian G, Gnanaprakasam JR, Wang T, Wu R, Chen X, Liu L, et al. Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. eLife. 2018;7:e36158. [DOI] [PMC free article] [PubMed]
- 325.Yan Z, Garg SK, Banerjee R. Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells. J Biol Chem. 2010;285:41525–32. doi: 10.1074/jbc.M110.189944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sofi MH, Wu Y, Schutt SD, Dai M, Daenthanasanmak A, Heinrichs Voss J, et al. Thioredoxin-1 confines T cell alloresponse and pathogenicity in graft-versus-host disease. J Clin Investig. 2019;129:2760–74. doi: 10.1172/JCI122899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Chakraborty P, Chatterjee S, Kesarwani P, Thyagarajan K, Iamsawat S, Dalheim A, et al. Thioredoxin-1 improves the immunometabolic phenotype of antitumor T cells. J Biol Chem. 2019;294:9198–212. doi: 10.1074/jbc.RA118.006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mougiakakos D, Johansson CC, Kiessling R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress–induced cell death. Blood. 2009;113:3542–5. doi: 10.1182/blood-2008-09-181040. [DOI] [PubMed] [Google Scholar]
- 329.Wakasugi N, Tagaya Y, Wakasugi H, Mitsui A, Maeda M, Yodoi J, et al. Adult T-cell leukemia-derived factor/thioredoxin, produced by both human T-lymphotropic virus type I- and Epstein-Barr virus-transformed lymphocytes, acts as an autocrine growth factor and synergizes with interleukin 1 and interleukin 2. Proc Natl Acad Sci USA. 1990;87:8282–6. doi: 10.1073/pnas.87.21.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Rosén A, Lundman P, Carlsson M, Bhavani K, Srinivasa BR, Kjellström G, et al. A CD4+ T cell line-secreted factor, growth promoting for normal and leukemic B cells, identified as thioredoxin. Int Immunol. 1995;7:625–33. doi: 10.1093/intimm/7.4.625. [DOI] [PubMed] [Google Scholar]
- 331.Fruman D, Limon J. Akt and mTOR in B cell activation and differentiation. Front Immunol. 2012;3:228. [DOI] [PMC free article] [PubMed]
- 332.Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol. 2014;192:3626–36. doi: 10.4049/jimmunol.1302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Doughty CA, Bleiman BF, Wagner DJ, Dufort FJ, Mataraza JM, Roberts MF, et al. Antigen receptor–mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–65. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Dufort FJ, Bleiman BF, Gumina MR, Blair D, Wagner DJ, Roberts MF, et al. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J Immunol. 2007;179:4953–7. doi: 10.4049/jimmunol.179.8.4953. [DOI] [PubMed] [Google Scholar]
- 335.Jellusova J. The role of metabolic checkpoint regulators in B cell survival and transformation. Immunol Rev. 2020;295:39–53. doi: 10.1111/imr.12855. [DOI] [PubMed] [Google Scholar]
- 336.Jellusova J, Cato MH, Apgar JR, Ramezani-Rad P, Leung CR, Chen C, et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol. 2017;18:303–12. doi: 10.1038/ni.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Blair D, Dufort FJ, Chiles TC. Protein kinase Cβ is critical for the metabolic switch to glycolysis following B-cell antigen receptor engagement. Biochemical J. 2012;448:165–9. doi: 10.1042/BJ20121225. [DOI] [PubMed] [Google Scholar]
- 338.Tsui C, Martinez-Martin N, Gaya M, Maldonado P, Llorian M, Legrave NM, et al. Protein kinase C-β dictates B cell fate by regulating mitochondrial remodeling, metabolic reprogramming, and heme biosynthesis. Immunity. 2018;48:1144–59.e5. doi: 10.1016/j.immuni.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Raybuck AL, Cho SH, Li J, Rogers MC, Lee K, Williams CL, et al. B cell-intrinsic mTORC1 promotes germinal center-defining transcription factor gene expression, somatic hypermutation, and memory B cell generation in humoral immunity. J Immunol. 2018;200:2627–39. doi: 10.4049/jimmunol.1701321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Gaudette BT, Jones DD, Bortnick A, Argon Y, Allman D. mTORC1 coordinates an immediate unfolded protein response-related transcriptome in activated B cells preceding antibody secretion. Nat Commun. 2020;11:723. doi: 10.1038/s41467-019-14032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Iwata TN, Ramírez-Komo JA, Park H, Iritani BM. Control of B lymphocyte development and functions by the mTOR signaling pathways. Cytokine Growth Factor Rev. 2017;35:47–62. doi: 10.1016/j.cytogfr.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Breda CNDS, Davanzo GG, Basso PJ, Saraiva Câmara NO, Moraes-Vieira PMM. Mitochondria as central hub of the immune system. Redox Biol. 2019;26:101255. doi: 10.1016/j.redox.2019.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ersching J, Efeyan A, Mesin L, Jacobsen JT, Pasqual G, Grabiner BC, et al. Germinal center selection and affinity maturation require dynamic regulation of mTORC1 kinase. Immunity. 2017;46:1045–58.e6. doi: 10.1016/j.immuni.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Jayachandran N, Mejia EM, Sheikholeslami K, Sher AA, Hou S, Hatch GM, et al. TAPP adaptors control B cell metabolism by modulating the phosphatidylinositol 3-kinase signaling pathway: a novel regulatory circuit preventing autoimmunity. J Immunol. 2018;201:406–16. doi: 10.4049/jimmunol.1701440. [DOI] [PubMed] [Google Scholar]
- 345.Xie J-H, Li Y-Y, Jin J. The essential functions of mitochondrial dynamics in immune cells. Cell Mol Immunol. 2020;17:712–21. doi: 10.1038/s41423-020-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Brookens SK, Cho SH, Basso PJ, Boothby MR. AMPKα1 in B cells dampens primary antibody responses yet promotes mitochondrial homeostasis and persistence of b cell memory. J Immunol. 2020;205:3011–22. [DOI] [PMC free article] [PubMed]
- 347.Brookens SK, Boothby MR. AMPK metabolism in the B lineage modulates humoral responses. Immunometabolism. 2021;3:e210011. doi: 10.20900/immunometab20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wheeler ML, DeFranco AL. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J Immunol. 2012;189:4405–16. doi: 10.4049/jimmunol.1201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Angajala A, Lim S, Phillips JB, Kim J-H, Yates C, You Z, et al. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front Immunol. 2018;9:1605. [DOI] [PMC free article] [PubMed]
- 350.Irish JM, Czerwinski DK, Nolan GP, Levy R. Kinetics of B cell receptor signaling in human B cell subsets mapped by phosphospecific flow cytometry. J Immunol. 2006;177:1581–9. doi: 10.4049/jimmunol.177.3.1581. [DOI] [PubMed] [Google Scholar]
- 351.Price MJ, Patterson DG, Scharer CD, Boss JM. Progressive upregulation of oxidative metabolism facilitates plasmablast differentiation to a T-independent antigen. Cell Rep. 2018;23:3152–9. doi: 10.1016/j.celrep.2018.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Vené R, Delfino L, Castellani P, Balza E, Bertolotti M, Sitia R, et al. Redox remodeling allows and controls B-cell activation and differentiation. Antioxid Redox Signal. 2010;13:1145–55. doi: 10.1089/ars.2009.3078. [DOI] [PubMed] [Google Scholar]
- 353.Jang K-J, Mano H, Aoki K, Hayashi T, Muto A, Nambu Y, et al. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nat Commun. 2015;6:6750. doi: 10.1038/ncomms7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ogura M, Inoue T, Yamaki J, Homma MK, Kurosaki T, Homma Y. Mitochondrial reactive oxygen species suppress humoral immune response through reduction of CD19 expression in B cells in mice. Eur J Immunol. 2017;47:406–18. doi: 10.1002/eji.201646342. [DOI] [PubMed] [Google Scholar]
- 355.Padilla ND, Ciurana C, van Oers J, Ogilvie AC, Hack CE. Levels of natural IgM antibodies against phosphorylcholine in healthy individuals and in patients undergoing isolated limb perfusion. J Immunol Methods. 2004;293:1–11. doi: 10.1016/j.jim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 356.Ajeganova S, Fiskesund R, de Faire U, Hafstrom I, Frostegard J. Effect of biological therapy on levels of atheroprotective antibodies against phosphorylcholine and apolipoproteins in rheumatoid arthritis – a one year study. Clin Exp Rheumatol. 2011;29:942–50. [PubMed] [Google Scholar]
- 357.Muri J, Thut H, Heer S, Krueger CC, Bornkamm GW, Bachmann MF, et al. The thioredoxin-1 and glutathione/glutaredoxin-1 systems redundantly fuel murine B-cell development and responses. Eur J Immunol. 2019;49:709–23. doi: 10.1002/eji.201848044. [DOI] [PubMed] [Google Scholar]
- 358.Lugade AA, Vethanayagam RR, Nasirikenari M, Bogner PN, Segal BH, Thanavala Y. Nrf2 regulates chronic lung inflammation and B-cell responses to nontypeable Haemophilus influenzae. Am J Respir Cell Mol Biol. 2011;45:557–65. doi: 10.1165/rcmb.2010-0321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Yi X, Zhao Y, Xue L, Zhang J, Qiao Y, Jin Q, et al. Expression of Keap1 and Nrf2 in diffuse large B-cell lymphoma and its clinical significance. Exp Ther Med. 2018;16:573–8. doi: 10.3892/etm.2018.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Jang JW, Lee JW, Yoon YD, Kang JS, Moon EY. Bisphenol A and its substitutes regulate human B cell survival via Nrf2 expression. Environ Pollut. 2020;259:113907. [DOI] [PubMed]
- 361.Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124. [DOI] [PMC free article] [PubMed]
- 362.Zwirner NW, Ziblat A. Regulation of NK cell activation and effector functions by the IL-12 family of cytokines: the case of IL-27. Front Immunol. 2017;8:25. [DOI] [PMC free article] [PubMed]
- 363.Kwon H-J, Choi G-E, Ryu S, Kwon SJ, Kim SC, Booth C, et al. Stepwise phosphorylation of p65 promotes NF-κB activation and NK cell responses during target cell recognition. Nat Commun. 2016;7:11686. [DOI] [PMC free article] [PubMed]
- 364.Rajasekaran K, Kumar P, Schuldt KM, Peterson EJ, Vanhaesebroeck B, Dixit V, et al. Signaling by Fyn-ADAP via the Carma1–Bcl-10–MAP3K7 signalosome exclusively regulates inflammatory cytokine production in NK cells. Nat Immunol. 2013;14:1127–36. doi: 10.1038/ni.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Rajasekaran K, Chu H, Kumar P, Xiao Y, Tinguely M, Samarakoon A, et al. Transforming growth factor-β-activated kinase 1 regulates natural killer cell-mediated cytotoxicity and cytokine production. J Biol Chem. 2011;286:31213–24. doi: 10.1074/jbc.M111.261917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Martínez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res. 2015;21:5047–56. doi: 10.1158/1078-0432.CCR-15-0685. [DOI] [PubMed] [Google Scholar]
- 367.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869. [DOI] [PMC free article] [PubMed]
- 368.Poznanski SM, Ashkar AA. What defines NK cell functional fate: phenotype or metabolism? Front Immunol. 2019;10:2278. [DOI] [PMC free article] [PubMed]
- 369.Terrén I, Orrantia A, Vitallé J, Astarloa-Pando G, Zenarruzabeitia O, Borrego F, Modulating NK. cell metabolism for cancer immunotherapy. Semin Hematol. 2020;57:213–24. doi: 10.1053/j.seminhematol.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 370.Assmann N, O'Brien KL, Donnelly RP, Dyck L, Zaiatz-Bittencourt V, Loftus RM, et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat Immunol. 2017;18:1197–206. doi: 10.1038/ni.3838. [DOI] [PubMed] [Google Scholar]
- 371.Keppel MP, Saucier N, Mah AY, Vogel TP, Cooper MA. Activation-specific metabolic requirements for NK cell IFN-γ production. J Immunol. 2015;194:1954–62. doi: 10.4049/jimmunol.1402099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Mao Y, van Hoef V, Zhang X, Wennerberg E, Lorent J, Witt K, et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood. 2016;128:1475–89. doi: 10.1182/blood-2016-02-698027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yang C, Malarkannan S. Transcriptional regulation of NK cell development by mTOR complexes. Front Cell Dev Biol. 2020;8:566090. [DOI] [PMC free article] [PubMed]
- 374.Donnelly RP, Loftus RM, Keating SE, Liou KT, Biron CA, Gardiner CM, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193:4477–84. doi: 10.4049/jimmunol.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Keating SE, Zaiatz-Bittencourt V, Loftus RM, Keane C, Brennan K, Finlay DK, et al. Metabolic reprogramming supports IFN-γ production by CD56bright NK cells. J Immunol. 2016;196:2552–60. doi: 10.4049/jimmunol.1501783. [DOI] [PubMed] [Google Scholar]
- 376.Loftus RM, Assmann N, Kedia-Mehta N, O’Brien KL, Garcia A, Gillespie C, et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat Commun. 2018;9:2341. [DOI] [PMC free article] [PubMed]
- 377.Cluff ER, Nolan J, Collins C, Varadaraj A, Rajasekaran N. Hypoxia-inducible factor-1α is upregulated in natural killer cells by interleukin-2 and hypoxia via PI3K/mTOR signaling pathway. J Immunol. 2019;202(1 Supplement):194. [Google Scholar]
- 378.Coulibaly A, Bettendorf A, Kostina E, Figueiredo AS, Velásquez SY, Bock H-G, et al. Interleukin-15 signaling in HIF-1α regulation in natural killer cells, insights through mathematical models. Front Immunol. 2019;10:2401. doi: 10.3389/fimmu.2019.02401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Domagala J, Lachota M, Klopotowska M, Graczyk-Jarzynka A, Domagala A, Zhylko A, et al. The tumor microenvironment-a metabolic obstacle to NK cells' activity. Cancers. 2020;12:3542. [DOI] [PMC free article] [PubMed]
- 380.Chambers AM, Matosevic S. Immunometabolic dysfunction of natural killer cells mediated by the hypoxia-CD73 axis in solid tumors. Front Mol Biosci. 2019;6:60. doi: 10.3389/fmolb.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Kotsafti A, Scarpa M, Castagliuolo I, Scarpa M. Reactive oxygen species and antitumor immunity-from surveillance to evasion. Cancers. 2020;12:1748. doi: 10.3390/cancers12071748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Lee S-H, Almutairi S, Ali AK. Reactive oxygen species modulate immune cell effector function. J Immunol. 2017;198(1 Supplement):222. [Google Scholar]
- 383.Boss AP, Freeborn RA, Duriancik DM, Kennedy RC, Gardner EM, Rockwell CE. The Nrf2 activator tBHQ inhibits the activation of primary murine natural killer cells. Food Chem Toxicol. 2018;121:231–6. doi: 10.1016/j.fct.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kumar P, Rajasekaran K, Nanbakhsh A, Gorski J, Thakar MS, Malarkannan S. IL-27 promotes NK cell effector functions via Maf-Nrf2 pathway during influenza infection. Sci Rep. 2019;9:4984. doi: 10.1038/s41598-019-41478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Millman AC, Salman M, Dayaram YK, Connell ND, Venketaraman V. Natural killer cells, glutathione, cytokines, and innate immunity against Mycobacterium tuberculosis. J Interferon Cytokine Res. 2008;28:153–65. doi: 10.1089/jir.2007.0095. [DOI] [PubMed] [Google Scholar]
- 386.Vojdani A, Mumper E, Granpeesheh D, Mielke L, Traver D, Bock K, et al. Low natural killer cell cytotoxic activity in autism: the role of glutathione, IL-2 and IL-15. J Neuroimmunol. 2008;205:148–54. doi: 10.1016/j.jneuroim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 387.Morris G, Anderson G, Galecki P, Berk M, Maes M. A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med. 2013;11:64. doi: 10.1186/1741-7015-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Mimura K, Kua LF, Shimasaki N, Shiraishi K, Nakajima S, Siang LK, et al. Upregulation of thioredoxin-1 in activated human NK cells confers increased tolerance to oxidative stress. Cancer Immunol Immunother. 2017;66:605–13. doi: 10.1007/s00262-017-1969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Yang Y, Neo SY, Chen Z, Cui W, Chen Y, Guo M, et al. Thioredoxin activity confers resistance against oxidative stress in tumor-infiltrating NK cells. J Clin Investig. 2020;130:5508–22. doi: 10.1172/JCI137585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Aydin E, Johansson J, Nazir FH, Hellstrand K, Martner A. Role of NOX2-derived reactive oxygen species in NK cell–mediated control of murine melanoma metastasis. Cancer Immunol Res. 2017;5:804–11. doi: 10.1158/2326-6066.CIR-16-0382. [DOI] [PubMed] [Google Scholar]
- 391.Thorén FB, Betten Å, Romero AI, Hellstrand K. Cutting edge: antioxidative properties of myeloid dendritic cells: protection of T cells and NK cells from oxygen radical-induced inactivation and apoptosis. J Immunol. 2007;179:21–5. doi: 10.4049/jimmunol.179.1.21. [DOI] [PubMed] [Google Scholar]
- 392.Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, et al. Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor–mediated natural killer cell function. Clin Cancer Res. 2018;24:1891–904. doi: 10.1158/1078-0432.CCR-17-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Morris G, Berk M, Galecki P, Maes M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs) Mol Neurobiol. 2014;49:741–56. doi: 10.1007/s12035-013-8553-0. [DOI] [PubMed] [Google Scholar]
- 394.Morris G, Puri BK, Olive L, Carvalho AF, Berk M, Maes M. Emerging role of innate B1 cells in the pathophysiology of autoimmune and neuroimmune diseases: association with inflammation, oxidative and nitrosative stress and autoimmune responses. Pharmacol Res. 2019;148:104408. doi: 10.1016/j.phrs.2019.104408. [DOI] [PubMed] [Google Scholar]
- 395.Weismann D, Binder CJ. The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta. 2012;1818:2465–75. doi: 10.1016/j.bbamem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Rhoads JP, Major AS. How oxidized low-density lipoprotein activates inflammatory responses. Crit Rev Immunol. 2018;38:333–42. doi: 10.1615/CritRevImmunol.2018026483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Gounopoulos P, Merki E, Hansen LF, Choi SH, Tsimikas S. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 2007;55:821–37. [PubMed] [Google Scholar]
- 398.Summerhill VI, Grechko AV, Yet SF, Sobenin IA, Orekhov AN. The atherogenic role of circulating modified lipids in atherosclerosis. Int J Mol Sci. 2019;20:3561. [DOI] [PMC free article] [PubMed]
- 399.Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res. 2006;47:1975–83. doi: 10.1194/jlr.M600064-JLR200. [DOI] [PubMed] [Google Scholar]
- 400.Al Gadban MM, Smith KJ, Soodavar F, Piansay C, Chassereau C, Twal WO, et al. Differential trafficking of oxidized LDL and oxidized LDL immune complexes in macrophages: impact on oxidative stress. PLoS One. 2010;5:e12534. [DOI] [PMC free article] [PubMed]
- 401.Lopes-Virella MF, Virella G. Pathogenic role of modified LDL antibodies and immune complexes in atherosclerosis. J Atheroscler Thromb. 2013;20:743–54. doi: 10.5551/jat.19281. [DOI] [PubMed] [Google Scholar]
- 402.Huang YH, Ronnelid J, Frostegard J. Oxidized LDL induces enhanced antibody formation and MHC class II-dependent IFN-gamma production in lymphocytes from healthy individuals. Arterioscler Thromb Vasc Biol. 1995;15:1577–83. doi: 10.1161/01.ATV.15.10.1577. [DOI] [PubMed] [Google Scholar]
- 403.Perrin-Cocon L, Coutant F, Agaugue S, Deforges S, Andre P, Lotteau V. Oxidized low-density lipoprotein promotes mature dendritic cell transition from differentiating monocyte. J Immunol. 2001;167:3785–91. doi: 10.4049/jimmunol.167.7.3785. [DOI] [PubMed] [Google Scholar]
- 404.Rhoads JP, Lukens JR, Wilhelm AJ, Moore JL, Mendez-Fernandez Y, Kanneganti T-D, et al. Oxidized low-density lipoprotein immune complex priming of the nlrp3 inflammasome involves TLR and FcγR cooperation and is dependent on CARD9. J Immunol. 2017;198:2105–14. [DOI] [PMC free article] [PubMed]
- 405.Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thrombo Vasc Biol. 2015;35:280–7. doi: 10.1161/ATVBAHA.114.303568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Ou HX, Guo BB, Liu Q, Li YK, Yang Z, Feng WJ, et al. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol Sin. 2018;39:1249–58. doi: 10.1038/aps.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Obama T, Ohinata H, Takaki T, Iwamoto S, Sawada N, Aiuchi T, et al. Cooperative action of oxidized low-density lipoproteins and neutrophils on endothelial inflammatory responses through neutrophil extracellular trap formation. Front Immunol. 2019;10:1899. doi: 10.3389/fimmu.2019.01899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Awasthi D, Nagarkoti S, Kumar A, Dubey M, Singh AK, Pathak P, et al. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic Biol Med. 2016;93:190–203. doi: 10.1016/j.freeradbiomed.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 409.Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18:553–66. doi: 10.1038/s41573-019-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Schnack L, Sohrabi Y, Lagache SMM, Kahles F, Bruemmer D, Waltenberger J, et al. Mechanisms of trained innate immunity in oxLDL primed human coronary smooth muscle cells. Front Immunol. 2019;10:13. doi: 10.3389/fimmu.2019.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Sohrabi Y, Lagache SMM, Schnack L, Godfrey R, Kahles F, Bruemmer D, et al. mTOR-dependent oxidative stress regulates oxLDL-induced trained innate immunity in human monocytes. Front Immunol. 2018;9:3155. doi: 10.3389/fimmu.2018.03155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Ganss R. Keeping the balance right: regulator of G protein signaling 5 in vascular physiology and pathology. Prog Mol Biol Transl Sci. 2015;133:93–121. doi: 10.1016/bs.pmbts.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 413.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–9. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.van Bergen LA, Roos G, De Proft F. From thiol to sulfonic acid: modeling the oxidation pathway of protein thiols by hydrogen peroxide. J Phys Chem A. 2014;118:6078–84. doi: 10.1021/jp5018339. [DOI] [PubMed] [Google Scholar]
- 415.Poole LB. The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med. 2015;80:148–57. doi: 10.1016/j.freeradbiomed.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Bretón-Romero R, Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–34. doi: 10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Chauvin JR, Pratt DA. On the reactions of thiols, sulfenic acids, and sulfinic acids with hydrogen peroxide. Angew Chem. 2017;56:6255–9. doi: 10.1002/anie.201610402. [DOI] [PubMed] [Google Scholar]
- 418.Nakamura T, Lipton SA. Protein S-nitrosylation as a therapeutic target for neurodegenerative diseases. Trends Pharmacol Sci. 2016;37:73–84. doi: 10.1016/j.tips.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Morris G, Carvalho AF, Anderson G, Galecki P, Maes M. The many neuroprogressive actions of tryptophan catabolites (TRYCATs) that may be associated with the pathophysiology of neuro-immune disorders. Curr Pharm Des. 2016;22:963–77. doi: 10.2174/1381612822666151215102420. [DOI] [PubMed] [Google Scholar]
- 420.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282:30667–72. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 421.Marshall HE, Hess DT, Stamler JS. S-nitrosylation: physiological regulation of NF-kappaB. Proc Natl Acad Sci USA. 2004;101:8841–2. doi: 10.1073/pnas.0403034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, et al. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci USA. 2004;101:8945–50. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–93. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 424.Kelleher ZT, Sha Y, Foster MW, Foster WM, Forrester MT, Marshall HE. Thioredoxin-mediated denitrosylation regulates cytokine-induced NF-κB activation. J Biol Chem. 2014;289:3066–72. [DOI] [PMC free article] [PubMed]
- 425.Xiong H, Zhu C, Li F, Hegazi R, He K, Babyatsky M, et al. Inhibition of interleukin-12 p40 transcription and NF-kappaB activation by nitric oxide in murine macrophages and dendritic cells. J Biol Chem. 2004;279:10776–83. doi: 10.1074/jbc.M313416200. [DOI] [PubMed] [Google Scholar]
- 426.Schroeder RA, Cai C, Kuo PC. Endotoxin-mediated nitric oxide synthesis inhibits IL-1β gene transcription in ANA-1 murine macrophages. Am J Physiol. 1999;277:C523–30. doi: 10.1152/ajpcell.1999.277.3.C523. [DOI] [PubMed] [Google Scholar]
- 427.Yu Z, Kuncewicz T, Dubinsky WP, Kone BC. Nitric oxide-dependent negative feedback of PARP-1 trans-activation of the inducible nitric-oxide synthase gene. J Biol Chem. 2006;281:9101–9. doi: 10.1074/jbc.M511049200. [DOI] [PubMed] [Google Scholar]
- 428.Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, Ayasolla K, et al. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 2005;25:177–92. doi: 10.1038/sj.jcbfm.9600012. [DOI] [PubMed] [Google Scholar]
- 429.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circulation Res. 2007;100:1589–96. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 431.Zheng Z-K, Wang J-J, Hu H, Jiang K, Nie J, Zhang J, et al. Short-term inhalation of nitric oxide inhibits activations of toll-like receptor 2 and 4 in the lung after ischemia-reperfusion injury in mice. J Huazhong Univ Sci Technolog Med Sci. 2013;33:219–23. doi: 10.1007/s11596-013-1100-4. [DOI] [PubMed] [Google Scholar]
- 432.Park HS, Huh SH, Kim MS, Lee SH, Choi EJ. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc Natl Acad Sci USA. 2000;97:14382–7. doi: 10.1073/pnas.97.26.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Lander HM, Ogiste JS, Pearce SF, Levi R, Novogrodsky A. Nitric oxide-stimulated guanine nucleotide exchange on p21ras. J Biol Chem. 1995;270:7017–20. doi: 10.1074/jbc.270.13.7017. [DOI] [PubMed] [Google Scholar]
- 434.Nikitovic D, Holmgren A, Spyrou G. Inhibition of AP-1 DNA binding by nitric oxide involving conserved cysteine residues in Jun and Fos. Biochem Biophys Res Commun. 1998;242:109–12. doi: 10.1006/bbrc.1997.7930. [DOI] [PubMed] [Google Scholar]
- 435.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Um HC, Jang JH, Kim DH, Lee C, Surh YJ. Nitric oxide activates Nrf2 through S-nitrosylation of Keap1 in PC12 cells. Nitric Oxide. 2011;25:161–8. doi: 10.1016/j.niox.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 437.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem. 2010;285:8463–71. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Nakamura M, Yamanaka H, Oguro A, Imaoka S. Bisphenol A induces Nrf2-dependent drug-metabolizing enzymes through nitrosylation of Keap1. Drug Metab Pharmacokinet. 2018;33:194–202. [DOI] [PubMed]
- 439.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, et al. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–8. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 441.Yasinska IM, Sumbayev VV. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett. 2003;549:105–9. doi: 10.1016/S0014-5793(03)00807-X. [DOI] [PubMed] [Google Scholar]
- 442.Numajiri N, Takasawa K, Nishiya T, Tanaka H, Ohno K, Hayakawa W, et al. On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN) Proc Natl Acad Sci USA. 2011;108:10349–54. doi: 10.1073/pnas.1103503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Kwak YD, Ma T, Diao S, Zhang X, Chen Y, Hsu J, et al. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegener. 2010;5:49. doi: 10.1186/1750-1326-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Gupta A, Anjomani-Virmouni S, Koundouros N, Dimitriadi M, Choo-Wing R, Valle A, et al. PARK2 depletion connects energy and oxidative stress to PI3K/Akt activation via PTEN S-nitrosylation. Mol Cell. 2017;65:999–1013.e7. doi: 10.1016/j.molcel.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Lopez-Rivera E, Jayaraman P, Parikh F, Davies MA, Ekmekcioglu S, Izadmehr S, et al. Inducible nitric oxide synthase drives mTOR pathway activation and proliferation of human melanoma by reversible nitrosylation of TSC2. Cancer Res. 2014;74:1067–78. doi: 10.1158/0008-5472.CAN-13-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Lee M, Choy JC. Positive feedback regulation of human inducible nitric oxide synthase expression by Ras S-nitrosylation. J Biol Chem. 2013;288:15677–86. [DOI] [PMC free article] [PubMed]
- 447.Park DW, Jiang S, Liu Y, Siegal GP, Inoki K, Abraham E, et al. GSK3beta-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L735–45. doi: 10.1152/ajplung.00165.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Suzuki T, Bridges D, Nakada D, Skiniotis G, Morrison SJ, Lin JD, et al. Inhibition of AMPK catabolic action by GSK3. Mol Cell. 2013;50:407–19. doi: 10.1016/j.molcel.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Oka S-i, Hirata T, Suzuki W, Naito D, Chen Y, Chin A, et al. Thioredoxin-1 maintains mechanistic target of rapamycin (mTOR) function during oxidative stress in cardiomyocytes. J Biol Chem. 2017;292:18988–9000. doi: 10.1074/jbc.M117.807735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Yoshida S, Hong S, Suzuki T, Nada S, Mannan AM, Wang J, et al. Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J Biol Chem. 2011;286:32651–60. doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, et al. A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev. 2017;74(Pt A):1–20. doi: 10.1016/j.neubiorev.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 452.Trümper V, Wittig I, Heidler J, Richter F, Brüne B, von Knethen A. Redox regulation of PPARγ in polarized macrophages. PPAR Res. 2020;2020:8253831. doi: 10.1155/2020/8253831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kim T, Yang Q. Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J Cardiol. 2013;5:164–74. doi: 10.4330/wjc.v5.i6.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Kakkar P, Singh BK. Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem. 2007;305:235–53. doi: 10.1007/s11010-007-9520-8. [DOI] [PubMed] [Google Scholar]
- 455.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Lucas K, Morris G, Anderson G, Maes M. The toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS Neurol Disord Drug Targets. 2015;14:838–54. doi: 10.2174/1871527314666150317224645. [DOI] [PubMed] [Google Scholar]
- 457.Pope S, Land JM, Heales SJ. Oxidative stress and mitochondrial dysfunction in neurodegeneration; cardiolipin a critical target? Biochim Biophys Acta. 2008;1777:794–9. doi: 10.1016/j.bbabio.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 458.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Mitochondrial dysfunction in brain aging: role of oxidative stress and cardiolipin. Neurochemistry Int. 2011;58:447–57. doi: 10.1016/j.neuint.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 459.Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res. 2018;341:154–75. doi: 10.1016/j.bbr.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 460.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 461.Litvinova L, Atochin DN, Fattakhov N, Vasilenko M, Zatolokin P, Kirienkova E. Nitric oxide and mitochondria in metabolic syndrome. Front Physiol. 2015;6:20. [DOI] [PMC free article] [PubMed]
- 462.Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci. 2005;26:190–5. doi: 10.1016/j.tips.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 463.Mailloux RJ. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid Med Cell Longev. 2018;2018:7857251. doi: 10.1155/2018/7857251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–33. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 465.Morris G, Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015;13:68. doi: 10.1186/s12916-015-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Wu YT, Wu SB, Lee WY, Wei YH. Mitochondrial respiratory dysfunction-elicited oxidative stress and posttranslational protein modification in mitochondrial diseases. Ann N Y Acad Sci. 2010;1201:147–56. doi: 10.1111/j.1749-6632.2010.05631.x. [DOI] [PubMed] [Google Scholar]
- 467.Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med. 2013;238:450–60. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]
- 468.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–70. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy: the yin and yang of cell death control. Circulation Res. 2012;111:1208–21. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Ronchi JA, Francisco A, Passos LA, Figueira TR, Castilho RF. The contribution of nicotinamide nucleotide transhydrogenase to peroxide detoxification is dependent on the respiratory state and counterbalanced by other sources of NADPH in liver mitochondria. J Biol Chem. 2016;291:20173–87. doi: 10.1074/jbc.M116.730473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Lopert P, Patel M. Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system. J Biol Chem. 2014;289:15611–20. doi: 10.1074/jbc.M113.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Santos LRB, Muller C, de Souza AH, Takahashi HK, Spégel P, Sweet IR, et al. NNT reverse mode of operation mediates glucose control of mitochondrial NADPH and glutathione redox state in mouse pancreatic β-cells. Mol Metab. 2017;6:535–47. doi: 10.1016/j.molmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Montano SJ, Lu J, Gustafsson TN, Holmgren A. Activity assays of mammalian thioredoxin and thioredoxin reductase: fluorescent disulfide substrates, mechanisms, and use with tissue samples. Anal Biochem. 2014;449:139–46. doi: 10.1016/j.ab.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 474.Cheng Q, Antholine WE, Myers JM, Kalyanaraman B, Arner ES, Myers CR. The selenium-independent inherent pro-oxidant NADPH oxidase activity of mammalian thioredoxin reductase and its selenium-dependent direct peroxidase activities. J Biol Chem. 2010;285:21708–23. doi: 10.1074/jbc.M110.117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Berkholz DS, Faber HR, Savvides SN, Karplus PA. Catalytic cycle of human glutathione reductase near 1 A resolution. J Mol Biol. 2008;382:371–84. doi: 10.1016/j.jmb.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Kamerbeek NM, van Zwieten R, de Boer M, Morren G, Vuil H, Bannink N, et al. Molecular basis of glutathione reductase deficiency in human blood cells. Blood. 2007;109:3560–6. doi: 10.1182/blood-2006-08-042531. [DOI] [PubMed] [Google Scholar]
- 477.Leung JH, Schurig-Briccio LA, Yamaguchi M, Moeller A, Speir JA, Gennis RB, et al. Structural biology. Division of labor in transhydrogenase by alternating proton translocation and hydride transfer. Science. 2015;347:178–81. doi: 10.1126/science.1260451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Padayatti PS, Leung JH, Mahinthichaichan P, Tajkhorshid E, Ishchenko A, Cherezov V, et al. Critical role of water molecules in proton translocation by the membrane-bound transhydrogenase. Structure. 2017;25:1111–9.e3. doi: 10.1016/j.str.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Hoxhaj G, Ben-Sahra I, Lockwood SE, Timson RC, Byles V, Henning GT, et al. Direct stimulation of NADP+ synthesis through Akt-mediated phosphorylation of NAD kinase. Science. 2019;363:1088–92. doi: 10.1126/science.aau3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Ohashi K, Kawai S, Koshimizu M, Murata K. NADPH regulates human NAD kinase, a NADP(+)-biosynthetic enzyme. Mol Cell Biochem. 2011;355:57–64. doi: 10.1007/s11010-011-0838-x. [DOI] [PubMed] [Google Scholar]
- 481.Ho H-Y, Lin Y-T, Lin G, Wu P-R, Cheng M-L. Nicotinamide nucleotide transhydrogenase (NNT) deficiency dysregulates mitochondrial retrograde signaling and impedes proliferation. Redox Biol. 2017;12:916–28. doi: 10.1016/j.redox.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Zhang LQ, Van Haandel L, Xiong M, Huang P, Heruth DP, Bi C, et al. Metabolic and molecular insights into an essential role of nicotinamide phosphoribosyltransferase. Cell Death Dis. 2017;8:e2705. doi: 10.1038/cddis.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Pittelli M, Felici R, Pitozzi V, Giovannelli L, Bigagli E, Cialdai F, et al. Pharmacological effects of exogenous NAD on mitochondrial bioenergetics, DNA repair, and apoptosis. Mol Pharmacol. 2011;80:1136–46. doi: 10.1124/mol.111.073916. [DOI] [PubMed] [Google Scholar]
- 484.Burgos ES. NAMPT in regulated NAD biosynthesis and its pivotal role in human metabolism. Curr Med Chem. 2011;18:1947–61. doi: 10.2174/092986711795590101. [DOI] [PubMed] [Google Scholar]
- 485.Jayaram HN, Kusumanchi P, Yalowitz JA. NMNAT expression and its relation to NAD metabolism. Curr Med Chem. 2011;18:1962–72. doi: 10.2174/092986711795590138. [DOI] [PubMed] [Google Scholar]
- 486.Bradshaw PC. Cytoplasmic and mitochondrial NADPH-coupled redox systems in the regulation of aging. Nutrients. 2019;11:504. doi: 10.3390/nu11030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell. 2014;55:253–63. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Hsieh J-Y, Shih W-T, Kuo Y-H, Liu G-Y, Hung H-C. Functional roles of metabolic intermediates in regulating the human mitochondrial NAD(P)+-dependent malic enzyme. Sci Rep. 2019;9:9081. doi: 10.1038/s41598-019-45282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Yamada S, Kotake Y, Demizu Y, Kurihara M, Sekino Y, Kanda Y. NAD-dependent isocitrate dehydrogenase as a novel target of tributyltin in human embryonic carcinoma cells. Sci Rep. 2014;4:5952. doi: 10.1038/srep05952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Zeng C, Aleshin AE, Hardie JB, Harrison RW, Fromm HJ. ATP-binding site of human brain hexokinase as studied by molecular modeling and site-directed mutagenesis. Biochemistry. 1996;35:13157–64. doi: 10.1021/bi960750e. [DOI] [PubMed] [Google Scholar]
- 491.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–57. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 492.John S, Weiss JN, Ribalet B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS One. 2011;6:e17674. doi: 10.1371/journal.pone.0017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Rassaf T, Luedike P. Between nitros(yl)ation and nitration: regulation of thioredoxin-1 in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;49:343–6. doi: 10.1016/j.yjmcc.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 494.Yin T, Hou R, Liu S, Lau WB, Wang H, Tao L. Nitrative inactivation of thioredoxin-1 increases vulnerability of diabetic hearts to ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;49:354–61. doi: 10.1016/j.yjmcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 495.Wang YT, Piyankarage SC, Williams DL, Thatcher GR. Proteomic profiling of nitrosative stress: protein S-oxidation accompanies S-nitrosylation. ACS Chem Biol. 2014;9:821–30. doi: 10.1021/cb400547u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Hashemy SI, Holmgren A. Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J Biol Chem. 2008;283:21890–8. doi: 10.1074/jbc.M801047200. [DOI] [PubMed] [Google Scholar]
- 497.Cruz-Tapias P, Agmon-Levin N, Israeli E, Anaya JM, Shoenfeld Y. Autoimmune (auto-inflammatory) syndrome induced by adjuvants (ASIA)-animal models as a proof of concept. Curr medicinal Chem. 2013;20:4030–6. doi: 10.2174/09298673113209990253. [DOI] [PubMed] [Google Scholar]
- 498.Garcia-Nogales P, Almeida A, Bolanos JP. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J Biol Chem. 2003;278:864–74. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- 499.Savvides SN, Scheiwein M, Bohme CC, Arteel GE, Karplus PA, Becker K, et al. Crystal structure of the antioxidant enzyme glutathione reductase inactivated by peroxynitrite. J Biol Chem. 2002;277:2779–84. doi: 10.1074/jbc.M108190200. [DOI] [PubMed] [Google Scholar]
- 500.Circu ML, Stringer S, Rhoads CA, Moyer MP, Aw TY. The role of GSH efflux in staurosporine-induced apoptosis in colonic epithelial cells. Biochem Pharmacol. 2009;77:76–85. doi: 10.1016/j.bcp.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Franco R, Cidlowski JA. Glutathione efflux and cell death. Antioxid Redox Signal. 2012;17:1694–713. doi: 10.1089/ars.2012.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Hammond CL, Madejczyk MS, Ballatori N. Activation of plasma membrane reduced glutathione transport in death receptor apoptosis of HepG2 cells. Toxicol Appl Pharmacol. 2004;195:12–22. doi: 10.1016/j.taap.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 503.Sodani K, Patel A, Kathawala RJ, Chen Z-S. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31:58–72. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Liu Q, Gao Y, Ci X. Role of Nrf2 and its activators in respiratory diseases. Oxid Med Cell Longev. 2019;2019:17. doi: 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Cebula M, Schmidt EE, Arner ES. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid Redox Signal. 2015;23:823–53. doi: 10.1089/ars.2015.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Schmidt EE. Interplay between cytosolic disulfide reductase systems and the Nrf2/Keap1 pathway. Biochem Soc Trans. 2015;43:632–8. doi: 10.1042/BST20150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: a key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000;20:8972–9. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Hiller S, DeKroon R, Hamlett ED, Xu L, Osorio C, Robinette J, et al. Alpha-lipoic acid supplementation protects enzymes from damage by nitrosative and oxidative stress. Biochim Biophys Acta. 2016;1860(1 Pt A):36–45. doi: 10.1016/j.bbagen.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Adam-Vizi V, Tretter L. The role of mitochondrial dehydrogenases in the generation of oxidative stress. Neurochem Int. 2013;62:757–63. doi: 10.1016/j.neuint.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 510.Tortora V, Quijano C, Freeman B, Radi R, Castro L. Mitochondrial aconitase reaction with nitric oxide, S-nitrosoglutathione, and peroxynitrite: mechanisms and relative contributions to aconitase inactivation. Free Radic Biol Med. 2007;42:1075–88. doi: 10.1016/j.freeradbiomed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 511.Han D, Canali R, Garcia J, Aguilera R, Gallaher TK, Cadenas E. Sites and mechanisms of aconitase inactivation by peroxynitrite: modulation by citrate and glutathione. Biochemistry. 2005;44:11986–96. doi: 10.1021/bi0509393. [DOI] [PubMed] [Google Scholar]
- 512.Yang ES, Richter C, Chun JS, Huh TL, Kang SS, Park JW. Inactivation of NADP(+)-dependent isocitrate dehydrogenase by nitric oxide. Free Radic Biol Med. 2002;33:927–37. doi: 10.1016/S0891-5849(02)00981-4. [DOI] [PubMed] [Google Scholar]
- 513.Lee JH, Yang ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. Implications for cytotoxicity and alcohol-induced liver injury. J Biol Chem. 2003;278:51360–71. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 514.Kil IS, Park JW. Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J Biol Chem. 2005;280:10846–54. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 515.Jiang P, Du W, Mancuso A, Wellen KE, Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature. 2013;493:689–93. doi: 10.1038/nature11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Chang Y-L, Gao H-W, Chiang C-P, Wang W-M, Huang S-M, Ku C-F, et al. Human mitochondrial NAD(P)+–dependent malic enzyme participates in cutaneous melanoma progression and invasion. J Investig Dermatol. 2015;135:807–15. doi: 10.1038/jid.2014.385. [DOI] [PubMed] [Google Scholar]
- 517.Hurd TR, Collins Y, Abakumova I, Chouchani ET, Baranowski B, Fearnley IM, et al. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. J Biol Chem. 2012;287:35153–60. doi: 10.1074/jbc.M112.400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D. Alpha-ketoglutarate: physiological functions and applications. Biomol Ther (Seoul) 2016;24:1–8. doi: 10.4062/biomolther.2015.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond Ser B Biol Sci. 2005;360:2335–45. doi: 10.1098/rstb.2005.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Feingold KR, Grunfeld C. Effect of inflammation on HDL structure and function. Curr Opin Lipidol. 2016;27:521–30. doi: 10.1097/MOL.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 521.Kim SY, Yu M, Morin EE, Kang J, Kaplan MJ, Schwendeman A. High-density lipoprotein in lupus: disease biomarkers and potential therapeutic strategy. Arthritis Rheumatol. 2020;72:20–30. doi: 10.1002/art.41059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Guirgis FW, Dodani S, Leeuwenburgh C, Moldawer L, Bowman J, Kalynych C, et al. HDL inflammatory index correlates with and predicts severity of organ failure in patients with sepsis and septic shock. PLoS One. 2018;13:e0203813. doi: 10.1371/journal.pone.0203813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Smith JD. Myeloperoxidase, inflammation, and dysfunctional high-density lipoprotein. J Clin Lipido. 2010;4:382–8. doi: 10.1016/j.jacl.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009;284:30825–35. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, et al. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–13. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- 526.Kumon Y, Suehiro T, Ikeda Y, Hashimoto K. Human paraoxonase-1 gene expression by HepG2 cells is downregulated by interleukin-1beta and tumor necrosis factor-alpha, but is upregulated by interleukin-6. Life Sci. 2003;73:2807–15. doi: 10.1016/S0024-3205(03)00704-5. [DOI] [PubMed] [Google Scholar]
- 527.Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157–62. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Gkolfinopoulou C, Stratikos E, Theofilatos D, Kardassis D, Voulgari PV, Drosos AA, et al. Impaired antiatherogenic functions of high-density lipoprotein in patients with ankylosing spondylitis. J Rheumatol. 2015;42:1652–60. doi: 10.3899/jrheum.141532. [DOI] [PubMed] [Google Scholar]
- 529.Liao KP, Playford MP, Frits M, Coblyn JS, Iannaccone C, Weinblatt ME, et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc. 2015;4:e001588. doi: 10.1161/JAHA.114.001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Jakob P, Luscher TF. Dysfunctional HDL and inflammation: a noxious liaison in adolescents with type 1 diabetes. Eur Heart J. 2019;40:3567–70. doi: 10.1093/eurheartj/ehz502. [DOI] [PubMed] [Google Scholar]
- 531.de la Llera Moya M, McGillicuddy FC, Hinkle CC, Byrne M, Joshi MR, Nguyen V, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222:390–4. doi: 10.1016/j.atherosclerosis.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Farid AS, Horii Y. Modulation of paraoxonases during infectious diseases and its potential impact on atherosclerosis. Lipids Health Dis. 2012;11:92. doi: 10.1186/1476-511X-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: relevance to cancer-related fatigue. Cancer. 2015;121:2129–36. doi: 10.1002/cncr.29302. [DOI] [PubMed] [Google Scholar]
- 534.Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-α-induced depression. J Psychiatry Neurosci. 2004;29:11–7. [PMC free article] [PubMed] [Google Scholar]
- 535.Wirthgen E, Hoeflich A. Endotoxin-induced tryptophan degradation along the kynurenine pathway: the role of indolamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immunosuppressive effects in endotoxin tolerance and cancer and its implications for immunoparalysis. J Amino Acids. 2015;2015:973548. doi: 10.1155/2015/973548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–90. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011;8:388–403. doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Testa U, Pelosi E, Castelli G, Labbaye C. miR-146 and miR-155: two key modulators of immune response and tumor development. Noncoding RNA. 2017;3:22. [DOI] [PMC free article] [PubMed]
- 539.Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 540.Ogawa H, Rafiee P, Heidemann J, Fisher PJ, Johnson NA, Otterson MF, et al. Mechanisms of endotoxin tolerance in human intestinal microvascular endothelial cells. J Immunol. 2003;170:5956–64. doi: 10.4049/jimmunol.170.12.5956. [DOI] [PubMed] [Google Scholar]
- 541.Parker LC, Jones EC, Prince LR, Dower SK, Whyte MK, Sabroe I. Endotoxin tolerance induces selective alterations in neutrophil function. J Leukoc Biol. 2005;78:1301–5. doi: 10.1189/jlb.0405236. [DOI] [PubMed] [Google Scholar]
- 542.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–87. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 543.Ishiyama K, Ohdan H, Tokita D, Shishida M, Tanaka Y, Irei T, et al. Induction of endotoxin tolerance inhibits alloimmune responses. Transpl Immunol. 2006;16:158–65. doi: 10.1016/j.trim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 544.Lauw FN, ten Hove T, Dekkers PE, de Jonge E, van Deventer SJ, van Der Poll T. Reduced Th1, but not Th2, cytokine production by lymphocytes after in vivo exposure of healthy subjects to endotoxin. Infect Immun. 2000;68:1014–8. doi: 10.1128/IAI.68.3.1014-1018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Domogalla MP, Rostan PV, Raker VK, Steinbrink K. Tolerance through education: how tolerogenic dendritic cells shape immunity. Front Immunol. 2017;8:1764. doi: 10.3389/fimmu.2017.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Raker VK, Domogalla MP, Steinbrink K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front Immunol. 2015;6:569. doi: 10.3389/fimmu.2015.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Cabrera-Perez J, Condotta SA, Badovinac VP, Griffith TS. Impact of sepsis on CD4 T cell immunity. J Leukoc Biol. 2014;96:767–77. doi: 10.1189/jlb.5MR0114-067R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Strother RK, Danahy DB, Kotov DI, Kucaba TA, Zacharias ZR, Griffith TS, et al. Polymicrobial sepsis diminishes dendritic cell numbers and function directly contributing to impaired primary CD8 T cell responses in vivo. J Immunol. 2016;197:4301–11. doi: 10.4049/jimmunol.1601463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Cao C, Ma T, Chai Y-F, Shou S-T. The role of regulatory T cells in immune dysfunction during sepsis. World J Emerg Med. 2015;6:5–9. doi: 10.5847/wjem.j.1920-8642.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Chiche L, Forel JM, Thomas G, Farnarier C, Vely F, Blery M, et al. The role of natural killer cells in sepsis. J Biomed Biotechnol. 2011;2011:986491. doi: 10.1155/2011/986491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Pan A, Deng Y, Yang T, Zhang L, Shao M, Zhou S, et al. [Phenotype and functions of natural killer cells in septic patients and its clinical significance] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:827–31. doi: 10.3760/cma.j.issn.2095-4352.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 552.Forel JM, Chiche L, Thomas G, Mancini J, Farnarier C, Cognet C, et al. Phenotype and functions of natural killer cells in critically-ill septic patients. PLoS One. 2012;7:e50446. doi: 10.1371/journal.pone.0050446. [DOI] [PMC free article] [PubMed] [Google Scholar]