Abstract
The cmpABCD operon of Synechococcus sp. strain PCC 7942, encoding a high-affinity bicarbonate transporter, is transcribed only under CO2-limited conditions. In Synechocystis sp. strain PCC 6803, the slr0040, slr0041, slr0043, and slr0044 genes, forming an operon with a putative porin gene (slr0042), were identified as the cmpA, cmpB, cmpC, and cmpD genes, respectively, on the basis of their strong similarities to the corresponding Synechococcus cmp genes and their induction under low CO2 conditions. Immediately upstream of and transcribed divergently from the Synechocystis cmp operon is a gene (sll0030) encoding a homolog of CbbR, a LysR family transcriptional regulator of the CO2 fixation operons of chemoautotrophic and purple photosynthetic bacteria. Inactivation of sll0030, but not of another closely related cbbR homolog (sll1594), abolished low CO2 induction of cmp operon expression. Gel retardation assays showed specific binding of the Sll0030 protein to the sll0030-cmpA intergenic region, suggesting that the protein activates transcription of the cmp operon by interacting with its regulatory region. A cbbR homolog similar to sll0030 and sll1594 was cloned from Synechococcus sp. strain PCC 7942 and shown to be involved in the low CO2-induced activation of the cmp operon. We hence designated the Synechocystis sll0030 gene and the Synechococcus cbbR homolog cmpR. In the mutants of the cbbR homologs, upregulation of ribulose-1,5-bisphosphate carboxylase/oxygenase operon expression by CO2 limitation was either unaffected (strain PCC 6803) or enhanced (strain PCC 7942), suggesting existence of other low CO2-responsive transcriptional regulator(s) in cyanobacteria.
Cyanobacteria fix CO2 efficiently despite the low affinity and selectivity of their ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) for CO2, because they possess a CO2-concentrating mechanism (CCM) to elevate the CO2 concentration around the active site of Rubisco (11, 21). The CCM involves the abilities to actively transport HCO3− into the cell, to convert CO2 to HCO3− intracellularly, and to effectively convert HCO3− into CO2 in carboxysomes, the polyhedral inclusion bodies to which Rubisco is localized. It is supposed that the conversion of CO2 to HCO3− in the cytoplasm not only helps to maintain high intracellular HCO3− concentrations but also allows diffusion of CO2 from external medium into the cytoplasm (10). Biosynthesis of the components of CCM, including Rubisco, is supposed to be controlled by CO2 availability (11, 21); however, detailed studies on the transcriptional regulation of the CCM-related genes are yet to be performed, and the underlying molecular mechanism is unknown.
Among the CCM-related genes, the cmp operon of Synechococcus sp. strain PCC 7942, encoding a high-affinity bicarbonate transporter (12, 18), is known to be a typical low CO2-inducible transcription unit; the cmp operon mRNA and the CmpA protein, which is by far the most abundant protein among the proteins encoded by the operon, are undetectable in cells grown under high CO2 conditions (1 to 5%, vol/vol) and accumulate to a high level when the cells are transferred to CO2-limited growth conditions (17, 18). To initiate studies on the regulation of the CCM-related genes, we identified a gene involved in the induction of cmp operon expression. Since genome sequence information is available for Synechocystis sp. strain PCC 6803 (9), we first identified the cmp operon and its regulator in this strain and then cloned and characterized the corresponding regulatory gene in Synechococcus sp. strain PCC 7942. It is shown that a homolog of cbbR (designated cmpR) plays an essential role in the low CO2 induction of the cmp operon in the two strains of cyanobacteria. From the persistence of the low CO2-responsive activation of Rubisco operon expression in the cmpR mutants, the existence of another mechanism for low CO2-responsive gene activation in cyanobacteria is deduced.
MATERIALS AND METHODS
Strains and growth conditions.
Synechocystis sp. strain PCC 6803 and Synechococcus sp. strain PCC 7942, and mutants derived therefrom, were grown photoautotrophically under continuous illumination provided by fluorescent lamps (70 μmol of photons m−2 s−1) at 30°C. The medium used was prepared by supplementing a nitrogen-free medium, obtained by modification of BG11 medium (27) as previously described (28), with 15 mM KNO3. The medium was buffered with 20 mM HEPES-KOH (pH 8.0). When appropriate, kanamycin and spectinomycin were added to the medium at 15 μg ml−1. The cultures were routinely maintained under high CO2 conditions, i.e., aeration with 2% (vol/vol) CO2 in air. For induction of cmp operon transcription, cells grown to the mid-logarithmic phase of growth were collected by centrifugation at 5,000 × g for 5 min at 25°C, washed twice with growth medium by resuspension and recentrifugation, inoculated into fresh medium, and aerated with air containing ca. 0.005% (vol/vol) CO2 under the same general conditions as before.
Insertional mutagenesis of Synechocystis cbbR homologs.
A DNA fragment carrying the entire sll0030 coding region (nucleotides +1 to +937 with respect to the translation start site) was amplified by PCR using the Synechocystis chromosomal DNA as the template and cloned into pT7Blue T-Vector. The forward primer used carried two additional nucleotides at the 5′ end, which created a BspHI recognition sequence at the translational start site so that the cloned sll0030 gene can be excised and used for construction of a translational fusion with maltose-binding protein (see below). After verification of the nucleotide sequence, a spectinomycin resistance cassette, which had been excised from plasmid pRL463 (3) by digestion with XbaI, was inserted at the NheI site in the cloned sll0030 gene. Wild-type Synechocystis sp. strain PCC 6803 was transformed with the resulting plasmid to spectinomycin resistance through homologous recombination between the wild-type copy of sll0030 on the genome and the interrupted copy of the gene, which resulted in replacement of the former with the latter to give rise to an sll0030 insertional mutant (designated MR1) (Fig. 1B).
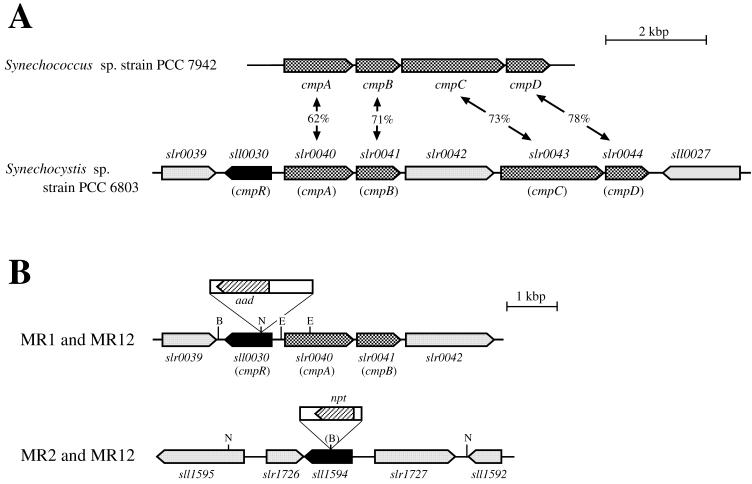
FIG. 1.
Gene organization in the cmp genomic region of Synechocystis sp. strain PCC 6803 and construction of insertional mutants of the cbbR homologs. (A) Map of the cmp region of the genome of Synechococcus sp. strain PCC 7942 and Synechocystis sp. strain PCC 6803, showing the extent of identity of the deduced amino acid sequences of the corresponding cmp genes. (B) Structure of the sll0030 genomic region of the MR1 and MR12 mutants and that of the sll1594 genomic region of the MR2 and MR12 mutants of Synechocystis sp. strain PCC 6803. The gene organization in Synechocystis was obtained from Cyanobase (http://www.kazusa.or.jp/cyano/cyano.html). The cmp genes are indicated by stippled bars, and the cbbR homologs (sll0030 and sll1594) are indicated by filled bars. Open bars represent the antibiotic resistance gene cassettes, with hatched bars showing the locations and orientations of the kanamycin resistance gene (npt) and the spectinomycin resistance gene (aad). Abbreviations for restriction endonuclease sites: B, BglII; E, EcoRV; N, NheI; D, DraI.
For construction of insertional mutants of sll1594, a DNA fragment carrying the entire sll1594 coding region (nucleotides −1 to +962 with respect to the translation start site) with a base substitution from T to A at position +462 was generated by overlap extension PCR using oligonucleotide primers carrying mismatches with the wild-type sequence (8). The base substitution created a BglII recognition sequence in the sll1594 coding region. After cloning of the DNA fragment into pT7Blue T-Vector and confirmation of the nucleotide sequence, a kanamycin resistance cassette excised from plasmid pUC4K (30) with BamHI was inserted into the engineered BglII site to interrupt the cloned sll1594 gene. The resulting plasmid was used to transform the wild-type Synechocystis strain and the MR1 mutant to kanamycin resistance through homologous recombination to obtain an sll1594 insertional mutant (MR2) and an sll0030 sll1594 double mutant (MR12), respectively (Fig. 1B).
Identification, nucleotide sequence analysis, and insertional mutagenesis of a Synechococcus cbbR homolog.
A 0.6-kbp fragment of a cbbR homolog was amplified from chromosomal DNA of Synechococcus sp. strain PCC 7942 by PCR, using degenerate oligonucleotides synthesized according to the amino acid sequences conserved in the two Synechocystis CbbR homologs encoded by sll0030 and sll1594: 5′-TT(CT)AC(CGT)A(AG)(AG)GC(AGCT)GC(AGCT) GA(AG)GA-3′ and 5′-TT(CT)AC(CGT)CG(AGCT)GC(AGCT)GC(AGCT)GA(AG)GA-3′ for FT(RK)AAEE (forward primer), and 5′-GC(CT)TG(CT)TT(AG)AT(ACGT)GC(CT)TC(AG)TT-3′ for NEAIKQA (reverse primer). For amplification of the DNA regions contiguous to the 0.6-kbp internal segment of the cbbR homolog, 1-μg aliquots of Synechococcus chromosomal DNA were digested with BamHI and NheI, respectively, the resulting fragments were circularized by self-ligation, and the circularized DNA fragments were used as the templates for inverse PCR. DNA fragments of 6 and 2.5 kbp were obtained from the BamHI and NheI digests, respectively, and used for determination of the nucleotide sequences of the 5′ and 3′ portions of the cbbR homolog and its 5′ and 3′ flanking regions.
For insertional interruption of the cbbR homolog in Synechococcus sp. strain PCC 7942, a DNA fragment carrying nucleotides −260 to +739 with respect to the translation start site was amplified by PCR and cloned into pT7Blue T-Vector. After confirmation of the nucleotide sequence, the spectinomycin resistance cartridge from pRL463 was inserted into the MscI site to interrupt the coding region. The resulting plasmid was used to transform the wild-type Synechococcus strain to spectinomycin resistance through homologous recombination to yield an insertional mutant (MR4) of the cbbR homolog.
Preparation of the Synechocystis CmpR protein and gel shift assay.
The PCR-amplified sll0030 gene cloned into pT7Blue T-Vector (see above) was excised from the plasmid with BspHI and BamHI and, after blunting of the termini, cloned into the SalI site in the polylinker of the expression vector pMAL-c2 (22). The resulting plasmid, designated pMR1, carried a chimeric gene encoding a translational fusion of the maltose-binding protein with CmpR. Cells of Escherichia coli NM522 transformants carrying pMR1 were grown in Luria-Bertani medium. Expression of the chimeric gene was induced by 1 mM isopropyl-B-d-thiogalactopyranoside (IPTG), and the recombinant protein was purified on amylose resin (22). The purified fusion protein was cleaved with factor Xa and used for gel retardation assays.
The DNA fragment used as the probe, carrying the entire intergenic region between cmpA and cmpR (nucleotides −252 to −1 with respect to the cmpA start codon), was labeled at both termini with 32P, using the Klenow fragment of DNA polymerase I and [α-32P]dCTP. Gel retardation assays were performed essentially as described by Buratowski and Chodosh (2). Gels containing 4% polyacrylamide were used for separation of the free probe and the protein-DNA complexes. Gels were dried, and the signals were detected by autoradiography.
Isolation and analysis of DNA and RNA.
Chromosomal DNA was extracted and purified from Synechocystis sp. strain PCC 6803 and Synechococcus sp. strain PCC 7942 cells as described by Williams (31). Manipulations and analyses of DNA were performed according to standard protocols (24). For Southern hybridization analysis of the genomic DNA digests, the following 32P-labeled double-stranded DNA probes were used: a 0.92-kbp entire coding region of sll0030; a 0.95-kbp entire coding region of sll1594; a 0.59-kbp HincII fragment of Synechococcus cmpR; a 2-kbp spectinomycin resistance cassette; and a 1.3-kbp kanamycin resistance cassette. Total RNA was extracted and purified from the cyanobacterial cells by the method of Aiba et al. (1). For Northern hybridization analysis, RNA samples (10 μg per lane) were denatured by treatment with formaldehyde, fractionated by electrophoresis in 1.2% agarose gels that contained formaldehyde, transferred to positively charged nylon membranes (Hybond N+; Amersham), and hybridized with the following gene-specific probes: a 737-bp PCR-amplified rbcL fragment of strain PCC 6803, carrying bases +50 to +786 of the coding region; a 3.8-kbp PCR-amplified fragment of strain PCC 6803 DNA, carrying 1,147 bases of cmpA, the entire cmpB gene, and 1,704 bp of slr0042 (bases +213 to +4079 with respect to the translation start site of cmpA); a 0.71-kbp SalI-SphI fragment of cmpC of strain PCC 7942; a 17-mer oligonucleotide complementary to bases +1566 through +1582 of the rbcL coding region of strain PCC 7942; and a 288-bp PCR-amplified ccmK fragment of strain PCC 7942, carrying bases +11 to +298 of the coding region. The double-stranded DNA probes were labeled with 32P as described by Feinberg and Vogelstein (4). The oligonucleotide probe was labeled with 32P, using [γ-32P]ATP and T4 polynucleotide kinase. The radioactivity of the probes that hybridized to mRNA was quantified with a Bio-Image analyzer (Fuji Photo Film).
Transformation of cyanobacteria.
Transformation of Synechocystis and Synechococcus was performed as described by Williams (31). The transformants were allowed to grow on solid medium supplemented with kanamycin and/or spectinomycin at 15 μg ml−1 (see above). After three serial streak purifications to segregate homozygous mutants (31), genomic DNA was isolated from the selected clones and analyzed by Southern hybridization to confirm the presence and position of the antibiotic resistance gene.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases with accession number AB047379.
RESULTS
Construction of Synechocystis mutants lacking the cbbR homologs.
The cmpA, cmpB, cmpC, and cmpD genes of Synechococcus sp. strain PCC 7942, encoding the high-affinity bicarbonate transporter BCT1, form a low CO2-inducible operon (18). These genes are similar, respectively, to the nrtA, nrtB, nrtC, and nrtD genes of the same cyanobacterium, which encode the nitrate/nitrite transporter (15, 16). Among the genes of Synechocystis sp. strain PCC 6803 (9), slr0040, slr0041, slr0043, and slr0044 are the most similar to cmpA, cmpB, cmpC, and cmpD, respectively (Fig. 1A). They are more similar to the cmp genes than to the nrt genes of Synechococcus, although they were initially designated “nrt” genes in Cyanobase (http: //www.kazusa.or.jp/cyano/cyano.html). On the other hand, another set of Synechocystis “nrt” genes in Cyanobase, sll1450, sll1451, sll1452, and sll1453, having the second-best similarities to the cmp genes among the Synechocystis genes, are more similar to the nrt genes than to the cmp genes of Synechococcus and are in fact involved in nitrate/nitrite uptake (M. Kobayashi and T. Omata, unpublished results). Northern hybridization analysis showed that slr0040, slr0041, slr0043, and slr0044 constitute a low CO2-inducible operon (see below) with a putative porin gene, slr0042 (6). On the basis of these observations, we have identified slr0040, slr0041, slr0043, and slr0044 as the cmpA, cmpB, cmpC, and cmpD genes, respectively, of the Synechocystis strain. While there is no potential protein-coding region within 700 bases upstream from the cmp operon of Synechococcus (11), a gene (sll0030) coding for a protein 31 to 51% identical to bacterial CbbR (RbcR) proteins is located 253 bases upstream of the Synechocystis cmp operon, being oriented divergently from the cmp operon (Fig. 1A). CbbR is a LysR family protein involved in transcriptional activation of the Rubisco operon in chemoautotrophic and phototrophic bacteria (5, 26). Since many of the LysR family proteins activate the divergently transcribed operon located upstream (7, 25), sll0030 was likely to be involved in activation of the cmp operon in Synechocystis.
To determine the role of sll0030, we constructed an insertional mutant of this gene. Since the Synechocystis genome contains two other cbbR homologs, sll1594 and sll0998, encoding proteins 53 and 33% identical to the Sll0030 protein, respectively, insertional mutagenesis was attempted also for these two genes. In the case of sll0030 and sll1594, the insertionally inactivated genes segregated to give rise to homozygous mutants MR1 and MR2 (Fig. 1B), respectively, as determined by Southern hybridization analysis (data not shown). By inactivating sll1594 in MR1, an sll0030 sll1594 double mutant MR12 was also constructed (Fig. 1B). Since segregation of the mutant genome, carrying the interrupted copy of the gene, was not attained for sll0998 (data not shown), the mutants of sll0030 and sll1594 (MR1, MR2, and MR12) were further characterized.
Under photoautotrophic conditions, the three cbbR mutants grew slightly more slowly than the wild-type strain in the presence of both high (2%) and low (0.035%) CO2 (data not shown). The final cell density in the mutant cultures was close to that in the cultures of the wild-type strain, indicating that sll0030 and sll1594 are not essential for growth of Synechocystis under the given conditions.
Activation of the cmp operon in the Synechocystis mutants.
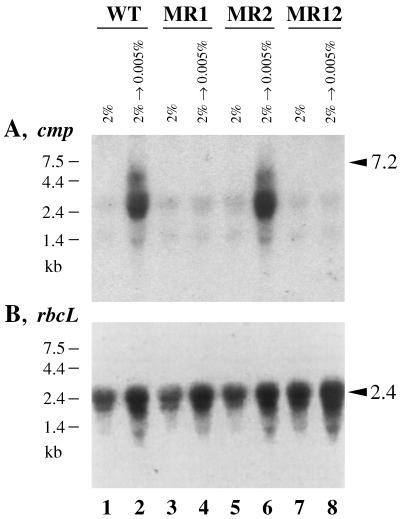
Figure 2 shows the effects of CO2 conditions on transcription of the cmp operon and rbc operon in the wild-type and mutant Synechocystis strains. In the case of the wild-type strain, transfer of the cells to low CO2 conditions induced accumulation of the cmp operon transcript (Fig. 2A, lanes 1 and 2). The hybridization profile showed a smeary signal ranging from 2 to 7 kb in size, indicating that the primary transcript is rapidly turned over. The low CO2-induced accumulation of the cmp transcript was abolished in the MR1 and MR12 mutants lacking sll0030 (lanes 3, 4, 7, and 8), but the MR2 mutant, which is defective solely in sll1594, showed normal low CO2-induced accumulation of the cmp operon transcript (lanes 5 and 6). These results indicated that sll0030 but not sll1594 is essential for the low CO2-responsive activation of the cmp operon in Synechocystis. On the basis of these results, we named sll0030 as cmpR.
FIG. 2.
Northern blot analysis of total RNA from the wild-type strain (WT; lanes 1 and 2), the sll0030 insertional mutant (MR1; lanes 3 and 4), the sll1594 insertional mutant (MR2; lanes 5 and 6), and the sll0030 sll1594 double mutant (R12; lanes 7 and 8) of Synechocystis sp. strain PCC 6803, showing the effects of CO2 conditions on expression of the cmp and rbc operons. Cells were grown under high CO2 conditions (2% CO2 in air) and transferred to low CO2 conditions (0.005% CO2 in air); total RNA samples (10 μg per lane) were extracted from the cells before (lanes 1, 3, 5, and 7) and 90 min after (lanes 2, 4, 6, and 8) the transfer and analyzed, using probes specific to cmpA-cmpB-slr0042 (A) and rbcL (B). Arrowheads indicate calculated sizes (in kilobases) of the full-length mRNA of the cmp operon (A) and the rbc operon (B).
Transfer of the wild-type Synechocystis cells to low CO2 conditions also caused an increase in the abundance of the mRNA for the rbcLXS (slr0009-slr0011-slr0012) operon (Fig. 2B, lanes 1 and 2). The extent of the increase in the mRNA abundance was 60 to 70% in three separate experiments (data not shown). The low CO2-induced increase in the rbcLXS mRNA abundance was observed also in the sll0030 and sll1594 mutants (lanes 3 to 8; 60 to 70% increase in MR1 and MR2 and 55 to 80% increase in MR12 in three separate experiments). Thus, neither gene was likely to be involved in regulation of rbcLXS expression.
Gel shift assay.
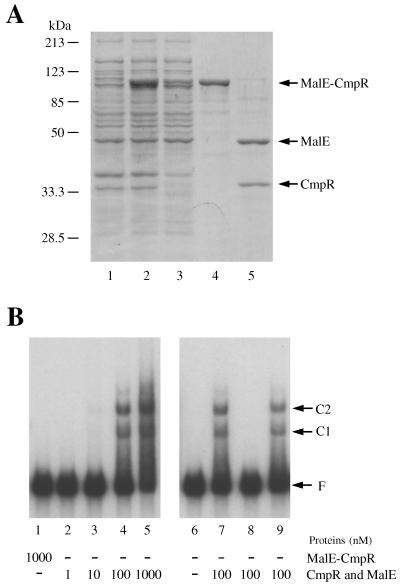
To examine whether CmpR binds to the cmpA operon regulatory region, gel shift assays were performed with CmpR, using a DNA fragment carrying the cmpR-cmpA intergenic region as the probe. Since His6-tagged CmpR was insoluble and could not be easily solubilized, CmpR was expressed in a soluble form as a MalE-CmpR fusion in E. coli (Fig. 3A, lanes 1 and 2), purified to near homogeneity (lanes 3 and 4), cleaved from MalE with factor Xa (lane 5), and used for the experiments (Fig. 3B). While addition of the MalE-CmpR fusion up to a concentration of 1 μM did not affect the electrophoretic mobility of the DNA probe (Fig. 3B, lane 1), addition of the CmpR-MalE mixture to give CmpR and MalE concentrations higher than 10 nM yielded two retarded bands representing DNA-protein complexes (lanes 3, 4, 5, and 7). Addition of an excess amount of cold DNA probe prevented binding of the labeled probe to the protein (lane 8), but addition of a nonhomologous DNA fragment had no effect thereon (lane 9). No change in mobility of the DNA probe was observed when a MalE-LacZα mixture, obtained by cleavage of the MalE-LacZα fusion expressed from the vector (pMAL-c2), was used in place of the MalE-CmpR mixture for the experiments (data not shown). These results showed that the CmpR protein specifically binds to the cmpR-cmpA intergenic region. Thus, CmpR is likely to activate transcription of the target operon by interacting with its promoter region, as other LysR-type transcription activators do (25).
FIG. 3.
Preparation of recombinant CmpR and the mobility shift assays. (A) Expression in E. coli and purification of the MalE-CmpR fusion (lanes 1 to 4) and cleavage of the MalE-CmpR fusion with factor Xa (lane 5). Proteins were separated on a sodium dodecyl sulfate–10% polyacrylamide gel and stained with Coomassie brilliant blue. Lane 1, total protein from the E. coli expression strain before IPTG treatment; lane 2, total protein from the expression strain after 2-h treatment with IPTG; lane 3, soluble fraction from the IPTG-induced expression strain; lane 4, the protein purified on amylose resin; and lane 5, the MalE-CmpR fusion cleaved with factor Xa. (B) Mobility shift assays showing retardation in a 4% polyacrylamide gel of the 32P-labeled cmpR-cmpA intergenic segment by CmpR. Samples of the MalE-CmpR fusion (lane 1) and the MalE-CmpR fusion cleaved with factor Xa (lane 2 to 9) were added to the reaction mixtures to give the indicated final concentrations; 100-fold-excess amounts of nonlabeled cmpR-cmpA intergenic segment and a 0.7-kb segment of rbcL coding region were added to lanes 8 and 9, respectively, as competitors. C1 and C2, DNA-protein complexes; F, free probe.
Identification and nucleotide sequence determination of a cbbR homolog of Synechococcus sp. strain PCC 7942.
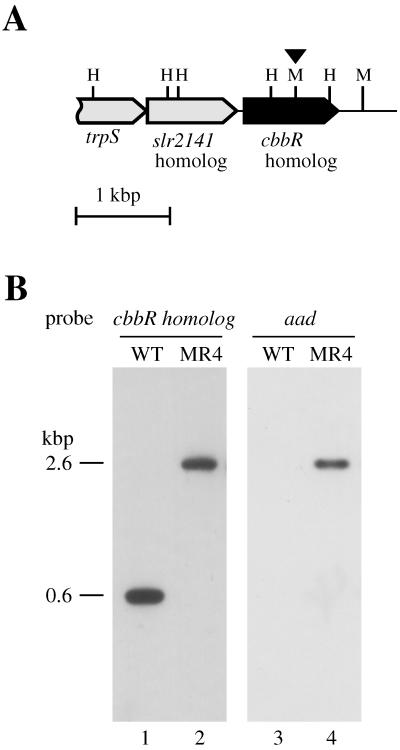
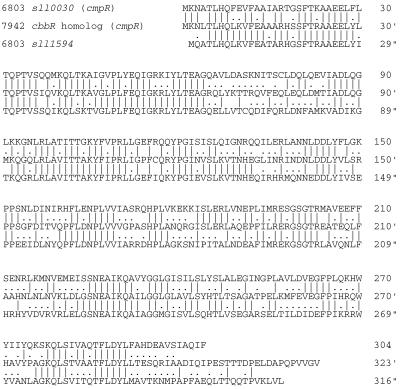
Although functionally related genes are usually clustered on the genome of Synechococcus sp. strain PCC 7942, no cmpR-like gene was found in the region upstream of its cmp operon (Fig. 1A). This raised a question as to whether a cbbR homolog is involved in regulation of the cmp operon in this strain of cyanobacterium. Using degenerate oligonucleotides synthesized according to the amino acid sequences conserved in the proteins encoded by sll0030 and sll1594 (see Materials and Methods), a 0.6-kbp fragment was amplified from chromosomal DNA of Synechococcus sp. strain PCC 7942 by PCR and shown to encode a CbbR homolog, as confirmed by cloning and nucleotide sequence determination, verifying the existence of a cbbR-like gene in Synechococcus sp. strain PCC 7942. Using the set of primers, only one species of cbbR homolog was amplified. A 2.5-kbp NheI fragment and a 6-kbp BamHI fragment of Synechococcus DNA, carrying the regions contiguous to the PCR-amplified 0.6-kb segment of the cbbR homolog, were subsequently amplified by inverse PCR and used for nucleotide sequence determination of the entire cbbR homolog and its flanking regions. Figure 4A shows the map of the DNA region around the Synechococcus cbbR homolog thus obtained. A homolog of slr2141, a Synechocystis gene of unknown function, was found to be located 71 bases upstream from the cbbR homolog. The open reading frame located farther upstream, which overlaps the slr2141 homolog by four bases, was partially sequenced from its 3′ end and tentatively identified as a tryptophanyl-tRNA synthetase gene (trpS) of Synechococcus sp. strain PCC 7942, because the encoded amino acid sequence was 73% identical to the C-terminal portion of tryptophanyl-tRNA synthetase from Synechocystis sp. strain PCC 6803. There was no potential protein-coding region within 600 bases downstream from the cbbR homolog. These results suggested that the cbbR homolog is the last gene of an operon. The organization of genes around the Synechococcus cbbR homolog is thus totally different from those in Synechocystis (Fig. 1B). The open reading frame of the cbbR homolog starts with a GTG codon and encodes a protein of 323 amino acids (see entry AB047379 in DDBJ, EMBL, and GenBank nucleotide sequence databases) which is 54.2 and 59.4% identical to the proteins encoded by sll0030 and sll1594, respectively (Fig. 5).
FIG. 4.
(A) Map of the genomic region of the cbbR homolog in Synechococcus sp. strain PCC 7942. The filled bar represents the Synechococcus cbbR homolog. The triangle above the map indicates the MscI site where a 2.0-kbp gene cassette carrying the spectinomycin resistance gene (aad) was inserted to construct the MR4 mutant. Abbreviations for restriction endonuclease sites: H, HincII; M, MscI. (B) Southern blot analysis of DNA from the wild-type strain (WT; lanes 1 and 3) and the MR4 mutant (lanes 2 and 4) of Synechococcus sp. strain PCC 7942. DNA samples (5 μg per lane) were digested with HincII, fractionated on a 0.7% agarose gel, transferred to a positively charged nylon membrane (Hybond N+; Amersham), and hybridized with the 32P-labeled gene-specific probes as indicated.
FIG. 5.
Alignment of the deduced amino acid sequence of the cbbR homolog of Synechococcus sp. strain PCC 7942 with those of the proteins encoded by sll0030 (cmpR) and sll1594 of Synechocystis sp. strain PCC 6803. The alignments were optimized by the FASTA program (19). Vertical lines indicate aligned and identical amino acid residues between adjacent sequences; dots indicate conservative replacements of amino acid residues.
Functional analysis of the cbbR homolog of Synechococcus.
To determine whether the Synechococcus cbbR homolog is involved in regulation of the cmp operon, a mutant (MR4) was constructed by inserting a spectinomycin resistance cassette in the gene (Fig. 4). Similar to the case of Synechocystis, a homozygous mutant lacking the wild-type gene was obtained (Fig. 4B). The mutant grew as fast as the wild-type strain under both high (2%) and low (0.035%) CO2 conditions (data not shown), indicating that the cbbR homolog of Synechococcus is not essential for growth of the cyanobacterium under the given conditions.
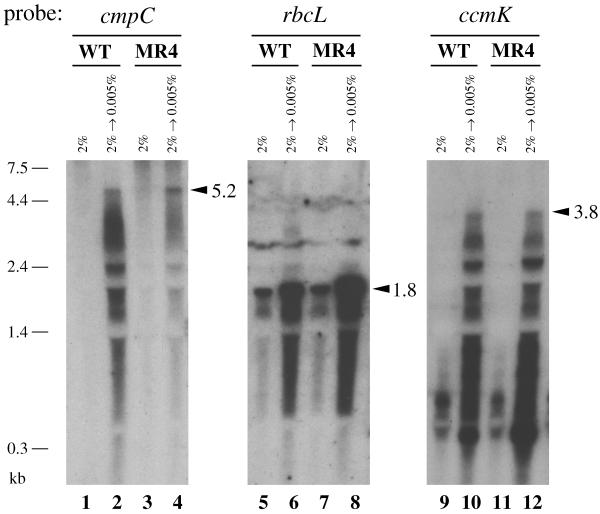
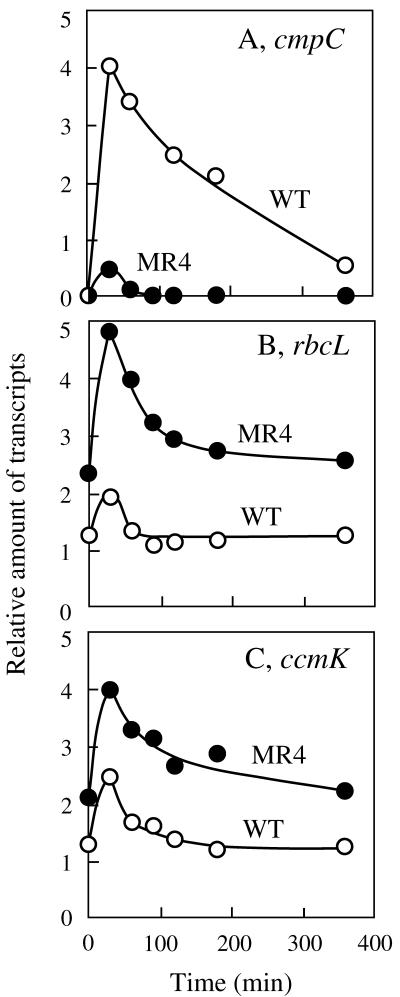
Figure 6 compares the effects of CO2 conditions on expression of the three transcription units of Synechococcus, i.e., the cmp operon, the rbc operon, and the carboxysome-related gene cluster ccmKLMNO, in the wild-type strain and the MR4 mutant. In the case of the wild-type strain, transfer of the cells to low CO2 conditions induced accumulation of the cmp operon transcript as previously shown (18) (lanes 1 and 2). The mRNA from the rbcLS operon was detected in high CO2-grown cells, and its abundance was increased by transfer of the cells to low CO2 conditions (lanes 5 and 6). While only low-molecular-size signals of <1.4 kb were detected with the ccmK-specific probe in the mRNA samples from high CO2-grown cells (lane 9), CO2 limitation caused accumulation of abundance of larger signals (lane 10). The maximum size of the hybridization signal, 3.8 kb (lane 10), corresponded to the entire size of the ccmKLMNO gene cluster, verifying that the five genes are transcribed as an operon at least under the CO2-limited conditions. Unlike the cmpR mutants (MR1 and MR12) of Synechocystis, the Synechococcus MR4 mutant accumulated the cmp operon transcript in response to CO2 limitation (compare lanes 3 and 4), but the level of the transcript was much lower than that in the wild-type strain (lane 2). On the other hand, the abundance of the transcripts from the rbc operon and the ccm operon was greater in the mutant than in the wild-type strain after induction (compare lanes 6 and 10 with lanes 8 and 12, respectively). The extent of low CO2-responsive increase in levels of the cmp, rbc, and ccm transcripts was variable in different experiments, but that of the rbc and ccm transcripts was always larger in the MR4 mutant than in the wild-type strain (Fig. 7B and C), and the maximum level of cmp operon transcript in the mutant was insignificant compared to that in the wild-type strain (Fig. 7A), being 10 to 25% of the wild-type level. It should also be noted that the mutant often accumulated higher levels of rbc and ccm operon transcripts than the wild-type strain even under the high CO2 conditions (Fig. 7B and C). The specific reduction in the level of cmp operon expression in the MR4 mutant indicated that the cbbR homolog of Synechococcus is involved in activation of the cmp operon. We therefore identified this gene as the cmpR gene of Synechococcus sp. strain PCC 7942.
FIG. 6.
Northern blot analysis of total RNA from the wild-type strain (WT; lanes 1, 2, 5, 6, 9, and 10) and the insertional mutant of the cbbR homolog (MR4, lanes 3, 4, 7, 8, 11, and 12) of Synechococcus sp. strain PCC 7942, showing the effects of CO2 conditions on expression of the cmp, rbc, and ccm operons. Synechococcus cells were grown under high CO2 conditions (2% CO2 in air) and transferred to low CO2 conditions (0.005% CO2 in air), and total RNA was extracted from the cells before (lanes 1, 3, 5, 7, 9, and 11) and 30 min after (lanes 2, 4, 6, 8, 10, and 12) the transfer. RNA samples (10 μg per lane) were denatured, fractionated by electrophoresis, transferred to positively charged nylon membranes, and hybridized with probes specific to cmpC (lanes 1 to 4), rbcL (lanes 5 to 8), and ccmK (lanes 9 to 12). Arrowheads indicate calculated sizes (in kilobases) of the full-length mRNA of the cmp (A), rbc (B), and ccm (C) operons.
FIG. 7.
Changes in relative abundance of the cmpC, rbcL, and ccmK transcripts after transfer of the wild-type strain and the cmpR insertional mutant (MR4) of Synechococcus sp. strain PCC 7942 from high (2%) to low (0.005%) CO2 conditions. Results of the dot hybridization analysis with 2.5 μg of RNA per dot were quantified and plotted. A representative of three sets of essentially the same results, obtained with three independent sets of cultures, is shown.
DISCUSSION
The CbbR protein of chemoautotrophic and photosynthetic bacteria is required for activation of the operons encoding the enzymes of the Calvin-Benson-Bassham (CBB) cycle (5, 26). As is usually the case with the LysR family transcription regulator proteins (7, 25), the genes encoding CbbR are located immediately upstream of their target operons (5). While a single cbbR gene is present in most of these bacteria (5), the genome sequencing project of the cyanobacterium Synechocystis sp. strain PCC 6803 revealed the presence of three genes coding for CbbR-like proteins, two of which (sll0030 and sll1594) are more similar to the cbbR genes of chemoautotrophic and phototrophic bacteria (31 to 54% identity at the amino acid sequence level) than the third one (sll0998) is (25 to 33% identity) (9). None of the cyanobacterial cbbR homologs is, however, clustered with other cbb genes, and their functions remain to be determined. The present results indicate that the sll0030 gene product activates the divergently transcribed operon located upstream, encoding the bicarbonate transporter, presumably by binding to its regulatory region (Fig. 3). Identification of a Synechococcus cbbR homolog involved in activation of the cmp operon (Fig. 4 to 7) suggests that cyanobacteria commonly utilize a CbbR homolog for the low CO2 induction of the bicarbonate transporter operon. Since the transporter is not a component of the CBB cycle, we have named the sll0030 gene and the cbbR homolog of Synechococcus sp. strain PCC 7942 as cmpR.
Rubisco activity (20) and the cellular carboxysome content (14, 29) have been shown to increase during adaptation of Synechococcus strains to low CO2 conditions, and elevated expression of the rbcLS and ccmNO genes following transition from high to low CO2 conditions has been reported in strain PCC 7942 (23). The present results confirm the latter observation and further show that the ccmKLMNO genes form an operon (Fig. 6). The upregulation of the rbcLS and ccmKLMNO operons was not abolished by inactivation of cmpR but rather enhanced by the mutation (Fig. 6 and 7). Low CO2-induced increase in the abundance of rbcLXS mRNA was observed also in Synechocystis sp. strain PCC 6803 and was found to be unaffected by the mutation of cmpR and sll1594 (Fig. 2). These findings suggest the presence of a cmpR-independent (and, in Synechocystis, sll1594-independent as well) mechanism for low CO2-induced activation of the rbc and ccm operons. The transcriptional regulator(s) involved in this process is not known, but the third cbbR homolog of Synechocystis sp. strain PCC 6803 (sll0998), which could not be completely deleted from the cell, is a good candidate for the regulator, because its close homolog has been found not only in the cyanobacteria Synechococcus sp. strain PCC 7942 (78% identity at the amino acid sequence level [T. Omata and S. Gohta, unpublished results, GenBank accession no. AB053349]), Anabaena sp. strain PCC 7120 (82% identity [Cyanobase]), Nostoc punctiforme (83% identity [http://www.jgi.doe.gov/JGI_microbial/html/nostoc_homepage.html]), and Synechococcus sp. strain WH8102 (64% identity [http://www.jgi.doe.gov/JGI_microbial/html/synechococcus.html]) but also in cyanelle and chloroplast DNA from all known eukaryotes having rbcS in an operon with rbcL (51 to 72% identity) (summarized in reference 13; see GenBank entry NC001675 for the cyanelle sequence). Much more work is required to obtain a comprehensive view of the mechanism of low CO2-induced gene regulation in cyanobacteria.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid for Scientific Research (09640768) and Grants-in-Aid for Scientific Research in Priority Areas (09274101 and 09274103) to T.O. from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 2.Buratowski S, Chodosh L A. Mobility shift DNA-binding assay using gel electrophoresis. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: Greene Publishing and Wiley-Interscience; 1996. p. 12.2.1-12.2.11. [Google Scholar]
- 3.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg A, Vogelstein B. A technique for radiolabeling DNA restriction endonulease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 5.Gibson J L, Tabita F R. The molecular regulation of the reductive pentose phosphate pathway in proteobacteria and cyanobacteria. Arch Microbiol. 1996;166:141–150. doi: 10.1007/s002030050369. [DOI] [PubMed] [Google Scholar]
- 6.Hansel A, Pattus F, Jürgens U J, Tadros M H. Cloning and characterization of the genes coding for two porins in the unicellular cyanobacterium Synechococcus PCC 6301. Biochim Biophys Acta. 1998;1399:31–39. doi: 10.1016/s0167-4781(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 7.Henikoff S, Haughn G W, Calvo J M, Wallace J C. A large family of bacterial activator proteins. Proc Natl Acad Sci USA. 1988;85:6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan A, Reinhold L. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:539–570. doi: 10.1146/annurev.arplant.50.1.539. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan A, Schwarz R, Lieman-Hurwitz J, Ronen-Tarazi M, Reinhold L. Physiological and molecular studies on the response of cyanobacteria to changes in the ambient inorganic carbon concentration. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 469–485. [Google Scholar]
- 12.Maeda S-I, Price G D, Badger M R, Enomoto C, Omata T. Bicarbonate binding activity of the CmpA protein of the cyanobacterium Synechococcus sp. strain PCC 7942 involved in active transport of bicarbonate. J Biol Chem. 2000;275:20551–20555. doi: 10.1074/jbc.M003034200. [DOI] [PubMed] [Google Scholar]
- 13.Maier U-G, Fraunholz M, Zauner S, Penny S, Douglas S. A nucleomorph-encoded CbbX and the phylogeny of Rubisco regulators. Mol Biol Evol. 2000;17:576–583. doi: 10.1093/oxfordjournals.molbev.a026337. [DOI] [PubMed] [Google Scholar]
- 14.McKay R M L, Gibbs S P, Espie G S. Effect of dissolved inorganic carbon on the expression of carboxysomes, localization of Rubisco and the mode of inorganic carbon transport in cells of the cyanobacterium Synechococcus UTEX 625. Arch Microbiol. 1993;159:21–29. [Google Scholar]
- 15.Omata T. Cloning and characterization of the nrtA gene that encodes a 45-kDa protein involved in nitrate transport in the cyanobacterium Synechococcus PCC 7942. Plant Cell Physiol. 1991;32:151–157. [Google Scholar]
- 16.Omata T, Andriesse X, Hirano A. Identification and characterization of a gene cluster involved in nitrate transport in the cyanobacterium Synechococcus sp. PCC 7942. Mol Gen Genet. 1993;236:193–202. doi: 10.1007/BF00277112. [DOI] [PubMed] [Google Scholar]
- 17.Omata T, Ogawa T. Biosynthesis of a 42-kD polypeptide in the cytoplasmic membrane of the cyanobacterium Anacystis nidulans strain R2 during adaptation to low CO2 concentration. Plant Physiol (Rockville) 1986;80:525–530. doi: 10.1104/pp.80.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omata T, Price G D, Badger M R, Okamura M, Gohta S, Ogawa T. Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc Natl Acad Sci USA. 1999;96:13571–13576. doi: 10.1073/pnas.96.23.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price G D, Coleman J R, Badger M R. Association of carbonic anhydrase activity with carboxysomes isolated from the cyanobacterium Synechococcus PCC7942. Plant Physiol. 1992;100:784–793. doi: 10.1104/pp.100.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price G D, Sültemeyer D, Klughammer B, Ludwig M, Badger M R. The functioning of the CO2 concentrating mechanism in several cyanobacterial strains: a review of general physiological characteristics, genes, proteins, and recent advances. Can J Bot. 1998;76:973–1002. [Google Scholar]
- 22.Riggs P. Expression and purification of maltose-binding protein fusion. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: Greene Publishing and Wiley-Interscience; 1994. p. 16.6.1-16.6.14. [Google Scholar]
- 23.Ronen-Tarazi M, Schwarz R, Bouevich A, Lieman-Hurwitz J, Erez J, Kaplan A. Response of photosynthetic microorganisms to changing ambient concentration of CO2. In: Joint I, editor. Molecular ecology of aquatic microbes. NATO ASI Series vol. G38. Berlin, Germany: Springer-Verlag; 1995. pp. 323–334. [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 26.Shively J M, van Keulen G, Meijer W G. Something from almost nothing: carbon dioxide fixation in chemoautotrophs. Annu Rev Microbiol. 1998;52:191–230. doi: 10.1146/annurev.micro.52.1.191. [DOI] [PubMed] [Google Scholar]
- 27.Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki I, Kikuchi H, Nakanishi S, Fujita Y, Sugiyama T, Omata T. A novel nitrite reductase gene from the cyanobacterium Plectonema boryanum. J Bacteriol. 1995;177:6137–6143. doi: 10.1128/jb.177.21.6137-6143.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turpin D H, Miller A G, Canvin D T. Carboxysome content of Synechococcus leopoliensis (Cyanophyta) in response to inorganic carbon. J Phycol. 1984;20:249–253. [Google Scholar]
- 30.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 31.Williams J G K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988;167:766–778. [Google Scholar]